Abstract
Nearly 50% of women experience at least one urinary tract infection (UTI) in their lifetime. Studies with mice have revealed that uropathogenic Escherichia coli (UPEC) isolates invade superficial umbrella cells that line the bladder, allowing them to find a safe haven and subvert clearance by innate host responses. Rapid intracellular replication results in the formation of distinctive intracellular bacterial communities (IBCs). In this study, we evaluated whether UPEC strains cultured from the urine of women and classified as causing acute cystitis, recurrent cystitis, asymptomatic bacteriuria, or pyelonephritis could progress through the IBC cascade in a well-characterized mouse model of cystitis. Of 18 UPEC isolates collected from women, 15 formed IBCs. Variations in the size, number, and kinetics of IBC formation were observed with strains isolated from women with different clinical syndromes. Two of the three isolates that did not form IBCs when inoculated alone were able to do so when coinoculated with an isolate that was capable of generating IBCs. The mixed infections dramatically altered the behavior of the coinfecting bacteria relative to their behavior in a single infection. The study also showed that mice with five different genetic backgrounds can support IBC formation. Although UPEC isolates differ genetically in their virulence factors, the majority of UPEC isolates from different types of UTI proceed through the IBC pathway, confirming the generality of IBCs in UTI pathogenesis in mice.
Urinary tract infections (UTIs) account for nearly 7 million office visits per year in the United States and cost $2 billion annually (4). The majority of community-acquired UTIs occur in women and are caused by uropathogenic Escherichia coli (UPEC) (7). More than 40% of women who experience acute cystitis develop recurrent UTIs (rUTIs) (12). Uropathogens causing UTIs as well as asymptomatic bacteriuria (ASB) are generally thought to emanate from the distal gut, colonize the vagina, and ascend to the bladder via the urethra (4). Likewise, acute pyelonephritis is assumed to be the result of an ascending infection from the bladder to the kidney. However, this widely accepted paradigm does not adequately address questions raised by epidemiologic data. Why does an initial UTI predispose a woman to an increased likelihood of a recurrent infection? Why are up to 68% of rUTIs caused by E. coli identical to the original infecting strain (23)? Why are recurrent uropathogens not more representative of the diversity of E. coli isolates present within an individual's gastrointestinal tract (2, 21)?
Although it is believed that bladder infection in humans does not involve the bladder wall, previous studies of human bladders indicated a potential intracellular component of UTI (3). Cultures of bladder biopsies taken from women with sterile urine cultures with various urinary tract symptoms revealed the presence of bacteria. Studies of mice have revealed that bacterial invasion of the epithelium lining the bladder lumen (urothelium) is an essential component of lower UTI.
In mice, adhesive bacterial fibers known as type 1 pili are necessary for invasion into the superficial umbrella cells of the urothelium (17, 18). Entry into these host cells initiates a program that has been extensively characterized in mice using the human cystitis isolate UTI89 and that results in the formation of intracellular bacterial communities (IBCs). Early IBCs are seen within 6 h of infection and consist of loosely packed rod-shaped bacteria (19). Over the next 6 h, they expand into tightly packed consortia of coccoid-shaped bacteria (mid-IBC) (15). Sixteen hours after infection, bacteria detach from the late IBC, elongate into a filamentous form, escape from the host epithelial cell, and spread in the bladder to reinitiate the IBC cascade in other host superficial umbrella cells (15, 19). Subsequent rounds of IBC formation allow E. coli cells to build up large populations within the bladder. Interestingly, within 12 h of infection, virtually all bacteria in the bladder are intracellular, implying that luminal bacteria are rapidly eliminated (18). One consequence of bacterial invasion is the establishment of a quiescent intracellular reservoir (QIR) in the bladder that can persist for months even in the face of antibiotic therapy (25). In addition, bacteria in this QIR can revert to an active metabolic state that is initiated by unknown signals: rapid intracellular bacterial replication ensues, reinitiating additional rounds of IBC formation, inflammation, and spread of bacterial infection throughout the urinary tract (20). This model argues that a portion of rUTIs in humans could be seeded from bacteria present in a QIR in the bladder.
In this study, we investigated the ability of multiple clinical isolates to form IBCs in the mouse model. We found that (i) the majority of UPEC isolates proceed through the IBC pathway; (ii) although isolates from women with ASB, acute UTI, rUTI, and pyelonephritis all form IBCs, IBCs from acute UTI isolates are significantly smaller; and (iii) isolates unable to from IBCs alone are able to do so in mixed infections where the second isolate was an IBC-competent strain. Together, these results confirm that the ability to form IBCs is a common attribute of UPEC isolates.
MATERIALS AND METHODS
Patients and strains.
Clinical studies were approved by the University of Washington Institutional Review Board, and written informed consent was obtained from all participants.
Five isolates causing ASB and four isolates causing first-episode acute cystitis (acute UTI) were collected from women enrolled in a previously described study at the University of Washington (5). Briefly, healthy women between 18 and 40 years old who were about to start or had started a new method of contraception and who had no more than one UTI in the previous year were eligible for enrollment. The study excluded a woman if she was pregnant, planned to become pregnant, had a chronic illness, had used systemic antimicrobial agents within 14 days, or had a known anatomical or functional abnormality of the urinary tract. Enrollees were monitored for 6 months, during which time urine was collected at monthly clinic visits and whenever patients had symptoms suggestive of a UTI. Asymptomatic bacteriuria was defined as >105 CFU of a uropathogen per milliliter of midstream urine documented in at least two separate cultures obtained at least 24 h apart. A symptomatic, culture-confirmed episode of cystitis was defined as ≥102 CFU of a uropathogen per milliliter of midstream urine from a patient with dysuria, frequency, and/or urgency.
E. coli isolates from five women with recurrent cystitis were collected from another study of healthy women between 18 and 45 years of age who had acute cystitis and a history of at least one previous UTI in the past year, who were not pregnant and did not plan to become pregnant, and who had no chronic health problems (T. M. Hooton, unpublished data). Approximately 110 women have been enrolled in this ongoing prospective study investigating the temporal associations between bacteriuria and clinical manifestations of recurrent UTI. The women were treated and then monitored by using daily urine cultures for 3 months, during which time they were seen in the clinic on a regular basis and whenever they had symptoms suggestive of a UTI. rUTI isolates used in that study were the initial isolate from women with “same-strain” recurrence. “Same-strain” isolates were identified by chromosomal restriction fragment length polymorphism analysis using pulsed-field gel electrophoresis (23).
