Abstract
Bactericidal/permeability-increasing (BPI) protein has been shown to play an important role in innate immunity to gram-negative bacteria, by direct microbicidal as well as endotoxin-neutralizing action. Here we examined potential interactions between a recombinant 21-kDa bioactive fragment of BPI, rBPI21, and the gram-positive pathogen Streptococcus pneumoniae. rBPI21 bound to pneumococci and pneumolysin (Ply) in a direct and specific fashion. We observed an enhanced inflammatory response in mouse macrophages when rBPI21 was combined with killed pneumococci or supernatant from overnight growth of pneumococci. In addition, rBPI21 augmented the proapoptotic activity of Ply+ (but not Ply−) pneumococci in TLR4-defective murine macrophages (known to be defective also in their apoptotic response to pneumolysin) in a tumor necrosis factor alpha-dependent manner. rBPI21 also enhanced the association of pneumococci with murine macrophages. In a model of invasive pneumococcal disease in TLR4-defective mice, the intranasal administration of rBPI21 following intranasal inoculation of Ply+ pneumococci both enhanced upper respiratory tract cell apoptosis and prolonged survival. We have thus discovered a novel interaction between pneumococcus and rBPI21, a potent antimicrobial peptide previously considered to target only gram-negative bacteria.
The innate immune response provides rapid and usually effective defense against microbial pathogens. This response involves recognition of pathogen-associated molecules, triggering production and release of inflammatory mediators, recruitment of leukocytes, and activation of antimicrobial effectors. The Toll-like receptors (TLRs), of which at least 11 have been described for mammals (2), are capable of discriminating among a wide variety of pathogen-associated molecules and eliciting protective responses. The first molecule to be identified as a Toll agonist in mammalian cells was the lipopolysaccharide (LPS) of gram-negative bacteria, through a complex consisting of LPS binding protein (LBP), CD14, and MD-2, which is essential for LPS-related signal transduction (1, 18, 39, 41). TLR4 was thus recognized as a sensor of LPS (2).
Reports soon followed showing that TLR4 could also recognize microbial products from organisms other than gram-negative bacteria, such as the F protein of respiratory syncytial virus (17) and cholesterol-dependent cytolysins (CDC) of gram-positive bacteria (6, 21, 29, 39). Specifically, our group showed that the inflammatory and apoptotic responses to pneumolysin (Ply), the CDC from Streptococcus pneumoniae, are mediated by TLR4 and that these responses protect mice against invasive pneumococcal disease (21, 39). More recently, another component of the LPS recognition complex, LBP, has been implicated in the inflammatory response to pneumococcal peptidoglycan (43). It is thus clear that components of the innate immune system that participate in the recognition of LPS also have a role in the recognition of gram-positive organisms.
Another molecule shown to have important activity against LPS is bactericidal/permeability-increasing protein (BPI), a cationic protein present in the azurophilic granules of polymorphonuclear leukocytes (44) and expressed in mucosal epithelia (8). BPI binds with high affinity to the lipid A moiety of LPS and exerts its antimicrobial activity on gram-negative bacteria by sequentially permeabilizing the outer and inner membranes of gram-negative bacteria, causing progressive membrane lysis (23), and by neutralizing LPS (24). The structural similarities between BPI and LBP (3, 4) led us to examine whether a recombinant 21-kDa bioactive fragment of BPI, rBPI21, plays any role in the recognition of pneumococcus. Here we show that rBPI21 binds to pneumococci, potentiates the inflammatory and apoptotic responses to pneumolysin-expressing pneumococci, enhances the association of pneumococci with macrophages, and confers a significant survival advantage on TLR4-defective mice after mucosal pneumococcal challenge.
MATERIALS AND METHODS
Reagents and bacterial strains.
rBPI21, a 21-kDa fragment of BPI (which was used in clinical trials for efficacy against meningococcal sepsis [5]); the diluent in which it was prepared; rabbit anti-BPI antibody; and LBP were all kindly provided by XOMA Corporation LLC (Berkeley, CA). Endotoxin-free human serum albumin (HSA) was from Gemini Bio-Products (Woodland, CA). Rat anti-tumor necrosis factor alpha (anti-TNF-α) and rat immunoglobulin G1 antibodies were purchased from Southern Biotech (Birmingham, AL). High-titer polyclonal rabbit antipneumolysin antibody was a gift from Wyeth-Lederle Vaccines and Pediatrics. Escherichia coli strain JM83 (a kind gift of Anders Hakansson, Children's Hospital, Boston, MA) was grown using standard methods and killed by being heated at 56°C for 1 h. Pseudomonas aeruginosa strain PAO1 and its AlgC and GalU mutants (33, 34) were a kind gift of Gregory Priebe (Children's Hospital, Boston, MA); these strains were grown using standard methods, frozen with 15% glycerol, and then thawed and washed just before use. S. pneumoniae type 6 strain 0603; type 3 strains A66.1, WU2, and WU2-PLA (isogenic pneumolysin-deficient strain); and the type 2 strain PLNA (pneumolysin-negative variant of strain D39) were prepared as described previously (21). Ethanol-killed pneumococci were prepared as follows: bacterial strains were grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) without shaking at 37°C with 5% CO2 to mid-logarithmic phase (A650, ≈0.3), harvested by centrifugation, killed in 70% (vol/vol) ethanol in phosphate-buffered saline (PBS) for 1 h on ice, washed, and resuspended in LPS-free PBS. CFU were determined before killing, and dry weights of the final preparations were measured to normalize bacterial numbers across different strain preparations.
