Abstract
The differential diagnosis of renal and supra-renal masses firstly depends on the age of the child. Neuroblastoma (NBL) may be seen antenatally or in the newborn period; this tumour has a good prognosis unlike NBL seen in older children (particularly NBL in those aged 2–4 years). Benign renal masses predominate in early infancy but beyond the first year of life Wilms' tumour is the most common renal malignancy, until adolescence when renal cell carcinoma has similar or increased frequency as children get older. Adrenal adenomas and carcinomas also occur in childhood; these tumours are indistinguishable on imaging but criteria for the diagnosis of adrenal carcinoma include size larger than 5 cm, a tendency to invade the inferior vena cava and to metastasise. The most topical dilemmas in the radiological assessment of renal and adrenal tumours are presented. Topics covered include a proposed revision to the staging of NBL, the problems inherent in distinguishing nephrogenic rests from Wilms' tumour and the current recently altered approach regarding small lung nodules in children with Wilms' tumour.
Keywords: Paediatric; neuroblastoma, Wilms' tumour, nephroblastoma, adrenal carcinoma, adrenal adenoma, mesoblastic nephroma, phaeochromocytoma imaging
Neuroblastoma
Neuroblastoma (NBL) along with ganglioneuroblastoma and ganglioneuroma constitute a group of ganglion cell origin tumours that originate from primordial neural crest cells, which are the precursors of the sympathetic nervous system[1]. The degree of malignancy is designated by the degree of cellular and extra-cellular maturation of these tumours. The most undifferentiated and aggressive NBL presents in young children (median age ≤2 years). The more mature tumour type is ganglioneuroma which affects older age groups. NBL accounts for 8–10% of childhood cancers. The two main prognostic factors are age and stage of disease at presentation[2]. Localised NBL and those arising in infants have a 90% survival rate except in cases with Myc-N amplification where survival is below 30%[2,3]. Approximately 50% of NBL occurring in children older than 18 months of age are metastatic at diagnosis.
NBL is the second most common abdominal neoplasm in children following Wilms' tumour and overall the third most common paediatric malignancy, after leukaemia and central nervous system tumours[4]. It accounts for almost 15% of childhood cancer fatalities[5], a number that reflects its aggressive nature and frequency of metastatic disease. Most NBL deaths occur within 2 years of diagnosis. More than 90% of the diagnosed cases are children aged less than 5 years. NBL protocols in Europe come under the new SIOPEN-R-NET (Research-Network), which is attempting the remote uploading of DICOM files onto a server for later central review. Infant NBL, localised unresectable NBL and high-risk (metastatic or MycN positive tumours) studies are currently in progress.
NBLs arise from the adrenal glands, the organ of Zuckerkandl or follow the distribution of the sympathetic ganglia along para-spinal areas from the neck to the pelvis. The most common primary site for NBL development is the retroperitoneum, the adrenal medulla (35%) and the extra-adrenal paraspinal ganglia (30–35%), followed by the mediastinum in 20%[1]. Less common sites are the pelvis (2–3%) and the neck (1–5%). Occasionally, in the presence of metastatic disease, no definite primary tumour can be found.
Clinical features
The most common (30–50%) malignancy encountered in the first month of life is NBL. The majority of children with NBL however, present between 1 and 5 years of age with a palpable abdominal mass. This may be an incidental finding in an otherwise healthy child or in a child clearly unwell (from widespread dissemination of tumour). NBL has overall a wide spectrum of clinical symptoms which depend on the site, extent and the biological features of the primary tumour, and the presence of distant metastatic disease. In half of the patients with intra-spinal tumour extension, peripheral neurologic deficits and neurological symptoms from compression of the nerve roots or the cord may be present[4,5]. Subcutaneous dark purple or blue metastatic nodules, the so-called “blueberry muffin” skin, may be visible. Metastatic involvement of the periorbital bones and soft tissues results in ecchymosed orbital proptosis, which is also described as “raccoon eyes” and may be misinterpreted as non-accidental injury. Compression of the optic nerves by metastases may cause blindness. Massive hepatic metastases can cause increased intra-abdominal pressure and death from respiratory insufficiency. Bilateral cystic NBL and acute intracystic haemorrhage in infancy have also been reported. Encephalopathic symptoms may be encountered. Cervical NBLs usually manifest as an isolated neck mass, stridor, or dysphagia. Horner's syndrome may be present at presentation or develop post-operatively from disruption of the sympathetic chain in the neck[4]. In less than 2%, NBLs can present with para-neoplastic syndromes: opsoclonus-myoclonus-ataxia syndrome or watery diarrhoea[5]. NBLs may also be discovered incidentally during scanning for other reasons, e.g. antenatal ultrasound, chest radiograph for pneumonia or screening protocols.
Infant screening
Screening with measurements of urinary vanillylmandelic acid (VMA) and homovanillic acid (HVA) levels in 6-month-old infants began in Japan in 1973[6]. Unfortunately, epidemiologic analyses showed that this did not alter the incidence of tumour in older children nor did it improve cure rates. However, it was soon realised that the incidence of stage 1 disease dramatically increased, and that the tumours discovered at screening were of low stage and favourable histological characteristics. It is assumed that similar numbers of NBL tumours occur in unscreened infants most of which regress spontaneously. Consequently, widespread screening in other countries has not been adopted and screening for NBL has been discontinued in Japan. The impact of screening in children >1 year of age, however, remains unknown[4].
Staging
The formal clinical staging system for NBL, the International Neuroblastoma Staging System (INSS), was framed from a consensus international group in 1986 and revised in 1993 (Table 1). It is widely accepted, uses the pattern of disease spread as determined by radiographic and scintigraphic studies, surgical operability, lymph node and bone marrow involvement, and is useful for tumour prognostication and comparison of treatment results. The new so-called Whistler criteria for International NBL Risk Grouping (INRG) may nevertheless replace the standard INSS staging for NBL in childhood. These modifications, currently being finalised, will categorise NBL into low, intermediate and high risk groups. High risk groups will include metastatic tumours (other than infantile 4S disease) and all Myc-N amplified tumours irrespective of age. The age cut-off for 4S disease will probably be increased to 18 months. Risk grouping will be dependent on pre-operative imaging rather than post-surgical staging (H. Brisse, personal communication). Invasive or infiltrative localised tumour will be designated as L2 (Fig. 1). Locoregional tumour not invading adjacent organs will be classified as L1 disease.
