Abstract
Francisella tularensis is a highly infectious bacterial pathogen, responsible for the zoonotic disease tularemia. We screened a bank of transposon insertion mutants of F. tularensis subsp. holarctica LVS for colony morphology alterations and selected a mutant with a transposon insertion in wbtA, the first gene of the predicted lipopolysaccharide O-antigen gene cluster. Inactivation of wbtA led to the complete loss of O antigen, conferred serum sensitivity, impaired intracellular replication, and severely attenuated virulence in the mouse model. Notably, this mutant afforded protection against a challenge against virulent LVS.
Francisella tularensis is a small nonmotile gram-negative bacterium. It causes the zoonotic disease tularemia in large numbers of animals and is one of the most infectious human bacterial pathogens (14). F. tularensis is an intracellular pathogen replicating and disseminating in vivo mainly inside macrophages. However, little is known about the molecular mechanism of entry and survival within its cellular vehicle. An approximately 30-kb pathogenicity island was recently discovered in F. tularensis species (12, 13) which comprises several genes that are essential for the intracellular survival of the bacterium (for reviews, see references 18 and 19). Only a few other virulence determinants have been identified thus far, and surface structures, such as the lipopolysaccharide (LPS) and the capsule (6, 17), have been considered as potential virulence factors.
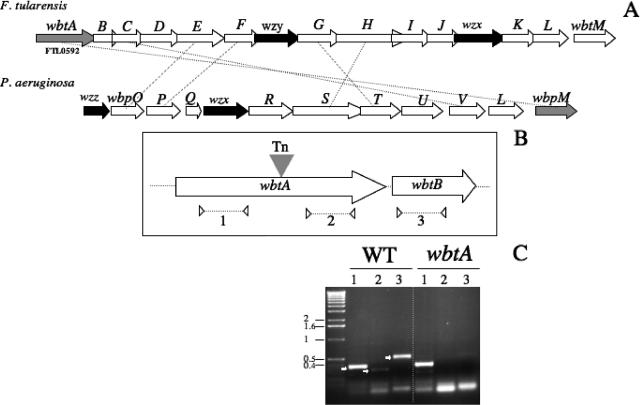
F. tularensis possesses a very peculiar LPS with weak endotoxinic activity that seems to be required for growth inside macrophages and for resistance to the bactericidal effect of the serum (6). The structural composition of the O antigen has been experimentally established in F. tularensis subsp. tularensis, F. tularensis subsp. holarctica, and Francisella novicida (4, 15, 21-23). In parallel, the wbt gene cluster supposedly involved in LPS O-antigen biogenesis has been identified by in silico analysis of the F. tularensis subsp. tularensis Schu S4 genome (15). This locus (FTL0592 to FTL0606; highly conserved in the LVS genome) is flanked by two transposases, suggesting a horizontal mode of acquisition (Fig. 1).
FIG. 1.
Genetic organization of the F. tularensis wbt locus and transcriptional analysis. (A) Schematic organization of the locus. The arrows indicate approximate sizes and orientations of the different wbt genes. The wzx (determining the cytoplasmic membrane O-antigen transporter), wzy, and wzz (involved in O-antigen polymerization) genes are indicated The LVS wbt locus is shown above the P. aeruginosa O6 wbp locus. Orthologous genes are connected by dotted lines. (B) The transposon insertion site. The filled triangle indicates the transposon (Tn) insertion site in wbtA. The three pairs of primers used for RT-PCR analysis are shown: two pairs in wbtA (to amplify region 1, upstream of the transposon insertion, and region 2, downstream) and one pair in wbtB (region 3). The dotted arrows below indicate the positions of the primers and approximate sizes of the PCR products used in the RT-PCR analysis. (C) RT-PCR. The amplified products were subjected to Tris-acetate-EDTA-agarose gel electrophoresis. RT-PCR was performed on RNA of the LVS wild-type strain (WT) and on RNA of the wbtA mutant. Lane numbers correspond to the regions indicated in panel B. Numbers to the left indicate the molecular mass standards (in kb).
