Abstract
Neurological manifestations of Lyme disease are usually accompanied by inflammatory changes in the cerebrospinal fluid (CSF) and the recruitment of activated T cells into the CSF compartment. In order to characterize the phenotype and identify target antigens of CSF-infiltrating T cells in early neuroborreliosis with central nervous system (CNS) involvement, we combined T-cell cloning, functional testing of T-cell responses with positional scanning synthetic combinatorial peptide libraries, and biometric data analysis. We demonstrate that CD4+ gamma interferon-producing T cells specifically responding to Borrelia burgdorferi lysate were present in the CSF of a patient with acute Lyme encephalitis. Some T-cell clones recognized previously uncharacterized B. burgdorferi epitopes which show a specific enrichment for lysine, such as the heat shock-induced chaperone HSP90. Degenerate T-cell recognition that included T-cell responses to borrelia-specific and CNS-specific autoantigens derived from the myelin protein 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) could be demonstrated for one representative clone. Our results show that spirochetal antigen-specific and Th1-polarized CD4+ lymphocytes infiltrate the CSF during monophasic CNS symptoms of Lyme disease and demonstrate that cross-recognition of CNS antigens by B. burgdorferi-specific T cells is not restricted to chronic and treatment-resistant manifestations.
Lyme disease is the most frequent vector-borne infection in the United States and is also endemic in Europe and parts of Asia (44). Transmitted by Ixodes ticks, its causative agent, Borrelia burgdorferi, initially propagates locally in the skin before it disseminates hematogenously to other organ systems. Neurological involvement, referred to as neuroborreliosis (NB), occurs in up to 20% of patients and presents as cranial neuritis, meningoradiculitis, or encephalitis (4). It is usually accompanied by a vigorous immune response against the spirochete (42). Typical inflammatory changes in the cerebrospinal fluid (CSF) involve disruption of the blood-brain barrier and pleocytosis consisting predominantly of lymphocytes (17, 46). After antibiotic treatment, most patients recover from acute disease, but in some cases, a chronic inflammation of the brain and spinal cord develops despite a dramatic drop in spirochetal numbers, or even in the absence of bacterial persistence (32, 46). Studies of Lyme arthritis and chronic neuroborreliosis suggest that spirochete-specific T cells cross-reacting with self-antigens may be at least partly responsible for autoimmune mechanisms possibly leading to chronic disease manifestations (8, 12, 25).
The innate and adaptive immune responses to B. burgdorferi have been studied extensively in infectious animal models. In susceptible rodents, infection with B. burgdorferi leads to a variety of organ manifestations, including myocarditis and arthritis (33). Central nervous system (CNS) infection, however, has only been observed in immunosuppressed monkeys that do not exhibit cardinal features of acute human neuroborreliosis (31). Due to the lack of appropriate animal models, the immune response in acute neuroborreliosis is much less defined. Previous studies of European patients with acute neuroborreliosis indicated that activated T cells are recruited into the CSF compartment during acute infection of the nervous system (16, 39, 40) and that a fraction of these cells are B. burgdorferi specific (13, 16, 25, 36). The antigen fine specificities of CSF-infiltrating T lymphocytes, however, were not determined.
In contrast to the case in Europe, where a transient lymphomeningoradiculitis (Bannwarth syndrome) is the typical and, by far, most common clinical manifestation of neuroborreliosis, patients from North America occasionally show a mild encephalitis without characteristic radiculitic symptoms and therefore differ significantly in their clinical phenotype (10). These differences might be due to distinct tropisms of different B. burgdorferi sensu lato species (i.e., parenchyma versus meninges and roots [22, 30]) or to different host response patterns to the invading pathogen (e.g., transient CNS tissue injury by infiltrating autoantigen-specific lymphocytes or different cytokine pattern of CSF-infiltrating T cells).
In the present study, we analyzed the phenotype and specificities of CSF-infiltrating T lymphocytes in a patient showing the classical phenotype of acute Lyme neurological disease (9, 10) by employing a previously successfully applied unbiased and systematic approach to isolate and expand T-cell clones (TCC) from the CSF and to identify target antigens and molecular mimics, using positional scanning synthetic combinatorial peptide libraries (PS-SCL) combined with a biometric search strategy (12, 43).
MATERIALS AND METHODS
Case report.
A 59-year-old white male who lives in an area in the United States where Lyme disease is endemic and who is an avid gardener presented in mid-June with a 10-day history of an expanding erythematous rash on his left wrist, which enlarged to 20 cm in diameter. He did not recall a tick bite. Three days after noticing the rash, the patient developed fevers (up to 103°F), myalgias, neck pain, scalp hyperesthesia, and severe headaches. During this time, he also presented with an encephalitic syndrome, including changes in personality, forgetfulness, and omissions of tasks at work, which were highly unusual for him. On presentation, he showed 12 separate homogeneous erythematous rashes, varying in diameter from 3 to 10 cm. There was no neck stiffness, and the patient did not show any focal neurological deficit in a neurological examination. CSF showed 10 white blood cells per microliter (10% polymorphonuclear cells, 61% lymphocytes, and 29% monocytes; normal range, <5 white blood cells per microliter), with 0 red blood cells. The CSF glucose and protein levels, immunoglobulin G (IgG) index, and albumin quotient were within normal limits. Routine fungal and B. burgdorferi CSF cultures and CSF Borrelia PCR were negative. While both serum and CSF were positive for anti-Borrelia burgdorferi antibodies, there was no intrathecal antibody production, as determined by a capture enzyme immunoassay, as frequently observed in early stages of acute neuroborreliosis (9, 47). B. burgdorferi serologies were positive by ELISA and IgG Western blotting using CDC criteria. An IgM immunofluorescence assay of the serum was also positive. The patient was treated with intravenous ceftriaxone at 2 g every 24 h for 28 days, with complete recovery. In parallel with this patient, we established two TCC from the CSF of an untreated patient with relapsing-remitting multiple sclerosis (MS) who showed an exacerbation at the time of lumbar puncture. These studies were performed under protocols approved by the National Institute of Allergy and Infectious Diseases and the National Institute of Neurological Disorders and Stroke Institutional Review Boards. The volunteers gave informed consent.