E. coli isolates collected from four women between 18 and 45 years of age who had a clinical diagnosis of pyelonephritis were also included in our analysis (T. M. Hooton and W. E. Stamm, unpublished data). Women were eligible for this study if they had fever, back pain and/or costovertebral angle tenderness (CVAT), and a positive urine culture, as defined above.
Virulence factor PCR.
Bacterial chromosomal DNA was isolated from each UPEC strain after growth overnight in Luria broth (LB) by using the Wizard genomic DNA purification kit (Promega). PCRs to detect virulence factors were performed as described previously by Johnson and Stell (14).
Western blotting for detecting PapA and FimH proteins.
For immunoblot analysis to detect the PapA protein in the pyelonephritis isolates, bacteria were grown on tryptic soy agar plates at 37°C for 36 h, scraped, and suspended into 1 ml of phosphate-buffered saline (PBS). Resuspended cells were heated at 65°C to remove bound pili (9), and the supernatants containing pili were heated in sodium dodecyl sulfate (SDS) sample buffer (0.25 M Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 50 mM dithiothreitol, bromophenol blue)-4 M urea at 95°C and analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblotting with antibodies to the P pilus (11).
For immunoblot analysis of FimH expression, bacteria were grown statically in LB at 37°C for 24 h. Equivalent numbers of cells (optical density at 600 nm of ∼1.0) from each isolate were suspended in SDS sample buffer, 1 M HCl was added dropwise until a pH indicator (bromophenol blue) turned yellow, and solution was heated at 95°C for 5 min. The samples were then neutralized and analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblotting with antibodies to FimH (raised in rabbits using purified FimH protein). Specific FimH proteins were visualized on nitrocellulose membranes by using the BCIP/NBT liquid substrate system (Sigma) and analyzed for intensity using ImageJ software (http://rsb.info.nih.gov/ij/).
HA assays for detecting bacterial adhesins.
Bacteria were grown statically in LB for 24 h to induce expression of type 1 pili. Hemagglutination (HA) assays with guinea pig erythrocytes were performed using well-established protocols (10, 26).
Mouse strains and infections.
All studies using mice were approved by the Animal Studies Committee of Washington University. Eight-week-old female mice belonging to the inbred C3H/HeN strain were anesthetized and inoculated with a 50-μl suspension of ∼107 CFU of a given clinical isolate (in PBS) via transurethral catheterization (19). Three, 6, 12, 16, and/or 24 h after inoculation, animals were euthanized, and their bladders harvested, divided in half, opened, and pinned onto dissecting wax. Bladders were washed three times with PBS to eliminate loosely associated bacteria not likely involved in infection. The splayed bladders were then incubated for 20 min at room temperature with (i) wheat germ agglutinin (WGA) (1:1,000 in PBS; Molecular Probes) to stain their luminal surfaces and (ii) SYTO 61 red (1:1,000 in PBS; Molecular Probes) to stain IBCs as well as host urothelial cell nuclei. (Individual bacteria could be visualized in the densely packed IBCs, thus distinguishing them from the nuclei.) Trolox (10 μM; Fluka) was added to the splayed bladders to reduce photobleaching (24). The total number of IBCs was scored by confocal microscopy (see below). If all infections were not successful, five successfully infected mice were used for further evaluation. The diameters of a maximum of 25 IBCs (five IBCs/bladder/isolate [n = 5 bladders/isolate]) were measured across the width of each IBC and averaged, and standard deviations were calculated to estimate differences in the sizes of IBCs. If less than 25 IBCs were observed for a given isolate, the total number of IBCs observed was used to calculate the average diameter and standard deviation. If IBCs were not observed in all five bladders inoculated with a clinical isolate, more than five IBCs were measured per bladder containing IBCs to obtain 25 measurements. After microscopy, bladders were homogenized in 1 ml of 0.025% Triton X-100-PBS, and bacterial titers were determined by plating serial dilutions of the homogenates onto LB agar plates. Standard gentamicin assays were done to define the number of intracellular bacteria in the bladder (19).
For single infections with the two laboratory strains, strains UTI89 and CFT073 were genetically engineered to express green fluorescent protein (GFP). Eight-week-old female mice belonging to inbred C3H/HeN, C3H/HeJ, C57BL/6J, CBA/J, and FVB/NJ strains were inoculated with UTI89-GFP and CFT073-GFP as described above. At 6 and 24 h after inoculation, bladders were harvested as described above; however, the splayed bladders were fixed and visualized without additional staining due to GFP-expressing UTI89 and CFT073.
Coinoculation of clinical isolate and UTI89.
Equal amounts of each clinical isolate and UTI89-GFP (cystitis strain engineered to express GFP) were mixed to obtain a 50-μl bacterial suspension of ∼107 CFU. The suspension was then inoculated into C3H/HeN mice, and infected bladders were harvested and visualized as described above. UTI89-gfp contains the kanamycin resistance cassette and is thus Kanr, whereas the clinical isolate was Kans. Serially diluted bladder homogenates were plated onto LB agar plates and LB agar plates containing kanamycin (50 μg/ml) and grown overnight at 37°C. The number of CFU of the clinical isolate was determined by subtracting the number of kanamycin-resistant CFU of strain UTI89 from the total number of CFU on LB medium lacking kanamycin.
Confocal scanning laser microscopy.
Bladders were examined with a Zeiss LSM410 confocal laser scanning microscope under a 63× objective. WGA and SYTO 61 were excited at 488 and 633 nm, respectively. In coinoculation experiments using GFP-expressing UTI89 and a clinical isolate that did not form IBCs alone, GFP was excited at 488 nm, the clinical isolate was stained red by SYTO 61, and wheat germ agglutinin was omitted.
Statistical analysis.
Observed differences in IBC diameters between isolates representing the four different types of UTI were analyzed for significance using the nonparametric Mann-Whitney U test (InStat; GraphPad Software).
RESULTS
Virulence genotypes of the clinical isolates.