To identify genes possibly involved in cell wall and/or capsule biogenesis, we created a bank of mutants using the commercial EZ::TN KAN-2 transposon mutagenesis system (Epicentre, Madison, WI), essentially as described previously (10) (Table 1). The transposon insertion site was determined (by genomic DNA sequencing) for several mutants that showed altered colony morphologies when spotted on Congo red agar plates. Congo red is a vital dye that binds to lipoproteins or lipids present on the bacterial surface and is classically used to characterize modifications affecting the cell wall.
TABLE 1.
Primers and strains used in this study
| Primer or strain | Sequence (5′→3′) or description | Source |
|---|---|---|
| Primers | ||
| wbtA1-5 | 5′-GCT TCT GCT GGT TAT CGC ATC-3′ | |
| wbtA2-3 | 5′-CTA CCT CCA GCT CCT GTG AC-3′ | |
| wbtA3-5 | 5′-GTG CTT GGT AGT AGT GGC AG-3′ | |
| wbtA4-3 | 5′-TCG GTA CTA ACA TCA TCT TC-3′ | |
| wbtB5-5 | 5′-ATG GGG TTG TTG TTA TTA AGT A-3′ | |
| wbtB6-3 | 5′-GTA CCA CCC TCA ACG ACG C-3′ | |
| Kan-2 FP-1a | 5′-ACC TAC AAC AAA GCT CTC ATC AAC C-3′ | |
| Kan-2 FRP-1a | 5′-GCA ATG TAA CAT CAG AGA TTT TGA G-3′ | |
| KanF | 5′-CTC AAA ATC TCT GAT GTT ACA TTG C-3′ | |
| KanR | 5′-GGT TGA TGA GAG CTT TGT TGT AGG T-3′ | |
| Strains | ||
| Escherichia coli DH5 | F− φ80lacZ ΔM15 endA1 recA1 hsdR17 supE44 thi-1 gyrA96 relA1 (lacZYA-argF)U169 | Invitrogen |
| F. tularensis | ||
| LVS | A. Sjostedt | |
| LVS wbtA | LVS wbtA::Tn5 | This work |
Provided in the Epicentre transposon kit.
One mutant, designated LVS wbtA, formed lawns with more regular edges than the parental LVS strain and was chosen for further analyses (Fig. 1A). In this mutant, the transposon is inserted in the middle of the wbtA gene (Fig. 1B). WbtA of LVS shares 41.7% amino acid identity with WbpM of Pseudomonas aeruginosa O6. WbpM is likely to be involved in the formation of 6-deoxy-N-acetylglucosamine, which is thought to be the first sugar attached to the core LPS. Notably, a P. aeruginosa wbpM knockout mutant no longer produces the O-antigen B-band form (1).
Reverse transcription-PCR (RT-PCR) analysis showed that both the wbtA and wbtB genes were transcribed in LVS (Fig. 1B and C). In contrast, in the wbtA mutant strain the gene wbtB and the region of wbtA downstream of the transposon were not transcribed, suggesting a polar effect of the insertion on the expression of the downstream sequences.
Growth of the wbtA mutant showed a slight delay in the complex Schaedler K3 medium (bioMerieux SA, Marcy l'Etoile, France), whereas in defined medium (2), the growth of the mutant was indistinguishable from that of the LVS strain (Fig. 2B). We evaluated the resistance to the bactericidal effect of human serum of the wbtA mutant. The sensitivity assay was performed essentially as described previously (17). LVS survived incubation for 1 h at 37°C with nondecomplemented human serum (PAA Laboratories GmbH, Pasching, Austria) (0 to 20%; data not shown), whereas the wbtA mutant was totally eliminated after 2 to 3 min of incubation with 2% serum, revealing an extreme sensitivity to serum (Fig. 2C). The bactericidal effect of the serum was suppressed by preincubation at 56°C for 30 min (data not shown). We also tested sensitivity to the detergent deoxycholate (0.01 and 2%) as described previously (6). LVS and the wbtA mutant were equally resistant to the detergent (data not shown). The impact of the mutations on LPS biogenesis was finally evaluated by Western blotting on whole-cell lysates (Fig. 2D) using LVS-specific anti-LPS antibodies. A ladder typical of LPS O antigen was detected in extracts of the LVS strain. In contrast, the wbtA mutant did not produce any detectable O-antigen ladder.