CSF-infiltrating T-cell clones.
TCC were established from the CSF derived from the patients characterized above before initiation of treatment. Cells were cloned by limiting dilution at 0.3, 3, and 10 cells/well with 2 × 105 autologous, irradiated (6,000 rad) peripheral blood mononuclear cells (PBMC) and either 2.5 μg/ml phytohemagglutinin (PHA; Sigma, St. Louis, MO) or 1 μg/ml low-passage sonicated B. burgdorferi lysate (Biosource) in RMPI 1640 containing 100 U/ml penicillin-streptomycin, 50 μg/ml gentamicin, 2 mM l-glutamine (BioWhittaker, Gaithersburg, MD), and 10% heat-decomplemented human AB serum (Gemini Bio-Products, Woodland, CA). After 24 h, 20 U/ml of human recombinant interleukin-2 (hrIL-2; National Cancer Institute, National Institutes of Health, Bethesda, MD) was added. Cells were restimulated every 2 weeks with their initial stimulus and autologous irradiated PBMC. Twenty units of hrIL-2 per milliliter was added every 3 to 4 days.
Flow cytometry.
Growing T-cell cultures were subsequently characterized for clonality by staining with a panel of 22 anti-T-cell receptor Vβ (anti-TCRBV) fluorescein-conjugated monoclonal antibodies (Beckman Coulter, Somerset, NJ) as previously described (12). CD4 and CD8 monoclonal antibodies (Becton Dickinson, Franklin Lakes, NJ) were used to further characterize cells. Fluorescence intensity was measured in a FACSCalibur flow cytometer and analyzed using CellQuest software (both from Becton Dickinson).
Peptide combinatorial libraries and individual peptides.
The decapeptide PS-SCL was synthesized by the simultaneous multiple peptide synthesis method (15), with the N-terminal end being N-acetylated and the C-terminal end being amidated (35). The PS-SCL consists of 200 mixtures in the OX9 format, where O represents one of the 20 l-amino acids, except cysteine. Each OX9 mixture consists of 3.2 × 1011 (199) different decamer peptides at an equimolar concentration, resulting in a 0.26 fM individual peptide concentration when tested at a final concentration of 100 μg/ml. Predicted peptides were synthesized (Sigma Genosys, St. Louis, MO) and tested for their stimulatory potential as described below.
Proliferation assays and cytokine production.
TCC proliferation in response to the PS-SCL or individual decapeptides was tested by seeding 2 × 104 T cells with 1 × 105 irradiated PBMC (6,000 rad) (12). PHA stimulation of the TCC with unknown specificity was used as a positive control. Proliferation was measured by [methyl-3H]thymidine (Amersham Biosciences, United Kingdom) incorporation. The stimulatory index for a PS-SCL mixture (SIPS-SCL) with an amino acid defined at one position was calculated as follows: SIPS-SCL = SI′/mean of all SI′ in the library, where SI′ = mean of duplicate counts per minute (cpm) (mixture) − mean cpm (background). Responses to mixtures were considered positive when SIs were >2 (43). [The SIs for individual peptides were calculated as follows: SI = mean of duplicate cpm (peptide)/mean cpm (background).] Responses to individual peptides were considered positive when the SIs were >2 and cpm were >1,000 and at least 3 standard deviations above the average background cpm. Supernatants were collected from cultures after 48 h, and IFN-γ as well as IL-4 levels were determined by enzyme-linked immunosorbent assay following the manufacturer's protocol (Biosource, Camarillo, CA).
Biometric analysis and database searches.
The responses of TCC to the decapeptide PS-SCL were analyzed as previously described (50). A positional scoring matrix was generated by assigning a value of the stimulatory potential to each of the 20 defined amino acids at each of the 10 positions. The predicted stimulatory score of a given peptide is the sum of the stimulatory potentials of all amino acids contained in the peptide at each position. Using a web-based search tool (50), a 10-mer peptide window was moved over all available amino acid sequences of human and borrelial proteins in the GenPept database (version 136).
RESULTS
Generation and characterization of CSF-infiltrating T-cell clones.
Assuming that CSF-infiltrating T lymphocytes are involved in the pathogenesis of acute Lyme encephalitis, we isolated CSF cells from the patient before therapy was initiated and cloned them by limiting dilution, using either PHA or low-passage B. burgdorferi lysate (B. burgdorferi sensu stricto strain B31) as the stimulus. Growing colonies were characterized for CD4, CD8, and TCR Vβ chain expression by flow cytometry. T-cell cultures that contained more than one T-cell receptor Vβ clonotype were characterized as T-cell lines (TCL), whereas cultures stained with either exclusively one or none of the TCRBV antibodies were considered TCC.
As summarized in Table 1, eight TCC and four TCL containing exclusively CD4+ lymphocytes could be expanded from the CSF. TCRBV 2 was detected in five T-cell cultures and represented the most frequently expressed TCR Vβ chain. All of the TCC and TCL, with one exception (X4), secreted substantial amounts of IFN-γ following stimulation with PHA or borrelial lysate. None of the T-cell cultures, aside from one TCL (X5), produced detectable amounts of IL-4. Thus, CSF-infiltrating lymphocytes which can be expanded in acute Lyme encephalitis are CD4+, respond specifically to B. burgdorferi lysate, and produce large amounts of T-helper 1 cytokines.