We studied 18 clinical isolates collected from women enrolled in the three different studies described above (Table 1). To characterize the isolates, they were first analyzed in vitro for the presence of 29 putative virulence factors by multiplex PCR, as previously described (14). The level of type 1 pilus expression was then assessed with hemagglutination assays and immunoblotting using FimH antisera. The isolates were then analyzed in a murine model to assess their ability to form IBCs within the bladder.
TABLE 1.
Characterization of UTI isolates collected
| Strain | Patient characteristicsa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Enrollment studyb | Duration of follow-up (wk) | Clinical classification | Symptoms | Duration of symptoms (days) | Age at first UTI (yr) | No. of prior lifetime UTIs | Age (yr) | Demographicc | |
| ASB1 | PUTS | 24 | ASB | None | NA | NA | 0 | 39 | W/sep/15 |
| ASB2 | PUTS | 24 | ASB | None | NA | NA | 0 | 22 | W/NM/16 |
| ASB3 | PUTS | 24 | ASB | None | NA | NA | 0 | 21 | W/NM/13-15 |
| ASB4 | PUTS | 20 | ASB | None | NA | NA | 0 | 28 | W/div/13-15 |
| ASB5 | PUTS | 16 | ASB | None | NA | NA | 0 | 18 | W/NM/12 |
| Acute1 | PUTS | 24 | First event | D, F, H | NR | NA | 0 | 23 | W/NM/17+ |
| Acute2 | PUTS | 24 | First event | D, F, H | NR | NA | 0 | 23 | W/NM/17+ |
| Acute3 | PUTS | 25 | First event | D, U | 4 | NA | 0 | 19 | W/NM/12 |
| Acute4 | PUTS | 24 | First event | F, U, N | <1 | NA | 0 | 18 | Hisp/NM/12 |
| rUTI1 | TOP | 12 | Recurrent | D, F | 1 | 23 | 5 | 27 | W/NM/17+ |
| rUTI2 | TOP | 12 | Recurrent | D, F | 1 | 13 | 10 | 25 | W/NM/17+ |
| rUTI3 | TOP | 12 | Recurrent | D, F | 2 | 18 | 3 | 22 | A/NM/13-15 |
| rUTI4 | TOP | 12 | Recurrent | D, F, U | 2 | 19 | 1 | 21 | A/NM/12 |
| rUTI5 | TOP | 12 | Recurrent | D, F, U | 1 | 6 | 20 | 23 | W/NM/13-15 |
| Pyelo1 | Pyelo | 20 | Pyelonephritis | F, N, S, O, LBP, FE, C | 16 | 19 | 3 | 21 | W/NM/15 |
| Pyelo2 | Pyelo | 4 | Pyelonephritis | D, F, U, N, S, O, LBP, FE, C | 1 | 24 | 3 | 24 | Other/NM/18 |
| Pyelo3 | Pyelo | 16 | Pyelonephritis | D, F, U, N, S, LBP, FE, LAP | 3 | 19 | 1 | 25 | W/div/21 |
| Pyelo4 | Pyelo | 4 | Pyelonephritis | U, LBP, FE, C, LAP, I | 2 | NA | 0 | 20 | Other/NM/13 |
Abbreviations: D, dysuria; F, frequency; H, hematuria; U, urgency; N, nocturia; S, urinating in small amounts; O, urine odor; LBP, lower back pain; FE, fever; C, chills; LAP, lower abdominal pain; I, incontinence; NA, not applicable; NR, not recorded; W, white; Hisp, Hispanic; A, Asian; sep, separated; NM, not married; div, divorced.
Enrollment criteria were as follows: for predisposition to UTI study (PUTS), women aged 18 to 40 years starting a new birth control method (may be pill, diaphragm, cervical cap, or foam and condoms) with no history of more than one UTI in the past year; for transcience of pathogens (TOP), women aged 18 to 45 years who were not pregnant, were taking contraception, had symptoms for less than 7 days, had no chronic health problems, and had no CVAT; for pyelonephritis study (Pyelo), women aged 18 to 45 years with a clinical diagnosis of pyelonephritis with fever, back pain, and/or CVAT.
Race/marital status/years of education.
UPEC virulence factors are typically involved in the colonization and invasion of the host and evasion of the immune responses. Each isolate had a unique combination of virulence factors, and their prevalence was similar to that reported in a previous study that analyzed 63 UPEC isolates (13), suggesting that our small sample size was representative of a larger, diverse UPEC group (Table 2). The most prevalent virulence factors were fimH (100% of isolates), fyuA (yersiniabactin receptor; 80%), traT (serum resistance; 70%), the pathogenicity-associated island marker (CFT073 pathogenicity-associated island marker for a series of genes that provide a mechanism for horizontal transfer of virulence factor genes; 70%), kpsMTII (group 2 capsule; 70%), sfa/focDE (S/F1 fimbriae; 60%), and hlyA (hemolysin; 50%). The presence or absence of individual virulence factors could not be correlated with a particular clinical syndrome.
TABLE 2.