A recombinant Rx1 pneumococcal strain expressing six-His-Ply was constructed by replacing the genomic copy of pneumolysin with a six-His-tagged gene fusion; integration at the correct genomic locus was confirmed by PCR. This strain was grown in Todd-Hewitt broth with 0.5% yeast (THY) at 37°C in 5% CO2 to an A650 of ≈0.9 (late-log phase, prior to induction of autolysis); pneumococci were harvested and lysed in 0.1% sodium deoxycholate for 20 min at 37°C in 5% CO2. The bacterial lysate was cleared by centrifugation, and six-His-Ply was purified using nickel-nitrilotriacetic acid chromatography (QIAGEN, Valencia, CA) using LPS-free buffers and plasticware. To remove residual LPS, the pure protein solution was treated with End-X endotoxin affinity resin (Associates of Cape Cod, East Falmouth, MA) and stored in 50% glycerol at −20°C. This preparation of six-His-Ply has the same bioactivity—hemolytic, proinflammatory, and proapoptotic—as that of the recombinant six-His-Ply purified from E. coli (39).
Stimulation of mouse macrophages and human epithelial cells.
Four- to six-week-old male C3H/HeOuJ and C3H/HeJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Thioglycolate-induced mouse macrophages were collected by peritoneal lavage and maintained in DMEM plus 10% FBS and 10 μg/ml of ciprofloxacin (Invitrogen, CA). Cells were plated at 105 cells/well for 96-well plates and 106 cells/well for 24-well plates and allowed to adhere for 24 h before stimulation. For costimulations, rBPI21 was coincubated with pneumococci and/or pure pneumolysin for 30 min at 37°C before being applied to the cells. Culture supernatants were assayed for TNF-α by enzyme-linked immunosorbent assay (ELISA; DuoSet; R&D Systems, Minneapolis, MN). All stimulations were performed in triplicate, and results are expressed as the means and standard deviations. Results shown are representative of three or more experiments. Induction of apoptosis was examined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining (In Situ cell death detection kit; fluorescein; Roche, Indianapolis, IN) as previously described (39).
For stimulation with pneumococcal culture supernatants, WU2 pneumococci were grown overnight at 37°C and 5% CO2 in DMEM plus 10% FBS; bacteria were separated by centrifugation, and the culture supernatants were sterile filtered with an 0.22-μm filter. Glycerol was added to 50%, and aliquots were frozen at −80°C. RAW264.7 mouse macrophages were stimulated with dilutions of these supernatants, and TNF-α was quantified as described above.
Human epithelial cells (HEK-293) transfected with genes coding for human TLR2 or TLR4 (a kind gift of Douglas Golenbock, University of Massachusetts, Worcester) were maintained in DMEM supplemented with 10% fetal calf serum, ciprofloxacin, and G418 as previously described (21). One day prior to stimulation, cells were seeded in 24-well plates (at a concentration of 5 × 105 cells/ml). Eighteen hours after stimulation, supernatants were collected and assayed for interleukin-8 (IL-8) concentration by ELISA (human IL-8 DuoSet; R&D Biosystems, Minneapolis, MN).
rBPI21 and pneumococcal interaction ELISA.
To examine rBPI21 binding to pneumococci, Nunc Immulon 2HB plates were coated with 100 μl/well of 108 CFU/ml of ethanol-killed pneumococcus Rx1 or its isogenic pneumolysin-negative mutant Rx1ply− in PBS overnight at 4°C and then washed and blocked with 0.05% casein in PBS (300 μl/well) for 2 h at room temperature. One hundred microliters/well of rBPI21 was added in an eight-point curve using 10-fold serial dilutions in PBS starting at 100 μg/ml protein, and plates were incubated at 37°C for 1 h in a humid chamber. After extensive washing, bound rBPI21 was detected using polyclonal rabbit anti-rBPI21 (1/20,000 dilution) followed by horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antibody at 1/10,000 (Santa Cruz Biotechnology, CA). Similar experiments were performed with live pneumococci (with strains Rx1 and WU2) and pseudomonal strains (strain PAO1 and isogenic AlgC and GalU mutants as described above); the protocol was similar except that the duration of coating was reduced to 2 h and all incubations were performed at 4 to 8°C.