TABLE 1.
TCRBV chain usage and cytokine production of T-cell lines and clones isolated from CSF
| TCL or TCC | TCRBV usage | Limiting-dilution stimulus | IFN-g concn (pg/ml) | IL-4 concn (pg/ml)a |
|---|---|---|---|---|
| X1 | 2, 21 | B. burgdorferi lysate | 675 | <DL |
| X2 | No staining | B. burgdorferi lysate | 1,780 | <DL |
| X3 | No staining | PHA | 525 | <DL |
| X4 | 2 | PHA | 8 | <DL |
| X5 | 2, 5, 6.7, 7, 13, 22 | PHA | 823 | 26 |
| X6 | 16 | PHA | 609 | <DL |
| X7 | 3 | PHA | 1,017 | <DL |
| X8 | 17 | B. burgdorferi lysate | 664 | <DL |
| X9 | 6.7 | B. burgdorferi lysate | 2,136 | <DL |
| X10 | No staining | PHA | 22 | <DL |
| X11 | 2, 13, 21 | PHA | 1,418 | 6.8 |
| X12 | 2, 3, 6.7, 14 | B. burgdorferi lysate | 1,855 | <DL |
<DL, below detection limit.
Prediction of candidate T-cell epitopes by positional scanning peptide libraries.
PS-SCL theoretically represent all possible peptides of a given length in systematically arranged mixtures (34). We previously showed that a single decapeptide mixture containing 300 billion (199) peptides can activate TCC and that the analysis of the T-cell response to the PS-SCL, using a mathematical approach that is based on a model of independent contributions of individual amino acids to peptide antigen recognition, can lead to the identification of novel candidate (auto)antigens in immune-mediated diseases (11, 12, 14, 50).
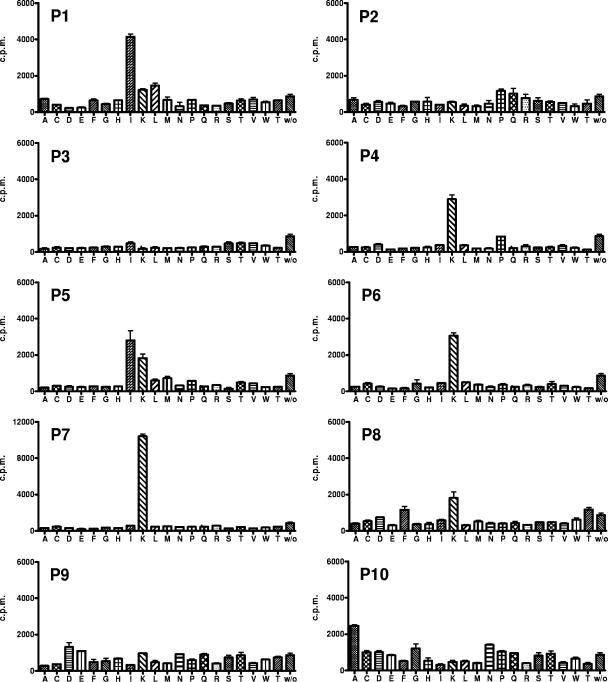
In a first screening step, for practical reasons, all eight TCC were tested with only 2 of the 10 positions of the decapeptide PS-SCL, using autologous PBMC as antigen-presenting cells. TCC X8 showed reproducible strong responses to the peptide libraries and was subsequently tested with a complete PS-SCL (200 mixtures). Each mixture has one defined amino acid at one position, whereas the other positions contain all 20 amino acids, except cysteine, incorporated randomly.
For the X8 clone, only a few mixtures of the PS-SCL elicited a response at each position. We identified the hypothetical peptide with the highest stimulatory score. This score results from adding the stimulatory values for the most stimulatory amino acids at each position. The predicted most stimulatory peptide mixture for TCC X8 contained lysine as the defined amino acid at five different positions, suggesting that lysine is critical for TCR recognition (Fig. 1).
FIG. 1.
Proliferative response of TCC X8 to a decapeptide PS-SCL in which each position has one defined amino acid (20 amino acids were used for each of the 10 positions [P1 to P10]). Horizontal axis, single-letter amino acid code; w/o, without any peptide mixture; vertical axis, proliferation quantified as cpm induced by each mixture of the PS-SCL (means and standard deviations for duplicate wells). Data represent one experiment of two. The predicted most stimulatory peptide mixture for the TCC X8 contained lysine as the defined amino acid at five different positions, suggesting that lysine is critical for TCR recognition.
Identification of biologically relevant T-cell epitopes.
In order to identify naturally occurring T-cell epitopes for TCC X8, we performed a systematic search of borrelial and human proteins by scanning all overlapping decapeptides in the GenPept database, using a scoring matrix that assigned a numerical value for the stimulatory potency of every amino acid at each position. Based on previous experiences, only peptides with scores of >70% of the maximum score were considered. The highest-scoring borrelial (n = 16), human (n = 16), and human myelin (n = 8) peptides were synthesized and tested for their actual stimulatory potencies at 1 μmol and 10 μmol, using autologous PBMC as antigen-presenting cells. Responses were considered positive if an SI of 2 and a cpm of at least 3 standard deviations above the background cpm could be achieved. From a total of 40 predicted stimulatory peptides, a positive proliferative response was observed with 13 peptides (7 borrelial, 3 human, and 3 human myelin peptides) at 1 μmol (32.5%) and with 15 peptides (7 borrelial, 3 human, and 5 human myelin peptides) at 10 μmol (37.5%). All of the stimulatory peptides showed an enrichment for lysine, as defined by the presence of more than two lysine residues per decapeptide.
