Abstract
AcpA of Francisella spp. is a respiratory-burst-inhibiting acid phosphatase that also exhibits phospholipase C activity. To better understand the molecular basis of AcpA in virulence, a deletion of acpA was constructed in Francisella novicida. The phosphatase and lipase activities were reduced 10-fold and 8-fold, respectively, in the acpA mutant compared to the wild type and were found mostly associated with the outer membrane. The acpA mutant was more susceptible to intracellular killing than the wild-type strain in the THP-1 human macrophage-like cell line. In addition, mice infected with the acpA mutant survived longer than the wild-type strain and were less fit than the wild-type strain in competition infection assays. Transmission electron microscopy showed that the acpA mutant was delayed in escape from macrophage phagosomes, as more than 75% of acpA mutant bacteria could still be found inside phagosomes after 12 h of infection in THP-1 cells and human monocyte-derived macrophages, whereas most of the wild-type bacteria had escaped from the phagosome by 6 h postinfection. Thus, AcpA affects intracellular trafficking and the fate of Francisella within host macrophages.
Francisella tularensis is a gram-negative, facultative intracellular pathogen that causes tularemia in humans and other mammals including rodents (38). The two primary human pathogens are F. tularensis subsp. tularensis (type A strain) and F. tularensis subsp. holarctica (type B strain). The type A strain is found predominantly in North America, is highly infectious, and causes a life-threatening disease in humans, especially when inhaled (10). Type B strains are found primarily in Europe and are considered to be less virulent for mammals than the type A strain (10). An attenuated live vaccine strain (LVS) was derived from a type B strain (12), and it elicits a protective response in humans, monkeys, guinea pigs and mice against systemic challenge with virulent type A F. tularensis (9, 12, 13, 18, 35, 36). Francisella novicida, a close relative of type A F. tularensis, is a much less frequent cause of infection in humans than either type A or type B (19). Nevertheless, F. novicida, F. tularensis subsp. tularensis, and F. tularensis subsp. holarctica all cause a lethal systemic infection in mice when inoculated by most routes (19).
The virulence mechanisms of this bacterium are not clear, although the products of several genes such as mglA and the pathogenicity island genes iglC, iglD, and pdpA-D that help Francisella to survive inside macrophages have been identified (6, 15, 17). However, the exact functions of these genes are not known. MglA shares homology with the SspA of Escherichia coli, which regulates stationary-phase gene transcription by interacting with RNA polymerase (40). MglA regulates several virulence factors within the pathogenicity island, including iglC (25), which is important for the ability of Francisella spp. to escape from the phagosome. Several studies have shown that F. tularensis resides inside a membrane-bound phagosome during its initial growth in a macrophage and that it is released into the cytoplasm during a later phase of growth (2, 11, 16).
Acid phosphatases are ubiquitous in nature and are present in almost all bacteria. These enzymes have been identified and characterized for many eukaryotes and prokaryotes and are divided into subgroups according to their substrate specificities, molecular weights, and sensitivities to known inhibitors (30). Acid phosphatases catalyze the hydrolysis of phosphomonoesters at an acidic pH. In several species, they have been implicated as virulence factors and help the bacteria to survive inside phagocytes (4, 7, 14, 23, 27, 28, 31), often by inhibiting the respiratory burst (4, 20, 23, 29, 31).
The published genome sequence of F. tularensis Schu 4 revealed the presence of four acid phosphatases (acpA [FTT0221], acpB [FTT0156], acpC [FTT0620], and hap [FTT1662c] [a pseudogene in Schu 4 but not LVS]) (21). AcpA (57 kDa) is a polyspecific periplasmic acid phosphatase that is highly expressed by F. tularensis (7, 27) and shows no significant global amino acid sequence similarity with any protein in the Protein Data Bank (8). This protein is also unusual in that it exhibits phospholipase C activity (27). Previous studies reported that Francisella AcpA has respiratory-burst-inhibiting properties and broad substrate specificity (27). It has also been shown that a transposon insertion in the 3′ region of the acpA open reading frame did not result in an intramacrophage survival defect or a loss of virulence (7). In the present study, we constructed a deletion of the entire acpA gene in F. novicida and analyzed its role in intracellular trafficking in macrophages and virulence in mice.
MATERIALS AND METHODS
Bacterial strains, plasmid construction, and molecular biology techniques.
