Abstract
Gastrointestinal nematode infections are prevalent worldwide and are potent inducers of T helper 2 responses with the capacity to modulate the immune response to heterologous antigens. Parasitic helminth infection has even been shown to modulate the immune response associated with autoimmune diseases. Nonobese diabetic (NOD) mice provide a model for studying human autoimmune diabetes; as in humans, the development of diabetes in NOD mice has been linked to the loss of self-tolerance to beta cell autoantigens. Previous studies with the NOD mouse have shown that helminth and bacterial infection appears to inhibit type 1 diabetes by disrupting the pathways leading to the Th1-mediated destruction of insulin-producing beta cells. The aim of our study was to examine whether infection with the gastrointestinal helminths Trichinella spiralis or Heligmosomoides polygyrus could inhibit the development of autoimmune diabetes in NOD mice and to analyze the mechanisms involved in protection and the role of Th2 responses. Protection from diabetes was afforded by helminth infection, appeared to inhibit autoimmune diabetes by disrupting pathways leading to the destruction of beta cells, and was mediated by seemingly independent mechanisms depending on the parasite but which may be to be related to the capacity of the host to mount a Th2 response.
Helminth infections are a worldwide phenomenon but are becoming increasingly less common in developed countries (49). These infections have been shown to influence the outcome of parasitic, viral, bacterial, and autoimmune diseases (2, 4, 9, 17, 50). Helminth parasites are strong inducers of T helper 2 (Th2) cell-associated responses such as eosinophilia, interleukin-4 (IL-4), IL-5, IL-13, and immunoglobulin E (IgE) production (20, 29). In addition to the Th2 polarizing response, the induction of regulatory responses is also a common feature of the immune response induced following exposure to parasitic helminth antigens (6, 30, 49).
The incidence of autoimmune type 1 diabetes is on the increase in developed countries while remaining relatively uncommon in the developing world. This trend coincides with a decrease in helminth infection (22). Autoimmune diabetes affects approximately 1 in 300 children and is of considerable cost to health care systems (8). Experimentally, helminths have been associated with protection against a number of autoimmune disorders, including inflammatory bowel disease and diabetes. Administration of schistosome eggs has been shown to reduce the severity of experimental autoimmune encephalomyelitis (42) as well as preventing the development of both trinitrobenzene sulfonic acid-induced colitis (18) and diabetes in the nonobese diabetic (NOD) mouse model (12, 14). The administration of Trichuris suis ova to patients with inflammatory bowel disease that had been nonresponsive to conventional therapy has been shown to reduce the inflammatory response and improve clinical scores (45). Furthermore, infection with helminths also modulates allergic responses, since Heligmosomoides polygyrus infection has recently been shown to suppress allergic airway inflammation (48).
Previous studies have shown that infection with the trematode parasite Schistosoma mansoni can significantly inhibit or delay the development of diabetes in NOD mice (13). This appeared to be due to a skewing of the diabetes-associated Th1 response towards protective Th2 responses including IL-4, IL-5, IL-10, and IL-13 production (50). The breakdown of multiple tolerance pathways leads to destruction of the insulin-producing beta cells (3). The development of diabetes in the NOD mouse shows a role for CD4+ and CD8+ T-cell-mediated beta cell destruction (27). The aim of this study was to establish whether infection with the gastrointestinal (GI) helminths H. polygyrus and Trichinella spiralis could alter the responses leading to the development of diabetes in the NOD mouse. Both H. polygyrus and T. spiralis are known to produce a marked Th2 response in their host and have previously shown the ability to modulate responses to unrelated conditions (1, 4, 7, 9, 11, 21). We sought to establish the role that parasite-induced responses may play in the development of autoimmune diabetes and whether this effect can be attributed to Th2, regulatory, or suppressor functions by the parasite. This study demonstrated that these small-intestine-dwelling helminths altered the immune response responsible for the progression to autoimmune diabetes by distinct mechanisms and that both regulation and suppression of the immune response leading to beta cell destruction coincided with the induction of a Th2 response.
MATERIALS AND METHODS
Animals.
Female mice BALB/c were bred and maintained at the University of Strathclyde animal facility. Female NOD mice were bred and obtained from the animal facilities of the University of Cambridge and maintained under barrier conditions at the University of Strathclyde animal facility. All animals were used in accordance with local and Home Office regulations and approved by the University Ethical Review Committee. All animals used were 6 to 8 weeks of age.
Infection of animals.
Animals were inoculated with either 300 Heligmosomoides polygyrus larvae or 400 Trichinella spiralis larvae suspended in 0.2 ml 0.05% agarose directly into the stomach lumen via a blunt 21-gauge needle as described previously (4). T. spiralis larval homogenate antigen (Ag) and H. polygyrus adult homogenate Ag were prepared as described previously (7).
Recovery of adult worms.
Animals were killed by CO2 inhalation, and the small intestines were removed, opened longitudinally, and placed into a piece of nylon mesh, which allowed the adult worms to migrate out. The mesh was then incubated in Hanks balanced salt solution (Gibco BRL) in a 37°C water bath for 1 h. The parasites were counted under a binocular dissecting microscope.
H. polygyrus fecal egg counts.
Fecal egg counts were carried out by the standard zinc floatation method (4). After floatation in a standard McMaster counting slide, the eggs were counted under a microscope and the quantity expressed as the number of eggs per gram of feces.
Assessment of blood glucose levels.
Blood glucose levels were evaluated using a standard blood glucose monitor (LifeScan) at regular intervals from blood taken from the tail vein of mice.
Measurement of antibody responses.
Total serum IgE levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) technique as described previously (7). Anti-mouse IgE was used as the capture antibody, and IgE was detected using biotinylated anti-mouse IgE (PharMingen). An IgE monoclonal antibody specific for trinitrophenyl was used as a standard (PharMingen).
Anti-insulin antibodies were evaluated using standard ELISA methods. In brief, plates were coated with bovine insulin at 10 μg/ml, and a goat anti-mouse polyvalent Ig (detecting IgM, IgG, and IgA), anti-mouse IgG2a, or anti-mouse IgG1 conjugated to alkaline phosphatase (Sigma, Poole, United Kingdom) was used as the developing antibody.
