Abstract
Severe malarial anemia (SMA), caused by Plasmodium falciparum infections, is one of the leading causes of childhood mortality in sub-Saharan Africa. Although the molecular determinants of SMA are largely undefined, dysregulation in host-derived inflammatory mediators influences disease severity. Macrophage migration inhibitory factor (MIF) is an important regulator of innate inflammatory responses that has recently been shown to suppress erythropoiesis and promote pathogenesis of SMA in murine models. To examine the role of MIF in the development of childhood SMA, peripheral blood MIF production was examined in Kenyan children (aged <3 years, n = 357) with P. falciparum malarial anemia. All children in the study were free from bacteremia and human immunodeficiency virus type 1. Since deposition of malarial pigment (hemozoin [Hz]) contributes to suppression of erythropoiesis, the relationship between MIF concentrations and monocytic acquisition of Hz was also examined in vivo and in vitro. Circulating MIF concentrations declined with increasing severity of anemia and significantly correlated with peripheral blood leukocyte MIF transcripts. However, MIF concentrations in peripheral blood were not significantly associated with reticulocyte production. Multivariate regression analyses, controlling for age, gender, and parasitemia, further revealed that elevated levels of pigment-containing monocytes (PCM) was associated with SMA and decreased MIF production. In addition, PCM levels were a better predictor of hemoglobin and MIF concentrations than parasite density. Additional experiments in malaria-naive individuals demonstrated that hemozoin caused both increased and decreased MIF production in cultured peripheral blood mononuclear cells (PBMC) in a donor-specific manner, independent of apoptosis. However, PBMC MIF production in children with acute malaria progressively declined with increasing anemia severity. Results presented here demonstrate that acquisition of hemozoin by monocytes is associated with suppression of peripheral blood MIF production and enhanced severity of anemia in childhood malaria.
Annually, there are 350 to 500 million clinical cases of malaria that result in over 1 million deaths (69). The majority of malaria-related mortalities are due to infections with Plasmodium falciparum and occur in immune-naive infants and young children in sub-Saharan Africa, accounting for 18% of all deaths before 5 years of age (69). Severe malarial anemia (SMA) accounts for the greatest proportion of malaria-related morbidity and mortality worldwide (13). In holoendemic P. falciparum transmission areas, SMA is the commonest clinical presentation of severe malaria in children less than 3 years of age, with cerebral malaria occurring only in rare cases (44, 51).
Markedly reduced hemoglobin (Hb) concentrations in children with SMA result from overlapping but distinct processes, including direct and indirect destruction of parasitized red blood cells (pRBC), increased clearance of uninfected erythrocytes, and suppression of erythropoiesis (1, 65, 68). Recent studies in western Kenya illustrate that children with SMA (Hb < 6.0g/dl) have lower peripheral parasite densities than parasitemic children without anemia (Hb ≥ 11.0 g/dl) (44, 51), suggesting that acute hemolysis of red blood cells (RBC) is not likely responsible for the low Hb levels observed in children with SMA in this holoendemic region. It is well-documented that children with SMA have a number of bone marrow abnormalities, including dyserythropoiesis, ineffective erythropoiesis, and reduced proliferation of erythroid colonies (1, 64, 65, 68). These findings, along with studies showing that the reticulocyte response is reduced relative to the degree of anemia in children with malarial anemia, even in the presence of elevated levels of erythropoietin (14, 36, 49), suggest that suppression of erythropoiesis may play a central role in the development of SMA.
Although the molecular mechanisms that regulate suppression of erythropoiesis are only partially defined, overproduction of proinflammatory mediators is thought to play a prominent role in conditioning the severity of childhood malarial anemia (25, 35, 38, 42, 52, 55). Recent investigations by our laboratory (7, 33, 50, 67) and others (41, 43) have focused on defining the role of proinflammatory mediators such as macrophage migration inhibitory factor (MIF) in the immunopathogenesis of malarial anemia, since MIF plays a pivotal role in regulating the innate immune response to invading pathogens (17). MIF is a pleiotropic cytokine released by several cell types, including monocytes/macrophages (15), T cells (8, 26), and cells of the anterior pituitary gland (11). Although MIF has potent proinflammatory properties that protect against Salmonella enterica serovar Typhimurium (34), Leishmania major (31, 58, 70), and Trypanosoma cruzi (57), elevated levels of circulating MIF are also associated with enhanced pathogenesis of bacterial sepsis (11, 12, 16), suggesting that increased MIF production can elicit both protective and pathogenic responses in different infectious diseases.
Previous investigations have observed elevated levels of MIF protein in blood vessel walls of Malawian children with cerebral malaria (23, 24) and intervillous blood mononuclear cells from women with placental malaria (20, 21). Studies in murine models of malaria show that elevated plasma MIF concentrations are associated with suppression of erythropoiesis and enhanced severity of anemia (41, 43). However, our recent investigations, which were the first to report circulating MIF levels in children with malaria, demonstrated that peripheral blood MIF concentrations and peripheral blood mononuclear cell (PBMC) MIF mRNA expression were reduced in Gabonese children with mild-to-moderate forms of malarial anemia and hyperparasitemia (7). In contrast, subsequent studies have shown that plasma MIF levels are elevated in Zambian children with malarial anemia (43).
Although the host-parasite interactions that mediate MIF production are largely undefined, studies from our laboratory (32, 33, 54),and others (5, 43, 56, 61) show that ingestion of malarial pigment, hemozoin (Hz), by phagocytic cells causes dysregulation in cytokine production. Hz is a coordinated aggregation polymer of heme generated by plasmodia during digestion of host Hb (28). During a malaria infection, Hz is acquired by leukocytes through direct phagocytosis of pRBC and free Hz released upon pRBC rupture (60). Recent studies have demonstrated that Hz contributes to the pathogenesis of SMA by suppressing erythropoiesis both directly and in synergy with proinflammatory cytokines, such as tumor necrosis factor alpha (18). In addition, acquisition of Hz by murine macrophages and human monocytes is associated with enhanced MIF production (41, 43).
To investigate the role of MIF in the immunopathogenesis of malarial anemia, peripheral blood MIF concentrations and leukocyte MIF transcript levels were measured in infants and young children residing in a holoendemic P. falciparum transmission area where severe anemia is the primary clinical manifestation of severe malaria. In addition, to examine host-parasite interactions that may be important for regulating MIF production, the relationship between circulating MIF levels and monocytic acquisition of Hz was determined. The direct effect of Hz on MIF production was also investigated in cultured PBMC from malaria-naive individuals. In vivo and in vitro results presented here demonstrate that MIF is suppressed in children with SMA and that monocyte-acquired P. falciparum-derived Hz (pfHz) plays an important role in promoting SMA and decreasing MIF production.
(A portion of this work was presented at the fourth Multilateral Initiative on Malaria [MIM] Pan-African malaria conference held in Yaoundé, Cameroon, 13 to 18 November 2005.)
MATERIALS AND METHODS
Study site.
Children (n = 357, age 3 to 31 months) were recruited at the pediatric ward of the Siaya District Hospital (SDH), Nyanza Province, western Kenya. In this region, malaria is holoendemic with entomological inoculation rates of up to 300 per annum (9). Approximately one-third of all pediatric admissions to SDH are due to SMA, which accounts for ∼50% of all pediatric deaths at the hospital (37). A detailed description of the study site and cohort is provided in our recent report (44, 51).
Study participants.