FIG. 2.
Characteristics of LVS and a wbtA mutant. (A) Morphology on Congo red plates. Overnight cultures were spotted (5 μl) onto solid medium containing Congo red dye (at a final concentration of 100 μg/ml). Morphology alterations were monitored after several (5 to 7) days at 37°C. (B) Growth curves of LVS and mutants in Schaedler K3 broth and in chemically defined medium (2) at 37°C with agitation. (C) Killing of LVS and mutants by nonimmune human serum. Bacteria were incubated in 2% serum; at selected intervals samples were collected, and the number of viable bacteria was determined by plating onto solid medium. (D) Western immunoblotting of whole-cell lysates of LVS and the wbtA mutant. A nitrocellulose membrane was probed with LVS-specific anti-LPS mouse monoclonal T14 antibody at a final dilution of 1:1,000 (left). Numbers to the left correspond to molecular mass markers in kDa. A Coomassie blue-stained gel of the same samples is also shown (right).
Intracellular survival and multiplication were monitored in the murine macrophage-like cell line J774. Macrophage monolayers were infected with stationary-phase bacteria at a multiplicity of infection of 10 for 90 min. As shown in Fig. 3, the parental LVS strain and the wbtA mutant infected the cells at similar levels. However, while the LVS strain multiplied efficiently in macrophages during the 24-h period of the assay, replication of the wbtA mutant was totally abolished, and the number of wbtA mutant bacteria remained the same after 4 h and 24 h of infection.
FIG. 3.
Kinetics of intracellular multiplication. Invasiveness of LVS and the wbtA mutant were evaluated in J774 macrophages. Values and error bars represent the means and standard deviations of the number (in log10) of bacteria per well (three wells per assay). The assay was repeated three times (P = 0.028 at 2 h and 3.6 × 10−5 at 4 h, as determined by a Student's t test).
Virulence of the mutant was evaluated in the mouse model, by the intraperitoneal (i.p.) route of infection, and compared to that of parental LVS. Groups of five pathogen-free 6- to 8-week-old female BALB/c mice (Janvier) were inoculated i.p. with bacterial suspension (0.2 ml in 0.15 M NaCl). Mortality was followed for 9 days (Fig. 4A). All mice infected with the highest dose of LVS bacteria (104 CFU) died within 7 days, whereas 80% of the mice infected with the lowest dose (101 CFU) survived. In contrast, only the mice infected with the highest dose (108 CFU) of the wbtA mutant bacteria became sick, and one mouse died. Using the graphic Probit method (16), the 50% lethal dose of LVS was determined to be 101.5 while that of the LVS wbtA mutant was >8 × 107, revealing a strong attenuation of virulence.
FIG. 4.
Virulence of the wbtA mutant. (A) Survival of mice was followed for 9 days after i.p. inoculation with F. tularensis LVS or wbtA mutant bacteria. Groups of five mice were infected with approximately 101, 102, 103, and 104 CFU of LVS or approximately 105, 106, 107, and 108 CFU of the wbtA mutant. (B) Protection of mice against F. tularensis LVS after immunization with the F. tularensis wbtA mutant. Naïve mice or mice immunized 39 days earlier by the i.p. route with 105 CFU of the wbtA mutant were challenged with approximately 104 CFU of LVS, and survival was monitored for 10 days. (C) Growth of F. tularensis LVS and the wbtA mutant in spleen and liver of BALB/c mice. Mice were inoculated by the i.p. route with approximately 104 CFU (five mice per group), and bacterial burdens in spleen and liver were determined 2, 3, 4, and 7 days after infection. †, all mice died. The numbers above the squares indicate the number of mice (in a group of five) in which bacteria were detected (P < 0.001 at all times, as determined by a Student's t test). The arrow indicates the number of CFU used for inoculation.