Lymphocyte isolation and in vitro culture.
Single-cell suspensions were prepared from spleens. Cells were washed in RPMI 1640 (Sigma) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 μM 2-mercaptoethanol, 1.25 μg/ml amphotericin B, 10 mM HEPES, and 10% fetal calf serum (FCS; Gibco-BRL) and then resuspended in RPMI 1640 plus supplements. The number of viable cells was counted using a trypan blue exclusion assay and adjusted to give 1 × 107 cells per 100 μl. The cells were incubated at 37°C in 5% CO2 for 24 h in triplicate cultures either in media alone or in the presence of 10 μg/ml concanavalin A (ConA) (Sigma), 100 μg/ml T. spiralis Ag, or 50 μg/ml H. polygyrus Ag.
Proliferation assay.
Cells were prepared as described above and incubated in triplicate cultures for 72 h. [3H]thymidine (Amersham Pharmacia Biotech) was added at 0.5 μCi per well for the last 8 h of culture. The cells were harvested onto filters (Biological Instrumentation Services Ltd.) using a cell harvester (Skatron Instrument, Inc.). Incorporated [3H]thymidine was measured with a scintillation counter (Beckman).
Cytokine ELISA.
Sandwich ELISAs were used to measure IL-4, IL-10, and gamma interferon (IFN-γ). Kits were purchased from Biosource, and assays were carried out according to the manufacturer's instructions.
Flow cytometric analysis.
Each pancreas was harvested individually and torn into pieces in cold phosphate-buffered saline (PBS) containing 5% FCS, 56 mM glucose (Sigma), and Complete Mini protease inhibitors (Roche). The tissues were washed twice in cold PBS prior to incubation in 2 ml prewarmed PBS containing 15% FCS and Liberase CI (Boehringer Mannheim). After digestion, the tissues were washed, and cell suspensions were prepared by being forced through a cell strainer. Suspensions were twice left to settle and the supernatants decanted to remove stromal debris before the cells were washed and used for fluorescence-activated cell sorter (FACS) analysis or intracellular cytokine staining as below (51). Cells were resuspended in FACS staining buffer (PBS-3% FCS) to 1 × 107 cells per ml, and 50 μl of the cell suspension was added to FACS tubes (BD). Nonspecific binding was blocked by incubation with antibody clone 2.4G2 (from the tissue culture supernatant) and neutravidin (Molecular Probes). Cells were washed and resuspended in staining buffer (PBS containing 2% bovine serum albumin and 0.05% NaN3). As an additional step for dendritic cell (DC) subset analysis, lymph nodes were cut into small fragments and digested with frequent mixing for 25 min at 37°C in medium containing Liberase CI. DC-T cell complexes were then disrupted by the addition of EDTA (0.1 M, pH 7.3). Cells were stained with the appropriate combinations of fluorescein isothiocyanate, phycoerythrin (PE), or peridinin chlorophyll protein conjugates (all from BD Pharmingen) of anti-CD3, anti-CD4, anti-CD8α, anti-B220, anti-CD11c, anti-CD49b (DX5), anti-major histocompatibility complex class II (OX6hiGeoMFI), anti-CD25, and anti-CD62. Washed cells were fixed overnight in 1% formaldehyde prior to analysis using a FACSCanto flow cytometer with FACSDiva software (Becton Dickinson).
Intracellular cytokine staining.
Cells were incubated in medium containing 50 ng/ml phorbol myristate acetate (Sigma) and 500 ng/ml ionomycin (Sigma) for 4 h, with the addition of 5 μl/ml brefeldin A (Sigma) after 2 h. Cells were then washed and blocked in 2.4G2 before staining with anti-CD3 fluorescein isothiocyanate and anti-CD4 peridinin chlorophyll protein. After washing, cells were incubated for 15 min at room temperature in fixation medium (Caltag) before being stained with anti-IFN-γ-PE, anti-IL-10-PE, anti-IL-4-PE, or the appropriate isotype control (all BD Pharmingen) in permeabilization buffer (Caltag). Cells were then washed and analyzed as described above.
Statistical analysis.
Results are presented as mean values from individual animals ± standard errors of the means (SEM) for groups of animals undergoing uniform treatment. Differences between groups were analyzed using the Mann-Whitney test. In all cases, a probability value (P) of less than 0.05 was considered significant.
RESULTS
Helminth infection of NOD mice alters the incidence of diabetes.
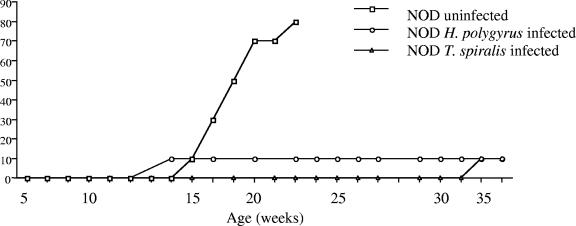
To determine if the Th2-inducing ability of helminth infection could alter the autoimmune destruction of insulin-producing pancreatic beta cells, levels of blood glucose present in the sera of infected and control NOD mice were measured. Progression to diabetes was determined as blood glucose levels reaching 12 mmol/liter and above. Uninfected NOD mice began to develop blood glucose levels of ≥12 mmol/liter from 14 to 15 weeks of age (Fig. 1); the incidence of disease increased within this group until 80% were diabetic by 22 to 23 weeks of age. NOD mice infected with H. polygyrus remained free of diabetes over the entire experimental time course (36 to 37 weeks). The 10% incidence of diabetes at 12 to 13 weeks in this group represents one mouse that was uninfected, as determined by intestinal worm burden. NOD mice infected with T. spiralis also remained diabetes free until an incidence of 10% was seen at week 36 to 37.
FIG. 1.
Infection with gastrointestinal helminths prevents the development of diabetes in NOD mice. NOD mice (aged 4 to 6 weeks) were infected with H. polygyrus (n = 10) or T. spiralis (n = 10) or left uninfected. Blood glucose levels were monitored at weekly intervals, and a level of over 12 mmol/liter was considered to indicate diabetes. Data are from one experiment, representative of two repeat studies.