A questionnaire and medical informatics system were used to recruit children at their first hospital contact for the treatment of malaria. After obtaining informed written consent from the parents/guardians of children presenting at SDH with the signs and symptoms of malaria, heel/finger-prick blood (<100 μl) was used to determine parasitemia and Hb concentrations. Children with P. falciparum parasitemia (any density) were categorized according to the following criteria: uncomplicated malaria (UM), Hb ≥ 11.0 g/dl, n = 26; mild malarial anemia (MlMA), 8.0 ≤ Hb < 11.0 g/dl, n = 75; moderate malarial anemia (MdMA), 6.0 ≤ Hb < 8.0 g/dl, n = 98; SMA, Hb < 6.0 g/dl, n = 119. Case definitions of anemia were based on previous studies in western Kenya examining >10,000 longitudinal Hb measurements in an age- and geographically matched population (44). Healthy, aparasitemic children (AC, Hb ≥ 11.0 g/dl, n = 39) presenting at SDH for routine immunizations were recruited as controls. In addition, only those children that were afebrile for ≥2 weeks were included in the AC group. None of the children in the current study had any signs/symptoms of cerebral malaria. Since human immunodeficiency virus type 1 (HIV-1) and bacteremia are common copathogens that influence anemia status in children with malaria (2, 10, 29, 53) and could influence MIF production, all study participants were screened for HIV-1 and/or bacteremia, and those children found to have copathogens were excluded from all analyses. Pre- and posttest HIV counseling was provided to parents/guardians of all children enrolled. The study was approved by the Ethics Committees of the Kenya Ministry of Health and the University of Pittsburgh Institutional Review Board.
Sample collection.
Prior to administration of antimalarials and/or any other treatment interventions, venous blood (<3 ml, a volume determined to be safe based on size, weight, and anemia status) was collected, and plasma was isolated according to our previous methods (44, 51). Leukocytes were obtained from the buffy coat by lysing RBC followed by storage in RNAlater (Ambion) at −20°C until use.
Laboratory evaluation.
Giemsa-stained blood smears were used to determine parasitemia and pigment-containing monocytes and neutrophils. The numbers of RBC infected with asexual P. falciparum parasites were determined per 300 leukocytes, and the peripheral parasite density/μl of blood was calculated using the white blood cell count/μl obtained on a Coulter AcT diff2 (Beckman Coulter Corp.). A total of 30 monocytes and 100 neutrophils were examined per thin smear, and the number of pigment-containing monocytes (PCM) or pigment-containing neutrophils (PCN) was expressed as a percentage of the total number of monocytes or neutrophils, respectively (39, 48). The number of PCM/μl of blood was calculated by multiplying the percentage of PCM by the absolute number of monocytes/μl obtained from the Coulter AcT diff2. Reticulocyte counts (percent) were determined using new methylene blue-stained slides, and the absolute reticulocyte number (ARN) was calculated by multiplying the reticulocyte percentage/100 by total RBC counts. HIV-1 status was determined per our previously described HIV-1 serology and PCR methods (53), while bacteremia was determined using the Wampole ISOLATOR 1.5-ml microbial system (Inverness Medical).
Determination of plasma MIF and leukocyte MIF transcript levels.
To avoid the possible influence of MIF released from lysis of erythrocytes (47) during blood clotting, circulating MIF levels were determined in plasma rather than serum samples. In addition, visibly hemolyzed samples were excluded from measurements. Plasma and culture supernatant MIF concentrations were determined by enzyme-linked immunosorbent assay (ELISA) with a matched anti-MIF antibody pair (R&D Systems). All samples were assayed at 1:5 and 1:10 dilutions in duplicate and according to manufacturer's recommendations. The limit of detection was >31.25 pg/ml. For determination of MIF transcript levels, total RNA was extracted from leukocyte pellets using the guanidinium isothiocyanate method as described previously (22). Fluorogenic primer/probe sets specific for MIF and the housekeeping gene, β-actin (assay identifiers Hs00236988_g1 and 4326315E, respectively; Applied Biosystems) were used for real-time reverse transcription-PCR on an ABI Prism 7700 sequence detection system (Applied Biosystems). MIF mRNA levels were normalized by expressing transcripts as change relative to β-actin mRNA (2−ΔCT, where ΔCT = critical threshold cycle of MIF − critical threshold cycle for β-actin), as described previously (7).
PBMC cultures.
Venous blood was obtained from healthy, malaria-naive U.S. donors (n = 15) and children with acute malaria (n = 94). PBMC were isolated using Ficoll-Hypaque according to previous methods (66). To ensure complete removal of RBC, PBMC were treated with RBC lysis buffer (BioWhittaker) for 5 min and then washed prior to culture. pfHz was isolated from laboratory-cultivated P. falciparum (PfD6), and synthetic Hz (sHz) was prepared from hemin chloride (Sigma) as described previously (32). Endotoxin levels in all pfHz and sHz preparations were determined to be <0.125 U/ml (i.e., <0.025 ng/ml; Limulus amebocyte lysate test; BioWhittaker). PBMC were plated at 1 × 106 cells/ml in Dulbecco's modified Eagle's medium containing HEPES buffer (25 mM), penicillin (100 U/ml)-streptomycin (100 μg/ml), and 10% heat-inactivated human serum from a nonmalarious region. Samples from children with acute malaria were cultured in media alone, while PBMC from U.S. donors were stimulated with media alone (unstimulated control), physiological concentrations of pfHz (10 μg/ml) (33), sHz (10 μg/ml), or lipopolysaccharide (LPS, 100 ng/ml; Alexis Corp.), and gamma interferon (IFN-γ, 200 U/ml; Biosource).
Cell viability and apoptosis assays.
The viability of cultured PBMC was determined using a methylthiazoletetrazolium (MTT)-based assay according to the manufacturer's recommendations (Sigma). Cellular apoptosis was assessed by quantifying the concentrations of nucleosomes in cell lysates (early-stage apoptosis) and culture supernatants (late-stage apoptosis) using a cell death detection ELISA (Roche Diagnostics) according to the manufacturer's recommendations, with nucleosome concentrations in treated cells expressed as a percentage relative to untreated cells (control).
Statistical analyses.
Kruskal-Wallis tests were used to compare variables across three or more groups, and where significant differences were observed, Mann-Whitney U tests were conducted for pairwise comparisons. Statistical associations between variables were examined using Pearson's correlation tests and multivariate linear and logistic regression analyses, controlling for age and gender. Prior to performing Pearson's correlational analyses, the distributional characteristics of all variables were examined for departures from normality using the Kolmogorov-Smirnov test. Those variables with significant skewness were transformed toward normality. P values of less than 0.05 were considered statistically significant for all analyses.
RESULTS
Clinical, parasitological, and hematological characteristics of study participants.
To examine the role of MIF in the immunopathogenesis of malarial anemia, children (n = 357) were stratified into clinical anemia categories. The clinical, parasitological, and hematological characteristics of the study participants upon admission are summarized in Table 1. Across-group comparisons showed significant differences in age (P = 0.005) and axillary temperature (P < 0.001). Children in the UM group were significantly older relative to all other groups (P < 0.01 for all comparisons). Axillary temperature was elevated in all categories of children with acute malaria relative to the AC group (P < 0.001 for all groups). Mean peripheral parasitemia (P = 0.117), geometric mean parasitemia, and the proportions of children with high-density parasitemia (HDP [≥10,000 parasites/μl]; P = 0.656) were not significantly different among children with acute malaria (Table 1). Since anemia severity was the basis for classification, Hb and RBC numbers decreased across the groups (P < 0.001 for both comparisons). In contrast, the ARN increased with increasing severity of anemia (P < 0.001). Taken together, these data illustrate that the severity of anemia in this holoendemic P. falciparum transmission region is not significantly associated with peripheral parasite density.
TABLE 1.