Table 1.
International Neuroblastoma Staging System (INSS)
| Stage | Description |
|---|---|
| 1 | Localised tumour, complete gross excision; negative representative regional lymph nodes |
| 2A | Localised tumour, incomplete gross excision; identifiable lymph nodes negative (ipsi- and contralateral) |
| 2B | Localised tumour, complete or incomplete gross excision; ipsilateral positive regional lymph nodes, contralateral negative lymph nodes |
| 3 | Unresectable tumour infiltrating across the midline, with or without regional lymph node involvement OR midline tumour with bilateral extension by infiltration or by lymph node involvement |
| 4 | Any primary tumour with dissemination to distal lymph nodes, cortical bone, bone marrow, liver, or other organs (except as defined in stage 4S) |
| 4S | Localised primary tumour, as defined for stage 1 or 2, with dissemination to liver, skin, or bone marrow. Only applies in infants <1 year of age |
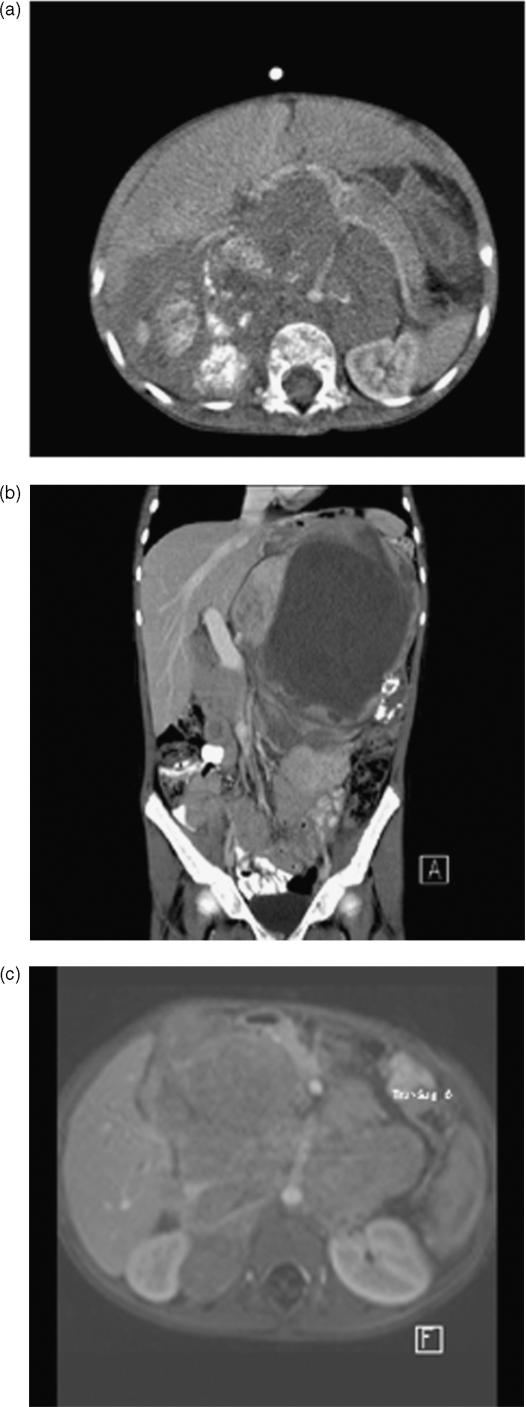
Figure 1.
Neuroblastoma. (a) Axial post-contrast CT section showing a typical heterogenous calcified supra-renal mass encasing the retroperitoneal vasculature and displacing the pancreas anteriorly. (b) Coronal CT showing a large mass of mixed attenuation and calcification in the left upper abdomen, abutting the portal vein. Complete encirclement of the portal vein renders the tumour inoperable. (c) Axial fat-suppressed post-gadolinium enhanced MRI showing an infiltrative retroperitoneal mass again with vascular encasement typical of an NBL. Although this is a single image from a fast gradient sequence which has reduced resolution in comparison to CT, repeated MRIs have a number of advantages including a lack of irradiation.
Localised tumours are currently divided into stages 1 and 2, based on regional lymph node status (contralateral nodes being a criterion for stage 3 disease). Unresectable tumours which extend across the midline at least as far as the contralateral pedicle of the vertebral column are classified as stage 3 disease[5]. Midline extension is often a feature of large, locally invasive tumour that encases vital vascular or neural structures. Stage 4 refers to all patients diagnosed with distant disease (lymph nodes, bones, bone marrow, liver and other organs). A special category in INSS is stage 4S disease, where S stands for special. It refers to infants <1 year of age with small, localised primary tumour and dissemination limited to the liver, skin or bone marrow but no distant osseous metastases. In bone marrow aspirates tumour cells are rare (<10%). Nevertheless, the distinction between the stage 4 types is somewhat arbitrary and can be confusing in children <1 year of age. As mentioned above, the age cut-off of 12 months may soon be changed to 18 months regarding 4S disease. Quantification of marrow and liver involvement (e.g. differentiation between “diffuse heterogeneous” in 4S and “numerous” liver metastases in the poorer forms of stage 4) is difficult, resulting in a grey area of overlapping radiological findings[7]. Despite metastatic spread, 4S tumours virtually always have favourable biologic behaviour and survival rate >90%. A more recently recognised variant of NBL, stage 4N has been described and is not included in the INSS classification. This has been proposed for children with distant nodal spread, but no cortical involvement, on account of their seemingly better prognosis.
Neonatal suprarenal masses
Antenatal suprarenal masses may be congenital NBL but the sonographic appearances are variable and differential diagnosis includes mesoblastic nephroma, extra-lobar pulmonary sequestration and adrenal haemorrhage[5]. A right-sided location, diagnosis in the third trimester, and cystic or mixed echogenicity, often help differentiate NBL from an intra-abdominal sequestration. Without pathologic proof, it is not possible to differentiate an adrenal haemorrhage from a spontaneously resolving NBL and non-invasive monitoring of these small supra-renal lesions with repeat ultrasound examinations and measurement of urinary catecholamine levels are advocated. Fetal NBL has a very good prognosis, and treatment may be conservative.