We further determined the bacterial burden in the spleen and liver of BALB/c mice 2, 3, 4, and 7 days after infection with ∼104 CFU of LVS or LVS wbtA bacteria (Fig. 4C). After 2 days of infection, the number of LVS bacteria in both spleen and liver had increased to about 106, whereas only about 1% of the wbtA mutant bacteria used for the infection could be recovered. After 4 days, the number of F. tularensis LVS bacteria recovered from the spleen and liver had increased to ∼109 CFU, and none of the mice survived for 7 days. In contrast, the number of wbtA bacteria recovered remained low (1 × 102 to 5 × 102) throughout the course of the infection. Furthermore, at day 2 of infection we were able to recover wbtA bacteria from all five mice in the group, but at later time points we only recovered bacteria from a subset of the five mice. However, in the mice with detectable numbers of wbtA bacteria, the amount stayed relatively constant in both organs. These results point to the critical importance of the wbt locus in the virulence of F. tularensis.
We also evaluated the capacity of the wbtA mutant to induce a protective immune response against virulent LVS. For this, a group of mice immunized 39 days earlier i.p. with ∼105 CFU of wbtA mutant bacteria were challenged, by the same route, with ∼104 CFU of LVS (i.e., with 100- to 1,000-fold the 50% lethal dose). Control naive mice died within 7 days after infection. In contrast, the mice immunized with the LVS wbtA mutant did not show any symptom of illness, and all of them survived, thus reflecting complete protection (Fig. 4B).
Interestingly, several transposon mutants defective in LPS biosynthesis have been previously isolated in F. novicida (6). In that study, one mutant showed a complete loss of reactivity with anti-Francisella LPS monoclonal antibody (mutant SC4). Our sequence comparison revealed that, in mutant SC4, the transposon insertion occurred within the wbtA gene orthologue of F. novicida. In contrast to the LVS wbtA mutant, the F. novicida wbtA mutant appeared to grow normally in mouse macrophages and was only moderately more sensitive to serum than the parental strain. This illustrates the different properties of the LPS O antigen in the two Francisella species and is in agreement with immunological studies (5, 11) that demonstrated the different natures and characteristics of the LPS O antigen from F. tularensis subsp. tularensis and F. novicida.
In another study (7), two classes of opacity variants (designated LVSG and LVSR) with a modified LPS were isolated. The LVSG strain was resistant to serum killing, whereas the LVSR strain appeared to be sensitive. Pseudo-revertants of LVSR and LVSG isolated after intracellular growth produced an LPS antigenically similar to that of parental LVS, prompting the authors to evoke a phase variation mechanism to account for these changes. A more recent study (9) focused on the antigenic and immunological differences between blue and spontaneous gray variants (GV) of LVS. The GV lacked any LPS O antigen and were sensitive to serum killing. In the murine model, they were nonpathogenic and failed to induce antibodies to F. tularensis, suggesting a very rapid in vivo elimination. Moreover, no revertant could be recovered from these GV, indicating that these GV are structurally and functionally totally distinct from those reported by Cowley et al. (7). The mutational events that resulted in the appearance of the opacity variants have not been elucidated. The establishment of the genetic bases of LPS O-antigen variations is a prerequisite for proper mechanistic interpretations.