NOD mice were less efficient at expelling adult H. polygyrus worms but showed no differences from BALB/c mice in their ability to control T. spiralis infection.
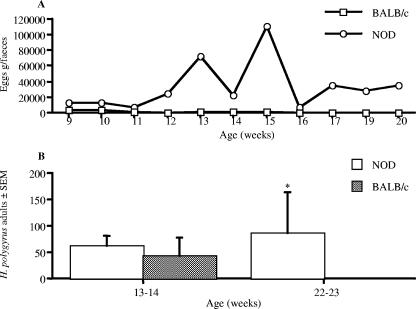
In conjunction with investigating the effect of infection on diabetes, the rate at which the parasites were expelled from the host animals was examined (Fig. 2). The intestinal parasite H. polygyrus is associated with chronic infection that can last several months, while T. spiralis infection is characterized by rapid expulsion followed by deposition of muscle larvae which persist for many months. We examined infection with H. polygyrus in both NOD and BALB/c mice by the release of parasite eggs in the feces and the presence of adult parasites in the small intestine. Parasites in BALB/c mice ceased all egg production by 15 to 16 weeks, while those in NOD mice continued egg production until the experimental end point (Fig. 2A). The number of adult H. polygyrus worms present in the small intestine of NOD and BALB/c mice was measured at 13 to 14 and 22 to 23 weeks (Fig. 2B). At 13 to 14 weeks of age both BALB/c and NOD mice had a similar worm burden, and while BALB/c mice had lost all their adult H. polygyrus worms by 22 to 23 weeks of age, NOD mice still harbored a significant worm burden.
FIG. 2.
NOD mice demonstrated a reduced capacity to expel H. polygyrus. NOD and BALB/c mice were infected with H. polygyrus. (A) Production of eggs in the feces of H. polygyrus-infected mice was assessed at weekly intervals. (B) The number of adult H. polygyrus worms in the small intestine was assessed at 13 to 14 and 22 to 23 weeks old. *, significant difference between NOD and BALB/c mice (P < 0.05). Data are from one experiment (means ± SEM), representative of two repeat studies.
Expulsion of T. spiralis adult worms by BALB/c mice is usually completed by 14 days postinfection, and unsurprisingly, at both time points, no adult T. spiralis worms were present in the small intestines of either NOD or BALB/c mice (data not shown). Additionally, at both 13 to 14 and 22 to 23 weeks, both NOD and BALB/c mice had similar T. spiralis muscle larva burdens (data not shown).
Helminth infection enhances the level of total IgE in NOD and BALB/c mice over the experimental time course.
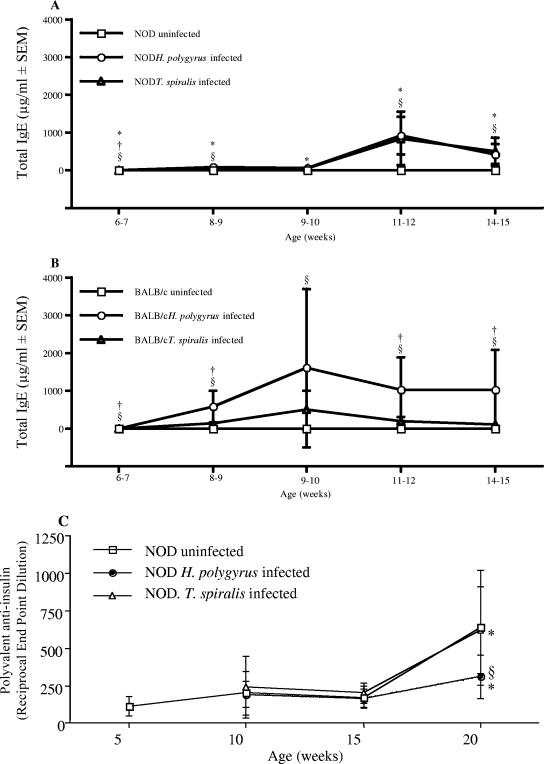
The production of IgG1 and IgE antibody isotypes is associated with a Th2 response and is a common feature of helminth infection (7, 29). NOD mice spontaneously develop antibodies to insulin (26). Total serum IgE and insulin-specific IgG1 and IgG2a were measured at various time points over the course of the experiment. In both NOD and BALB/c mice, the level of total IgE produced was enhanced by infection with H. polygyrus or T. spiralis, although levels in NOD mice were significantly lower than those observed in BALB/c mice (Fig. 3A and B). Titers of total immunoglobulin insulin-specific antibodies increased significantly in both uninfected and T. spiralis-infected NOD mice; however, titers in H. polygyrus infected mice were significantly lower (Fig. 3C). Infection with either helminth did not affect the levels of insulin-specific IgG1 or IgG2a (data not shown).
FIG. 3.
Infection with GI helminths elevates IgE levels. The levels of total serum IgE were determined by ELISA at intervals following infection of NOD (A) and BALB/c (B) mice with H. polygyrus or T. spiralis. (C) Levels of spontaneous polyvalent anti-insulin autoantibodies in NOD mice. §, significant difference between uninfected and infected animals (P < 0.05); *, significant difference between NOD and BALB/c mice (P < 0.05); †, significant difference between H. polygyrus- and T. spiralis-infected mice. (P < 0.05). Data are from one experiment, representative of two repeat studies.
Proliferative responses are increased by helminth infection in both NOD and BALB/c mice.