Clinical, parasitological, and hematological characteristics of study participants
| Parameter | Mean (SEMa) for group:
|
P value | ||||
|---|---|---|---|---|---|---|
| AC | UM | MlMA | MdMA | SMA | ||
| No. of children | 39 | 26 | 75 | 98 | 119 | |
| Age (mo) | 9.1 (1.2) | 14.0 (1.9) | 12.2 (0.8) | 11.5 (0.6) | 10.2 (0.6) | 0.005b |
| Axillary temp (°C) | 36.8 (0.2) | 37.6 (0.2) | 37.6 (0.1) | 37.7 (0.1) | 37.6 (0.1) | <0.001b |
| No. of parasites/μl | 0 | 84,118 (22,734) | 44,168 (6,092) | 48,697 (5,925) | 74,490 (10,443) | 0.117b |
| Geometric mean no. of parasites/μl | 0 | 29,689 | 21,020 | 18,623 | 31,458 | |
| No. (%) of children with HDP | 0 | 20 (77) | 53 (71) | 71 (72) | 93 (78) | 0.656c |
| Hemoglobin (g/dl) | 11.7 (0.1) | 11.8 (0.2) | 9.2 (0.1) | 6.9 (0.1) | 4.7 (0.1) | <0.001b |
| % Hematocrit | 35.2 (0.3) | 34.5 (1.1) | 28.0 (0.4) | 21.7 (0.2) | 15.1 (0.3) | <0.001b |
| RBC (106/μl) | 4.91 (0.08) | 4.78 (0.13) | 4.15 (0.07) | 3.23 (0.06) | 2.18 (0.05) | <0.001b |
| ARN (103/μl) | 97.5 (18.7) | 105.7 (16.5) | 110.6 (8.7) | 147.3 (11.6) | 140.7 (10.4) | <0.001b |
| % PCM | 0.8 (0.8) | 3.1 (1.0) | 7.6 (1.4) | 15.2 (1.6) | <0.001b | |
| PCM (103/μl) | 0.01 (0.01) | 0.04 (0.02) | 0.09 (0.02) | 0.23 (0.03) | <0.001b | |
Data are presented as means with standard errors of the means indicated in parentheses except where otherwise indicated. Comparisons of parasitemia were among parasitemic groups only.
Kruskal-Wallis test.
Chi-square test.
Circulating MIF levels progressively decline with increasing severity of malarial anemia.
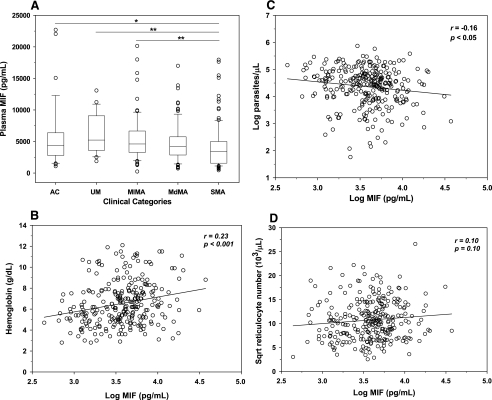
Our previous investigations showed that peripheral blood MIF concentrations were suppressed in a small cohort of children with acute malaria (7). In contrast, additional studies (also limited by sample size) illustrated that plasma MIF concentrations were elevated in children with acute malaria (43). To more fully investigate the role of MIF in the immunopathogenesis of childhood malaria, children were stratified according to malarial anemia status, and circulating MIF levels were examined in the UM (n = 23), MlMA (n = 71), MdMA (n = 94), and SMA (n = 109) groups and healthy AC (n = 39). As shown in Fig. 1A, MIF concentrations progressively declined in parasitemic children with increasing severity of anemia. Children with SMA (median [interquartile range], 3,422 pg/ml [1,566 to 4,993 pg/ml]) had lower plasma MIF levels than children in the AC (4,383 pg/ml [2,807 to 6,376 pg/ml]; P < 0.05), UM (5,225 pg/ml [3,615 to 9,071 pg/ml]; P < 0.001), and MlMA (4,611 pg/ml [3,270 to 6,665 pg/ml]; P < 0.001) groups (Fig. 1A). In addition, there was a significant positive correlation between circulating MIF levels and Hb concentrations (r = 0.23; P < 0.001) and a significant inverse correlation between plasma MIF levels and parasitemia (r = −0.16; P < 0.05) among children with malaria (Fig. 1B and C). However, there was a nonsignificant correlation between circulating MIF concentrations and the absolute reticulocyte number (r = 0.10; P = 0.10) (Fig. 1D). Multiple regression analysis, controlling for age and gender, further revealed that MIF was a significant positive predictor of Hb concentration (standardized partial regression coefficient [β-weight] = 0.153; P < 0.005) and a negative predictor of parasitemia (β-weight = −0.096; P = 0.067). These results demonstrate that SMA is characterized by reduced peripheral blood MIF concentrations and that pRBC and/or a parasitic product(s) either directly or indirectly promotes suppression of MIF concentrations.
FIG. 1.
Relationship of plasma MIF with anemia, parasite density, and absolute reticulocyte number. Plasma levels of MIF in children with malaria and controls were determined by ELISA. (A) Data are presented according to the following anemia categories: UM (Hb ≥ 11.0 g/dl, n = 23), MlMA (8.0 ≤ Hb < 11.0 g/dl, n = 71), MdMA (6.0 ≤ Hb < 8.0 g/dl, n = 94), and SMA (Hb < 6.0 g/dl, n = 109). AC (n = 39) with Hb levels of ≥11.0 g/dl were used as a reference group. Boxes represent the interquartile range, the line through the box is the median, whiskers show 10th and 90th percentiles, and symbols are outliers. Median (interquartile range) levels of MIF were as follows (in pg/ml): AC, 4,383 (2,807 to 6,376); UM, 5,225 (3,615 to 9,071); MlMA, 4,611 (3,270 to 6,665); MdMA, 4,197 (2,862 to 5,743); SMA, 3,422 (1,566 to 4,993). *, P < 0.05; **, P < 0.001. Mann-Whitney U tests conducted after analysis of variance (Kruskal-Wallis test) revealed significant differences across groups. Linear relationships of plasma MIF levels with hemoglobin concentrations (B), parasite density (C), and ARN (D) in children with malaria (n = 298) are shown as scatter plots. MIF concentrations and parasitemia were log transformed, while the ARN was square root (sqrt) transformed for normality. Statistical associations were determined by Pearson's correlation tests.
Peripheral blood leukocyte MIF transcripts as a source of circulating MIF.
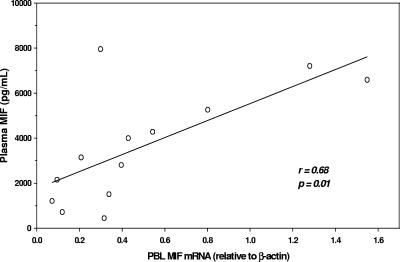
Although MIF is produced by a number of different cell types (11, 15, 17), our recent investigations of Gabonese children with malaria demonstrated that suppression of plasma MIF concentrations was associated with decreased PBMC MIF transcripts (7). Based on the number of different experimental measures performed with limited blood sample volumes from children with malarial anemia, we opted to measure MIF transcripts in peripheral blood leukocytes (PBL) from a subset of children in which matched samples were available. These experiments revealed that MIF transcripts closely paralleled circulating MIF concentrations (r = 0.68; P = 0.01) (Fig. 2), suggesting that MIF gene expression in PBL may be an important source of circulating MIF levels.
FIG. 2.
Correlation of PBL MIF mRNA with circulating MIF. Levels of MIF mRNA in PBL (n = 13) were determined by real-time reverse transcription-PCR and expressed relative to endogenous β-actin mRNA levels. The scatter plot shows the correlation of PBL MIF mRNA with circulating MIF levels in matched samples. Statistical association was determined by Pearson's rank correlation test.
MIF production from cultured PBMC progressively declines with increasing anemia severity.