Several reports have described a capsular structure on F. tularensis (3, 8, 17, 20). However, other reports failed to visualize this capsule, and its precise nature is currently unknown. In spite of that, the term “capsule-negative mutant” is regularly found in the literature and refers, in some instances, only to serum-sensitive F. tularensis mutants. The distinction between LPS O antigen and capsular antigen might sometimes be complicated, in terms of both functional definition and chemical composition. Our attempts to visualize the capsule of F. tularensis LVS by transmission electron microscopy have thus far been unsuccessful. In silico analysis of the F. tularensis genome identified two genes (FTT0805 and FTT0806) encoding homologues of the CapB and CapC proteins of Bacillus anthracis, suggesting the presence of poly-d-glutamate in an F. tularensis capsule (12). However, we were unable to detect poly-d-glutamate in F. tularensis using an antibody against B. anthracis poly-γ-d-glutamate in a dot blot assay (data not shown). At this stage, it cannot be excluded that the LPS O antigen might constitute the only capsule-like structure. Alternatively, other types of capsular antigens might be expressed at the bacterial surface and, in particular, under defined environmental conditions such as during intracellular survival.
We showed here that inactivation of the wbtA gene leads to a severe attenuation of virulence of F. tularensis LVS in the mouse model. This is the first report that correlates a genetic alteration in the O-antigen biosynthetic gene cluster with the loss of O-antigen production and impaired virulence. In vitro, the wbtA mutant was able to survive in infected macrophages without multiplication. In vivo, in spite of a rapid elimination of most of the injected bacteria, the remaining bacteria were able to persist for at least 3 days in infected organs and triggered an efficient immune response. It remains to be determined whether the wbtA mutant protects against a challenge with a virulent F. tularensis subsp. tularensis strain and against challenges by different routes. Genetically defined attenuated derivatives of LVS lacking the O antigen, unable to replicate in infected hosts but promoting protection, might constitute safer live vaccines against F. tularensis than LVS.
Acknowledgments
We thank Anders Sjöstedt (Department of Clinical Microbiology, Umeå University) for providing the LVS strain. We thank Agnès Fouet (Institut Pasteur, Paris, France) for fruitful discussions and advice.
This work was supported by CNRS, INSERM, and Université Paris V. C.R. was supported by a fellowship from the INSERM. K.L.M's fellowship and part of these studies were supported by a grant from Sanofi-Aventis France and Bayer Pharma as part of a multi-institutional call for proposals.
Editor: D. L. Burns
Footnotes
Published ahead of print on 9 October 2006.
REFERENCES
- 1.Belanger, M., L. L. Burrows, and J. S. Lam. 1999. Functional analysis of genes responsible for the synthesis of the B-band O antigen of Pseudomonas aeruginosa serotype O6 lipopolysaccharide. Microbiology 145:3505-3521. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain, R. E. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherwonogrodzky, J. W., M. H. Knodel, and M. R. Spence. 1994. Increased encapsulation and virulence of Francisella tularensis live vaccine strain (LVS) by subculturing on synthetic medium. Vaccine 12:773-775. [DOI] [PubMed] [Google Scholar]
- 4.Conlan, J. W., H. Shen, A. Webb, and M. B. Perry. 2002. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 20:3465-3471. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, J. W., E. Vinogradov, M. A. Monteiro, and M. B. Perry. 2003. Mice intradermally inoculated with the intact lipopolysaccharide, but not the lipid A or O-chain, from Francisella tularensis LVS rapidly acquire varying degrees of enhanced resistance against systemic or aerogenic challenge with virulent strains of the pathogen. Microb. Pathog. 34:39-45. [DOI] [PubMed] [Google Scholar]