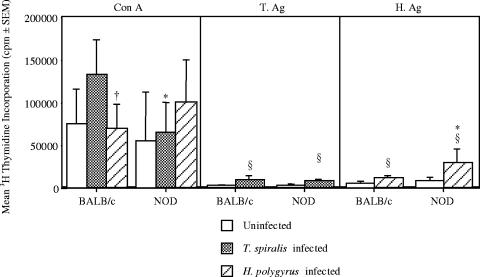
To study the effect of helminth infection on the lymphoproliferative response to various stimuli, splenocytes were removed from 22- to 23-week-old NOD and BALB/c mice and restimulated in vitro with ConA or parasite antigen. Responses to ConA were similar in both uninfected and T. spiralis-infected BALB/c and NOD mice, while responses in H. polygyrus-infected BALB/c mice were significantly greater than those in NOD mice (Fig. 4A). As expected, there was an increase in proliferation of splencocytes to T. spiralis Ag following infection of BALB/c mice with T. spiralis; interestingly, this was not echoed in the NOD mice (Fig. 4B). Splenocytes from both BALB/c and NOD mice infected with H. polygyrus demonstrated a moderate increase in the proliferative response to H. polygyrus Ag response, with the response of NOD mice being significantly greater (Fig. 4C). The lack of proliferative responses seen in response to T. spiralis Ag is likely to be due to the time point examined (17 to 18 weeks postinfection), as peak proliferative responses following infection with T. spiralis are observed at 5 to 8 days postinfection, with negligible responses observed after 18 days postinfection (29a). Overall, the results suggest that GI helminth infection of NOD and BALB/c mice shows an increase in proliferative responses.
FIG. 4.
Infection with gastrointestinal helminths enhances the proliferative responses of BALB/c and NOD mice. Splenocytes from 22- to 23-week-old NOD and BALB/c mice infected with H. polygyrus or T. spiralis were cultured in the presence or absence of ConA (A), T. spiralis antigen (T.Ag) (B), or H. polygyrus antigen (H.Ag) (C) for 72 h, and the incorporation of tritiated thymidine in the final 8 h was assessed. §, significant difference between uninfected and infected animals (P < 0.05); *, significant difference between NOD and BALB/c mice (P < 0.05); †, significant difference between H. polygyrus- and T. spiralis-infected mice (P < 0.05). Results are means ± SEM for data generated from means of five sets of triplicates set up from individual mice. Data are from one experiment, representative of two repeat studies.
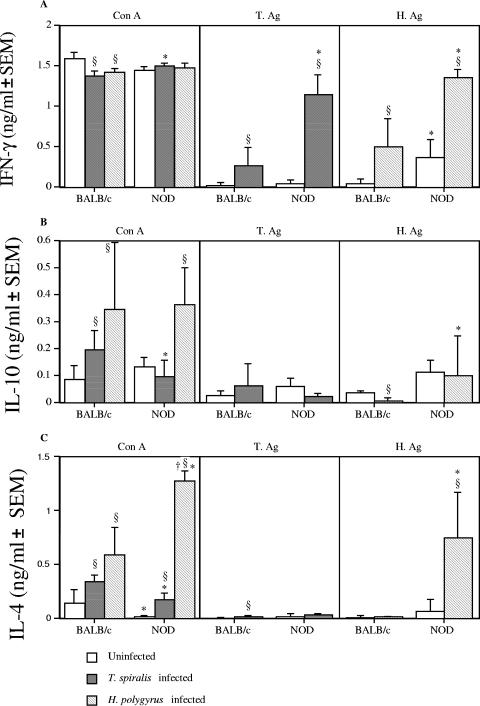
Helminth infection skews the Th1/Th2 cytokine balance in NOD and BALB/c mice.
To understand whether the inhibition of diabetes in the helminth-infected NOD mice was due to an alteration in Th1/Th2-associated responses, splenocytes were restimulated in vitro and cytokine production was measured. The Th1-associated cytokine IFN-γ accompanies the development of diabetes in NOD mice (15). In response to ConA, production of IFN-γ by splenocytes was reduced in infected BALB/c mice but remained the same in infected NOD mice compared to uninfected mice. In response to either parasite antigen, IFN-γ was enhanced in infected NOD and BALB/c mice; NOD mice also demonstrated significantly greater IFN-γ production than did BALB/c mice (Fig. 5A). These results indicate that the presence of helminth infection does not necessarily lead to a reduction in Th1-associated IFN-γ.
FIG. 5.
Infection of NOD mice with gastrointestinal helminths results in the induction of a profound Th2 response. Splenocytes from 22- to 23-week-old NOD and BALB/c mice infected with H. polygyrus or T. spiralis were cultured in the presence or absence of ConA, T. spiralis antigen (T.Ag), or H. polygyrus antigen (H.Ag) for 24 h. Levels of cytokines in supernatants were assessed by sandwich ELISA. (A) IFN-γ; (B) IL-10; (C) IL-4. §, significant difference between uninfected and infected animals (P < 0.05); *, significant difference between NOD and BALB/c mice (P < 0.05); †, significant difference between H. polygyrus- and T. spiralis-infected mice. (P < 0.05). Data are from one experiment, representative of two repeat studies.
IL-10 has been associated with regulatory responses responsible for protection against diabetes (24). In response to ConA, infection with both helminths increased splenocyte IL-10 production in BALB/c mice, while in NOD mice, only H. polygyrus-infected mice demonstrated an increase in IL-10 levels. Helminth infection produced no effect on IL-10 production to T. spiralis antigen in either BALB/c or NOD mice. In response to H. polygyrus antigen, however, H. polygyrus-infected BALB/c mice displayed a reduction in IL-10 production, while production in NOD mice was unaltered (Fig. 5B).
Finally we sought to investigate the effect of helminth infection on the presence of the Th2-associated cytokine IL-4. Our results demonstrated that production of IL-4 by splenocytes was significantly enhanced in both T. spiralis- and H. polygyrus-infected NOD and BALB/c mice, and in H. polygyrus-infected NOD mice, this was significantly higher than in T. spiralis-infected mice. In response to T. spiralis antigen, T. spiralis-infected BALB/c mice, but not NOD mice, displayed a slight, but significant, increase in IL-4, while in response to H. polygyrus antigen, only H. polygyrus-infected NOD mice demonstrated a significant increase in IL-4 (Fig. 5C). Overall, helminth infection increased the levels of mitogen-induced IL-4 production, and in NOD mice, H. polygyrus infection increased the level of IL-4 produced in response to H. polygyrus Ag.