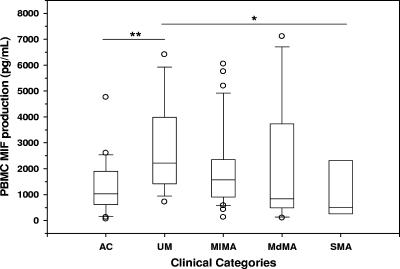
To further investigate MIF production in children with malarial anemia, PBMC MIF production was measured under baseline conditions in cultured cells isolated from the following groups of children with varying degrees of malarial anemia and healthy controls: AC (n = 24), UM (n = 15), MlMA (n = 37), MdMA (n = 13), and SMA (n = 5). MIF concentrations in culture supernatants were significantly increased in children with UM (median [interquartile range], 2,218 pg/ml [1,418 to 3,989 pg/ml]) relative to the AC group (1,034 pg/ml [615 to 1,902 pg/ml]; P < 0.005) (Fig. 3) at 48 h. However, MIF production progressively declined with increasing anemia severity, with the SMA group (511 pg/ml [264 to 2,312 pg/ml]) having lower culture supernatant MIF levels than the UM (P < 0.05), MlMA (1,609 pg/ml [940 to 2,439 pg/ml]; P = 0.08), and MdMA (842 pg/ml [495 to 3,737 pg/ml]; P = 0.43) groups (Fig. 3). MIF production did not significantly differ between the AC group and any of the malarial anemia groups (SMA, P = 0.51; MdMA, P = 0.87; MlMA, P = 0.06). Taken together, these in vitro experiments demonstrate that reduced MIF production from PBMC contributes to decreased circulating MIF levels in children with malarial anemia. Furthermore, increased MIF production in children with a favorable clinical outcome (i.e., children with uncomplicated malaria) suggests that increased MIF production during childhood malarial anemia may provide a protective response against severe disease.
FIG. 3.
MIF production from PBMC of children with acute malaria. PBMC were isolated from the following groups of children with acute malaria and healthy controls (AC) and cultured for 48 h: AC (n = 24), UM (n = 15), MlMA (n = 37), MdMA (n = 13), and SMA (n = 5). MIF concentrations in culture supernatants were determined by ELISA and presented as box plots in which the box represents the interquartile range, the line through the box is the median, whiskers show 10th and 90th percentiles, and symbols are outliers. Median (interquartile range) levels of MIF were as follows (in pg/ml): AC, 1,034 (615 to 1,902); UM, 2,218 (1,418 to 3,989); MlMA, 1,609 (940 to 2,439); MdMA, 842 (495 to 3,737); SMA, 511 (264 to 2,312). *, P < 0.05; **, P < 0.005. Mann-Whitney U tests conducted after analysis of variance (Kruskal-Wallis test) revealed significant differences between the groups.
In vivo acquisition of pfHz by monocytes is associated with enhanced SMA.
Recent studies of individuals with malaria suggest that phagocytosis of pfHz by monocytes and neutrophils is an important predictor of clinical severity (4, 18, 39, 48). Additional studies have demonstrated that sHz enhanced MIF production in mouse peritoneal macrophages and human mononuclear cells (43). To further examine the role of naturally acquired pfHz in conditioning disease outcomes in children with malarial anemia, the relationship between intracellular malarial pigment and malaria disease severity was determined. Percentages of PCM ranged from 0 to 90% (mean, 10%), while the percentages of PCN ranged from 0 to 9% (mean, 0.3%), with PCM present in 47% (144/306) and PCN present in 12% (37/306) of the study participants. Based on the higher abundance of PCM relative to PCN, we examined the impact of PCM on clinical outcomes. The proportion of pigment-containing monocytes (%PCM) and PCM/μl progressively increased across the acute malaria groups (P < 0.001 for both comparisons) (Table 1). In a multiple regression model controlling for age, gender, and parasitemia, PCM emerged as a strong predictor of Hb concentrations (β-weight = −0.434; P < 0.001).
To further investigate the role of PCM in conditioning the outcomes of acute malaria, children were stratified into three groups according to the distribution of PCM: no PCM (0% PCM), low PCM (PCM ≤ 10%), and high PCM (PCM > 10%). These analyses revealed that age was not significantly different across the groups (P = 0.645). Moreover, although parasitemia increased with elevated PCM across the three groups (P = 0.034) (Table 2), parasitemia and the percentage of HDP did not significantly differ between the high- and low-PCM groups (P = 0.181 and P = 0.086, respectively) (Table 2). Conversely, Hb concentrations decreased with increasing pfHz deposition (P < 0.001, across groups), with the high-PCM group having the highest percentage of SMA cases (Table 2). Multiple logistic regression analyses controlling for age, gender, and parasitemia demonstrated that the risk of SMA was increased for the low-PCM (odds ratio [OR] = 3.4; 95% confidence interval [CI], 1.8 to 6.6; P < 0.0001) and high-PCM (OR = 7.5; 95% CI, 4.1 to 14.0; P < 0.0001) groups relative to the no-PCM group (Table 2). There was also an increased risk of SMA in the high-PCM group compared to the low-PCM group (OR = 2.1; 95% CI, 1.1 to 4.3; P < 0.05). Thus, consistent with the demonstrated role of Hz in suppression of erythropoiesis during malarial anemia (18), these results show that deposition of pfHz in circulating monocytes is associated with an increased prevalence of SMA.
TABLE 2.
Relationship between monocyte acquisition of hemozoin and disease severity in children with malaria
| Parameter | Mean (SEMa) for PCM group:
|
P value | ||
|---|---|---|---|---|
| No PCM (0%) | Low PCM (≤10%) | High PCM (>10%) | ||
| No. (%) of children | 162 (53) | 62 (20) | 82 (27) | |
| Age (mo) | 11.3 (0.5) | 12.2 (0.9) | 11.1 (0.8) | 0.645b |
| No. of parasites/μl | 49,371 (5,088) | 60,141 (9,906) | 80,447 (13,266)c | 0.034b |
| No. (%) of children with HDP | 113 (70) | 50 (79) | 67 (82) | 0.086d |
| Hemoglobin (g/dl) | 7.8 (0.2) | 6.5 (0.2) | 5.7 (0.2)e | <0.001b |
| No. (%) of children with SMA | 34 (21) | 28 (46) | 53 (65)e | <0.001c |
| Odds of SMAf | 1 | 3.4 (1.8-6.6) | 7.5 (4.1-14.0) | |
Data are presented as means with standard errors of the means indicated in parentheses unless otherwise indicated.
Kruskal-Wallis test.
Significantly different from the no-PCM group but not from the low-PCM group.
Chi-square test.
Significantly different from the no-PCM and low-PCM groups.
Multivariate logistic regression using the no-PCM group as a reference and controlling for age, gender, and parasitemia. Data are presented as odds ratios with 95% confidence intervals indicated in parentheses; P < 0.0001 for both low- and high-PCM groups.
In vivo acquisition of pfHz by monocytes is associated with suppression of MIF.
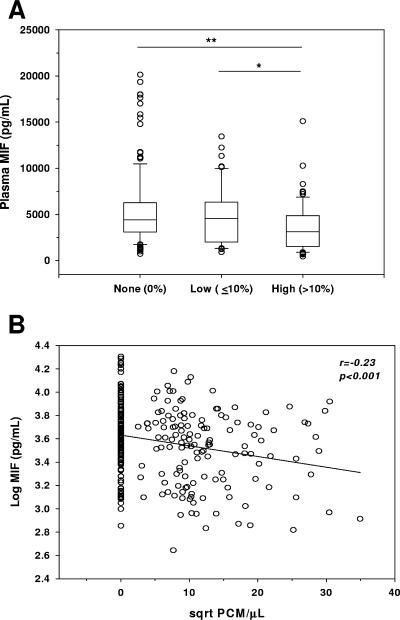
Since PCM was strongly correlated with SMA and MIF concentrations declined with increasing severity of anemia, the relationship between PCM and MIF was examined. The high-PCM group had lower MIF levels (median [interquartile range], 3,115 pg/ml [1,525 to 4,879 pg/ml]) than the no-PCM (4,417 pg/ml [3,112 to 6,266 pg/ml]; P < 0.005) and low-PCM (4,584 pg/ml [1,525 to 4,879 pg/ml]; P < 0.05) groups (Fig. 4A). Furthermore, circulating MIF concentrations were significantly inversely correlated with PCM levels (r = −0.23; P < 0.001) (Fig. 4B). Multiple linear regression analyses controlling for age and gender further revealed that PCM (β-weight = −0.238; P < 0.001) was a stronger predictor of MIF than parasitemia (β-weight = −0.087; P = 0.024), suggesting that monocytic acquisition of pfHz may contribute to suppression of MIF concentrations.