Imaging features
Ultrasonography (US) is the initial imaging modality to investigate an abdominal mass. On US, NBLs are heterogeneous solid lesions. Cystic anechoic areas are much less common in NBLs than in Wilms' tumour. Calcification is common but variable in appearance. The ipsilateral kidney is usually displaced by the large retroperitoneal tumour, and its identification facilitates differentiation from Wilms' tumour. The aorta and inferior vena cava (IVC) are usually displaced anteriorly and together with the portal vein, the celiac axis, the mesenteric and the renal vessels may be surrounded by the lesion. Vessel patency should be evaluated with colour Doppler US. Metastatic involvement or invasion of the liver can be detected with US. The typical finding suggesting invasion of the liver by the mass is the absence of differential movement between them.
NBL staging requires additional multi-modality imaging. CT or MRI, [123I]meta-iodobenzylguanidine (123I-MIBG), and laboratory investigations (bilateral bone marrow aspirates with histochemical tests and urine catecholamine level measurements) all need to be performed[4,7,8]. Additional routine 99mTc-methylene diphosphonate bone scan (99mTc-MDP) is also advocated by many. Overall MRI has become the most useful modality in staging of NBL. MRI is superior to CT in determining marrow infiltration and intra-spinal extension of tumour. Bone marrow disease is usually seen as diffuse infiltration but it may also present a nodular pattern with areas of low and high signal intensity on T1-weighted (T1W) and T2-weighted (T2W) images, respectively (Fig. 2).
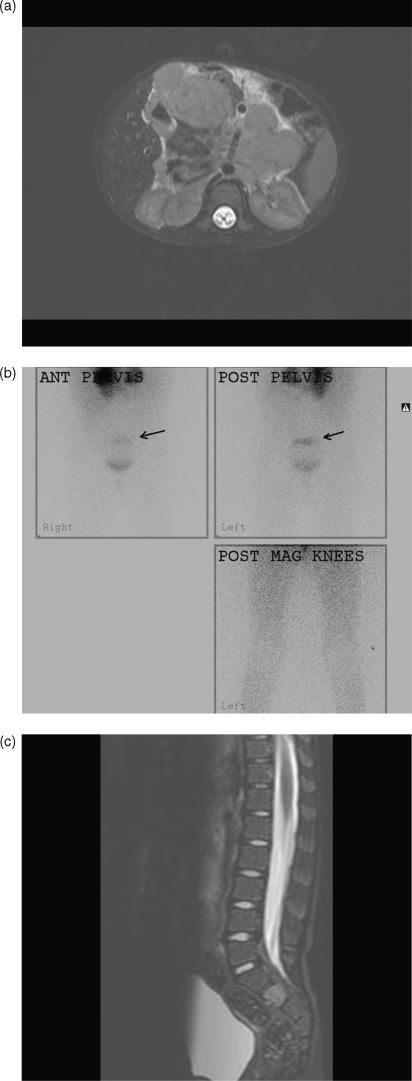
Figure 2.
Neuroblastoma. (a) Axial T2W MRI showing a large upper abdominal primary. (b) Planar MIBG scan showing a solitary metastasis in the sacrum superior to isotope in the bladder (arrows). Note the primary upper abdominal tumour is also MIBG avid. The lower limbs and knees are normal. (c) Sagittal T2W MRI showing abnormal signal in S2. This lesion was initially “hot” on positron emission tomography (PET)/CT, and later “cold” on PET/CT after chemotherapy, indicating a response to therapy, although it remained persistently MIBG avid throughout treatment. It is hoped the persisting MIBG avidity in this sacral lesion reflected differentiation into more mature disease.
MIBG shows almost similar results with bone marrow aspirates in detecting bone marrow infiltration and could be considered more sensitive since it has the advantage of depicting the whole skeleton. MIBG is probably as sensitive as magnetic resonance imaging (MRI) in detecting bone marrow involvement during initial staging but more specific than MRI in evaluating response to treatment (Fig. 2). Benign ganglioneuromas may also take up MIBG and cannot be differentiated from active NBL. MIBG scans offer additional prognostic information: a poor outcome has been associated with MIBG-positive scans in stage 4 patients >1 year after presentation, and in those patients remaining positive after induction chemotherapy. The same correlation was not seen with concurrent MDP bone scans[5]. Disappearance of all metastatic foci on MIBG scanning is commonly regarded as complete response (CR) in NBL staging and follow-up. Semi-quantitative MIBG scoring systems are now in place for many metastatic NBL studies. The achievement of a CR of the metastases is one of the strongest prognostic factors. When CR is achieved it is also common practice for the residual primary tumour to be resected when possible.
Treatment and prognosis
Chemotherapy is indicated in localised NBL, particularly with larger primary tumours, in order to attempt a safe surgical excision. Prognosis of NBL, which even includes a propensity to spontaneous regression in infancy, is influenced by several parameters, such as tumour proto-oncogenes, DNA content, and catecholamine synthesis. Use of these parameters enables tumour categorisation into low-, intermediate-, or high-risk. Therapeutic strategy strongly depends on initial staging with multi-modality imaging and constitutes surgery where possible, chemotherapy in the majority, while bone marrow transplant has been recently introduced[9]. High dose chemotherapy followed by haematopoietic stem cell transplantation, and maintenance therapy with retinoic acid, improves survival by 35% in children presenting with metastatic NBL, but the 5-year event-free survival remains below 50%[10]. Radiotherapy is now used in Europe for high-risk disease patients (treating only the primary site) after chemotherapy. It is also used occasionally as palliation for painful bone metastases and for hepatomegaly from 4S disease that compromises respiratory function. High-dose MIBG therapy is used in selected relapsed stage 4 patients. Children with stages 1, 2, and 4S tumours have 3-year event-free survival rates of 75–90%. Children older than 1 year with INSS stages 3 and 4 tumours have 3-year event-free survival rates of 50% and 15%, respectively[5].