Bacterial strains, plasmids, and primers used in this study are listed in Tables 1 and 2. F. novicida U112 was routinely grown at 37°C on cysteine heart agar (CHA) (Hi-Media Laboratories, India) and in modified tryptic soy broth (Difco Laboratories, Detroit, MI) containing 135 μg/ml ferric pyrophosphate and 0.1% cysteine hydrochloride. CHA containing 5% defibrinated sheep blood and 135 μg/ml ferric pyrophosphate was used for transformation studies (Hemostat Laboratories, Dixon, CA). When required, the growth medium for wild-type (WT) F. novicida was supplemented with kanamycin (25 μg/ml) or tetracycline (12.5 μg/ml). All manipulations with Francisella spp. were performed in a class II biological safety laboratory. E. coli DH5α was grown at 37°C aerobically in Luria-Bertani (LB) medium (Difco Laboratories, Detroit, MI) supplemented with kanamycin (15 μg/ml), tetracycline (12.5 μg/ml), or ampicillin (100 μg/ml) when required. All antibiotics and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype or relevant phenotype | Source or reference |
|---|---|---|
| Strains | ||
| Francisella | ||
| JSG1819 | Francisella novicida U112 | ATCC |
| JSG2660 | JSG1819 with ΔacpA::kan | This work |
| JSG2661 | JSG2660 complemented with acpA carried on pKK214pgroEL | This work |
| E. coli | ||
| DH5α | F− 80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17 deoR thi-1 supE441 gyrA96 relA1 | BRL |
| Plasmids | ||
| pUC18 | High-copy cloning vector | NEB |
| pKK214 | Low-copy expression vector with GroEL promoter of F. tularensis LVS | 39 |
| pAcpUp | pUC18-acpA upstream region | This work |
| pAcpUpDn | pUC18-acpA upstream and downstream region | This work |
| pAcpA-Kan | pAcpUpDN with Kan cassette | This work |
| pAcpA | pKK214 with acpA for complementation study | This work |
TABLE 2.
Oligonucleotide primers
| Primer | Sequence (5′-3′) |
|---|---|
| JG996 | CGAGCTCCATCACCTTCTTCATAATGAGAGAATTTCTTAAGCGCATGACC |
| JG997 | GGGGTACCATGATACCTTTAGTTGTTAGATTCAAAGGAAATATTAATAAC |
| JG998 | AACTGCAGCTCGGTAAGTTGCTTTAATCTAGTATTTTCGCTTATTGCCTCTTCTAAACTTAC |
| JG999 | ACATGCATGCCATTCAGATAGTAGTATAGCCTTAGAGTTAGCACAAGAGTTGTATAAAAATAAAGGTTAAGC |
| JG868 | CGGAATTCGGATCCCTGCAGATCGATTGTTGTTTCAAGTTTTGATAATGATTAAAAATAATAGGAGTTAAAAATGAGCCATATTCAACGGGAAACG |
| JG1048 | AACTGCAGTTAGAAAAACTCATCGAGCATCAAATGAAACTGC |
| JG1016a | AACTGCAGGCACTGCAGAGGAGGGTTTTTAATGAAGCTCAATAAAATTACTTTAGGAATTTTAAGTCTAAGTATCGC |
| JG1017a | CGGAATTCTTAGTTTAATTTATCCACTACTAATCCTGTCTTAGGGTCTAAAATC |
Chromosomal DNA preparation, ligation, E. coli transformation, and Southern blotting were performed according to methods described previously by Sambrook et al. (32). To construct plasmid pAcpA-Kan, upstream and downstream regions of acpA were generated by PCR with primer pairs JG996/JG997 and JG998/JG999, respectively, from WT genomic DNA. The 772-bp upstream fragment was digested with SacI and KpnI, cloned into dephosphorylated SacI- and KpnI-digested pUC18, and renamed pAcpUp. Similarly, the 871-bp downstream region of acpA was digested with PstI and SphI, cloned into dephosphorylated PstI- and SphI-digested plasmid pAcpUp, and renamed pAcpUpDn. A kanamycin (Kan) cassette driven by the F. tularensis outer membrane protein (GenBank accession number YP_169847) promoter was amplified from plasmid pDSK519 using primer pair JG868/JG1048, digested with KpnI and PstI, ligated into the KpnI/PstI-digested and dephosphorylated pAcpUpDn vector, and renamed pAcpA-Kan. Plasmid pAcpA-Kan was transformed into WT cells by cryotransformation. In brief, 1 μg of plasmid was mixed with WT cells (optical density at 600 nm of 0.5) in transformation buffer (10 mM MOPS [morpholinepropanesulfonic acid], 75 mM CaCl2, 10 mM RbCl2, and 15% glycerol, with the pH adjusted to 6.5 with 1 N KOH), incubated on ice for 30 min, and flash frozen in liquid nitrogen for 5 min. Cells were thawed at room temperature, plated onto CHA-Kan, and incubated at 37°C. This resulted in the creation of strain JSG2660 (ΔacpA::kan). Growth curves were identical for strain JSG2660 and the WT. For complementation in trans, plasmid pKK214 containing the groEL promoter of F. tularensis LVS was used (1). The acpA gene was generated by PCR using primer pair JG1016a/JG1017a and cloned into the EcoRI and PstI sites of pKK214groEL such that acpA was expressed from the groEL promoter. The resulting plasmid, pAcpA, was introduced into JSG2660 by cryotransformation as described above, creating strain JSG2661.