FIG. 4.
Relationship between plasma MIF levels and pigment-containing monocytes. Giemsa-stained blood smears obtained from children with malaria were examined for the presence of PCM. (A) Plasma levels of MIF are presented according to percentage of total monocytes containing pigment, as follows: none (no PCM observed, n = 149), low (≤10% PCM, n = 60), and high (>10% PCM, n = 79). Boxes represent the interquartile range, the line through the box is the median, whiskers show 10th and 90th percentiles, and symbols are outliers. Median (interquartile range) levels of MIF were as follows (in pg/ml): no PCM, 4,417 (3,112 to 6,266); low PCM, 4,584 (2,019 to 6,331); high PCM, 4,417 (3,112 to 6,266). *, P < 0.05; **, P < 0.005. Mann-Whitney U tests conducted after analysis of variance (Kruskal-Wallis test) revealed significant differences across groups. (B) Correlation between circulating MIF concentrations and PCM/μl of blood. MIF levels were log transformed, while PCM/μl values were square root (sqrt) transformed for normality, and Pearson's correlation test was used to examine statistical association.
pfHz and sHz decrease MIF production in cultured PBMC independent of apoptosis.
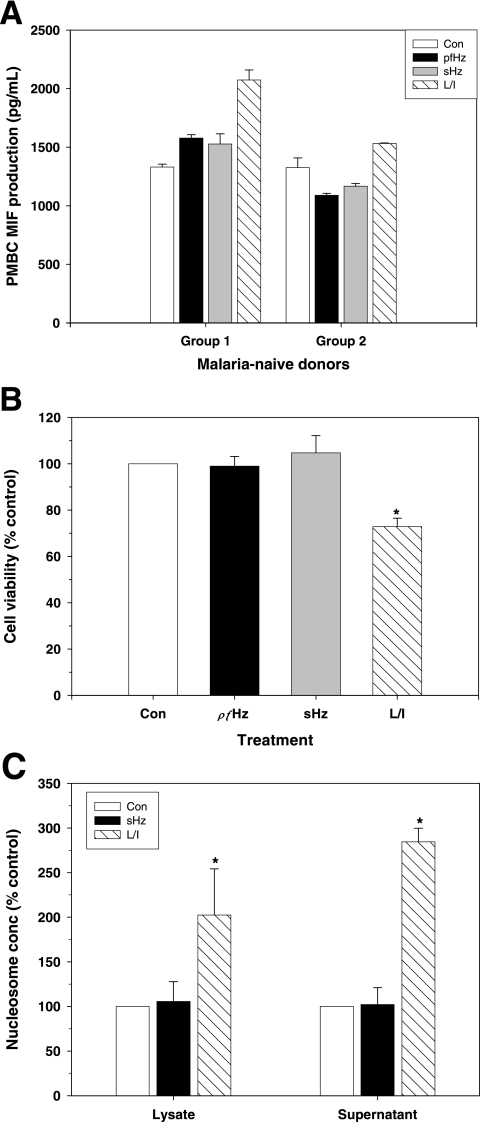
Since the in vivo results suggested that PCM may be an important factor for suppressing MIF production, the direct effect of malarial pigment on MIF production was examined in PBMC from healthy, malaria-naive U.S. donors. Cultures were stimulated with concentrations of pfHz or sHz comparable to those in children with severe malaria (i.e., 10 μg/ml) (33) and LPS and IFN-γ (L/I) as a positive control. Treatment of PBMC with pfHz or sHz resulted in a significant increase in MIF production in 3/15 donors (P < 0.05 for all comparisons; group 1) (Fig. 5A), while MIF production was significantly decreased in 11/15 donors (P < 0.05 for all comparisons; group 2) (Fig. 5A). In addition, there was no significant change in MIF production in pfHz- or sHz-stimulated PBMC from one donor (data not shown). Stimulation of PBMC with L/I increased MIF production in 9/15 donors (P < 0.05 for all comparisons) (Fig. 5A), while MIF production was not significantly altered in the PBMC of 6/15 donors (data not shown).
FIG. 5.
Effects of hemozoin on MIF production and apoptosis of cultured leukocytes. PBMC from malaria-naive donors were cultured (1 × 106 cells/ml) in the presence of media alone (Con), 10 μg/ml hemozoin (pfHz), 10 μg/ml β-hematin (sHz), or a combination of 100 ng/ml LPS (L) and 200 U/ml IFN-γ (I). (A) Supernatants were harvested after 48 h of incubation, and MIF concentrations were determined by ELISA. Data shown are for three donors representative of individuals for whom MIF increased (group 1) and three donors representative of individuals for whom MIF decreased (group 2) in response to stimulation with pfHz and sHz. Differences in MIF levels between treated cells and control conditions were statistically significant (P < 0.05 for all comparisons, Student's t test). (B) Cell viability was assessed in PBMC from 3 donors after 48 h of culture using a MTT assay and expressed as a percentage of the control result. (C) PBMC apoptosis was determined by detecting nucleosomes in cell lysates and supernatants at 48 h using ELISA. Data are presented as means (±standard errors of the means) of results from independent experiments. *, P < 0.05 compared to Con, Student's t test.
Since pfHz or sHz could alter MIF by influencing PBMC viability, cellular metabolism was investigated with the MTT assay. Relative to control conditions, stimulation with pfHz or sHz had no significant effect on cellular viability at 48 h (P = 0.601 and P = 0.522, respectively), while L/I reduced viability by 28% (P < 0.05) (Fig. 5B). Examination of cellular apoptosis by measuring nucleosome release (DNA fragmentation) revealed that both early- and late-stage apoptosis were unaffected by sHz treatment (P = 0.247 and P = 0.928, respectively). However, stimulation with L/I increased both early and late apoptosis (P < 0.001 for both comparisons) (Fig. 5C), which may explain the lack of significantly enhanced MIF production in some of the donors. Taken together, these results show that malarial pigment decreases MIF production from PBMC, independent of altered cell viability or apoptosis.
DISCUSSION
A number of studies in murine models of malaria suggest that there is a circulating host-derived inflammatory mediator(s) that inhibits the erythropoietic response (46, 62, 71), and recent evidence has identified MIF as the potential immune mediator that suppresses erythropoiesis during malaria (41, 43). However, the role of MIF in regulating anemia in childhood malaria remains unclear because of contradictory findings in small cohorts of children (7, 43). While our previous study demonstrated suppression of circulating MIF and ex vivo PBMC MIF transcripts in Gabonese children with acute malaria versus healthy, aparasitemic controls (7), subsequent investigations illustrated that plasma MIF levels were elevated in Zambian children with malarial anemia relative to healthy controls (43).
To further investigate the role of MIF in the immunopathogenesis of malarial anemia, the relationship between MIF production and anemia was investigated in a large cohort of clinically well-characterized children that were stratified according to the severity of malarial anemia. These studies confirmed our previous finding in Gabon (7) and extended this observation by demonstrating that circulating MIF levels and MIF production from PBMC isolated from children with acute malaria progressively declined with increasing severity of malarial anemia. Results presented here also illustrate that there was a significant positive correlation between circulating MIF levels and Hb concentrations. Moreover, the large sample size in the current study allowed us to confirm the significant positive association between MIF and Hb by conducting multivariate regression analyses that controlled for important confounders, such as age. Our previous studies in this cohort of children demonstrated that the reticulocyte response was inappropriate for the level of anemia and that children with malarial anemia had suppression of erythropoiesis (67). Correlation analyses examining the relationship between MIF and erythropoiesis revealed that MIF was not significantly associated with the absolute reticulocyte number, suggesting that circulating MIF may not be responsible for suppression of erythropoiesis.