Virulence genotypes and hemagglutination titers of clinical isolates
| Strain | Genotype
|
Titer
|
FimH immunoblotc | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| papACEFG | sfa/focDE | fimH | hlyA | fyuA | iutA | kpsMTII | kpsMTIII | K1 | K5 | ibeA | traT | PAIa | HAb | HA mannose | ||
| ASB1 | + | + | + | + | + | − | + | − | + | − | + | − | + | 128 | <2 | + |
| ASB2 | − | − | + | − | + | − | + | − | − | − | + | + | + | 64 | <2 | + |
| ASB3 | − | − | + | − | + | + | + | − | − | + | − | + | + | 128 | <2 | +++ |
| ASB4 | − | − | + | − | − | − | + | − | − | + | − | − | − | 32 | <2 | + |
| ASB5 | − | + | + | + | + | − | + | − | − | + | − | + | + | 128 | <2 | + |
| Acute1 | − | + | + | − | + | + | + | − | + | − | − | + | + | 16 | <2 | 0 |
| Acute2 | − | − | + | − | − | − | + | − | − | + | − | + | − | 64 | <2 | + |
| Acute3 | + | + | + | + | + | − | − | + | − | − | − | − | + | 64 | <2 | ++ |
| Acute4 | + | + | + | + | + | − | + | − | − | + | + | − | + | 32 | <2 | + |
| rUTI1 | − | − | + | − | − | − | − | − | − | − | − | − | − | 8 | 8 | 0 |
| rUTI2 | − | − | + | − | + | + | + | − | − | + | − | + | + | 2 | <2 | + |
| rUTI3 | + | + | + | + | + | − | − | + | − | − | − | + | + | 8 | 2 | + |
| rUTI4 | + | − | + | + | + | + | + | − | − | + | − | + | + | 2 | <2 | 0 |
| rUTI5 | + | + | + | + | + | + | + | − | − | + | − | + | + | 8 | 4 | + |
| Pyelo1 | − | − | + | + | + | − | − | − | − | − | − | + | − | 16 | 16 | + |
| Pyelo2 | − | + | + | − | − | − | − | − | − | − | − | − | − | 4 | 2 | + |
| Pyelo3 | − | + | + | − | + | + | + | − | − | + | − | + | + | 8 | 8 | + |
| Pyelo4 | − | + | + | − | + | + | + | − | + | − | + | + | − | 8 | 8 | + |
| UTI89 | + | + | + | + | + | − | + | − | + | − | + | + | + | 64 | 2 | ++ |
| CFT073 | + | + | + | + | + | + | − | − | − | − | − | − | + | 128 | 4 | ++ |
| % Prevalence | 40 | 60 | 100 | 50 | 80 | 40 | 70 | 10 | 20 | 45 | 25 | 70 | 70 | |||
PAI, pathogenicity-associated island marker.
HA, hemagglutination of guinea pig red blood cells. Values equal 2x, where x equals the last well of agglutination.
Percent expression relative to UTI89 (0 = 0% to 49%; + = 50% to 99%; ++ = 100% to 149%; +++ = 150% to 199%).
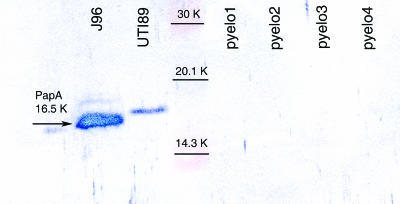
Surprisingly, none of the four pyelonephritis isolates had genes within the pap operon, which codes for the production of P pili. Due to the absence of the pap operon, anti-P-pilus antibodies failed to react with these isolates after growth on tryptic soy agar, which typically induces the production of P pili (Fig. 1).
FIG. 1.
Immunoblot of pyelonephritis isolates with antibodies against P pili. P pilus antibodies were raised against J96 P pili; thus, J96 and UTI89 were used as positive controls for P pilus production. Both isolates are positive for P pili due to a PapA-specific band at a molecular weight of 16,500 (16.5K). However, the P pilus antibody did not recognize the 16.5K protein for the four pyelonephritis isolates, confirming the lack of the pap operon seen with multiplex PCR. Thus, pyelo1, pyelo2, pyelo3, and pyelo4 do not contain the genes, nor do they produce protein associated with P pili, which are thought to be critical in kidney colonization.
Type 1 pilus production in clinical isolates.
HA of guinea pig red blood cells was performed as previously described (10) to determine the degree of type 1 pilus production by each clinical isolate. Type 1-mediated HA is specifically inhibited by the addition of exogenous mannose, and this reaction is referred to as mannose-sensitive HA (MSHA).
For this study, bacteria were grown in static culture at 37°C for 24 h to enhance type 1 production. After 24 h of static growth, UTI89, a prototypic cystitis reference strain that was shown to proceed through the IBC pathway, produced an MSHA titer of 64 (values equal 2x, where x equals the last well of agglutination). Comparable MSHA titers were seen with the ASB isolates, which ranged from 32 to 128, suggesting a level of type 1 production similar to that of strain UTI89 (Table 2). The first-event UTI strains (acute UTI isolates) produced MSHA titers ranging from 16 to 64. The rUTI isolates had HA titers ranging from 2 to 8 that could not be entirely inhibited by the addition of exogenous mannose. The persisting HA titers in the presence of mannose suggests the expression of a mannose-resistant adhesin system under these growth conditions. The pyelonephritis isolates had mannose-resistant HA (MRHA) titers ranging from 4 to 16.
To further confirm the results seen with HA titers, anti-FimH antibodies were used in immunoblot assays to assess the relative levels of FimH in all of the strains after 24 h of growth in static broth. The intensity of each band was quantitated and expressed as a value relative to the amount of FimH expressed by UTI89, which was set at 100% (Table 2). MC4100, which does not produce type 1 pili, was used as a negative control. The majority of isolates expressed type 1 pili at levels of 50% to 99% of the levels expressed by strain UTI89. Only one isolate, ASB3, expressed type 1 pili at a level substantially higher than that of UTI89, 150% to 199%. Strains acute1, rUTI1, and rUTI4 did not express detectable levels of type 1 pili, and acute3 and CFT073 expressed comparable levels of type 1 pili relative to that of UTI89 as determined by our immunoblot analysis. These results suggest that UPEC isolates from different clinical syndromes produce various degrees of type 1 pili in vitro.
IBC formation by UPEC strains isolated from women.
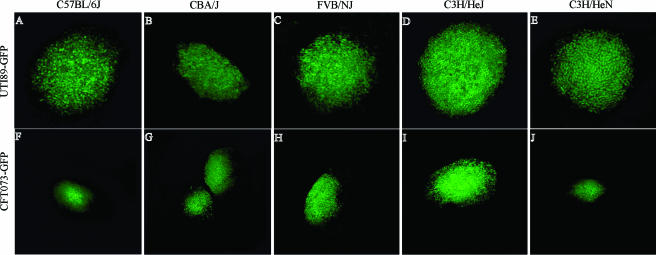
First, the commonality of the IBC pathway in the face of differing host responses was evaluated. To date, the IBC pathway has been best described using the C3H/HeN and C3H/HeJ mouse strains with UPEC strains UTI89 and NU14 (1, 15, 18). A previous study evaluated the level of infection and inflammation in different mouse genetic backgrounds but did not examine the IBC pathway (8). To determine whether the cystitis and pyelonephritis isolates UTI89 and CFT073, respectively, form IBCs in various host genetic backgrounds, three other mouse strains were infected, C57BL/6J, CBA/J, and FVB/NJ. Mouse strains for use in this study were chosen based on their previous use as models for host responses to UPEC infection and/or identification of bacterial virulence factors (16, 18, 27). At 6 h postinfection, IBCs were detected in all mouse strains tested with both UPEC strains. IBCs from C3H/HeJ (Fig. 2D and I) and C3H/HeN (Fig. 2E and J) mice showed the expected IBC morphology inside superficial epithelial cells. Early IBCs were readily detected in C57BL/6J, CBA/J, and FVB/NJ (Fig. 2A to C and F to H) mice, which were visually indistinguishable from IBCs in the C3H genetic background. Occasionally, some IBCs in C57BL/6J mice had more loosely defined edges. The presence of IBCs at 6 h postinfection in five mouse strains after infection with UTI89 and CFT073 suggests that the mechanisms utilized for invasion and intracellular replication in vivo are a conserved feature in these strains.