For Southern blot analysis, PCR products were labeled with digoxigenin according to instructions provided by the manufacturer (Roche, Indianapolis, IN). Probes were hybridized to EcoRI-digested chromosomal DNA of WT F. novicida and the F. novicida ΔacpA strain followed by anti-digoxigenin-alkaline phosphatase conjugate antibody treatment. Membranes were developed with nitroblue tetrazolium/BCIP (5-bromo-4-chloro-3-indolylphosphate) solution.
Cell fractionation.
Stationary-phase cultures of wild-type (JSG1819) and mutant (JSG2660) bacteria grown overnight in tryptic soy broth-0.1% cysteine hydrochloride were centrifuged at 8,000 × g for 20 min at 4°C. The cell pellet was washed twice with phosphate-buffered saline (PBS) and sonicated at a constant output of 60 W for a total of 300 s. Cell debris and unbroken cells were removed by centrifugation at 3,000 × g for 15 min at 4°C. The supernatant was centrifuged at 100,000 × g for 24 h at 18°C in a gradient of 2.1 to 1.4 to 0.7 M sucrose to separate the outer and inner membrane fractions. The non-membrane-containing fraction was used as the cytosolic fraction. These fractions were assayed for phosphatase activity as described previously by Aragon et al. (3) Western blot analysis was performed on protein fractions using anti-AcpA polyclonal sera (gift of Tom Reilly) by standard protocols.
Intramacrophage survival assays.
The WT or the ΔacpA strain was used to infect J774.1 murine macrophages, NR8383 rat alveolar macrophages, and phorbol myristate acetate-induced (10 ng/ml) THP-1 human macrophages at a multiplicity of infection (MOI) of ∼50:1. Wells were seeded with ∼2 ×105 macrophages, and ∼1.0 × 107 bacteria were added to each well. After 2 h of incubation at 37°C and 5% CO2, gentamicin (50 μg/ml) was added to the medium to eliminate extracellular organisms. Wells were washed twice with PBS and incubated with their respective media supplemented with 10 μg/ml gentamicin. The macrophage cells were lysed with 0.1% sodium dodecyl sulfate at 2 h, 12 h, and 24 h postinfection, and the lysates were immediately serially diluted in PBS and plated onto CHA plates for determination of viable counts. Experiments were performed in triplicate on a minimum of three separate occasions with similar results.
Transmission electron microscopy.
Monolayers of monocyte-derived macrophages (MDMs) (24) or THP-1 cell lines were incubated with the WT (JSG1819) or the ΔacpA strain (JSG2660) at an MOI of 500:1 in a plastic four-well chamber slide. This high MOI was used to enhance the number of macrophages in the population that were infected, which in turn aided visualization. After 2, 6, and 12 h of incubation at 37°C and 5% CO2, the wells were washed and fixed immediately with 2.5% warmed glutaraldehyde for 5 min followed by a combination of 2.5% glutaraldehyde and 1% osmium tetroxide in 0.1 M sodium cacodylate, pH 7.3, for 15 min at 4°C (11). The cells were then stained with 0.25% uranyl acetate in 0.1 M sodium acetate buffer at pH 6.3 for 45 min. Monolayers were washed with ice-cold normal saline, and the chambers were then removed. Slides were dehydrated through a graded series of ethanol, rinsed in hydroxypropylmethylacrylate, and infiltrated with Polybed 812. They were embedded by up-ending resin-filled beam capsules over the cells and polymerized at 60°C for 24 h. Thin sections cut with a Leica EM UC6 ultramicrotome were collected onto Formvar-coated copper grids, stained with uranyl acetate and lead citrate, and viewed by transmission electron microscopy using a Philips CM12 transmission electron microscope at 60 kV. Multiple fields were examined for bacteria, and identified bacteria were determined to be intraphagosomal or intracytosolic. The criterion for intraphagosomal bacteria was the visualization of more than 50% of the phagosomal membrane surrounding the bacterium.
Mouse survival studies.
Groups of five female 4- to 6-week-old BALB/c mice (Harlan Sprague) were inoculated at a dose of ∼100 CFU delivered in 100 μl PBS by the intraperitoneal route. Actual bacterial counts delivered were determined by plate count from each inoculum. Mice were monitored for 4 days postinfection. For competition assays, wild-type (JSG1819) and mutant (JSG2660) bacteria were inoculated into BALB/c mice at a 1:1 ratio (100 CFU of each in 100 μl total), and at 4 days postinoculation, organs were harvested, macerated, and plated onto appropriate solid media to select for each of the competing strains. The competitive index was calculated as the number of mutant CFU/WT CFU recovered.