The basis of different MIF production patterns in Zambian children (43) versus those in Gabon (7) and in Kenyan children presented here remains to be defined. However, our studies in western Kenya and those of others (2, 10, 29, 53) have shown that children presenting with malarial anemia often have coinfections with bacteremia and HIV-1 which could potentially alter MIF production. Although children with coinfections were excluded from our analyses, it is not certain if such coinfected children were excluded from other studies. In addition, since RBC contain substantial quantities of MIF (47), the use of plasma from hemolyzed blood could yield falsely elevated MIF concentrations. For analysis of circulating MIF in the current study, all plasma samples were examined by an individual who was blinded to the clinical categories of study participants, and those samples with evidence of hemolysis were excluded from measurement. Furthermore, it is well documented that malaria transmission intensity conditions the host immune response and age-specific pathophysiological manifestations of P. falciparum (40, 63). The relatively older children examined in Zambia (mean age, 25.5 months), where P. falciparum endemicity is lower, therefore, may have different immunopathogenic mechanisms responsible for the promotion of malarial anemia than the younger children examined here in western Kenya (Table 1) residing in a holoendemic transmission area.
Recent investigations illustrated that MIF suppresses erythropoietin-dependent colony formation in cultured erythroid progenitor cells and Hb synthesis in murine and human cell lines (43). Additional studies showed that elevated MIF concentrations are present in bone marrow lysates of Plasmodium chabaudi-infected mice (41). However, measurement of MIF concentrations in the bone marrow of children with SMA will be important for understanding the role of MIF in human malaria, since contrasting roles of MIF in murine studies (41, 43) versus those in human studies is not unprecedented. For example, previous investigations revealed that MIF production in response to glucocorticoids differs in murine and human systems (3, 30). Furthermore, while anemia in murine models of malaria closely correlate with peripheral parasitemia and RBC hemolysis, data presented here illustrate that parasite density and anemia severity are unrelated in children with SMA. It is also important to note that MIF production during malaria appears to be both tissue and compartment specific (19, 20, 23). For example, previous studies of women with placental malaria showed increased MIF locally in intervillous blood in the presence of decreased peripheral blood MIF concentrations (20). Therefore, our findings do not exclude the possibility that MIF may be increased in the local milieu within the bone marrow of the children in our cohort despite the reduced concentrations of MIF in the peripheral circulation.
Previous studies in malaria-endemic regions illustrate that phagocytosis of pfHz by monocytes and neutrophils is a better index of disease severity than peripheral parasitemia (4, 18, 39, 45, 48). This may be related to the fact that concomitant peripheral parasitemia does not account for parasite sequestration within microvascular networks and/or the duration of infection. In this study, there was a lower prevalence of PCM and PCN than previously observed by others (4, 39, 45). This may be because children in the present study were younger and, therefore, likely more immune naive to P. falciparum. Importantly, the clearance kinetics of PCM and PCN differ considerably, with median PCM and PCN clearance times of approximately 10 and 4 days, respectively (27). Increased abundance of PCM relative to PCN in the current cohort suggests that malarial anemia results from prolonged (chronic) malaria infections. Although Hz has been shown to regulate MIF production in vitro (6, 43), the relationship between MIF production and Hz levels in vivo has not been examined previously. Multivariate analyses controlling for age, gender, and parasitemia revealed a significant association between increased levels of PCM, SMA, and decreased circulating MIF levels, suggesting that acquisition of pfHz by monocytes may promote SMA and suppress peripheral MIF production.
Based on the statistical relationship between elevated PCM and decreased MIF, malarial pigment was investigated as a parasitic source responsible for MIF suppression in children with SMA. A series of experiments in cultured PBMC from malaria-naive donors identified two main types of responders: those who increased MIF production and those who decreased MIF production in response to stimulation by physiological doses (33) of pfHz or sHz. Cell viability experiments further demonstrated that reductions in MIF production were not mediated by a loss in cell viability or increased apoptosis. Identical results observed with pfHz and sHz demonstrate that the core ferriprotoporphyrin IX structure, rather than adherent host and/or parasitic proteins, lipids, or nucleic acids, is responsible for altering MIF production. Recent studies in cultured mononuclear cells from individuals with low (5-CATT/5-CATT)- and high (6-CATT/6-CATT and 6-CATT/7-CATT)-expression MIF-794 alleles show that MIF production in response to sHz stimulation was genotype dependent (43). Although the genetic backgrounds of the donors examined in the present study were unknown, subsequent investigations in our laboratories have revealed that PBMC from individuals with the GG genotype at MIF-173 have increased MIF production, while GC individuals have decreased MIF production following stimulation with pfHz (6). It is, therefore, not surprising that circulating MIF concentrations were generally inversely correlated with PCM levels in the study cohort, since the C allele is predominant in this cohort of children (6). The molecular mechanism(s) by which acquisition of malarial pigment decreases MIF production remains to be defined. However, previous studies demonstrated that ingestion of Hz causes impairment of several cellular functions, including cytokine secretion, phagocytosis, and antigen presentation (59, 60). It remains to be determined if suppression of MIF in response to Hz occurs through a direct or indirect mechanism following phagocytosis of Hz. Taken together, the results presented here, which represent the most comprehensive examination to date of the role of MIF in promoting childhood malarial anemia, suggest that MIF may not be responsible for enhanced anemia in pediatric populations with acute falciparum malaria and that monocytic ingestion of Hz is responsible, either directly or indirectly, for suppression of MIF.
Acknowledgments
We are very grateful to all the parents, guardians, and children from the Siaya District community and the U.S. donors for their participation in this study. We also thank the University of Pittsburgh/KEMRI staff and the Siaya District Hospital staff for their support during the study. We thank the Director of KEMRI for approving the manuscript for publication.
There is no conflict of interest for any of the authors of this report due to either commercial or other affiliations.
The study was funded by National Institutes of Health grant 1 R01 (to D.J.P.) and Fogarty International Center training grant 1 D43 (to D.J.P.).