To detect rBPI21 binding to pneumolysin, Nunc Maxisorp ELISA plates (Fisher, Pittsburgh, PA) were coated with 100 μl/well of 1.7-μg/ml antigen solution (rBPI21, diluent, LBP, and HSA) in PBS overnight at 4°C. Wells were blocked with 1% skim milk (Difco) in PBS (300 μl/well) for 2 h at room temperature. Wells were washed four times with PBS-Tween 20 between steps. After blocking, 100 μl of pneumolysin (six-His-pneumolysin purified from a recombinant pneumococcal strain) was added in a 12-point curve using twofold serial dilutions in 4.5% endotoxin-free HSA in PBS starting at 6 μg/well protein. Plates were incubated at 37°C for 1 h in a humid chamber. After washing, bound pneumolysin was detected using rabbit antipneumolysin (1/10,000) followed by HRP-conjugated donkey anti-rabbit antibody (1/10,000 dilution) in 1% HSA in PBS-Tween 20. In the reverse configuration, with rBPI21 as the ligand, plates were coated with 100 μl/well of pneumolysin at 1.7 μg/ml in PBS overnight at 4°C, washed with PBS Tween 20, and blocked with 0.05% casein in PBS (300 μl/well) for 2 h at room temperature. One hundred microliters/well of rBPI21 was added in a 12-point curve using twofold serial dilutions in PBS starting at 0.5 μg/well protein. Plates were incubated at 37°C for 1 h in a humid chamber. After washing, bound rBPI21 was detected using polyclonal rabbit anti-rBPI21 (1/20,000 dilution) followed by HRP-conjugated donkey anti-rabbit antibody at a 1/10,000 dilution. Plates were developed using SureBlue peroxidase substrate from Kierkegaard & Perry Laboratories (Gaithersburg, MD). The color reaction was terminated by addition of 2 N H2SO4, and absorbance was read at 450 nm. Formula weights used for pneumolysin and rBPI21 were 54,133 and 21,403, respectively. All binding assays were performed in duplicate, and results shown are representative of three or more experiments.
Association of pneumococci with murine macrophages.
To assess whether rBPI21 enhances the association of live pneumococci with macrophages, fluorescein isothiocyanate (FITC)-labeled pneumococci (strain 0603, a type 6B invasive pneumococcal isolate [22]; labeled strain provided by Debby Bogaert) were incubated with various concentrations of rBPI21 for 30 min at 37°C in the dark. Following this incubation, bacteria were coincubated with RAW macrophages (107 CFU to 106 macrophages; ratio, 10:1) for 30 min at 37°C with 5% CO2; cells were then extensively washed to remove nonadherent pneumococci and analyzed by flow cytometry. These experiments were performed three times.
Animal models of pneumococcal disease.
C3H/HeJ mice carry a mutant TLR4 gene (32) and are hypersusceptible to pneumococcal infection due to type 3 pneumococcus (21). In vivo apoptosis in the nasopharyngeal tissue of C3H/HeJ mice was assessed as previously described (39): briefly, mice (n = 10 per group) were restrained gently without anesthesia and inoculated intranasally with 20 μl (108 CFU) of strain WU2 (Ply+, type 3 strain). rBPI21 or diluent was administered intranasally in 10-μl (27-μg) doses at time zero and at 4, 8, and 16 h postinoculation for a total of four doses; mice in the two control groups (n = 2 per group) received rBPI21 or diluent alone. Twenty hours postinoculation, mice were sacrificed and retrograde tracheal washes were collected. Harvested cells were washed, fixed, permeabilized, and labeled according to the manufacturer's instructions (In Situ cell death detection kit; fluorescein; Roche, Indianapolis, IN) and finally analyzed by flow cytometry.
For sepsis experiments, C3H/HeJ mice (n = 12 per group) were restrained gently without anesthesia and inoculated intranasally with 20 μl (6 × 107 CFU) of strain A66.1 (Ply+, type 3). rBPI21 or diluent was administered intranasally in 10-μl (27-μg) doses at time zero and 4 h later and then every 6 to 8 h for 2 days postinoculation for a total of six doses. Mice were followed clinically for 7 days, at 12-hour intervals. All ill-appearing mice were immediately euthanized; in these cases, blood cultures that were performed confirmed the presence of pneumococcal bacteremia.
Statistical analysis.