FIG. 2.
UTI89 and CFT073 formed IBCs in multiple mouse strains. UTI89 and CFT073, two commonly used UTI isolates, were inoculated into five different mouse strains. At 6 h postinfection, each mouse strain was able to support IBC formation by each UTI isolate. A to E represent UTI89 IBCs, and F to J represent CFT073 IBCs.
Next, the commonality of IBC formation among different clinical isolates was evaluated. Eighteen clinical isolates recovered from women enrolled in three different UTI studies (Table 1) were evaluated for their ability to form IBCs. Each isolate was inoculated transurethrally into the bladders of young adult female C3H/HeN mice, and the bladders were harvested at 3, 6, 12, 16, and 24 h postinfection (n = 5 mice/time point/isolate) (Table 3 and Fig. 3).
TABLE 3.
IBC formation at different time points
| Isolate type | No. of IBC-forming isolates/total no. of isolatesa
|
||||
|---|---|---|---|---|---|
| 3 h | 6 h | 24 h | No IBCs | Avg IBC diameter (μm) | |
| ASB | 0/5 | 4/5 | 4/5 | 0/5 | 51 ± 20 |
| Acute | 1/4 | 1/4 | 0/4 | 1/4 | 20 ± 8 |
| Recurrent | 1/5 | 2/5 | 1/5 | 1/5 | 41 ± 14 |
| Pyelonephritis | 0/4 | 3/4 | 0/4 | 1/4 | 55 ± 10 |
n = 5 mice/time point. Five IBCs/bladder/isolate were measured (n = 5 bladders).
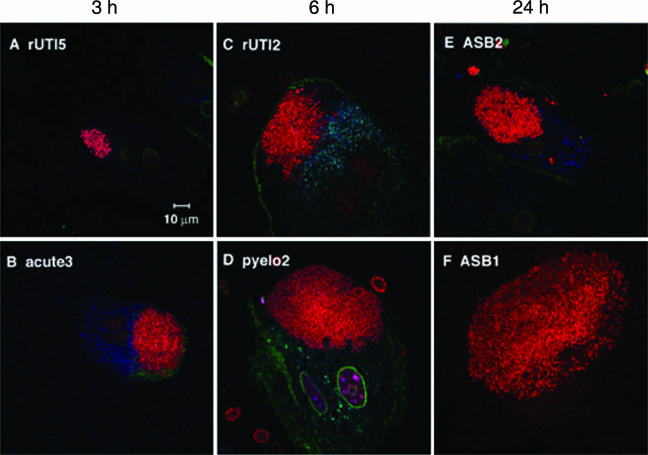
FIG. 3.
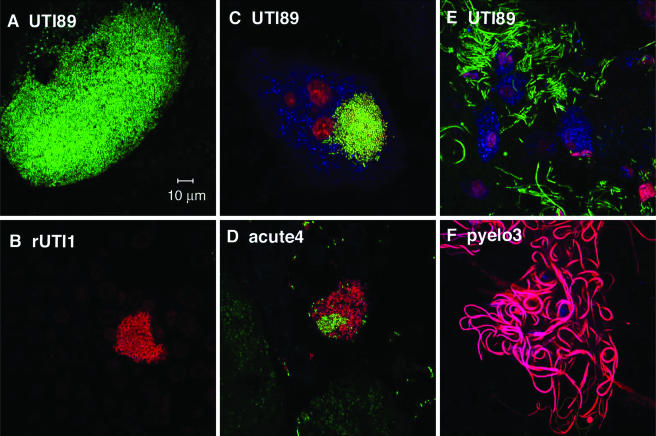
IBCs were formed by UPEC strains isolated from women. Clinical isolates (red) were inoculated into female C3H/HeN mice, and bladders were harvested at 3 h, 6 h, and 24 h. Fifteen isolates produced IBCs at either (A and B) 3 h, (C and D) 6 h, or (E and F) 24 h. Each clinical syndrome is represented at the top of each panel. The cell surface (green) was stained with WGA and can be seen bordering the umbrella cells in this cross-sectional view. While the majority of isolates produced IBCs, the sizes and densities of the IBCs varied. Bars, 10 μm.
All five ASB isolates formed IBCs, although the time course varied: IBCs were observed 6 h and 24 h after inoculation of strains ASB1, ASB2, and ASB3 (Fig. 3E and F), while IBCs were observed only at 6 h for ASB4 and at 24 h for ASB5. This suggests that although IBC formation is common, the rate at which the isolates proceed through the pathway is potentially strain specific. In UTI89, first-round IBCs were observed at 6 h postinfection and second-round IBCs were observed at 24 h postinfection despite asynchronous micturation among the mice. Since IBCs were not observed at 24 h in strain ASB4, it is possible that the second-round IBCs were either earlier or later and thus missed. The fact that ASB5 formed apparent IBCs only at 24 h postinoculation suggests that it either proceeds more slowly through the IBC pathway due to slow replication within the umbrella cells or invades the urothelium at later time points. ASB5 produces a high MSHA titer (128), and FimH was detectable in the immunoblots (Table 2). These piliation characteristics strongly argue that the observed delay in IBC formation is not related to type 1 pilus expression in the inoculum. The average diameter of ASB IBCs was 51 ± 20 μm (five IBCs/bladder), which is the same as that of the prototypic isolate, UTI89, which has an average IBC diameter of 51 ± 9 μm at 6 h postinfection.