The study was approved by the Ethics Committees of the Kenya Medical Research Institute (KEMRI), Kenyan Ministry of Health, and the University of Pittsburgh Institutional Review Board, and informed consent was obtained from all participants or the parents/legal guardians of all participating children.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Abdalla, S. H. 1990. Hematopoiesis in human malaria. Blood Cells 16:401-419. [PubMed] [Google Scholar]
- 2.Akpede, G. O., and R. M. Sykes. 1993. Malaria with bacteraemia in acutely febrile preschool children without localizing signs: coincidence or association/complication? J. Trop. Med. Hyg. 96:146-150. [PubMed] [Google Scholar]
- 3.Alourfi, Z., R. P. Donn, A. Stevens, A. Berry, A. McMaster, and D. W. Ray. 2005. Glucocorticoids suppress macrophage migration inhibitory factor (MIF) expression in a cell-type-specific manner. J. Mol. Endocrinol. 34:583-595. [DOI] [PubMed] [Google Scholar]
- 4.Amodu, O. K., A. A. Adeyemo, P. E. Olumese, and R. A. Gbadegesin. 1998. Intraleucocytic malaria pigment and clinical severity of malaria in children. Trans. R. Soc. Trop. Med. Hyg. 92:54-56. [DOI] [PubMed] [Google Scholar]
- 5.Arese, P., and E. Schwarzer. 1997. Malarial pigment (haemozoin): a very active ‘inert’ substance. Ann. Trop. Med. Parasitol. 91:501-516. [DOI] [PubMed] [Google Scholar]
- 6.Awandare, G. A., C. Ouma, C. C. Keller, T. Were, R. Otieno, Y. Ouma, G. C. Davenport, J. B. Hittner, J. M. Ong'echa, R. Ferrell, and D. J. Perkins. 2006. A macrophage migration inhibitory factor promoter polymorphism is associated with high-density parasitemia in children with malaria. Genes Immun. 7:568-575. (First published 24 August 2006; doi: 10.1038/sj.gene.6364332.) [DOI] [PubMed] [Google Scholar]
- 7.Awandare, G. A., J. B. Hittner, P. G. Kremsner, D. O. Ochiel, C. C. Keller, J. B. Weinberg, I. A. Clark, and D. J. Perkins. 2006. Decreased circulating macrophage migration inhibitory factor (MIF) protein and blood mononuclear cell MIF transcripts in children with Plasmodium falciparum malaria. Clin. Immunol. 119:219-225. [DOI] [PubMed] [Google Scholar]
- 8.Bacher, M., C. N. Metz, T. Calandra, K. Mayer, J. Chesney, M. Lohoff, D. Gemsa, T. Donnelly, and R. Bucala. 1996. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 93:7849-7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beier, J. C., C. N. Oster, F. K. Onyango, J. D. Bales, J. A. Sherwood, P. V. Perkins, D. K. Chumo, D. V. Koech, R. E. Whitmire, C. R. Roberts, et al. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 50:529-536. [DOI] [PubMed] [Google Scholar]
- 10.Berkley, J., S. Mwarumba, K. Bramham, B. Lowe, and K. Marsh. 1999. Bacteraemia complicating severe malaria in children. Trans. R. Soc. Trop. Med. Hyg. 93:283-286. [DOI] [PubMed] [Google Scholar]
- 11.Bernhagen, J., T. Calandra, R. A. Mitchell, S. B. Martin, K. J. Tracey, W. Voelter, K. R. Manogue, A. Cerami, and R. Bucala. 1993. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365:756-759. [DOI] [PubMed] [Google Scholar]
- 12.Bozza, M., A. R. Satoskar, G. Lin, B. Lu, A. A. Humbles, C. Gerard, and J. R. David. 1999. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breman, J. G., A. Egan, and G. T. Keusch. 2001. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 64:iv-vii. [DOI] [PubMed] [Google Scholar]
- 14.Burchard, G. D., P. Radloff, J. Philipps, M. Nkeyi, J. Knobloch, and P. G. Kremsner. 1995. Increased erythropoietin production in children with severe malarial anemia. Am. J. Trop. Med. Hyg. 53:547-551. [DOI] [PubMed] [Google Scholar]
- 15.Calandra, T., J. Bernhagen, R. A. Mitchell, and R. Bucala. 1994. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calandra, T., B. Echtenacher, D. L. Roy, J. Pugin, C. N. Metz, L. Hultner, D. Heumann, D. Mannel, R. Bucala, and M. P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164-170. [DOI] [PubMed] [Google Scholar]
- 17.Calandra, T., and T. Roger. 2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casals-Pascual, C., O. Kai, J. O. Cheung, S. Williams, B. Lowe, M. Nyanoti, T. N. Williams, K. Maitland, M. Molyneux, C. R. Newton, N. Peshu, S. M. Watt, and D. J. Roberts. 2006. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 108:2569-2577. (First published 27 June 2006; doi: 10.1182/blood-2006-05-018697.) [DOI] [PubMed] [Google Scholar]
- 19.Chaisavaneeyakorn, S., N. Lucchi, C. Abramowsky, C. Othoro, S. C. Chaiyaroj, Y. P. Shi, B. L. Nahlen, D. S. Peterson, J. M. Moore, and V. Udhayakumar. 2005. Immunohistological characterization of macrophage migration inhibitory factor expression in Plasmodium falciparum-infected placentas. Infect. Immun. 73:3287-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaisavaneeyakorn, S., J. M. Moore, C. Othoro, J. Otieno, S. C. Chaiyaroj, Y. P. Shi, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2002. Immunity to placental malaria. IV. Placental malaria is associated with up-regulation of macrophage migration inhibitory factor in intervillous blood. J. Infect. Dis. 186:1371-1375. [DOI] [PubMed] [Google Scholar]
- 21.Chaiyaroj, S. C., A. S. Rutta, K. Muenthaisong, P. Watkins, M. Na Ubol, and S. Looareesuwan. 2004. Reduced levels of transforming growth factor-beta1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta Trop. 89:319-327. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 23.Clark, I. A., M. M. Awburn, R. O. Whitten, C. G. Harper, N. G. Liomba, M. E. Molyneux, and T. E. Taylor. 2003. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar. J. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark, I. A., and W. B. Cowden. 2003. The pathophysiology of falciparum malaria. Pharmacol. Ther. 99:221-260. [DOI] [PubMed] [Google Scholar]
- 25.Clark, I. A., J. L. Virelizier, E. A. Carswell, and P. R. Wood. 1981. Possible importance of macrophage-derived mediators in acute malaria. Infect. Immun. 32:1058-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David, J. R. 1966. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day, N. P., T. D. Pham, T. L. Phan, X. S. Dinh, P. L. Pham, V. C. Ly, T. H. Tran, T. H. Nguyen, D. B. Bethell, H. P. Nguyan, T. H. Tran, and N. J. White. 1996. Clearance kinetics of parasites and pigment-containing leukocytes in severe malaria. Blood 88:4694-4700. [PubMed] [Google Scholar]
- 28.Goldie, P., E. F. Roth, Jr., J. Oppenheim, and J. P. Vanderberg. 1990. Biochemical characterization of Plasmodium falciparum hemozoin. Am. J. Trop. Med. Hyg. 43:584-596. [DOI] [PubMed] [Google Scholar]
- 29.Graham, S. M., A. L. Walsh, E. M. Molyneux, A. J. Phiri, and M. E. Molyneux. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans. R. Soc. Trop. Med. Hyg. 94:310-314. [DOI] [PubMed] [Google Scholar]
- 30.Isidori, A. M., G. A. Kaltsas, M. Korbonits, M. Pyle, M. Gueorguiev, A. Meinhardt, C. Metz, N. Petrovsky, V. Popovic, R. Bucala, and A. B. Grossman. 2002. Response of serum macrophage migration inhibitory factor levels to stimulation or suppression of the hypothalamo-pituitary-adrenal axis in normal subjects and patients with Cushing's disease. J. Clin. Endocrinol. Metab. 87:1834-1840. [DOI] [PubMed] [Google Scholar]
- 31.Juttner, S., J. Bernhagen, C. N. Metz, M. Rollinghoff, R. Bucala, and A. Gessner. 1998. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J. Immunol. 161:2383-2390. [PubMed] [Google Scholar]
- 32.Keller, C. C., J. B. Hittner, B. K. Nti, J. B. Weinberg, P. G. Kremsner, and D. J. Perkins. 2004. Reduced peripheral PGE2 biosynthesis in Plasmodium falciparum malaria occurs through hemozoin-induced suppression of blood mononuclear cell cyclooxygenase-2 gene expression via an interleukin-10-independent mechanism. Mol. Med. 10:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller, C. C., P. G. Kremsner, J. B. Hittner, M. A. Misukonis, J. B. Weinberg, and D. J. Perkins. 2004. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect. Immun. 72:4868-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koebernick, H., L. Grode, J. R. David, W. Rohde, M. S. Rolph, H. W. Mittrucker, and S. H. Kaufmann. 2002. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 99:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurtzhals, J. A., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 36.Kurtzhals, J. A., O. Rodrigues, M. Addae, J. O. Commey, F. K. Nkrumah, and L. Hviid. 1997. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br. J. Haematol. 97:169-174. [DOI] [PubMed] [Google Scholar]