Adrenal tumours
Phaeochromocytoma
Phaeochromocytomas are a type of paraganglioma which arise from the adrenal medulla. However, up to one-third of phaeochromocytomas (extra-adrenal paragangliomas) may occur in the sympathetic chain of the neck, mediastinum or abdomen. They are generally sporadic in childhood, usually occurring in adolescence. A minority are associated with multiple endocrine neoplasia syndromes (mostly type 2), von Hippel–Lindau syndrome or neurofibromatosis. Approximately 10% of sporadic tumours are multiple in location, but the incidence of multiple tumours rises to 30% in the inherited cancer syndromes. Ten percent of tumours are malignant. In children, hypertension is nearly always sustained rather than paroxysmal. Headaches, sweating, nausea and vomiting are common presenting features. Postural hypotension is also frequently encountered[11]. Diagnosis depends on raised plasma catecholamines and increased urinary catecholamines, and their metabolites (urinary epinephrine, metanephrine, HVA, VMA). Abdominal US should be the first radiologic examination. MIBG scanning must be performed next (Fig. 3). MIBG may be positive in the abdomen even when an US is negative and MIBG should always be done prior to surgery to detect or exclude multiple sites of disease. Computed tomography (CT), or preferably MRI, are useful for surgical planning after MIBG scintigraphy. Adrenergic blockade, to prevent a hypertensive crisis, is not required prior to non-ionic contrast administration during CT scanning. Phaeochromocytomas typically show intense enhancement after contrast administration at both CT and MRI. These tumours are also characteristically markedly hyperintense on T2W MRI (Fig. 3). Surgical resection is curative in benign disease.
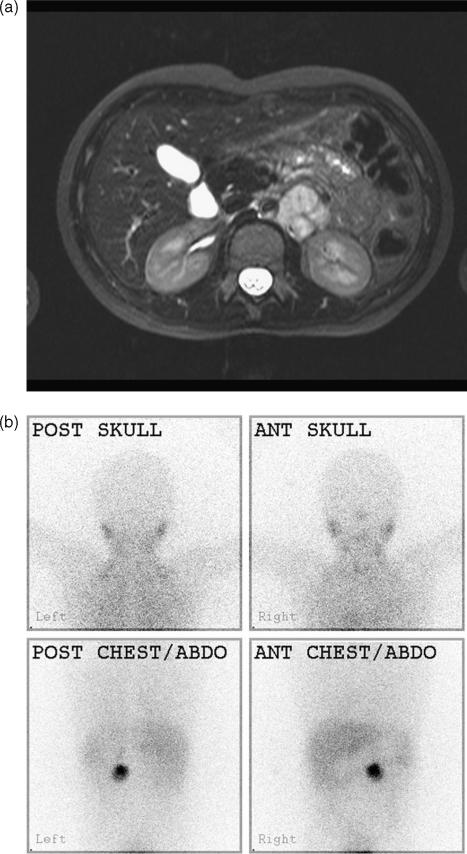
Figure 3.
Phaeochromocytoma. (a) Although this is probably not an adrenal primary, this left-sided phaeochromocytoma mass shows typical hyperintensity on T2W MRI. (b) The tumour also characteristically takes up MIBG. This was a solitary tumour.
Adrenocortical tumours
Adrenocortical tumours (ACT) are very rare in children with a worldwide annual incidence of 0.3 per million children below the age of 15 years[12]. Curiously there appears to be a 15 times higher frequency of ACTs in southern Brazil (most of these children have a p53 germline mutation)[13]. The incidence is higher in young girls with a female/male ratio of 2:1, whereas in adolescence the sex ratio is equal. Virilisation, with early onset of pubic hair, hypertrophy of the clitoris or penis, accelerated growth, gynaecomastia or acne, is the most common presentation. The second most common manifestation is with hypercortisolism (Cushing's syndrome), whilst presentation with a palpable abdominal mass is unusual. Cushing's syndrome is a relatively more common presentation in adolescents and young adults. Very few tumours are non-hormone secreting in contrast to ACTs in adults. Hypertension may be seen in up to 43% at diagnosis[14]. This may be due to either mineralocorticoid or glucocorticoid excess, increased aldosterone production or simply renal artery compression by the tumour, and the hypertension usually resolves after tumour resection. Diagnosis of an ACT is supported by raised levels of androstenedione, dehydroepiandrosterone sulphate (DHEAS), testosterone, and urinary steroids. These hormones are also useful markers for the detection of tumour recurrence during follow-up. Two syndromes have a clear association with this tumour: Li–Fraumeni syndrome is associated with mutations of the p53 gene, and Beckwith–Wiedeman which has mutations in the 11p15 region[15]. Ultrasound is the first line investigation and is particularly useful in evaluating for IVC tumour invasion. With a negative ultrasound and the appropriate clinical context an MRI should also be performed as left adrenal masses in particular may be difficult to visualise with US. Where MRI is unavailable CT may be performed (Fig. 4). Chest CT to exclude or detect pulmonary metastases should be performed at first diagnosis.
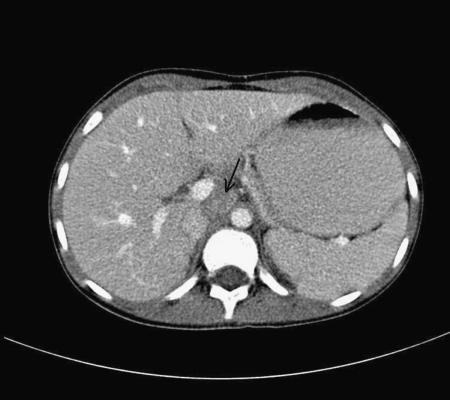
Figure 4.
Adrenocortical tumour (ACT). Axial CT section post-contrast enhancement shows a small soft tissue mass immediately to the right of the aorta (arrow), due to an unfortunate recurrence of an original right adrenal ACT.
The classification of benign from malignant ACTs is not clear cut and is the subject of much debate. Differentiation between adenoma and carcinoma is in practice somewhat arbitrary, and all patients (even those with a seemingly benign adenoma completely resected) require close follow-up initially. On occasions it is recommended to classify the tumour as an “atypical adrenocortical neoplasm” or “adrenocortical neoplasm of indeterminate malignant potential”. The majority of ACTs in children are interpreted pathologically as malignant in most studies[13]. Factors favouring malignancy include size over 5–10 cm, weight over 200 g, invasion into the periadrenal soft tissues or IVC.