Among the highest-scoring peptides, we focused on eight candidates which elicited strong proliferative responses (four borrelial, two human, and two human myelin protein-derived antigens) and tested them for their stimulatory potential in antigen dose-titration experiments. All eight lysine-enriched peptides elicited dose-dependent responses in TCC X8, as measured by proliferation and IFN-γ production. Table 2 displays proliferative potencies, expressed as EC50 values (i.e., the antigen concentration at which 50% of the maximal response can be obtained), and stimulation indices in relation to PHA.
TABLE 2.
Origin, sequence, potency, and function of stimulatory peptides for CSF TCC X8a
| Species | Peptide | Sequence | Potency (EC50 [μmol]) | Maximum SI compared to PHA (%) | Maximum IFN-γ production compared to PHA (%) | Protein | Molecular mass (kDa) | No. of amino acids | Function | Location | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi | Bb#1 | IQEIIKKYSN | 0.01-0.1 | 25 | 11 | Heat shock protein 90 | 71.2 | 616 | Heat shock-induced chaperone | Cytoplasm | 6a |
| Bb#2 | LPTKIKKYTV | 0.01-0.1 | 19 | 3 | 30S ribosomal protein | 11.8 | 103 | Ribonucleoprotein; binding of tRNA to ribosomes | Cytoplasm | 6a | |
| Bb#3 | IAKKIKKTIA | 0.1-1 | 35 | 5 | Periplasmic protein 1 | 78.2 | 670 | Porphrine biosynthesisb | Unknown | 6a | |
| Bb#4 | LFKKIKKYDG | 0.1-1 | 31 | 8 | Prolipoprotein diacylglyceryl transferase | 37.6 | 328 | Lipoprotein biosynthesis | Inner membrane | 6a | |
| Homo sapiens | Hu#1 | TYKKIKKDSA | 0.1-1 | 84 | 37 | UDP-glucuronosyltransferase | 59.6 | 533 | Transfer of hexosyl groups | Microsome | 49a |
| Hu#2 | IMKKIKKGDF | 0.1-1 | 24 | 7 | Nuclear mitogen- and stress-activated protein kinase 1 (MSK1) | 89.9 | 802 | ATP binding protein, histone phosphorylation | Nucleusc | 4a | |
| Hu#3 | GNHKAFKKEL | 0.1-1 | 21 | 12 | CNPase | 47.6 | 421 | Structural protein, synaptic transmission | CNS myelin membrane structures | 21a | |
| Hu#4 | SADDLKKLKP | 0.1-1 | 20 | 5 | CNPase | 47.6 | 421 | Structural protein, synaptic transmission | CNS myelin membrane structures | 21a |
Peptide-specific responses are compared to mitogenic stimulation with PHA (2.5 μg/ml), which yielded a mean cpm value of 51,345 (SI of 24) and mean IFN-g production of 1,143 pg/ml.
Inferred from sequence similarity searches.
Expressed at high levels in the brain, placenta, and heart.
Peptide antigens derived from cytoplasmic B. burgdorferi proteins, i.e., the heat shock-induced chaperone molecule HSP 90 and the ribosomal protein S10, showed the highest stimulatory potencies in terms of their EC50 values being <0.1 μmol. EC50 values for epitopes derived from human proteins, such as the CNS white matter membrane protein 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), were 1 log higher for the human peptides (0.1 to 1 μmol) than for the most stimulatory borrelial epitopes, indicating lower antigen avidities.
Taken together, the determination of TCR specificities by combinatorial peptide libraries and biometric data analysis for a TCC infiltrating the inflamed organ compartment of a patient with acute neuroborreliosis led to the identification of T-cell stimulatory epitopes derived from the causative agent of the disease. Furthermore, our results demonstrate degenerative T-cell recognition for a CSF-infiltrating T-cell clone that includes T-cell responses to foreign (borrelial) and self (CNPase) antigens.
Stimulatory potential of identified target epitopes for other CSF-infiltrating TCC.
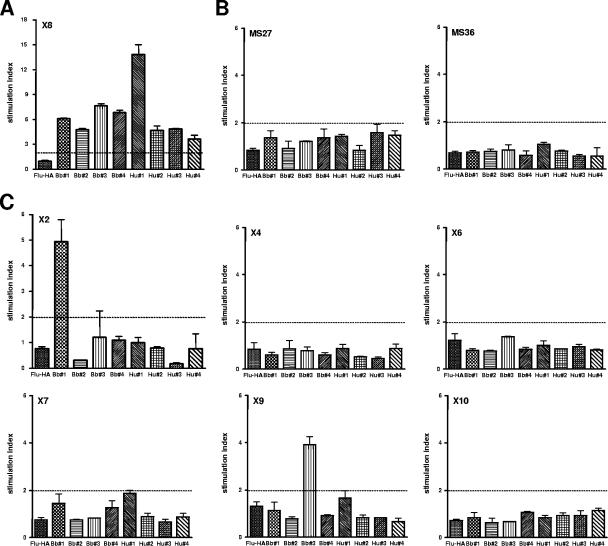
Next, we investigated whether the identified peptides that elicited strong responses in TCC X8 would also be recognized by other CSF-infiltrating TCC and TCL derived from the same patient. As demonstrated in Fig. 2, none of the peptides induced any response in CSF-infiltrating TCC derived from a control patient with relapsing-remitting multiple sclerosis. In contrast, two of the identified borrelial epitopes (derived from the heat shock-induced chaperone molecule HSP 90 and from periplasmic protein 1) were indeed capable of eliciting a proliferative response in two other TCC (X2 and X9). Both TCC were generated by using B. burgdorferi lysate as the limiting-dilution stimulus and produced high levels of IFN-γ (>1,000 pg/ml) but not IL-4 (Table 1). In contrast to TCC X8, no other identified borrelial peptides and none of the human peptides were recognized by TCC X2 and X9. The different TCRBV chain usage demonstrates that the three responding TCC were derived from distinct T cells (Table 1). Thus, we identified two borrelial antigens with reactivities that are shared by more than one CSF-infiltrating Th1 clone, suggesting that they may be potential candidate target epitopes, in a patient with Lyme encephalitis.