- 37.Lackritz, E. M., C. C. Campbell, T. K. Ruebush II, A. W. Hightower, W. Wakube, R. W. Steketee, and J. B. Were. 1992. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet 340:524-528. [DOI] [PubMed] [Google Scholar]
- 38.Luty, A. J., D. J. Perkins, B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, J. B. Weinberg, and P. G. Kremsner. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyke, K. E., D. A. Diallo, A. Dicko, A. Kone, D. Coulibaly, A. Guindo, Y. Cissoko, L. Sangare, S. Coulibaly, B. Dakouo, T. E. Taylor, O. K. Doumbo, and C. V. Plowe. 2003. Association of intraleukocytic Plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am. J. Trop. Med. Hyg. 69:253-259. [PubMed] [Google Scholar]
- 40.Marsh, K., and R. W. Snow. 1997. Host-parasite interaction and morbidity in malaria endemic areas. Philos. Trans. R. Soc. Lond. B 352:1385-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martiney, J. A., B. Sherry, C. N. Metz, M. Espinoza, A. S. Ferrer, T. Calandra, H. E. Broxmeyer, and R. Bucala. 2000. Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect. Immun. 68:2259-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDevitt, M. A., J. Xie, V. Gordeuk, and R. Bucala. 2004. The anemia of malaria infection: role of inflammatory cytokines. Curr. Hematol. Rep. 3:97-106. [PubMed] [Google Scholar]
- 43.McDevitt, M. A., J. Xie, G. Shanmugasundaram, J. Griffith, A. Liu, C. McDonald, P. Thuma, V. R. Gordeuk, C. N. Metz, R. Mitchell, J. Keefer, J. David, L. Leng, and R. Bucala. 2006. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J. Exp. Med. 203:1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McElroy, P. D., F. O. ter Kuile, A. A. Lal, P. B. Bloland, W. A. Hawley, A. J. Oloo, A. S. Monto, S. R. Meshnick, B. L. Nahlen, et al. 2000. Effect of Plasmodium falciparum parasitemia density on hemoglobin concentrations among full-term, normal birth weight children in western Kenya, IV. Am. J. Trop. Med. Hyg. 62:504-512. [DOI] [PubMed] [Google Scholar]
- 45.Metzger, W. G., B. G. Mordmuller, and P. G. Kremsner. 1995. Malaria pigment in leucocytes. Trans. R. Soc. Trop. Med. Hyg. 89:637-638. [DOI] [PubMed] [Google Scholar]
- 46.Miller, K. L., J. C. Schooley, K. L. Smith, B. Kullgren, L. J. Mahlmann, and P. H. Silverman. 1989. Inhibition of erythropoiesis by a soluble factor in murine malaria. Exp. Hematol. 17:379-385. [PubMed] [Google Scholar]
- 47.Mizue, Y., J. Nishihira, T. Miyazaki, S. Fujiwara, M. Chida, K. Nakamura, K. Kikuchi, and M. Mukai. 2000. Quantitation of macrophage migration inhibitory factor (MIF) using the one-step sandwich enzyme immunosorbent assay: elevated serum MIF concentrations in patients with autoimmune diseases and identification of MIF in erythrocytes. Int. J. Mol. Med. 5:397-403. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen, P. H., N. Day, T. D. Pram, D. J. Ferguson, and N. J. White. 1995. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans. R. Soc. Trop. Med. Hyg. 89:200-204. [DOI] [PubMed] [Google Scholar]
- 49.Nussenblatt, V., G. Mukasa, A. Metzger, G. Ndeezi, E. Garrett, and R. D. Semba. 2001. Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute, uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab Immunol. 8:1164-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochiel, D. O., G. A. Awandare, C. C. Keller, J. B. Hittner, P. Kremsner, J. B. Weinberg, and D. J. Perkins. 2005. Differential regulation of beta-chemokines in children with acute falciparum malaria. Infect. Immun. 73:4190-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ong'echa, J. M., C. C. Keller, T. Were, C. Ouma, R. O. Otieno, Z. Landis-Lewis, D. Ochiel, J. L. Slingluff, S. Mogere, G. A. Ogonji, A. S. Orago, J. M. Vulule, S. S. Kaplan, R. D. Day, and D. J. Perkins. 2006. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am. J. Trop. Med. Hyg. 74:376-385. [PubMed] [Google Scholar]
- 52.Othoro, C., A. A. Lal, B. Nahlen, D. Koech, A. S. Orago, and V. Udhayakumar. 1999. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279-282. [DOI] [PubMed] [Google Scholar]
- 53.Otieno, R. O., C. Ouma, J. M. Ong'echa, C. C. Keller, T. Were, E. N. Waindi, M. G. Michaels, R. D. Day, J. M. Vulule, and D. J. Perkins. 2006. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 20:275-280. [DOI] [PubMed] [Google Scholar]
- 54.Perkins, D. J., J. M. Moore, J. Otieno, Y. P. Shi, B. L. Nahlen, V. Udhayakumar, and A. A. Lal. 2003. In vivo acquisition of hemozoin by placental blood mononuclear cells suppresses PGE2, TNF-alpha, and IL-10. Biochem. Biophys. Res. Commun. 311:839-846. [DOI] [PubMed] [Google Scholar]
- 55.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988-992. [DOI] [PubMed] [Google Scholar]
- 56.Pichyangkul, S., P. Saengkrai, and H. K. Webster. 1994. Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am. J. Trop. Med. Hyg. 51:430-435. [PubMed] [Google Scholar]
- 57.Reyes, J. L., L. I. Terrazas, B. Espinoza, D. Cruz-Robles, V. Soto, I. Rivera-Montoya, L. Gomez-Garcia, H. Snider, A. R. Satoskar, and M. Rodriguez-Sosa. 2006. Macrophage migration inhibitory factor contributes to host defense against acute Trypanosoma cruzi infection. Infect. Immun. 74:3170-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satoskar, A. R., M. Bozza, M. Rodriguez Sosa, G. Lin, and J. R. David. 2001. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarzer, E., M. Alessio, D. Ulliers, and P. Arese. 1998. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect. Immun. 66:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwarzer, E., F. Turrini, D. Ulliers, G. Giribaldi, H. Ginsburg, and P. Arese. 1992. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp. Med. 176:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherry, B. A., G. Alava, K. J. Tracey, J. Martiney, A. Cerami, and A. F. Slater. 1995. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J. Inflamm. 45:85-96. [PubMed] [Google Scholar]
- 62.Silverman, P. H., J. C. Schooley, and L. J. Mahlmann. 1987. Murine malaria decreases hemopoietic stem cells. Blood 69:408-413. [PubMed] [Google Scholar]
- 63.Snow, R. W., J. A. Omumbo, B. Lowe, C. S. Molyneux, J. O. Obiero, A. Palmer, M. W. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650-1654. [DOI] [PubMed] [Google Scholar]
- 64.Srichaikul, T., M. Wasanasomsithi, V. Poshyachinda, N. Panikbutr, and T. Rabieb. 1969. Ferrokinetic studies and erythropoiesis in malaria. Arch. Intern. Med. 124:623-628. [PubMed] [Google Scholar]
- 65.Weatherall, D., and P. Abdalla. 1982. The anaemia of P. falciparum malaria. Br. Med. Bull. 38:147-151. [DOI] [PubMed] [Google Scholar]
- 66.Weinberg, J. B., J. J. Muscato, and J. E. Niedel. 1981. Monocyte chemotactic peptide receptor. Functional characteristics and ligand-induced regulation. J. Clin. Investig. 68:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Were, T., J. B. Hittner, C. Ouma, R. O. Otieno, A. S. S. Orago, J. M. Ong'echa, J. M. Vulule, C. C. Keller, and D. J. Perkins. 2006. Suppression of RANTES in children with Plasmodium falciparum malaria is associated with severe malarial anemia and suppression of erythropoiesis. Haematologica 91:1396-1399. [PubMed]
- 68.Wickramasinghe, S. N., and S. H. Abdalla. 2000. Blood and bone marrow changes in malaria. Baillieres Best Pract. Res. Clin. Haematol. 13:277-299. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization. 2005. World Malaria Report 2005. United Nations Children's Fund, World Health Organization, Geneva, Switzerland. http://www.rollbackmalaria.org/wmr2005/pdf/WMReport_lr.pdf.
- 70.Xu, D., S. J. McSorley, L. Tetley, S. Chatfield, G. Dougan, W. L. Chan, A. Satoskar, J. R. David, and F. Y. Liew. 1998. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-alpha, and IFN-gamma administered orally via attenuated Salmonella typhimurium. J. Immunol. 160:1285-1289. [PubMed] [Google Scholar]
- 71.Yap, G. S., and M. M. Stevenson. 1991. Production of soluble inhibitor of erythropoiesis during Plasmodium chabaudi AS infection in resistant and susceptible mice. Ann. N. Y. Acad. Sci. 628:279-281. [DOI] [PubMed] [Google Scholar]