Surgical resection is the mainstay of treatment. Lymph node biopsy should be performed in all cases and radical lymph node dissection may be necessary in some malignant cases[15]. The role of radiotherapy is uncertain. Similarly the role of chemotherapy is limited with many centres using mitotane, as in adult ACTs, but its efficacy in children has not been well studied. Younger children, particularly those less than 5 years, with pathologically malignant ACTs, have a significantly better prognosis than older children and adolescents.
Renal tumours
Nephroblastomatosis
The term “nephrogenic rest” implies persistence of embryonic renal parenchyma (metanephric blastema) beyond 36 weeks gestation. Nephroblastomatosis is the presence of multiple nephrogenic rests. These abnormal foci of persistent nephrogenic cells are regarded as precursor lesions. There is an increased incidence of Wilms' tumour (“nephroblastoma”) in children with nephroblastomatosis. The lesions are found in approximately 40% of unilateral Wilms' tumours, and 99% of multicentric or bilateral Wilms' tumours. The malignant potential of individual lesions is uncertain as only a minority of nephrogenic rests develop into Wilms' tumours and spontaneous regression may occur.
Imaging features
Nephroblastomatosis may either be diffuse or multifocal although a unifocal form may be found. The diffuse form typically manifests as a thick hypoechoic band on US (Fig. 5a). This abnormal tissue surrounds the renal periphery and is non-enhancing on CT and MRI (Fig. 5b,c). In the more common multifocal type, the nephrogenic rests resemble normal renal cortex on all modalities and can be scattered throughout the kidneys; they may be nodular or plaque-like and after contrast administration they become hypodense on CT and hypointense on MRI due to poor perfusion in relation to the highly vascular renal cortex.
Figure 5.
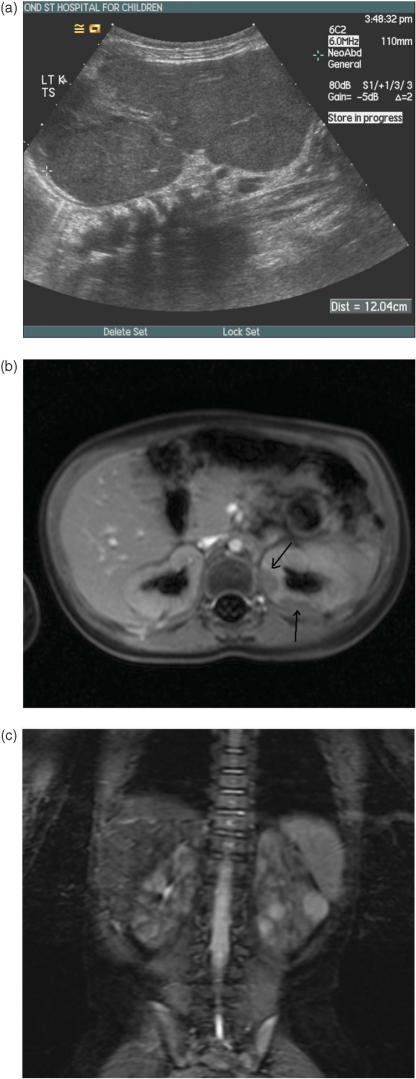
Nephroblastomatosis. (a) Longitudinal ultrasound showing marked renal enlargement in a 1-year-old with widespread hypoechoic change in the renal parenchyma due to diffuse nephroblastomatosis. (b) Fat-suppressed post-gadolinium enhanced MRI. The two plaque-like areas of hypointense, non-enhancement in the periphery of the left kidney are foci of nephroblastomatosis (arrows). (c) Coronal fat-suppressed T2W MRI demonstrating at least three hyperintense lesions in the left kidney. These lesions could be foci of Wilms' tumour or simply hyperplastic rests of nephroblatomatosis. They are indistinguishable and merit removal or at least close surveillance.
Nephroblastomatosis has variable signal intensity depending on cellularity and histologic characteristics on T2W images. In general, the signal intensity of nephroblastomatosis on all sequences, including gadolinium-enhanced images, tends to be relatively homogeneous in contrast to Wilms' tumours which are always heterogeneous in appearance. Contrast administration is mandatory in the assessment for nephroblastomatosis as the lesions are always much more conspicuous on post-contrast studies, most notably after gadolinium administration for MRI. Despite fairly characteristic imaging appearances, if a lesion enlarges then early histological evaluation is warranted and serial assessments are necessary because of the known malignant risk.
It is hypothesised that MRI may be able to distinguish a sclerotic from a hyperplastic nephrogenic rest[16]. Sclerotic rests are thought to be in a regressive phase and to thus lack the potential to develop into a Wilms' tumour. Sclerotic rests typically appear dark on T2W MRI, whilst hyperplastic rests are usually hyperintense on T2, similar to Wilms' tumour[16]. It is less important to distinguish a Wilms' tumour from a hyperplastic rest as the latter has the potential to develop into a tumour and also the capacity to enlarge. In the setting of bilateral nephroblastomatosis or bilateral Wilms' tumours on treatment, it is thought that hypointense lesions on T2 (sclerotic rests) may simply be observed, whereas hyperintense lesions on T2 may require further chemotherapy or local resection[16]. CT is unable to make this distinction.
Mesoblastic nephroma
Mesoblastic nephroma, also known as congenital mesoblastic nephroma (CMN), is the most common renal neoplasm in the first 3–6 months of life. It typically presents as an abdominal mass in a neonate. On US, the mass is solid but there may be hypoechoic areas due to cystic change or necrosis. Neither US nor CT can reliably distinguish CMN from Wilms' tumour. The benign CMN does not invade the vascular pedicle nor does it metastasise. Local recurrence may result from incomplete removal or capsular penetration. With complete removal there is an excellent prognosis.