RESULTS
Construction of a ΔacpA mutant.
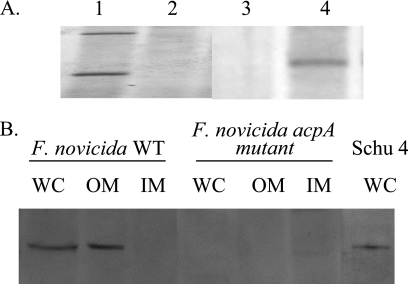
A modified cryotransformation technique that increased the efficiency of transformation 100- to 1,000-fold in comparison to the standard Francisella MgCl2-KCl cryotransformation or electroporation was developed. This technique was used to introduce pAcpA-Kan, a nonreplicating suicide vector carrying a DNA fragment with a kanamycin cassette in the place of a complete deletion of acpA, into WT cells. After 5 days of growth on CHA-Kan plates, several colonies were screened by PCR and Southern blotting, resulting in the identification of more than 85% of the clones with the correct deletion and loss of the vector sequences (Fig. 1A).
FIG. 1.
Construction and confirmation of the ΔacpA mutant. (A) Southern blot analysis of the chromosomal DNA of the ΔacpA mutant versus the WT. DNAs were digested with EcoRI and probed with the acpA ORF (lanes 1 and 2) or the kan cassette (lanes 3 and 4) showing the expected differential hybridization of the probe. An EcoRI site within the acpA ORF results in two hybridizing bands, while no EcoRI sites were found within the acpA ORF. Lanes 1 and 3, F. novicida; lanes 2 and 4, F. novicida ΔacpA. (B) Western blot analysis of whole-cell lysates (WC), outer membrane fractions (OM), and inner membrane fractions (IM) of the WT, the ΔacpA strain, and Schu 4 detecting the 57-kDa AcpA protein. Rabbit polyclonal anti-AcpA serum was used as the primary antibody.
Comparative measurements of acid phosphatase and lipase activity.
WT and ΔacpA cells were disrupted and separated by a sucrose gradient to recover inner and outer membrane fractions. A Western blot analysis was performed, which showed the presence of AcpA in whole-cell lysates and the outer membrane fraction of WT cells (Fig. 1B). AcpA was absent in all fractions analyzed from the ΔacpA strain. A single band of similar molecular weight was detected in a Schu 4 whole-cell lysate, demonstrating its expression in type A virulent Francisella. Acid phosphatase activities of the F. novicida cell extracts and inner and outer membrane fractions were measured. Using 6,8-difluoro-4-methylumbelliferyl phosphate as the substrate, we found no difference in the phosphatase activities of cell extracts/cytosolic fractions or inner membrane fractions between the mutant and wild-type strains but a 10-fold decrease in phosphatase activity of the mutant outer membrane fraction (Fig. 2A). This was consistent with the observed location of the enzyme in the outer membrane fraction (Fig. 1B). Complementation of the ΔacpA strain with a plasmid containing the acpA gene restored outer membrane phosphatase activity to the wild-type level (Fig. 2A). Using p-nitrophenyl palmitate as the substrate, we also measured an eightfold decrease in phospholipase activity recovered from the outer membrane fraction of the ΔacpA strain compared to the WT (Fig. 2B). These results are consistent with the predicted secretion of AcpA into the periplasmic space and suggest a tight association with the outer membrane. In addition, these data demonstrate that the ΔacpA strain has significantly reduced enzymatic activities that are associated with AcpA.
FIG. 2.
Acid phosphatase (A) and lipase (B) activities of inner membrane (IM), outer membrane (OM), and cytoplasmic (Cyt) fractions of the ΔacpA (gray bars) versus the ΔacpA complemented (white bars) and parental (black bars) strains. Data represent the means ± standard deviations of three independent experiments each with duplicate wells.
The ΔacpA strain is defective in intramacrophage survival/replication.
The primary cellular target of F. tularensis during infection is the macrophage (26, 39). To examine the role that AcpA may play in protecting Francisella from phagocytic killing and intracellular trafficking, we measured the survival of the ΔacpA strain in the THP-1 cell line. While the WT and the ΔacpA complemented strains replicated within the THP-1 cells, the ΔacpA strain showed about a half-log reduction over 12 h postinfection, with a slight recovery to the initial infection numbers by 24 h (Fig. 3). The experiment was not continued beyond 24 h due to a loss of macrophage viability. Similar results were observed in murine and rat macrophage cell lines (data not shown). These results demonstrate that AcpA plays a role in the intramacrophage survival and/or replication of WT F. novicida.