FIG. 2.
Stimulatory potentials of lysine-enriched borrelial and self-antigens predicted for CSF TCC X8 and of an influenza virus hemagglutinin-derived (Flu-HA) control peptide (amino acids 306 to 318) for (A) TCC X8, (B) two CSF-infiltrating TCC derived from a different patient with relapsing-remitting multiple sclerosis (MS27 and MS36) which were used as controls in this experiment, and (C) CSF TCC other than X8 derived from the Lyme encephalitis patient. TCC were stimulated using a peptide concentration of 1 μmol. The data depicted are mean stimulation indices and standard deviations. In addition to TCC X8, two other clones, X2 and X9, responded specifically to two borrelial antigens, derived from heat shock protein 90 and periplasmic protein 1 of B. burgdorferi, respectively.
DISCUSSION
The aim of this study was to characterize the phenotype, determine the antigen specificities, and estimate the degeneracy of CSF-infiltrating T cells in a patient with early Lyme encephalitis. We were particularly interested in investigating whether borrelia-specific T-cell clones are capable of cross-recognizing CNS-derived autoantigens. We generated different TCC from the CSF by using polyclonal stimuli and analyzed their TCR Vβ usage, their cytokine expression profiles, and their spectra of recognized antigens with PS-SCL-based biometric analysis, which integrates data generated from screening of combinatorial libraries in the positional scanning format with protein database analysis as described previously (12, 50).
The CSF-derived TCC and TCL showed a strong Th1 commitment, based on their production of large amounts of IFN-γ. Due to different effector functions that are elicited by either Th1 or Th2 cells, the enrichment of one particular lineage in affected tissues is generally believed to be critical for the clinical outcomes of infectious diseases. Th1 cells have consistently been shown to predominate in the joints of patients with chronic Lyme arthritis (8, 49). For animal models of Lyme disease, Th1 responses were frequently reported to be associated with the development of tissue destruction, whereas Th2 responses appeared to be protective (18, 19, 27). Likewise, B. burgdorferi-specific CD4+ TCC generated from the blood of patients with chronic NB and Lyme arthritis secreted large amounts of IFN-γ and, interestingly, IL-10 (36), a finding that could be confirmed in CSF-derived T cells from a patient with chronic NB (12). In contrast, adoptive transfer of IFN-γ-secreting CD4+ T cells to infected αβ T-cell receptor-deficient mice was reported to promote successful resolution of B. burgdorferi infection in a murine model of Lyme carditis, indicating a beneficial role for CD4+ T-helper 1 cells (2). While the impact of T-helper-cell signature cytokines on the development or resolution of Lyme disease is not well understood, our data indicate that Th1 commitment of organ-infiltrating T cells is not necessarily associated with long-lasting clinical deficits in neuroborreliosis.
In our study, we focused on CSF-derived T cells, since the CSF compartment was repeatedly shown to be enriched for disease-relevant cells in various infectious neurological diseases, including NB (6, 7, 16, 21, 24, 25). Using a flow cytometry-based serial TCRBV chain analysis of blood and CSF samples derived from patients with acute NB, it was previously demonstrated that both CD4+ and CD8+ T-cell clones accumulate in the CNS during the early phase of disease manifestation (16). The antigen specificities of these CSF-enriched T-cell species were, however, not determined. Proteins expressed by B. burgdorferi in culture differ significantly from proteins expressed in the human host, and the expression pattern changes during the course of infection (1, 3, 5, 37, 41). A clonal accumulation of T cells within the CSF in patients with NB suggests that the expression pattern of borrelial antigens might be different from that in extraneurological compartments.
In an attempt to identify the target antigens, we established eight CSF-infiltrating TCC and tested them for their response to the PS-SCL and the identified stimulatory peptides. A consistent proliferative response to the PS-SCL could be achieved with only one TCC, which is in line with previously reported response rates of 10 to 20% for peripheral blood-derived TCC (13), The TCC that was responsive to the complex peptide mixtures of the PS-SCL recognized several lysine-enriched borrelial and human epitopes. Such degenerate antigen recognition was previously described for a TCC derived from the CSF of a patient with chronic NB (12) and also for a patient with an acute exacerbation during relapsing-remitting multiple sclerosis (43). In the latter study, it was observed for the first time that multiple TCC recognized peptides enriched in a positively charged amino acid (R) derived from protein domains that are conserved in phylogeny and that serve important functions, such as nucleic acid binding domains or nuclear localization signals (43). In the present study, a central feature of the predicted TCR ligand for TCC X8 was an enrichment of lysine.