Wilms' tumour (nephroblastoma)
Wilms' tumour (nephroblastoma) is the most common malignant primary renal tumour in childhood. Wilms' tumour accounts for up to 12% of all childhood cancers with a peak incidence at around 3 years of age (Fig. 6). The commonest presentation is an asymptomatic abdominal mass. Haematuria, particularly after minor trauma, is another typical clinical manifestation; pain, fever or hypertension (in up to a quarter of cases) are unusual but recognised presenting features. Microscopic haematuria is present in 25% of cases. There is equal distribution between the sexes with the highest incidence being in the black population in the USA and Africa. Around 10% of Wilms' tumours are bilateral, of which two-thirds are synchronous and one-third metachronous.
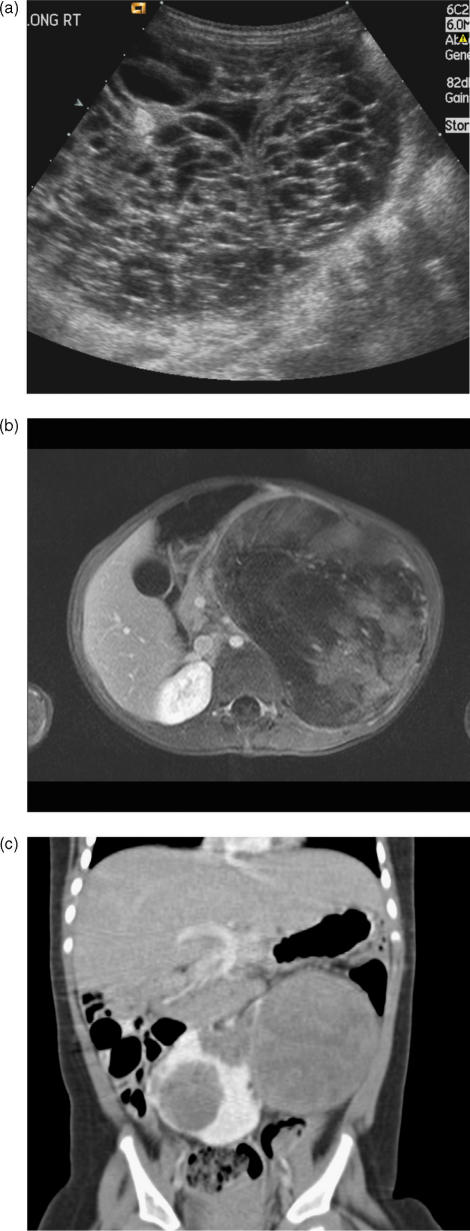
Figure 6.
Wilms' tumour. (a) Longitudinal ultrasound showing a very cystic Wilms' mass which had few solid components. (b) Axial T1W MRI after gadolinium-enhancement showing a large heterogenous left renal tumour, with a reasonably well-defined “capsule”. (c) Coronal CT demonstrating bilateral tumours in a horseshoe kidney.
Associated conditions
Associated congenital anomalies occur in 15% of children and include cryptorchidism and horseshoe kidney (Fig. 6c). Certain syndromes have a predisposition to Wilms' tumour. These include aniridia (absence of ophthalmic iris), Beckwith–Wiedemann (macroglossia, exomphalos, gigantism), hemihypertrophy, Denys–Drash (pseudohermaphroditism), Soto's (cerebral gigantism), Bloom's (immunodeficiency and facial telangiectasia) and Perlman's syndromes. In Denys–Drash syndrome, for example, most but not all patients will develop a Wilms' tumour, the median age at presentation being 18 months, and 20% of cases are bilateral. The role of routine US screening to detect tumours at an early stage in Beckwith–Wiedeman syndrome in particular is debated because interval tumours may occur between US studies, and tumours are always of favourable histology and chemosensitive. As screening in high risk cases has tended to be erratic in the UK (if not the entire world) a national screening programme has been instituted to screen children with a greater than 5% risk of developing a Wilms' tumour up to 7 years of age.
A small group of Wilms' cases are familial. Intralobar nephrogenic rests are commonly found in such patients but there is no association of familial tumours with bilaterality. Extra-renal Wilms' tumours are rare; the most common locations are the retroperitoneum, inguinal region and pelvis. Their histology and outlook are identical to that of renal Wilms' tumours.
Histopathology
Wilms' tumours are solid lesions with a fibrous pseudocapsule and have variable areas of haemorrhage and necrosis. The tumour may invade the renal vein and IVC with caval extension, often to the right atrium, seen in 4% of patients. Metastases to local para-aortic lymph nodes and haematogenous spread to the lungs and less commonly the liver are seen. Epithelial (tubular, glomerular), stromal (spindle, myxoid) and blastemal (small round cells) cell lines are the histologic components of nephrogenic rests, fetal kidneys and Wilms' tumours. When all three are present in malignant masses, the lesions are termed triphasic Wilms' tumours and are regarded as favourable histology. These lesions lack anaplastic changes. Unfavourable tumours comprise 6% of lesions and typically have hyperchromatic cells with large nuclei. The degree of anaplasia correlates with patient outcome. Minimally anaplastic tumours have a prognosis similar to favourable histology lesions.
Staging
The (post-surgical) staging of Wilms' tumour according to the North American National Wilms' Tumor Study Group is summarised below:
Stage I: tumour confined to the kidney without capsular or vascular invasion.
Stage II: tumour beyond renal capsule, vessel infiltration or intraoperative tumour rupture.
Stage III: positive lymph nodes in the abdomen or pelvis, peritoneal invasion or residual tumour at surgical margins.
Stage IV: metastatic disease outside the abdomen or pelvis.
Stage V: bilateral tumours at original diagnosis.
In Europe and elsewhere the International Society of Paediatric Oncology (SIOP) utilises the same staging system with one exception – masses that have been biopsied may be regarded as stage I disease when later excised (if confined to the kidney) but a biopsy upstages a tumour to stage III disease in North America. An additional disadvantage of renal biopsy is that many anaplastic tumours are undiagnosed until the time of nephrectomy, particularly those with focal anaplasia[17]. Whether a renal tumour merits biopsy prior to treatment is also debated but is routine practice prior to chemotherapy in the UK. Some European centres omit biopsy for presumed Wilms' tumours in early childhood, but at a cost of administering chemotherapy to 12% of children with renal masses who, after nephrectomy, are proven not to have a Wilms tumour[17].