Three of the four acute UTI isolates formed IBCs at the time points examined. Again, there were isolate-specific differences in the time course. IBCs were observed only at 3 h in acute3 (Fig. 3B), 6 h in acute2, and 12 h in acute1 (data not shown) (n = 5 mice for each group). IBCs in all acute UTI isolates were small, with an average diameter of 20 ± 8 μm (P < 0.01 compared to UTI89 and ASB isolates) and sparse (average of 3 IBCs/bladder compared to ∼20 to 100 IBCs/bladder with UTI89 and ASB isolates [n = 15 mice]). No IBCs were observed at any of the five time points surveyed for acute4. However, bacteria were visualized on the luminal surface of the bladder and quantitated by tissue titer to be greater than 102 CFU/bladder throughout the time course. With UTI89, by 12 h, virtually all of the bacteria entered an intracellular niche (19). The ability of acute4 to persist extracellularly up to 24 h emphasizes that different UTI strains may use alternative mechanisms to cause infection. Although acute4 produces type 1 pili in vitro, it is unable to form IBCs. Thus, additional factors must be critical for IBC formation independent of type 1 piliation. Acute4 was further analyzed in studies described below and in separate studies to investigate the mechanism by which it persists in the urinary tract.
IBCs were observed for four of the five recurrent cystitis isolates. IBCs were observed only at 3 h in rUTI5 (Fig. 3A), 6 h in rUTI2 (Fig. 3C) and rUTI3, and 24 h in rUTI4 (n = 5 mice for each group), again showing differences in the IBC pathway. The diameter of the IBCs observed in all four isolates was 41 ± 14 μm, a value that is significantly greater than those of acute UTI strain IBCs (P < 0.05) but not significantly different from those of UTI89 or ASB strain IBCs (P = 0.274). Similar to strain acute4, rUTI1 bacteria were present in the bladders throughout the time course, as evidenced by tissue titers (103 to 104), but no IBCs were observed by microscopy. However, in contrast to acute4, rUTI1 expressed little to no type 1 pili in vitro (Table 2) even though it contains the fimH gene. The inability to produce type 1 pili in vitro could possibly explain the inability of rUTI1 to invade (see below) and form IBCs at the time points tested. Whether type 1 pilus expression can be induced in vivo at later time points and lead to invasion and IBC formation will be the subject of future studies.
Of the four pyelonephritis isolates, three formed IBCs and did so by 6 h postinoculation (Fig. 3D), suggesting similar kinetics in their IBC pathways (n = 5 mice in each group). The IBC diameter (55 ± 10 μm) was significantly larger than that for acute UTI strains (P < 0.01). IBCs were not observed with pyelo3, and, similar to rUTI1, MSHA was not detected after growth in static broth in vitro (Table 2). As seen with the two other strains that were unable to form IBCs, acute4 and rUTI1, pyelo3 was unable to invade into the superficial umbrella cells of the bladder (see below).
Invasion defects in strains unable to form IBCs.
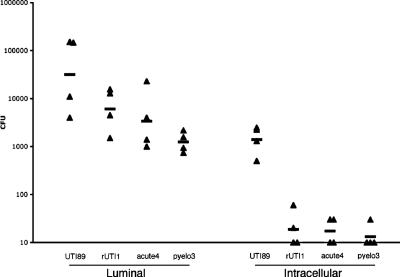
Since invasion is a prerequisite for IBC formation, we performed an in vivo gentamicin protection assay to assess whether any of the three isolates that did not form IBCs, acute4, rUTI1, and pyelo3, had invasion defects. PCR demonstrated that the fimH gene was present in all three strains, and HA assays revealed that acute4 produced type 1 pili, while rUTI1 and pyelo3 produced mannose-resistant fibers. The ability of UPEC to invade is thought to be dependent on type 1 pili (17). At 1 h postinfection, bladders were removed, bisected, and incubated in gentamicin to kill extracellular bacteria. Homogenates of the treated bladders were then cultured, since intracellular bacteria have been shown to survive this treatment (19). Bacterial titers revealed that the three non-IBC-forming isolates were deficient in invasion compared to UTI89 (Fig. 4). A total of 4.4% of adherent UTI89 bacteria (defined as bacteria removed from the lumen after multiple washes) were able to invade the urothelium (defined as bacteria surviving the gentamicin treatment). In contrast, the three isolates that did not form IBCs (acute4, rUTI1, and pyelo3) had invasion percentages of 0.3%, 0.5%, and 1.1%, respectively, thus potentially explaining their inability to form IBCs.
FIG. 4.
The three non-IBC-forming isolates, rUTI1, acute4, and pyelo3, were deficient in invasion in vivo. Female C3H/HeN mice were inoculated with UTI89, rUTI1, acute4, and pyelo3. At 1 h postinfection, bladders were removed and processed. The luminal fraction represents luminal bacteria, and the intracellular fraction represents invaded bacteria. One hour after inoculation, 4.4% of UTI89 bacteria invaded, whereas for rUTI1, acute4, and pyelo3, only 0.3%, 0.5%, and 1.1% of luminal bacteria invaded, respectively. Thus, the three non-IBC-forming isolates invade poorly relative to UTI89.
Mixed-infection model of UTI89 with a non-IBC-forming isolate.
Due to the complex nature of the intestinal, perianal, vaginal, and periurethral microbiota, it is likely that the initial inoculum in the human bladder is a mixture of bacterial strains. We sought to investigate whether one isolate could improve the fitness or outcompete another isolate in the bladder. Thus, we investigated the outcome of experiments where UTI89 was genetically engineered to express a readily detectable green fluorescent protein marker and then coinoculated with each of the three non-IBC-forming clinical isolates in equal concentrations. Although IBCs were not observed during single infections with acute4, when it was coinoculated with UTI89, acute4 was able to participate in IBC formation but only as a mixed IBC with UTI89-GFP IBCs (Fig. 5C and D). When rUTI1 was coinfected with UTI89, rUTI1 formed IBCs at 24 h (Fig. 5B) and caused UTI89 to form giant IBCs at 6 h (diameter, 130 ± 25 μm) (Fig. 5A). Pyelo3 was unable to form IBCs in the presence or absence of UTI89 and even prevented IBC formation in UTI89. In the UTI89/pyelo3 mixed infection, most of the extracellular (luminal) pyelo3 cells were filamentous, and this same overwhelming filamentation phenotype was induced in UTI89 (Fig. 5E and F). Interestingly, although two of the three clinical isolates altered the behavior of UTI89, they were present in the bladder in quantities that were 100- to 1,000-fold less than UTI89 at each time point tested (n = 5 mice in each group). Additionally, all three of the clinical isolates showed altered behavior in the presence of UTI89 relative to their behavior when inoculated individually. Clinically, a UTI is often defined as bacteria in the urine in quantities greater than 105, and mixed infections with different UPEC strains would typically not be detected. Thus, small amounts of a competing strain in the bladder could possibly have profound and distinct effects on the outcome of infection.