FIG. 3.
Intramacrophage survival assays performed in THP-1 human macrophage-like cell lines with the ΔacpA (squares), ΔacpA complemented (triangles), and parental (diamonds) strains. Data represent means ± standard deviations of three independent experiments each with duplicate wells for each time point. Asterisks denote significant differences for the ΔacpA mutant compared to the WT at 12 and 24 h postinfection (P < 0.005, Student's t test).
Electron microscopy demonstrates delayed phagosomal escape of the acpA mutant.
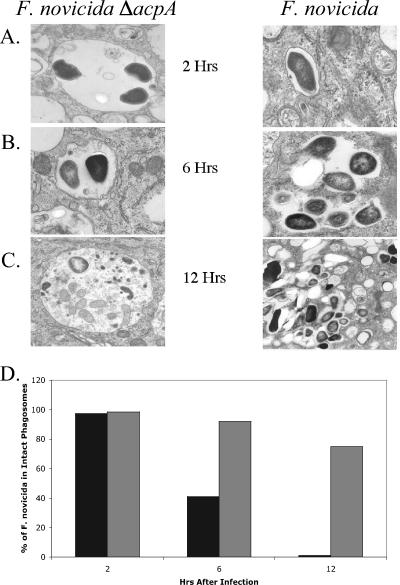
To further delineate the function of AcpA in intramacrophage survival, we used transmission electron microscopy to visualize phorbol myristate acetate-stimulated THP-1 cells and MDMs infected with either the WT or the ΔacpA strain (Fig. 4 and 5). At 2 h postinfection in both macrophages, nearly all of the mutant and wild-type bacteria were contained in membrane-bound vacuoles, consistent with the known early compartmentalization of the pathogen in phagosomes (Fig. 4A and 5A). After 6 h, over half of WT cells had escaped from phagosomes (Fig. 4B and 5B), and by 12 h postinfection, almost none of the wild-type pathogen was contained inside vacuoles with distinct membranes boundaries in THP-1 cells (Fig. 4C). Studies with MDMs revealed similar findings, except that the escape of WT cells at 12 h was slower, such that 40% of the cells were still in intact membranes (versus 75% of the ΔacpA cells) (Fig. 5C). In sharp contrast, we observed very little degradation of vacuoles containing ΔacpA cells during the 12-h timeframe of the experiments. After 12 h, approximately 75% of ΔacpA cells were still contained in vacuoles with distinct membrane borders (Fig. 4D and 5D). These results suggest that AcpA plays a role in intramacrophage survival/replication via the disruption of the phagosomal membrane that allows Francisella cells access to the host cell cytosol.
FIG. 4.
Transmission electron microscopy images of THP-1 cells infected with the acpA null strain of F. novicida (left panel) and the WT (right panel) obtained 2 h (A), 6 h (B), and 12 h (C) postinfection and (D) semiquantitative assessment of bacteria within/outside of phagosomes as determined by counting phagosomes in a minimum of 100 cross-sections/test group. Gray bars, ΔacpA; black bars, parental strain. The width of each panel in A, B, and C is 2.87 μm.
FIG. 5.
Transmission electron microscopy images of MDM cells infected with the acpA null strain of F. novicida (left panel) and WT F. novicida (right panel) obtained 2 h (A), 6 h (B), and 12 h (C) postinfection and (D) semiquantitative assessment of bacteria within/outside of phagosomes in a minimum of 100 cross-sections/test group. Gray bars, ΔacpA; black bars, parental strain. The width of each panel in A, B, and C is 1.5 μm.
The ΔacpA mutant has reduced virulence in the mouse model.
To examine the role of AcpA in an animal model of infection, we measured survival rates of mice infected with WT and ΔacpA cells. After 36 h following intraperitoneal infection, only 20% of the mice infected with the WT had survived, while 80% of the mice infected with the ΔacpA strain survived (Fig. 6). At the 48-h time point, all mice infected with the WT were dead, while 60% of those infected with the ΔacpA strain survived. However, by 72 h postinfection, all mice had died. Competition assays corroborated the apparent decrease in ΔacpA strain virulence, demonstrating a consistent competitive index of ∼0.17 in both liver and spleen. These data indicate that the loss of AcpA activity results in a less virulent pathogen, which is presumably due to decreased intramacrophage survival. Because mice infected with the ΔacpA strain eventually died, it is likely that AcpA acts in concert with other factors to disrupt normal intracellular trafficking and that the influence of AcpA is greatest in the initial stages of infection.
FIG. 6.