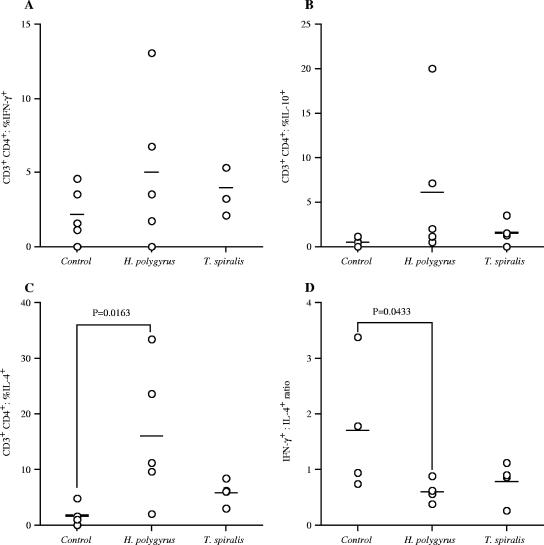
Infection with helminths alters the cell phenotype of infiltrating pancreatic lymphocytes in NOD mice.
The percentages of IFN-γ-, IL-10-, and IL-4-producing CD4+ T cells were evaluated in infiltrating pancreatic lymphocytes following infection of BALB/c and NOD mice with GI helminths (Fig. 6A to D). At 13 to 14 weeks of age, infected NOD mice showed no differences in comparison to uninfected controls in the percentage of IFN-γ-positive and IL-10-positive CD4+ cells (Fig. 6A and B). At 22 to 23 weeks of age, however, both the percentage of IFN-γ-positive and IL-10-positive cells had increased in NOD mice protected from diabetes by T. spiralis infection. The percentage of IL-4-positive cells significantly increased in H. polygyrus-infected NOD mice at 13 to 14 weeks of age, and at 22 to 23 weeks of age, the percentage of IL-4-positive cells were also significantly increased in T. spiralis-infected mice (Fig. 6C). By examining the ratio of IFN-γ-positive cells to IL-4-positive cells, it was observed that uninfected NOD mice displayed significantly more IFN-γ-positive cells in relation to IL-4-positive cells than the mice protected from diabetes by infection with H. polygyrus (Fig. 6D).
FIG. 6.
Infection with gastrointestinal helminths upregulates the percentage of Th2 cytokine producing cells in NOD mice. The percentage of intracellular IFN-γ (A), IL-10 (B), and IL-4 (C) production by infiltrating pancreatic CD4+ T cells in uninfected or H. polygyrus- or T. spiralis-infected NOD mice was assessed by flow cytometry at 13 to 14 weeks of age. (D) IFN-γ/IL-4 ratio. Data points are for individual mice, bars are mean values, and P values are by Mann-Whitney U test. Data are from one experiment, representative of two repeat studies.
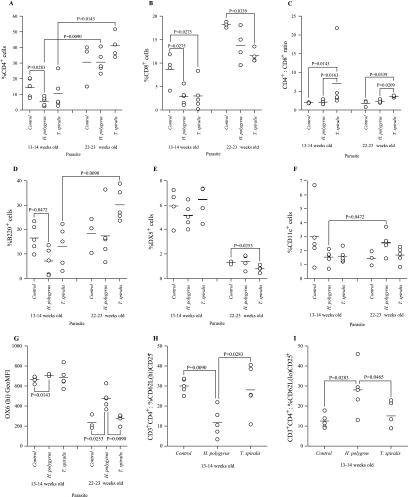
The percentages of CD4+ and CD8+ lymphocytes infiltrating the pancreas of uninfected and helminth-infected NOD mice were evaluated by flow cytometry. Despite a significantly lower level in the percentage of CD4+ cells in the H. polygyrus-infected mice at 13 to 14 weeks, at 22 to 23 weeks, the percentage of CD4+ lymphocytes in all groups had increased to similar levels (Fig. 7A). Similarly, reduced percentages of CD8+ lymphocytes in the pancreas in both infected groups at 13 to 14 weeks old in comparison to uninfected NOD mice were observed (Fig. 7B). Although the percentage of CD8+ cells had increased in all groups at 22 to 23 weeks of age, levels in helminth-infected mice were significantly lower than those in uninfected NOD mice. By examining the ratio of percentages of CD4+ to CD8+ cells, it was seen that, at both 13 to 14 weeks and 22 to 23 weeks, infection with T. spiralis but not H. polygyrus resulted in a greater proportional representation of CD4+ than CD8+ in NOD mice (Fig. 7C). Overall, examination of the percentage of CD4+ and CD8+ T cells has shown that the mice protected from diabetes by T. spiralis infection show the clearest protective shift, with increases in the percentage of infiltrating T cells expressing CD4 relative to those expressing CD8.
FIG.7.
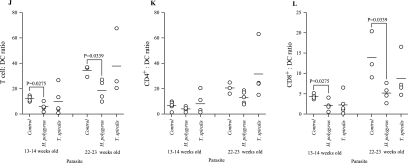
Infection with gastrointestinal helminths modulates the phenotype of cells infiltrating the pancreas of NOD mice. Cells infiltrating the pancreases of uninfected or H. polygyrus- or T. spiralis-infected NOD mice were assessed by flow cytometry at 13 to 14 and 22 to 23 weeks of age. (A) CD4+; (B) CD8+; (C) CD4+/CD8+ ratio; (D) B220+; (E) DX5+; (F) CD11c+; (G) OX6hiGeoMFI; (H) CD3+ CD4+ CD62Lhi CD25−; (I) CD3+ CD4+ CD62Llo CD25+; (J) T cell/DC ratio; (K) CD4+/DC ratio; (L) CD8+/DC ratio. Data points are for individual mice, bars are mean values, and P values are by Mann Whitney U test. Data are from one experiment, representative of two repeat studies.