Lysine contains a long flexible side chain with a positively charged end. The flexibility of the chain renders this amino acid suitable for binding to molecules with many negative charges on their surfaces, and the strong charge frequently positions this amino acid on the outer hydrophilic surfaces of proteins. DNA-binding proteins are among the molecules that are enriched for positively charged amino acids such as lysine. Consistent with this reasoning, three of the four identified borrelial epitopes exhibit nucleotide binding activities, whereas one is derived from a membrane protein involved in the biosynthesis of lipoproteins. Peptides derived from the heat shock-induced chaperone HSP 90 and the spirochetal periplasmic protein 1 elicited positive T-cell responses in two different clones. The former protein is involved in protein folding and stabilization. The exact location and function of the latter are unknown. Neither one has previously been associated with the cellular immune response to B. burgdorferi. Two of the identified human T-cell epitopes are derived from the third most abundant myelin protein, i.e., CNPase. Previous studies demonstrated the immunogenic potential of CNPase at the T-cell level in humans and mice (28, 29, 38). Muraro et al. identified two epitope clusters, CNP (343-373) and CNP (356-388), that were recognized in the context of HLA-DR2 and -DR4 molecules, which confer genetic susceptibility to multiple sclerosis, and found the strongest responses to CNP (343-373) in two MS patients with high disease activity (29). Our patient did not express MS-associated HLA class II alleles, and the two identified epitopes differed from the immunodominant clusters described in the aforementioned study. Moreover, the T-cell responses towards the CNPase peptides as well as to the third molecular mimic derived from a protein abundantly expressed in the human brain (i.e., MSK1) were less strong than those induced by borrelial antigens.
It is now generally accepted that degenerate antigen recognition is a fundamental feature of adaptive cellular immune responses. Mathematical models indicate that the TCR repertoire is not large enough to give functional protection against all possible foreign epitopes on the basis of a one-TCR-one-epitope model (26), and several groups consistently demonstrated that there can be considerable flexibility in TCR recognition of peptide-major histocompatibility complex (MHC) complexes (20, 23, 45, 48). Degenerate TCR recognition is considered to represent a compromise between the need to provide host protection against virtually any pathogen-derived epitope and, at the same time, the need to ensure thymic positive selection and peripheral maintenance of this T-cell repertoire via intermediate affinity recognition of self-peptides that are presented by self-MHC molecules. Such degenerate specificity, however, also carries a certain risk for autoimmunity under special circumstances, e.g., strong innate immune activation. The observation that the identified self-epitopes showed lower stimulatory potencies, as defined by their EC50 values, for CSF TCC X8 than did the pathogen-derived antigens is compatible with the concept of elimination via negative selection of T cells reactive with high affinity to self-peptides in the context of self-MHC class II molecules. Definite conclusions would require the knowledge of the HLA restriction element for this particular clone. The lower antigen affinity for self-antigens and the complete recovery of the patient once treated with antibiotics, however, do not argue for a major pathogenic role of T-cell cross-reactivity in the symptomatology observed.
Two other CSF-derived TCC showed positive responses to B. burgdorferi-derived peptides that were predicted for and stimulatory in CSF TCC X8. The observation that only one of the TCR ligands could elicit a response in each of these two clones indicates different levels of degeneracy. We previously showed that the flexibility in antigen recognition differs between TCC and suggested that the propensity of certain clones to respond to a larger number of self-peptides and pathogen-derived mimic peptides imposes a higher risk for clinically relevant autoimmune responses (12, 43).
Cross-recognition of self-antigens by B. burgdorferi-specific CD4+ T cells has been implicated in the pathogenesis of chronic manifestations of the disease. Patients with treatment-resistant Lyme arthritis, but not other forms of arthritis, expressing the rheumatoid arthritis-associated MHC allele HLA-DRB1*0401 generated clonal responses to both the immunodominant borrelial outer surface protein A and the human leukocyte function-associated antigen 1, which was tested as a candidate autoantigen (8). In a patient with chronic NB expressing the MS-associated HLA-DRB1*1501 allele, we previously showed that a single TCC preferentially recognizes different borrelial peptides but also responds to human autoantigenic mimics (12). Our data clearly indicate that degenerate antigen recognition that includes pathogen-derived and self-epitopes is not restricted to chronic and treatment-resistant manifestations of the disease. Molecular mimicry alone is thus not sufficient to induce chronic Lyme disease, and other genetic, environmental, and infectious factors must be involved. Probably the best risk-conferring candidate is the HLA haplotype, since both of the aforementioned studies were performed with patients who expressed HLA class II alleles associated with autoimmunity in the affected organ compartment. However, studies investigating the association between HLA haplotypes and chronic Lyme disease have up until now been lacking, and a clear association between certain HLA alleles and organ involvement other than the joints, e.g., the brain and/or spinal cord, has not yet been documented.