FIG. 5.
Coinfection with a clinical isolate and UTI89 altered the behavior of the bacteria. (A and B) rUTI1 (red) produced IBCs only when coinfected with UTI89 (green) and caused UTI89 to produce abnormally large IBCs (compare A to C). (C and D) acute4 (red) formed mixed IBCs with UTI89 (green), which also formed normal IBCs. (E and F) pyelo3 (red) formed large collections of filaments, induced UTI89 (green) to do the same, and prevented UTI89 IBCs. Bars, 10 μm.
DISCUSSION
The widely accepted paradigm of acute and recurrent infection is that both types of infection are due to the ascension of bacteria from the gut microbiota to the vagina and then the bladder. In addition, UTIs have clinically been thought to be due to simple extracellular colonization of the urothelium by UPEC. However, it has recently been shown that UPEC invades the urothelium in order to subvert innate defenses in mice (15, 17, 18). Likewise, intracellular bacteria have been found in the human bladder following UTI (3). The discovery and characterization of IBCs in the murine model of cystitis has challenged the traditional clinical paradigm of UTIs and revealed a mechanism by which UPEC isolates are able to build up in numbers in the bladder despite potent innate defenses. The IBC pathway has been well characterized with the cystitis isolate UTI89 in the C3H mouse model of cystitis. In this study, we have demonstrated that the IBC pathway is common to most UPEC strains isolated from humans with different UTI syndromes and from mice of different genetic backgrounds.
We found, through multiplex PCR virulence factor assays, that the isolates chosen for this study were representative of a larger pool of UPEC isolates (13). Surprisingly, none of our pyelonephritis isolates contained any genes within the pap operon and thus did not express P pili. The pap operon codes for P pili containing a distally located PapG adhesin that binds the globoside receptor in the human kidney, which has been shown to be critical for the ability of pyelonephritic strains to colonize the kidney (22). The four pyelonephritis strains in this study were obtained from patients that had classic symptoms of pyelonephritis, namely, fever and CVAT (Table 1). The absence of the pap operon in these strains is intriguing, but given the small sample size, further studies will need to be done to interpret the significance of this finding. In contrast, all of the pyelonephritis isolates, as well as the ASB, acute UTI, and rUTI isolates, had the fimH gene. However, expression of type 1 pili after growth in static LB differed among the strains. Strains with various levels of type 1 pili after in vitro growth were able to colonize the mouse bladder and form IBCs. It is possible that the in vivo environment in the bladder altered the type 1 pilus expression profile after inoculation, thus partially explaining the ability of strains with various levels of type 1 pili to colonize and form IBCs within the bladder.
In our mouse model of UTI, we demonstrated that 15 of 18 clinical isolates from various human clinical syndromes proceeded through the IBC pathway when inoculated as a single strain. However, IBC formation differed among the strains in terms of the kinetics by which bacterial communities formed, the size of the communities, and the number of communities. While isolates from each clinical syndrome formed IBCs, the acute UTI isolates formed significantly smaller and fewer IBCs relative to any other syndrome. In general, ASB, rUTI, and pyelonephritis strains are associated with longer-term persistence in the host. The ASB, rUTI, and pyelonephritis strains produced more IBCs/bladder, and the IBCs were of increased size compared to acute UTI strains. The differences in IBC behaviors of the acute strains compared to those of the other three groups could potentially explain why the acute strains are less persistent.
The behaviors of the three outlying strains (rUTI1, acute4, and pyelo3) required more research to better understand their apparent deviations in IBC behavior with respect to their group. rUTI1, acute4, and pyelo3 were unable to efficiently invade the superficial umbrella cells of the bladder and were thus unable to form IBCs. The lack of IBC formation in the three isolates could not be associated with a specific virulence factor due to their differing virulence factor profiles. Likely, there is a multitude of virulence factors contributing to IBC formation and perhaps some redundancy in the necessary factors. rUTI1 did not produce any detectable levels of type 1 pili in vitro, which thus most likely explains its inability to invade and form IBCs at the time points tested. Acute4 was able to produce type 1 pili in vitro at levels equivalent to or better than those of isolates that were able to form IBCs, suggesting that type 1 pilus expression is not sufficient for IBC formation and implicating the importance of other factors in invasion. Pyelo3 produced a small amount of type 1 pili as determined by immunoblotting with anti-FimH antisera. No MSHA was detected in vitro, possibly because of the presence of MRHA adhesins that would potentially mask type 1 pilus-mediated hemagglutination. The deficiency of in vivo invasion of pyelo3 could suggest that the MRHA adhesins somehow interfered with the process and/or that pyelo3 lacked other factors involved in invasion.
Given the diverse microbial communities in the rectum and vagina (2, 21), it is likely that multiple strains of bacteria are simultaneously introduced into the urinary tract of a woman during the early events that lead to a UTI, such as sexual intercourse. While mixed infections may select for bacteria with a distinct competitive advantage, they may also allow less virulent bacteria the opportunity to establish a niche in conjunction with more efficient UPEC strains. Coinoculation of UTI89, a known IBC-forming strain, with each of the three isolates that were deficient in IBC formation resulted in dramatic, synergistic effects. Of the three isolates for which IBC formation was not detected, two were able to do so in mixed infections with UTI89 (rUTI1 and acute4). This may explain the seemingly outlying behavior of these strains when analyzed in single infections. The diagnosis of UTIs rarely delineates whether multiple strains of E. coli are present. Thus, strains such as rUTI1 and acute4 could have “piggy-backed” with a more invasive IBC-forming strain that went undetected. UTI89 formed significantly larger IBCs in the presence of rUTI1. This may be due to potential host responses induced by rUTI1 that facilitate bacterial replication in the superficial umbrella cells, or possibly, rUTI1 induces signals that prevent or slow the dispersal and fluxing of the bacteria in the IBC. IBC formation is a clonal process in that each IBC arises from a single bacterium (P. C. Seed and S. J. Hultgren, unpublished data). Surprisingly, the mixed infection with acute4 and UTI89 resulted in nonclonal IBCs. Acute4 existed as a mixed IBC only with UTI89. The bacteria did not intermingle in the IBC, but instead, each strain seemed to group together in patches that melded into one IBC. These results argue that UTI89 was able to provide factors in trans that could support acute4 in the intracellular biofilm matrix.