A number of other cellular markers were examined in the pancreatic infiltrate of uninfected and helminth-infected NOD mice by flow cytometry. The percentage of B cells present was measured via expression of the pan-B-cell marker B220 and absence of CD11c (Fig. 7D). At 13 to 14 weeks old, H. polygyrus-infected NOD mice showed a decreased relative pancreatic infiltration by B cells. This was not repeated, however, at 22 to 23 weeks of age. The percentage of B cells was increased in the T. spiralis-infected mice at both 13 to 14 and 22 to 23 weeks of age. DX5 antibody was used as a marker for NK cells, as it binds CD49b (Fig. 7E). At 13 to 14 weeks of age, there were no changes in any of the groups. At 22 to 23 weeks, we saw a decrease in the percentage of NK cells in those mice protected from diabetes by T. spiralis infection. CD11c is a widely used pan marker for dendritic cells (Fig. 7F); although no changes among the groups was seen at either time point, there was an increase in CD11c+ expression in the H. polygyrus-infected animals between 13 to 14 weeks and 22 to 23 weeks of age. In conjunction with this, we also examined the expression of OX6 (Fig. 7G). OX6 binds major histocompatibility complex class II, which is expressed on classical antigen-presenting cells such as DC. H. polygyrus-infected mice showed an increase in OX6 expression at both time points. CD62L (l-selectin) is lost from T cells upon antigen activation, and thymus-derived naturally arising CD4+ regulatory T cells have been shown to express high levels of CD62L and CD25 (28, 38). Other populations of CD4+ regulatory T cells have been described that can be induced following exposure to IL-10 and antigen, and these induced T regulatory cells may be found in both the CD25+ and CD25− populations (46). A decrease in T cells that were CD62Lhi CD25− (Fig. 7H) and an increase in cells that were CD62Llo CD25+ was observed in H. polygyrus-infected mice at 13 to 14 weeks (Fig. 7I).
Antigen-presenting cells infiltrating the pancreas of 13- to 14- and 22- to 23-week-old uninfected and helminth-infected NOD mice were analyzed by flow cytometry. At both time points, we saw a decrease in the ratio of T cells to DCs (Fig. 7J). When the ratio of CD4+ and CD8+ cells was examined in comparison to DCs (Fig. 7K), we found no change in the CD4+/DC ratio but greater relative DC infiltration in H. polygyrus-infected NOD mice than CD8+ cells (Fig. 7L).
DISCUSSION
It has been well established that the balance of Th1/Th2 can affect the outcome of many diseases. In response to helminth infections, a Th2 response, characterized by increased IL-4, IL-5, and IL-13 cytokine production, is protective, while a Th1 response is associated with susceptibility (19, 20, 29). These parasites also demonstrate an ability to induce immunoregulatory responses that both suppress the responses that might lead to the parasite being expelled from the host but also alter the responses to other infectious organisms and heterologous antigens (7, 30, 48). The ability of these parasites to alter the host response may not be restricted to Th1/Th2 subversion, with the induction of regulatory T cells also potentially playing a role (48). The results of this study indicate that the induction and progression of diabetes in the NOD mouse model can be inhibited by gastrointestinal helminth infection and that the mechanisms induced were different for each parasite.
Infection of NOD and BALB/c mice with the GI helminths T. spiralis and H. polygyrus elicited a Th2 response, as demonstrated by increased IL-4 and IgE levels. This is in contrast to the Th1 response that accompanies the development of diabetes in NOD mice and suggests that the Th2 response induced by T. spiralis and H. polygyrus infection may have protected the mice from the effects of Th1-mediated beta cell destruction. At odds with this, however, our experiments demonstrated no decrease in the Th1-associated cytokine IFN-γ accompanying the enhanced Th2 response. The role of Th1 and Th2 cytokines in autoimmune diabetes can be paradoxical, exogenously administered IFN-γ does not influence type 1 diabetes development in NOD mice and even protects DP-BB rats from disease development (44). Accordingly, the onset of diabetes may be prevented in NOD mice and BB rats by systemically administering type 1 cytokines such as IL-1, tumor necrosis factor alpha, and IL-2 (33, 41). This therefore indicates that the induction of Th2 type responses may not be solely responsible for protection from diabetes.
Infection with T. spiralis induced an increase in CD4+ T cells and a decrease in the levels of both CD8+ T cells and NK cells in the pancreas; however, splenocyte proliferation following in vitro restimulation was not affected by infection. In contrast, previous work has also shown that proliferative responses are diminished in mice protected from diabetes by S. mansoni eggs (50). There are numerous examples where suppression of immune responses has been demonstrated following infection with helminths (1, 4, 7, 9, 11, 21). Although protection from diabetes is associated with the induction of IL-10 (35, 36, 50), infection with Salmonella enterica serovar Typhimurium could protect NOD mice from diabetes onset and was associated with an increase in IFN-γ and a decrease in IL-4 and IL-10 (51). Similarly, although infection with T. spiralis ameliorated the development of diabetes, there was no evidence of increased IL-10 production in NOD mice following this infection.
The “hygiene hypothesis” postulates that the increased incidence of autoimmune diseases such as diabetes in the developed world is the result of decreased exposure to infectious agents. Diabetes in animal models can be prevented by a variety of infectious agents, including helminths, bacteria, and viruses. However, the mechanisms of action vary from the induction of regulatory T cells to altered trafficking of diabetogenic T cells (12). Inoculation of NOD mice with complete Freund's adjuvant or the Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccine protects against diabetes in NOD mice (25, 39). Infection of NOD mice with Mycobacterium avium inhibits diabetes in a manner associated with increased production of IFN-γ and decreased production of IL-4. Additionally, in this system, increased FasL expression was observed on T cells, and Fas-FasL-mediated T-cell deletion was suggested as a mechanism for the protection from diabetes (31). Virus infection can prevent diabetes in both NOD mice and the BB rat (16, 47), and it has been suggested that these infections may alter the trafficking of autoreactive T cells, since infection with lymphocytic choriomeningitis virus enhances the expression of the chemokine IFN-γ-inducible protein 10 in the lymph node draining the pancreas, and it was proposed that this would lead to recruitment of T cells away from the pancreatic islets to the lymph node, where they would undergo apoptosis (10). Th2 cells induced following infection with gastrointestinal helminths result in an upregulation in expression of the integrin α4β7 which binds to the mucosal addressin MAdCAM (32). Since blockade of MAdCAM could prevent the development of diabetes in neonatal mice transferred with the islet-reactive T-cell clone BDC-2.5 (37), it is possible that alterations in the expression of MAdCAM by helminth infection could alter the development of cytotoxic responses and/or alter lymphocyte traffic of autoreactive T cells that result in damage to insulin-producing cells.