Survival of BALB/c mice following intraperitoneal infection with the F. novicida ΔacpA strain (squares), the ΔacpA complemented strain (triangles), and the parental strain (diamonds). A total of 10 mice/strain were used in two independent experiments. Asterisks denote significant differences for the ΔacpA mutant versus the WT at 36 and 48 h postinfection (P < 0.005, Student's t test).
DISCUSSION
Francisella AcpA possesses physical and chemical properties unlike other bacterial acid phosphatases (20, 31). This 57-kDa enzyme hydrolyzes a wide variety of physiologically meaningful substrates, including phosphorylated tyrosine peptides, inositol phosphates, phospholipid-like molecules, AMP, ATP, fructose-1,6-bisphosphate, glucose- and fructose-6-phosphosphates, NADP, and ribose-5-phosphate, but fails to possess significant similarity to other acid phosphatases in the protein database (27, 28). It has recently been crystallized (14), and the completed structure of this enzyme will likely provide important clues to its biological functions.
Many acid phosphatases have been shown or predicted to play a role in virulence, most often in intracellular pathogens, by the inhibition of a respiratory burst. Such activity has been reported for AcpA of the WT (27) as well as for Coxiella burnetii (4), Legionella spp. (31), and Leishmania (29). In this study, we described the role of Francisella AcpA, which has dual acid phosphatase and phospholipase C activities, in virulence and phagosomal trafficking in macrophages.
In this work, we constructed a complete deletion of the acpA open reading frame (ORF). A previous study of AcpA in the WT concluded that upon the chromosomal deletion of a 300-bp 3′ region and the replacement of this region by an erythromycin cassette, there was no effect upon intramacrophage survival or virulence (7). We were therefore somewhat surprised by the phenotypes observed due to the complete deletion of acpA. It is possible that since the entire acpA ORF was not deleted in the previous study, a functional truncated protein was still produced. With regard to acid phosphatase activity, this may be unlikely, as the peak of phosphatase activity in protein fractions associated with AcpA was absent in the previously described mutant. However, it is possible that the truncated protein retained phospholipase activity, and it is this activity that is important for phagosomal trafficking.
It was shown that the phosphatase and phospholipase activities of the ΔacpA mutant had 10-fold and 8-fold less activity than the WT, respectively, indicating that AcpA contributed to the overall activity but that it is not the only phosphatase or phospholipase in the WT. Indeed, enzyme assays have shown at least two peaks of phosphatase activity in crude protein fractions (7), and genome scanning shows three other acid phosphatases in the WT (unpublished observations). The future characterization and mutagenesis of these additional acid phosphatases will help to define the individual contributions of each enzyme to the biology of Francisella.
We determined the growth kinetics of the acpA mutant and compared them to those of the WT in various macrophage cell lines, where we showed that the acpA mutant exhibited a 10- to 20-fold reduction in survival in THP-1 cells. This phenotype was due to the loss of acpA, as the complemented mutant strain revealed intramacrophage growth kinetics identical to those of the WT strain. To explore the survival or growth of the acpA mutant in vivo, mice were infected intraperitoneally (and intranasally, with similar results) (data not shown). We found that mice infected with the acpA mutant survived longer than mice infected with the WT strain, and there was a corresponding reduction in organ bacterial load. When mice were infected with a mixture of WT and acpA mutant strains at a ratio of 1:1, the acpA mutant strain was less competitive than the WT strain (competitive index of ∼0.17 in both liver and spleen). These data suggest that AcpA contributes to virulence. It is possible that a greater virulence defect will be observed upon the deletion of multiple acid phosphatase genes.
It has been shown that Francisella spp. are initially contained within a phagosome but later escape into cytoplasm (11, 33, 34). The factors involved in and the mechanism of phagosomal escape are largely unknown, but IglC and MglA have been shown to play a role (33). We found that in THP-1 cells and MDMs, the ΔacpA mutant was still located primarily inside the phagosome at 6 h postinfection and only poorly escaped into the cytoplasm after 12 h of infection, whereas the WT was nearly 100% and 60% escaped from phagosomes by 12 h of infection in THP-1s and MDMs, respectively. These data suggest that AcpA plays a role in intramacrophage trafficking, and the inability of the mutant to escape from the phagosome likely contributes to its decreased intramacrophage survival and virulence.
Because AcpA has numerous enzymatic targets, a challenge for the future is to determine which of these enzymatic activities underlies the observed respiratory burst suppression and altered phagosomal escape due to AcpA. One possibility is that AcpA hydrolyzes phospholipids of the phagosomal inner membrane, which might compromise membrane integrity. Alternatively, AcpA might affect host signaling pathways by dephosphorylation of host proteins, inositol phosphates or phosphoinositides, with the latter being critically important for phagosome formation (37) and respiratory burst activation (5). A third hypothesis is that AcpA activity within the bacterium helps to induce or amplify the activity of other proteins that are essential for phagosome disruption, such as IglC and its regulator, MglA (22, 33). In this context, the polyspecificity and high abundance of AcpA may point to a phosphate scavenger role for the enzyme (41). Based on structural, enzymatic, and phenotypic properties, inhibitors of AcpA and other acid phosphatases may be developed that, when used in combination with other antimicrobial agents, would provide alternative therapy against F. tularensis infection.