Imaging features
US must be the first radiological method of assessment. The tumour typically is large with a mixture of solid hyperechoic masses and cystic areas with often the cystic components predominating. Normal native renal tissue can be difficult to detect and is typically stretched at the periphery of the lesion. Movement of the mass separate from adjacent organs such as the liver, indicating a lack of direct invasion, can be optimally assessed with US, a phenomenon that is difficult to evaluate on CT or MRI. The renal vein, IVC, liver and opposite kidney should be carefully assessed for spread of disease. US is also the most reliable method of excluding renal vein and IVC thrombus.
Contrast enhanced CT or MRI is generally deemed necessary for further delineation of tumour extent but it is noteworthy here that many leading European centres perform only US at diagnosis and follow-up in their children with renal tumours with no apparent detriment to patient care. On CT and MRI a tumour enhances to a lesser degree than normal renal parenchyma. A so-called claw or beak sign of normal renal tissue may be seen displaced by the tumour. Exclusion of adenopathy, liver abnormalities, peritoneal invasion and contralateral kidney changes should be undertaken. Contrast-enhanced images are required to assess the contralateral kidney for nephroblastomatosis in particular or another small Wilms' mass.
Pulmonary metastases
There is continuing uncertainty among the paediatric collaborative oncology groups regarding the optimum staging approach in patients with small lung lesions. Although many such small (5–10 mm) lesions are metastases, a substantial minority are not[16]. Until recently the commonly used staging systems for pulmonary metastases were based solely on chest radiographs, such that positive findings on CT were ignored if no lesions could be visualised on a chest x-ray. The rationale for this was that chemotherapy for local disease “mopped up” the possible small tumour burden within the chest. However, some limited evidence suggests those with CT positive nodules, negative on chest x-ray, have a higher relapse rate[16]. In addition, inconsistency in staging meant that in some centres when small nodules were identified on CT, these patients were more intensively treated as stage 4 disease, contrary to protocol. Local stage 1–2 disease merits only two-drug chemotherapy, whereas stage 4 disease warrants three drugs (with the addition of doxorubicin). But the optimal treatment paradigm for small bulk disease in the chest is unresolved also. The role of radiotherapy in particular is widely debated. Fewer relapse after lung irradiation but at a cost – more deaths from lung toxicity and more long-term morbidity. Within the latest North American (Children's Oncology Group (COG), from 2006) study the staging of metastatic disease will be done by central review. In the next COG Wilms' study, the initial response to therapy by lung nodules will determine subsequent pulmonary specific therapy. Lesions that disappear after 6 weeks of chemotherapy will not require irradiation. Persisting lesions after chemotherapy will be biopsied and if positive for tumour will receive radiation therapy. In the current SIOP Wilms' tumour study, lung CT is also now recommended. In that study, lesions must measure over 10 mm on CT to be defined as metastases (but whether these are to be measured on a typical lung or mediastinal window setting is not stipulated).
Treatment and prognosis
The treatment strategy of Wilms' tumours has become a model for the successful multidisciplinary approach to paediatric solid tumours. The prognosis for most children with Wilms' tumour is now so good that it is difficult to conduct a randomised trial with adequate power to detect a magnitude of difference that might be clinically significant[16]. North American practice is initial surgical removal of the tumour followed by adjuvant chemotherapy dictated by the staging found at surgery. European oncologists favour initial chemotherapy (after biopsy confirmation in the UK) with later resection. The optimal surgery includes a transperitoneal approach with biopsy of adjacent regional lymph nodes, whether enlarged or not. Nephrectomy is usually carried out except in the setting of bilateral Wilms' tumours where partial nephrectomies, when possible, are indicated to preserve as much normal kidney tissue with the hope of avoiding or delaying the subsequent onset of chronic renal failure. The prognosis for Wilms' tumour patients is excellent and there is little evidence to suggest that the overall relapse-free survival is adversely affected by either approach. The 4-year overall survival rate, and presumed cure, ranges between 86% and 96% for stages I–III disease, is up to 83% for stage IV and 70% for stage V disease. Patients with the much less common diffuse anaplastic Wilms' tumours have a much poorer outcome, however. Their 4-year survival figures are 45% for stage III and only 7% for stage IV disease.
Clear cell sarcoma of the kidney
Clear cell sarcoma of the kidney (CCSK) is a distinct entity accounting for 4% of all childhood renal neoplasms with a male preponderance. The peak age of incidence is similar to that of Wilms' tumour. There are no specific radiological features to help distinguish CCSK from a Wilms' tumour. Although this neoplasm may metastasise to the lungs, there is a particular predilection for skeletal metastases at diagnosis (over 20% risk), hence the other known term, bone metastasising tumour. Once diagnosed, 99mTc-MDP bone scintigraphy is indicated for staging purposes. The discovery of a lytic lesion in the skeleton in a child with a presumed diagnosis of Wilms' tumour should suggest that the primary diagnosis is incorrect and that the tumour is likely to be a CCSK, although ultimately diagnosis rests on histological evaluation. Curiously, although seen rarely at diagnosis, when relapse occurs with CCSK it seldom affects the skeleton but more commonly relapses are seen in the lungs or central nervous system.
Rhabdoid tumour of the kidney
Rhabdoid tumour is the most aggressive malignant renal tumour in childhood and accounts for about 1–2% of paediatric renal neoplasms. Most cases are diagnosed in the first year of life (Fig. 7). Like CCSK, the mass is indistinguishable from a Wilms' tumour on imaging. A peripheral fluid crescent sign on CT has been described but it is not pathognomic and is seldom seen. Invasion of the renal vein is common. Metastases to the lungs, liver and brain have been reported. There is also an association with simultaneous primitive neuroectodermal tumours usually in the posterior fossa. Consequently, cranial imaging is routinely recommended when this renal tumour is encountered.