The present study identified novel spirochetal antigens as potential target epitopes in a patient with acute Lyme encephalitis. Our results demonstrate the predictive power of PS-SCL and biometric analysis as an epitope-mapping strategy for a disease state induced by a defined infectious agent. More extensive studies are needed to investigate the biological importance of these peptides for other patients with early neuroborreliosis. Furthermore, we provided evidence that a degenerate T-cell response that includes pathogen-derived and self-epitopes is not restricted to treatment-resistant, presumably autoimmunity-mediated, manifestations of the disease. We suggest that these findings are relevant to our understanding of the pathogenesis of early versus late neuroborreliosis and important for our concept of infection-associated autoimmunity in humans.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, the National Institute of Allergy and Infectious Diseases, the National Institute of Neurological Disorders and Stroke, and the Gemeinnützige Hertie Stiftung. J.D.L. received a postdoctoral grant from the Deutsche Forschungsgemeinschaft (LU 900/1-1) and the Irvington Institute for Immunological Research. INiMS is supported by the Gemeinnützige Hertie Stiftung.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bockenstedt, L. K., I. Kang, C. Chang, D. Persing, A. Hayday, and S. W. Barthold. 2001. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 69:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle, P. K., and S. E. Schutzer. 2002. Neurologic aspects of Lyme disease. Med. Clin. N. Am. 86:261-284. [DOI] [PubMed] [Google Scholar]
- 4a.Deak, M., A. D. Clifton, L. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Embers, M. E., R. Ramamoorthy, and M. T. Philipp. 2004. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect. 6:312-318. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer, B., and H. W. Kreth. 1983. Clonal analysis of HLA-restricted virus-specific cytotoxic T lymphocytes from cerebrospinal fluid in mumps meningitis. J. Immunol. 130:2187-2190. [PubMed] [Google Scholar]
- 6a.C. M. Fraser, S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fuji, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 290:580-586. [DOI] [PubMed] [Google Scholar]
- 7.Giunti, D., G. Borsellino, R. Benelli, M. Marchese, E. Capello, M. T. Valle, E. Pedemonte, D. Noonan, A. Albini, G. Bernardi, G. L. Mancardi, L. Battistini, and A. Uccelli. 2003. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J. Leukoc. Biol. 73:584-590. [DOI] [PubMed] [Google Scholar]
- 8.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 9.Halperin, J. J. 2005. Central nervous system Lyme disease. Curr. Neurol. Neurosci. Rep. 5:446-452. [DOI] [PubMed] [Google Scholar]
- 10.Halperin, J. J. 1997. Neuroborreliosis: central nervous system involvement. Semin. Neurol. 17:19-24. [DOI] [PubMed] [Google Scholar]
- 11.Hemmer, B., B. T. Fleckenstein, M. Vergelli, G. Jung, H. McFarland, R. Martin, and K. H. Wiesmuller. 1997. Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J. Exp. Med. 185:1651-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmer, B., B. Gran, Y. Zhao, A. Marques, J. Pascal, A. Tzou, T. Kondo, I. Cortese, B. Bielekova, S. E. Straus, H. F. McFarland, R. Houghten, R. Simon, C. Pinilla, and R. Martin. 1999. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat. Med. 5:1375-1382. [DOI] [PubMed] [Google Scholar]
- 13.Hemmer, B., C. Pinilla, B. Gran, M. Vergelli, N. Ling, P. Conlon, H. F. McFarland, R. Houghten, and R. Martin. 2000. Contribution of individual amino acids within MHC molecule or antigenic peptide to TCR ligand potency. J. Immunol. 164:861-871. [DOI] [PubMed] [Google Scholar]
- 14.Hemmer, B., M. Vergelli, B. Gran, N. Ling, P. Conlon, C. Pinilla, R. Houghten, H. F. McFarland, and R. Martin. 1998. Predictable TCR antigen recognition based on peptide scans leads to the identification of agonist ligands with no sequence homology. J. Immunol. 160:3631-3636. [PubMed] [Google Scholar]
- 15.Houghten, R. A. 1985. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. USA 82:5131-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsen, M., D. Zhou, S. Cepok, S. Nessler, M. Happel, S. Stei, B. Wilske, N. Sommer, and B. Hemmer. 2003. Clonal accumulation of activated CD8+ T cells in the central nervous system during the early phase of neuroborreliosis. J. Infect. Dis. 187:963-973. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser, R. 1998. Neuroborreliosis. J. Neurol. 245:247-255. [DOI] [PubMed] [Google Scholar]
- 18.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 19.Keane-Myers, A., and S. P. Nickell. 1995. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 154:1770-1776. [PubMed] [Google Scholar]
- 20.Kersh, G. J., and P. M. Allen. 1996. Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands. J. Exp. Med. 184:1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kivisakk, P., D. J. Mahad, M. K. Callahan, C. Trebst, B. Tucky, T. Wei, L. Wu, E. S. Baekkevold, H. Lassmann, S. M. Staugaitis, J. J. Campbell, and R. M. Ransohoff. 2003. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA 100:8389-8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Kurihara, T., Y. Takahashi, A. Nishiyama, and T. Kumanishi. 1988. cDNA cloning and amino acid sequence of human brain 2′,3′-cyclic-nucleotide 3′-phosphodiesterase. Biochem. Biophys. Res. Commun. 152:837-842. [DOI] [PubMed] [Google Scholar]
- 22.Lunemann, J. D., S. Zarmas, S. Priem, J. Franz, R. Zschenderlein, E. Aberer, R. Klein, L. Schouls, G. R. Burmester, and A. Krause. 2001. Rapid typing of Borrelia burgdorferi sensu lato species in specimens from patients with different manifestations of Lyme borreliosis. J. Clin. Microbiol. 39:1130-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, R., B. Gran, Y. Zhao, S. Markovic-Plese, B. Bielekova, A. Marques, M. H. Sung, B. Hemmer, R. Simon, H. F. McFarland, and C. Pinilla. 2001. Molecular mimicry and antigen-specific T cell responses in multiple sclerosis and chronic CNS Lyme disease. J. Autoimmun. 16:187-192. [DOI] [PubMed] [Google Scholar]