- 6.Cowley, S. C., C. J. Gray, and F. E. Nano. 2000. Isolation and characterization of Francisella novicida mutants defective in lipopolysaccharide biosynthesis. FEMS Microbiol. Lett. 182:63-67. [DOI] [PubMed] [Google Scholar]
- 7.Cowley, S. C., S. V. Myltseva, and F. E. Nano. 1996. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity and nitric oxide production. Mol. Microbiol. 20:867-874. [DOI] [PubMed] [Google Scholar]
- 8.Geisbert, T. W., P. B. Jahrling, and J. W. Ezzell, Jr. 1993. Use of immunoelectron microscopy to demonstrate Francisella tularensis. J. Clin. Microbiol. 31:1936-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley, G., R. Taylor, J. Prior, S. Newstead, P. G. Hitchen, H. R. Morris, A. Dell, and R. W. Titball. 2006. Grey variants of the live vaccine strain of Francisella tularensis lack lipopolysaccharide O-antigen, show reduced ability to survive in macrophages and do not induce protective immunity in mice. Vaccine 24:989-996. [DOI] [PubMed] [Google Scholar]
- 10.Kawula, T. H., J. D. Hall, J. R. Fuller, and R. R. Craven. 2004. Use of transposon-transposase complexes to create stable insertion mutant strains of Francisella tularensis LVS. Appl. Environ. Microbiol. 70:6901-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieffer, T. L., S. Cowley, F. E. Nano, and K. L. Elkins. 2003. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 5:397-403. [DOI] [PubMed] [Google Scholar]
- 12.Larsson, P., P. C. Oyston, P. Chain, M. C. Chu, M. Duffield, H. H. Fuxelius, E. Garcia, G. Halltorp, D. Johansson, K. E. Isherwood, P. D. Karp, E. Larsson, Y. Liu, S. Michell, J. Prior, R. Prior, S. Malfatti, A. Sjostedt, K. Svensson, N. Thompson, L. Vergez, J. K. Wagg, B. W. Wren, L. E. Lindler, S. G. Andersson, M. Forsman, and R. W. Titball. 2005. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37:153-159. [DOI] [PubMed] [Google Scholar]
- 13.Nano, F. E., N. Zhang, S. C. Cowley, K. E. Klose, K. K. Cheung, M. J. Roberts, J. S. Ludu, G. W. Letendre, A. I. Meierovics, G. Stephens, and K. L. Elkins. 2004. A Francisella tularensis pathogenicity island required for intramacrophage growth. J. Bacteriol. 186:6430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 15.Prior, J. L., R. G. Prior, P. G. Hitchen, H. Diaper, K. F. Griffin, H. R. Morris, A. Dell, and R. W. Titball. 2003. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J. Med. Microbiol. 52:845-851. [DOI] [PubMed] [Google Scholar]
- 16.Roth, Z. 1961. A graphic Probit method for the calculation of LD50 and relative toxicity. Cesk. Fysiol. 10:408-422. (In Czech.) [PubMed] [Google Scholar]
- 17.Sandstrom, G., S. Lofgren, and A. Tarnvik. 1988. A capsule-deficient mutant of Francisella tularensis LVS exhibits enhanced sensitivity to killing by serum but diminished sensitivity to killing by polymorphonuclear leukocytes. Infect. Immun. 56:1194-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santic, M., M. Molmeret, K. E. Klose, and Y. Abu Kwaik. 2006. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 14:37-44. [DOI] [PubMed] [Google Scholar]
- 19.Sjostedt, A. 2006. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 8:561-567. [DOI] [PubMed] [Google Scholar]
- 20.Sorokin, V. M., N. V. Pavlovich, and L. A. Prozorova. 1996. Francisella tularensis resistance to bactericidal action of normal human serum. FEMS Immunol. Med. Microbiol. 13:249-252. [DOI] [PubMed] [Google Scholar]
- 21.Vinogradov, E., W. J. Conlan, J. S. Gunn, and M. B. Perry. 2004. Characterization of the lipopolysaccharide O-antigen of Francisella novicida (U112). Carbohydr. Res. 339:649-654. [DOI] [PubMed] [Google Scholar]
- 22.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 23.Vinogradov, E. V., A. S. Shashkov, Y. A. Knirel, N. K. Kochetkov, N. V. Tochtamysheva, S. F. Averin, O. V. Goncharova, and V. S. Khlebnikov. 1991. Structure of the O-antigen of Francisella tularensis strain 15. Carbohydr. Res. 214:289-297. [DOI] [PubMed] [Google Scholar]