Acknowledgments
This work was supported by National Institutes of Health grants U56AI057164 (Midwest Center of Excellence [MWCE] Transmission/Pathogenesis of Bioterrorism Agents to L.S.S.), U54AI057153 (Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium [GLRCE] to L.S.S. and J.S.G.), and U54AI057156 (Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases to J.S.G.) and an NIH institutional training grant, AI 0155411 (to A.B.).
We thank Karen Elkins and Fran Nano for providing guidance and Francisella strains and Jack Tanner and Tom Reilly for their advice and critical reading of the manuscript.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Abd, H., T. Johansson, I. Golovliov, G. Sandström, and M. Forsman. 2003. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 69:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, L. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragon, V., S. Kurtz, A. Flieger, B. Neumeister, and N. P. Cianciotto. 2000. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68:1855-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baca, O. G., M. J. Roman, R. H. Glew, R. F. Christner, J. E. Buhler, and A. S. Aragon. 1993. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect. Immun. 61:4232-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggiolini, M., and M. P. Wymann. 1990. Turning on the respiratory burst. Trends Biochem. Sci. 15:69-72. [DOI] [PubMed] [Google Scholar]
- 6.Baron, G. S., and F. E. Nano. 1998. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol. Microbiol. 29:247-259. [DOI] [PubMed] [Google Scholar]
- 7.Baron, G. S., T. J. Reilly, and F. E. Nano. 1999. The respiratory burst-inhibiting acid phosphatase AcpA is not essential for the intramacrophage growth or virulence of Francisella novicida. FEMS Microbiol. Lett. 176:85-90. [DOI] [PubMed] [Google Scholar]
- 8.Berman, H. M., J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov, and P. E. Boune. 2001. The Protein Data Bank. Nucleic Acids Res. 28:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, W., H. Shen, A. Webb, R. KuoLee, and J. W. Conlan. 2003. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 21:3690-3700. [DOI] [PubMed] [Google Scholar]
- 10.Chu, M. C., and R. Weyant. 2003. Francisella and Brucella, p. 789-797. In P. R. Murray, E. J. Baron, J. H. Jorgenson, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 11.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eigelsbach, H. T., and C. M. Downs. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J. Immunol. 87:415-425. [PubMed] [Google Scholar]
- 13.Eigelsbach, H. T., J. Tulis, E. L. Overholt, and W. R. Griffith. 1961. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc. Soc. Exp. Biol. Med. 108:732-734. [DOI] [PubMed] [Google Scholar]
- 14.Felts, R. L., T. J. Reilly, and J. J. Tanner. 2005. Crystallization of AcpA, a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. Biochim. Biophys. Acta 1752:107-110. [DOI] [PubMed] [Google Scholar]
- 15.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjostedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golovliov, I., A. Sjostedt, A. Mokrievich, and V. A. Pavlov. 2003. Method for allelic replacement in Francisella tularensis. FEMS Microbiol. Lett. 222:273-280. [DOI] [PubMed] [Google Scholar]
- 17.Gray, C. G., S. C. Cowley, K. K. Cheung, and F. E. Nano. 2002. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol. Lett. 215:53-56. [DOI] [PubMed] [Google Scholar]
- 18.Hodge, F. A., W. R. Leif, and M. S. Silverman. 1968. Susceptibility to infection with Pasteurella tularensis and the immune response of mice exposed to continuous low dose rate gamma radiation. Natl. Radiol. Defense Lab. Trans. 68:1-28. [PubMed] [Google Scholar]
- 19.Kieffer, T., S. Cowley, F. E. Nano, and K. L. Elkins. 2003. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 5:397-403. [DOI] [PubMed] [Google Scholar]
- 20.Kondo, E., T. Kurata, P. Naigowit, and K. Kanai. 1996. Evolution of cell-surface acid phosphatase of Burkholderia pseudomallei. Southeast Asian J. Trop. Med. Public Health 27:592-599. [PubMed] [Google Scholar]