The reasons why these two helminths induce different responses in NOD mice may be related to their different life cycle strategies. H. polygyrus has a strictly enteric life cycle and is generally considered a chronic infection, with infection lasting from 4 to 12 months depending on mouse strain. Conversely, T. spiralis is referred to as an acute infection, since the adult parasite is expelled after between 10 and 20 days; however, the larval stage in the muscle can persist for many years. While it is unequivocal that a Th2 response is induced following infection with either of these parasites, there are a number of subtle differences, most notably that T. spiralis induces a profound mastocytosis and intestinal inflammation, while H. polygyrus does not and can even suppress the inflammation induced by T. spiralis (23, 29). The immune response to the larval muscle stage of T. spiralis is also characterized by a Th2 response, and this is coupled with an intense IL-10 response which is crucial for controlling the accompanying inflammation (5). These two parasites are therefore present and manipulating the immune response for the duration of the study; it would therefore be of interest to determine if parasite products could elicit similar responses, as has been demonstrated both for diabetes and in other systems (1, 4, 6, 7, 9, 21, 48, 50).
In conclusion, the lack of parasite expulsion seen in NOD mice infected with H. polygyrus may coincide with the induction of a regulatory response in the host. This follows the model of an upregulation of regulatory responses occurring after long-term exposure to helminth antigens, rendering the immune system hyporesponsive to unrelated antigens (48). It would seem that helminth infection induces an anti-inflammatory state that enhances parasite survival but also spills over to modulate autoaggressive responses. This mirrors the recent study in which infection with H. polygyrus suppressed allergic airway inflammation by the induction of regulatory T cells (48). Furthermore, these infections may also alter the expression of integrins and/or chemokines that lead to the migration of cytotoxic cells away from the pancreas.
The study of the different mechanisms by which superficially similar helminth infections alter the development of diabetes may help to better understanding how regulatory mechanisms are induced and how they can be manipulated to control a variety of autoimmune diseases.
Acknowledgments
We thank the University of Strathclyde for studentship support for K.A.S.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Abou Afifi, M., M. M. Gamra, M. A. Moustafa, and L. M. el-Hoseiny. 2000. Modulation of the pathologic and apoptotic changes of experimental toxoplasmosis by concomitant infection with Trichinella spiralis. J. Egypt. Soc. Parasitol. 30:69-81. [PubMed] [Google Scholar]
- 2.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, M. S., and J. A. Bluestone. 2005. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23:447-485. [DOI] [PubMed] [Google Scholar]
- 4.Behnke, J. M., D. Wakelin, and M. M. Wilson. 1978. Trichinella spiralis: delayed rejection in mice concurrently infected with Nematospiroides dubius. Exp. Parasitol. 46:121-130. [DOI] [PubMed] [Google Scholar]
- 5.Beiting, D. P., S. K. Bliss, D. H. Schlafer, V. L. Roberts, and J. A. Appleton. 2004. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect. Immun. 72:3129-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boitelle, A., H. E. Scales, C. Di Lorenzo, E. Devaney, M. W. Kennedy, P. Garside, and C. E. Lawrence. 2003. Investigating the impact of helminth products on immune responsiveness using a TCR transgenic adoptive transfer system. J. Immunol. 171:447-454. [DOI] [PubMed] [Google Scholar]
- 7.Boitelle, A., C. Di Lorenzo, H. E. Scales, E. Devaney, M. W. Kennedy, P. Garside, and C. E. Lawrence. 2005. Contrasting effects of acute and chronic gastro-intestinal helminth infections on a heterologous immune response in a transgenic adoptive transfer model. Int. J. Parasitol. 35:765-775. [DOI] [PubMed] [Google Scholar]
- 8.Brown, A. 1998. Complications of diabetes: prevention and management. Pharm. J. 261:31-38. [Google Scholar]
- 9.Chowaniec, W., R. B. Wescott, and L. L. Congdon. 1972. Interaction of Nematospiroides dubius and influenza virus in mice. Exp. Parasitol. 32:33-44. [DOI] [PubMed] [Google Scholar]
- 10.Christen, U., D. Benke, T. Wolfe, E. Rodrigo, A. Rhode, A. C. Hughes, M. B. Oldstone, and M. G. von Herrath. 2004. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J. Clin. Investig. 113:74-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christie, P. R., D. Wakelin, and M. M. Wilson. 1979. The effect of the expulsion phase of Trichinella spiralis on Hymenolepis diminuta infection in rats. Parasitology 78:323-330. [DOI] [PubMed] [Google Scholar]
- 12.Cooke, A., P. Zaccone, T. Raine, J. M. Phillips, and D. W. Dunne. 2004. Infection and autoimmunity: are we winning the war, only to lose the peace? Trends Parasitol. 20:316-321. [DOI] [PubMed] [Google Scholar]
- 13.Cooke, A., P. Tonks, F. M. Jones, H. O'Shea, P. Hutchings, A. J. Fulford, and D. W. Dunne. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21:169-176. [DOI] [PubMed] [Google Scholar]
- 14.David, T., C. Thomas, P. Zaccone, D. W. Dunne, and A. Cooke. 2004. The impact of infection on the incidence of autoimmune disease. Curr. Top. Med. Chem. 4:521-529. [DOI] [PubMed] [Google Scholar]
- 15.Debray-Sachs, M., C. Carnaud, C. Boitard, H. Cohen, I. Gresser, P. Bedossa, and J. F. Bach. 1991. Prevention of diabetes in NOD mice treated with antibody to murine IFN gamma. J. Autoimmun. 4:237-248. [DOI] [PubMed] [Google Scholar]
- 16.Dyrberg, T., P. L. Schwimmbeck, and M. B. Oldstone. 1988. Inhibition of diabetes in BB rats by virus infection. J. Clin. Investig. 81:928-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott, D. E., J. Li, A. Blum, A. Metwali, K. Qadir, J. F. Urban, Jr., and J. V. Weinstock. 2003. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284:G385-G391. [DOI] [PubMed] [Google Scholar]