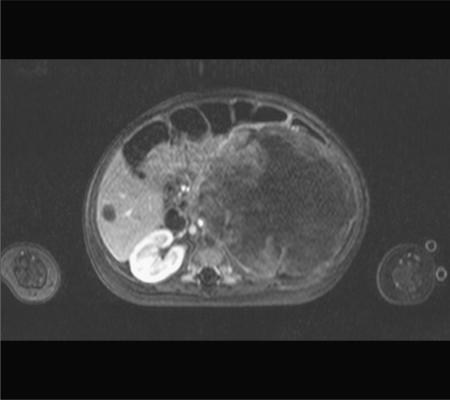
Figure 7.
Rhabdoid tumour. Axial T1W MRI post-gadolinium enhancement showing a large heterogenous left renal mass with a hypointense metastasis in the right lobe of the liver, due to metastatic rhabdoid tumour in an infant. This is usually a very aggressive neoplasm.
Renal cell carcinoma
Renal cell carcinoma rarely presents in the first two decades of life. Less than 1% of all cases occur in children. A mass or flank pain are common presenting features. Haematuria is encountered less frequently. The mean age of presentation in childhood is 9 years. A typically solid intrarenal mass cannot be distinguished from a Wilms' tumour although ring-like calcifications, unusual for a Wilms' tumour, may be present within the mass. The major discriminating feature from a possible Wilms' tumour is the older age of the patient. The frequency of this carcinoma in young patients may be increasing, paralleling an increased incidence in adults[18]. Locoregional nodal spread of disease, in the absence of distal metastatic spread, does not appear to confer as poor a prognosis in younger patients compared to adults[19]. Metastases to the lungs, liver, skeleton or brain are present in 20% of patients at diagnosis.
Lymphoma and leukaemia
Renal involvement with or without retroperitoneal adenopathy is seen in 12% of children with non-Hodgkin's lymphoma, most commonly B-cell Burkitt's lymphoma. Multiple, usually bilateral, nodules are typical although diffuse infiltration may be seen. There is generally widespread disease elsewhere. Renal enlargement on US with altered echo texture is characteristic of both renal lymphoma and leukaemia. The changes in the kidneys can be quite subtle on CT and are generally more conspicuous on MRI, particularly T1W images after gadolinium enhancement. US is recommended in all children with leukaemia/lymphoma prior to commencement of chemotherapy to detect tumour infiltration or calyceal dilatation. During initial chemotherapy the excretion of tumour metabolites may result in renal obstruction or uric acid nephropathy. Consequently children with leukaemic or lymphomatous involvement of the kidneys need careful nephrological monitoring.
References
- 1.Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics. 2003;23:29–43. doi: 10.1148/rg.231025050. [DOI] [PubMed] [Google Scholar]
- 2.Evans AE, d'Angio GJ, Propert K, et al. Prognostic factors in neuroblastoma. Cancer. 1987;59:1853–9. doi: 10.1002/1097-0142(19870601)59:11<1853::aid-cncr2820591102>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Rubie H, Hartmann O, Michon J, et al. N-Myc gene amplification is a major prognostic factor in localised neuroblastoma: results of the French NBL 90 study. J Clin Oncol. 1997;15:1171–82. doi: 10.1200/JCO.1997.15.3.1171. [DOI] [PubMed] [Google Scholar]
- 4.Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–88. [PubMed] [Google Scholar]
- 5.Hiorns MP, Owens CM. Radiology of neuroblastoma in children. Eur Radiol. 2001;11:2071–81. doi: 10.1007/s003300100931. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Kohno S, Mimaya J, et al. Neuroblastoma detected by mass screening: the Tumor Board's role in its treatment. Pediatr Surg Int. 2004;20:27–32. doi: 10.1007/s00383-003-1070-x. [DOI] [PubMed] [Google Scholar]
- 7.McHugh K, Pritchard J. Problems in the imaging of three common paediatric solid tumours. Eur J Radiol. 2001;37:72–8. doi: 10.1016/s0720-048x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- 8.Hugosson C, Nyman R, Jorulf H, et al. Imaging of abdominal neuroblastoma in children. Acta Radiol. 1999;40:534–42. doi: 10.3109/02841859909175580. [DOI] [PubMed] [Google Scholar]
- 9.Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002;22:911–34. doi: 10.1148/radiographics.22.4.g02jl15911. [DOI] [PubMed] [Google Scholar]
- 10.Matthay KK, Villablanca J, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation and 13-cis retinoic acid. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 11.Bhansali A, Rajput R, Behra A, et al. Childhood sporadic pheochromocytoma: clinical profile and outcome in 19 cases. J Pediatr Endocrinol Metab. 2006;19:749–56. doi: 10.1515/jpem.2006.19.5.749. [DOI] [PubMed] [Google Scholar]
- 12.Bonfig W, Bittman I, Bechtold S, et al. Virilising adrenocortical tumours in children. Eur J Pediatr. 2003;162:623–8. doi: 10.1007/s00431-003-1230-y. [DOI] [PubMed] [Google Scholar]
- 13.Dehner L. Pediatric adrenocortical neoplasms: on the road to some clarity. Am J Surg Pathol. 2003;27:1005–7. doi: 10.1097/00000478-200307000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumours: a report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–45. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JN, Flageole H, Kavan P. A surgical approach to adrenocortical tumors in children: the mainstay of treatment. J Pediatr Surg. 2004;39(5):759–63. doi: 10.1016/j.jpedsurg.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Grundy P, Perlman E, Rosen NS, et al. Current issues in Wilms tumor management. Curr Probl Cancer. 2005;29:221–60. doi: 10.1016/j.currproblcancer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Vujanic GM, Kelsey A, Mitchell C, et al. The role of biopsy in the diagnosis of renal tumors of childhood: results of the UKCCSG Wilms Tumor Study 3. Med Pediatr Oncol. 2003;40:18–22. doi: 10.1002/mpo.10216. [DOI] [PubMed] [Google Scholar]
- 18.Estrada CR, Suthar AM, Eaton SH, Cilento Jr BG. Renal cell carcinoma: Children's Hospital Boston experience. Urology. 2005;66:1296–300. doi: 10.1016/j.urology.2005.06.104. [DOI] [PubMed] [Google Scholar]
- 19.Geller JI, Dome JS. Local lymph node involvement does not predict poor outcome in pediatric renal cell carcinoma. Cancer. 2004;101:1575–83. doi: 10.1002/cncr.20548. [DOI] [PubMed] [Google Scholar]