- 21.Larsson, P., P. C. F. Oyston, P. Chain, M. C. Chu, M. Duffield, H. H. Fuxelius, E. Garcia, G. Hälltorp, D. Johansson, K. E. Isherwood, P. D. Karp, E. Larsson, Y. Liu, S. Michell, J. Prior, R. Prior, S. Malfatti, A. Sjöstedt, K. Svensson, N. Thompson, L. Vergez, J. K. Wagg, B. W. Wren, L. E. Lindler, S. G. E. Andersson, M. Forsman, and R. W. Titball. 2005. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37:153-159. [DOI] [PubMed] [Google Scholar]
- 22.Lauriano, C. M., J. R. Barker, S. S. Yoon, F. E. Nano, B. P. Arulanandam, D. J. Hassett, and K. E. Klose. 2004. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc. Natl. Acad. Sci. USA 101:4246-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markova, N., V. Kussovski, and T. Radoucheva. 1998. Killing of Pseudomonas pseudomallei by polymorphonuclear leukocytes and peritoneal macrophages from chicken, sheep, swine and rabbits. Zentralbl. Bakteriol. 288:103-110. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy, T. R., J. B. Torrelles, A. S. MacFarlane, M. Katawczik, B. Kutzbach, L. E. DesJardin, S. Clegg, J. B. Goldberg, and L. S. Schlesinger. 2005. Overexpression of Mycobacterium tuberculosis manB, a phosphomannomutase that increases phosphatidylinositol mannoside biosynthesis in Mycobacterium smegmatis and mycobacterial association with human macrophages. Mol. Microbiol. 58:774-790. [DOI] [PubMed] [Google Scholar]
- 25.Nano, F. E., N. Zhang, S. C. Cowley, K. E. Klose, K. K. M. Cheung, M. J. Roberts, J. S. Ludu, G. W. Letendre, A. I. Meierovics, G. Stephens, and K. L. Elkins. 2004. A Francisella tularensis pathogenicity island required for intramacrophage growth. J. Bacteriol. 186:6430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 27.Reilly, T. J., G. S. Baron, F. E. Nano, and M. S. Kuhlenschmidt. 1996. Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J. Biol. Chem. 271:10973-10983. [DOI] [PubMed] [Google Scholar]
- 28.Reilly, T. J., R. L. Felts, M. T. Henzl, M. J. Calcutt, and J. J. Tanner. 2006. Characterization of recombinant Francisella tularensis acid phosphatase A. Protein Expr. Purif. 45:132-141. [DOI] [PubMed] [Google Scholar]
- 29.Remaley, A. T., R. H. Glew, D. B. Kuhns, R. E. Basford, A. S. Waggoner, L. A. Ernst, and M. Pope. 1985. Leishmania donovani: surface membrane acid phosphatase blocks neutrophil oxidative metabolite production. Exp. Parasitol. 60:331-341. [DOI] [PubMed] [Google Scholar]
- 30.Rossolini, G. M., S. Schippa, M. L. Riccio, F. Berlutti, L. E. Macaskie, and M. C. Thaller. 1998. Bacterial nonspecific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell. Mol. Life Sci. 54:833-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha, A. K., J. N. Dowling, K. L. LaMarco, S. Das, A. T. Remaley, N. Olomu, M. T. Pope, and R. H. Glew. 1985. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch. Biochem. Biophys. 243:150-160. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 33.Santic, M., M. Molmeret, and Y. Abu Kwaik. 2005. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell. Microbiol. 7:957-967. [DOI] [PubMed] [Google Scholar]
- 34.Santic, M., M. Molmeret, K. E. Klose, S. Jones, and Y. A. Kwaik. 2005. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell. Microbiol. 7:969-979. [DOI] [PubMed] [Google Scholar]
- 35.Saslaw, S., H. T. Eigelsbach, H. E. Wilson, J. A. Prior, and S. Carhart. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Int. Med. 107:689-701. [DOI] [PubMed] [Google Scholar]
- 36.Shen, H., W. Chen, and J. W. Conlan. 2004. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine 22:2116-2121. [DOI] [PubMed] [Google Scholar]
- 37.Simonsen, A., A. E. Wurmser, S. D. Emr, and H. Stenmark. 2001. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13:485-492. [DOI] [PubMed] [Google Scholar]
- 38.Tärnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 39.Titball, R. W., A. Johansson, and M. Forsman. 2003. Will the enigma of Francisella tularensis virulence soon be solved? Trends Microbiol. 11:118-123. [DOI] [PubMed] [Google Scholar]
- 40.Williams, M. D., T. X. Ouyang, and M. C. Flickinger. 1994. Glutathione S-transferase-sspA fusion binds to E. coli RNA polymerase and complements delta sspA mutation allowing phage P1 replication. Biochem. Biophys. Res. Commun. 201:123-127. [DOI] [PubMed] [Google Scholar]
- 41.Yang, K., and W. W. Metcalf. 2004. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. Proc. Natl. Acad. Sci. USA 101:7919-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]