- 19.Else, K. J., and F. D. Finkelman. 1998. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28:1145-1158. [DOI] [PubMed] [Google Scholar]
- 20.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 21.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 22.Gale, E. A. 2002. The rise of childhood type 1 diabetes in the 20th century. Diabetes 51:3353-3361. [DOI] [PubMed] [Google Scholar]
- 23.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22:605-612. [DOI] [PubMed] [Google Scholar]
- 24.Hammond, K. J., L. D. Poulton, L. J. Palmisano, P. A. Silveira, D. I. Godfrey, and A. G. Baxter. 1998. alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp. Med. 187:1047-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada, M., Y. Kishimoto, and S. Makino. 1990. Prevention of overt diabetes and insulitis in NOD mice by a single BCG vaccination. Diabetes Res. Clin. Pract. 8:85-89. [DOI] [PubMed] [Google Scholar]
- 26.Hutchings, P., P. Tonks, and A. Cooke. 1997. Effect of MHC transgene expression on spontaneous insulin autoantibody class switch in nonobese diabetic mice. Diabetes 46:779-784. [DOI] [PubMed] [Google Scholar]
- 27.Kay, T. W., H. L. Chaplin, J. L. Parker, L. A. Stephens, and H. E. Thomas. 1997. CD4+ and CD8+ T lymphocytes: clarification of their pathogenic roles in diabetes in the NOD mouse. Res. Immunol. 148:320-327. [DOI] [PubMed] [Google Scholar]
- 28.Laouar, Y., and I. N. Crispe. 2000. Functional flexibility in T cells: independent regulation of CD4+ T cell proliferation and effector function in vivo. Immunity 13:291-301. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence, C. E. 2003. Is there a common mechanism of gastrointestinal nematode expulsion? Parasite Immunol. 25:271-281. [DOI] [PubMed] [Google Scholar]
- 29a.Lawrence, C. E., J. C. Paterson, L. M. Higgins, T. T. MacDonald, M. W. Kennedy, and P. Garside. 1998. IL-4-regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 28:2672-2684. [DOI] [PubMed] [Google Scholar]
- 30.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 31.Martins, T. C., and A. P. Aguas. 1999. Mechanisms of Mycobacterium avium-induced resistance against insulin-dependent diabetes mellitus (IDDM) in non-obese diabetic (NOD) mice: role of Fas and Th1 cells. Clin. Exp. Immunol. 115:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohrs, K., D. P. Harris, F. E. Lund, and M. Mohrs. 2005. Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J. Immunol. 175:5306-5313. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti, F., P. Zaccone, R. Di Marco, G. Magro, S. Grasso, F. Stivala, G. Calori, L. Mughini, P. L. Meroni, and G. Garotta. 1998. Paradoxical antidiabetogenic effect of gamma-interferon in DP-BB rats. Diabetes 47:32-38. [DOI] [PubMed] [Google Scholar]
- 34.Reference deleted.
- 35.Pennline, K. J., E. Roque-Gaffney, and M. Monahan. 1994. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin. Immunol. Immunopathol. 71:169-175. [DOI] [PubMed] [Google Scholar]
- 36.Phillips, J. M., N. M. Parish, M. Drage, and A. Cooke. 2001. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J. Immunol. 167:6087-6091. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, J. M., K. Haskins, and A. Cooke. 2005. MAdCAM-1 is needed for diabetes development mediated by the T cell clone, BDC-2.5. Immunology 116:525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raine, T., P. Zaccone, P. Mastroeni, and A. Cooke. 2006. Salmonella typhimurium infection in nonobese diabetic mice generates immunomodulatory dendritic cells able to prevent type 1 diabetes. J. Immunol. 177:2224-2233. [DOI] [PubMed] [Google Scholar]
- 39.Sadelain, M. W., H. Y. Qin, J. Lauzon, and B. Singh. 1990. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39:583-589. [DOI] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Satoh, J., H. Seino, T. Abo, S. Tanaka, S. Shintani, S. Ohta, K. Tamura, T. Sawai, T. Nobunaga, T. Oteki, K. Kumagai, and R. Toyota. 1989. Recombinant human tumor necrosis factor alpha suppresses autoimmune diabetes in nonobese diabetic mice. J. Clin. Investig. 84:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sewell, D., Z. Qing, E. Reinke, D. Elliot, J. Weinstock, M. Sandor, and Z. Fabry. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 15:59-69. [DOI] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Sobel, D. O., and J. Newsome. 1997. Gamma interferon prevents diabetes in the BB rat. Clin. Diagn. Lab. Immunol. 4:764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summers, R. W., D. E. Elliot, K. Qadir, J. F. Urban, Jr., R. Thompson, and J. V. Weinstock. 2003. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 98:2034-2041. [DOI] [PubMed] [Google Scholar]
- 46.Taams, L. S., and A. N. Akbar. 2005. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr. Top. Microbiol. Immunol. 293:115-131. [DOI] [PubMed] [Google Scholar]
- 47.Wilberz, S., H. J. Partke, F. Dagnaes-Hansen, and L. Herberg. 1991. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34:2-5. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, M. S., M. D. Taylor, A. Balic, C. A. Finney, J. R. Lamb, and R. M. Maizels. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yazdanbakhsh, M., P. G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science 296:490-494. [DOI] [PubMed] [Google Scholar]
- 50.Zaccone, P., Z. Fehervari, F. M. Jones, S. Sidobre, M. Kronenberg, D. W. Dunne, and A. Cooke. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33:1439-1449. [DOI] [PubMed] [Google Scholar]
- 51.Zaccone, P., T. Raine, S. Sidobre, M. Kronenberg, P. Mastroeni, and A. Cooke. 2004. Salmonella typhimurium infection halts development of type 1 diabetes in NOD mice. Eur. J. Immunol. 34:3246-3256. [DOI] [PubMed] [Google Scholar]