- 24.Martin, R., P. Marquardt, S. O'Shea, M. Borkenstein, and H. W. Kreth. 1989. Virus-specific and autoreactive T cell lines isolated from cerebrospinal fluid of a patient with chronic rubella panencephalitis. J. Neuroimmunol. 23:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, R., J. Ortlauf, V. Sticht-Groh, U. Bogdahn, S. F. Goldmann, and H. G. Mertens. 1988. Borrelia burgdorferi—specific and autoreactive T-cell lines from cerebrospinal fluid in Lyme radiculomyelitis. Ann. Neurol. 24:509-516. [DOI] [PubMed] [Google Scholar]
- 26.Mason, D. 1998. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 19:395-404. [DOI] [PubMed] [Google Scholar]
- 27.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris-Downes, M. M., K. McCormack, D. Baker, D. Sivaprasad, J. Natkunarajah, and S. Amor. 2002. Encephalitogenic and immunogenic potential of myelin-associated glycoprotein (MAG), oligodendrocyte-specific glycoprotein (OSP) and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in ABH and SJL mice. J. Neuroimmunol. 122:20-33. [DOI] [PubMed] [Google Scholar]
- 29.Muraro, P. A., M. Kalbus, G. Afshar, H. F. McFarland, and R. Martin. 2002. T cell response to 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in multiple sclerosis patients. J. Neuroimmunol. 130:233-242. [DOI] [PubMed] [Google Scholar]
- 30.Pachner, A. R., D. Dail, Y. Bai, M. Sondey, L. Pak, K. Narayan, and D. Cadavid. 2004. Genotype determines phenotype in experimental Lyme borreliosis. Ann. Neurol. 56:361-370. [DOI] [PubMed] [Google Scholar]
- 31.Pachner, A. R., H. Gelderblom, and D. Cadavid. 2001. The rhesus model of Lyme neuroborreliosis. Immunol. Rev. 183:186-204. [DOI] [PubMed] [Google Scholar]
- 32.Pfister, H. W., V. Preac-Mursic, B. Wilske, K. M. Einhaupl, and K. Weinberger. 1989. Latent Lyme neuroborreliosis: presence of Borrelia burgdorferi in the cerebrospinal fluid without concurrent inflammatory signs. Neurology 39:1118-1120. [DOI] [PubMed] [Google Scholar]
- 33.Philipp, M. T., and B. J. Johnson. 1994. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 2:431-437. [DOI] [PubMed] [Google Scholar]
- 34.Pinilla, C., J. R. Appel, P. Blanc, and R. A. Houghten. 1992. Rapid identification of high affinity peptide ligands using positional scanning synthetic peptide combinatorial libraries. BioTechniques 13:901-905. [PubMed] [Google Scholar]
- 35.Pinilla, C., J. R. Appel, and R. A. Houghten. 1994. Investigation of antigen-antibody interactions using a soluble, non-support-bound synthetic decapeptide library composed of four trillion (4 × 10(12) sequences. Biochem. J. 301:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pohl-Koppe, A., K. E. Balashov, A. C. Steere, E. L. Logigian, and D. A. Hafler. 1998. Identification of a T cell subset capable of both IFN-gamma and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J. Immunol. 160:1804-1810. [PubMed] [Google Scholar]
- 37.Ramamoorthy, R., L. Povinelli, and M. T. Philipp. 1996. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect. Immun. 64:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosener, M., P. A. Muraro, A. Riethmuller, M. Kalbus, G. Sappler, R. J. Thompson, R. Lichtenfels, N. Sommer, H. F. McFarland, and R. Martin. 1997. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: a novel candidate autoantigen in demyelinating diseases. J. Neuroimmunol. 75:28-34. [DOI] [PubMed] [Google Scholar]
- 39.Rupprecht, T. A., U. Koedel, B. Muhlberger, B. Wilske, A. Fontana, and H. W. Pfister. 2005. CXCL11 is involved in leucocyte recruitment to the central nervous system in neuroborreliosis. J. Neurol. 252:820-823. [DOI] [PubMed] [Google Scholar]
- 40.Rupprecht, T. A., H. W. Pfister, B. Angele, S. Kastenbauer, B. Wilske, and U. Koedel. 2005. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65:448-450. [DOI] [PubMed] [Google Scholar]
- 41.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigal, L. H. 1997. Immunologic mechanisms in Lyme neuroborreliosis: the potential role of autoimmunity and molecular mimicry. Semin. Neurol. 17:63-68. [DOI] [PubMed] [Google Scholar]
- 43.Sospedra, M., Y. Zhao, H. Zur Hausen, P. A. Muraro, C. Hamashin, E. M. de Villiers, C. Pinilla, and R. Martin. 2005. Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS Pathog. 1:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vergelli, M., B. Hemmer, U. Utz, A. Vogt, M. Kalbus, L. Tranquill, P. Conlon, N. Ling, L. Steinman, H. F. McFarland, and R. Martin. 1996. Differential activation of human autoreactive T cell clones by altered peptide ligands derived from myelin basic protein peptide (87-99). Eur. J. Immunol. 26:2624-2634. [DOI] [PubMed] [Google Scholar]
- 46.Weber, K., and H. W. Pfister. 1994. Clinical management of Lyme borreliosis. Lancet 343:1017-1020. [DOI] [PubMed] [Google Scholar]
- 47.Wilske, B. 2002. Microbiological diagnosis in Lyme borreliosis. Int. J. Med. Microbiol. 291(Suppl. 33):114-119. [DOI] [PubMed] [Google Scholar]
- 48.Wucherpfennig, K. W., and J. L. Strominger. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yssel, H., M. C. Shanafelt, C. Soderberg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49a.Zhang, T., P. Haws, and Q. Wu. 2004. Multiple variable first exons: a mechanism for cell- and tissue-specific gene regulation. Genome Res. 14:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao, Y., B. Gran, C. Pinilla, S. Markovic-Plese, B. Hemmer, A. Tzou, L. W. Whitney, W. E. Biddison, R. Martin, and R. Simon. 2001. Combinatorial peptide libraries and biometric score matrices permit the quantitative analysis of specific and degenerate interactions between clonotypic TCR and MHC peptide ligands. J. Immunol. 167:2130-2141. [DOI] [PubMed] [Google Scholar]