Pyelo3 was the only strain in our study that was unable to form IBCs under any of the conditions tested. Coinoculation of UTI89 and pyelo3 resulted in massive filamentation of both isolates. Based on previous work showing that filamentation occurs in response to an inflammatory response (15), further study is required to assess whether pyelo3 is triggering a more robust innate response that leads to the massive filamentation observed. The complexities that we have observed with coinfections between the bacteria and with the host might explain the occurrence of different clinical syndromes manifested by the same organism (6) or the differing behavior in single versus mixed infections as described above. Alternatively, it is possible that these isolates could represent a class of strains that do not use the IBC pathway but instead use a different extracellular strategy for persistence.
In summary, 15 of 18 isolates causing different clinical syndromes in humans and possessing various combinations of virulence factors were successful in invading the bladder epithelium of mice to form IBCs. Two of the remaining three isolates were also able to form IBCs in mixed infections with UTI89. These findings demonstrate that the IBC pathway is common to UPEC pathogenesis. Studies are in progress to determine whether UPEC forms IBCs in humans and whether they contribute to same-strain recurrences of UTIs. The murine model of IBC formation will allow investigators to study the intricacies of host-pathogen interactions in the bladder. Understanding the basis of the intracellular niche occupied by UPEC will lead to the better evaluation and treatment of UTIs and will foster the development of new therapeutic strategies.
Acknowledgments
This study was funded by NIH/NIDDKD grants DK 64540 ORWH, DK 51406, DK 40045, and DK 47549; NIH/NIAID grant AI029549; and an Infectious Diseases/Basic Microbial Pathogenesis Mechanisms training grant.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott, T. S., L. Reed, R. C. Slack, and M. C. Bishop. 1985. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J. Infect. 11:191-199. [DOI] [PubMed] [Google Scholar]
- 4.Foxman, B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl. 1A):5S-13S. [DOI] [PubMed] [Google Scholar]
- 5.Hooton, T. M., D. Scholes, J. P. Hughes, C. Winter, P. L. Roberts, A. E. Stapleton, A. Stergachis, and W. E. Stamm. 1996. A prospective study of risk factors for symptomatic urinary tract infection in young women. N. Engl. J. Med. 335:468-474. [DOI] [PubMed] [Google Scholar]
- 6.Hooton, T. M., D. Scholes, A. E. Stapleton, P. L. Roberts, C. Winter, K. Gupta, M. Samadpour, and W. E. Stamm. 2000. A prospective study of asymptomatic bacteriuria in sexually active young women. N. Engl. J. Med. 343:992-997. [DOI] [PubMed] [Google Scholar]
- 7.Hooton, T. M., and W. E. Stamm. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. N. Am. 11:551-581. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins, W. J., A. Gendron-Fitzpatrick, E. Balish, and D. T. Uehling. 1998. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 66:2798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoschützky, H., F. Lottspeich, and K. Jann. 1989. Isolation and characterization of the α-galactosyl-1,4-β-galactosyl-specific adhesin (P adhesin) from fimbriated Escherichia coli. Infect. Immun. 57:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultgren, S. J., J. L. Duncan, A. J. Schaeffer, and S. K. Amundsen. 1990. Mannose-sensitive haemagglutination in the absence of piliation in Escherichia coli. Mol. Microbiol. 4:1311-1318. [DOI] [PubMed] [Google Scholar]
- 11.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikäheimo, R., A. Siitonen, T. Heiskanen, U. Karkkainen, P. Kuosmanen, P. Lipponen, and P. H. Makela. 1996. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 22:91-99. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, J. R., M. A. Kuskowski, A. Gajewski, S. Soto, J. P. Horcajada, M. T. Jimenez de Anta, and J. Vila. 2005. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J. Infect. Dis. 191:46-50. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 15.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik, A. A., N. Radhakrishnan, K. Reddy, A. D. Smith, and P. C. Singhal. 2002. Morphine-induced macrophage apoptosis modulates migration of macrophages: use of in vitro model of urinary tract infection. J. Endourol. 16:605-610. [DOI] [PubMed] [Google Scholar]
- 17.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 19.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mysorekar, I. U., and S. J. Hultgren. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. USA 103:14170-14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, H. K., S. S. Shim, S. Y. Kim, J. H. Park, S. E. Park, H. J. Kim, B. C. Kang, and C. M. Kim. 2005. Molecular analysis of colonized bacteria in a human newborn infant gut. J. Microbiol. 43:345-353. [PubMed] [Google Scholar]
- 22.Roberts, J. A., B. I. Marklund, D. Ilver, D. Haslam, M. B. Kaack, G. Baskin, M. Louis, R. Mollby, J. Winberg, and S. Normark. 1994. The Gal(α1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA 91:11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo, T. A., A. Stapleton, S. Wenderoth, T. M. Hooton, and W. E. Stamm. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 172:440-445. [DOI] [PubMed] [Google Scholar]
- 24.Scheenen, W. J., L. R. Makings, L. R. Gross, T. Pozzan, and R. Y. Tsien. 1996. Photodegradation of indo-1 and its effect on apparent Ca2+ concentrations. Chem. Biol. 3:765-774. [DOI] [PubMed] [Google Scholar]
- 25.Schilling, J. D., R. G. Lorenz, and S. J. Hultgren. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 70:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slonim, L. N., J. S. Pinkner, C. I. Branden, and S. J. Hultgren. 1992. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 11:4747-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]