Differences between the two groups were evaluated by the t test or the Mann-Whitney and Wilcoxon tests depending on whether or not the data were normally distributed; survival curves were analyzed using the Kaplan-Meier log rank test. For all comparisons P < 0.05 was considered significant.
RESULTS
rBPI21 binds to pneumococci and pneumolysin.
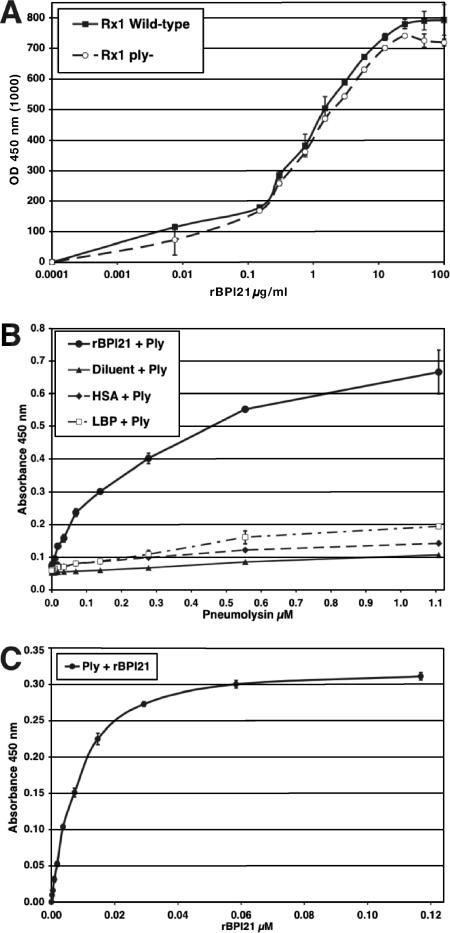
LBP, a homolog of BPI, binds to pneumococci (43). To test whether rBPI21 also binds to pneumococcus, 96-well ELISA plates were coated with ethanol-killed pneumococci (either pneumolysin-positive or -negative strains) and an increasing amount of rBPI21 was overlaid to allow binding. After extensive washing to remove unbound protein, the bound rBPI21 was detected by an anti-rBPI21 antibody. As shown in Fig. 1A, rBPI21 bound to immobilized pneumococci, in a dose-dependent and saturable manner. As controls for this binding assay, we also evaluated the binding of live pseudomonal strains of either smooth (PAO1) or rough (AlgC or GalU mutants of PAO1) phenotype (33, 34). Consistent with the findings of others (9, 45), strains of the rough phenotype bound significantly better to rBPI21 than the smooth strain (data not shown). There was no detectable difference between binding with pneumolysin-positive and that with the pneumolysin-negative strain as the coating reagent, suggesting that binding of rBPI21 to pneumococcus can occur independently of pneumolysin. Similar results, confirming binding of rBPI21 to pneumococcus, were obtained when live pneumococcus (strain WU2) was used as the coating antigen (data not shown).
FIG. 1.
Binding of rBPI21 to pneumococci and pneumolysin in a solid-phase binding assay. Ninety-six-well ELISA plates were coated with ethanol-killed pneumolysin-positive or -negative pneumococci (A); rBPI21, diluent, HSA, and LBP (B); and pneumolysin (C). After blocking, wells were overlaid with increasing amounts of purified native pneumolysin (B) or rBPI21 (A and C). After extensive washing, bound pneumolysin or rBPI21 was detected by anti-Ply or anti-BPI antibody. Results shown are representative of three or more experiments.
To evaluate more-specific binding of rBPI21 and pneumolysin, we asked whether a direct interaction between purified pneumolysin and rBPI21 could be demonstrated. Thus, a similar assay was developed: rBPI21 (or other control proteins) was applied as a coating onto 96-well ELISA plates and then native pneumolysin was overlaid to allow binding. As shown in Fig. 1B, pneumolysin demonstrated strong, reproducible, and dose-dependent binding to rBPI21. Pneumolysin showed no interaction either with the diluent in which rBPI21 is preserved or with HSA. Importantly, pneumolysin did not bind LBP, a related protein that also binds strongly to the lipid A portion of LPS and is predicted to be structurally similar to BPI (3, 4, 47), testifying to the specificity of the interaction between pneumolysin and rBPI21. Pneumolysin was also able to bind to the 55-kDa BPI holoprotein in this assay (data not shown).
This physical association between the two proteins was further examined using a similar approach but with proteins in the opposite configuration: wells were coated with pneumolysin and then overlaid with rBPI21; bound rBPI21 was then detected by a specific antibody. As shown in Fig. 1C, rBPI21 demonstrated strong, reproducible, and dose-dependent binding with pneumolysin. Taken together, these results strongly indicate that rBPI21 interacts both with whole pneumococcal cells and with pneumolysin and that these interactions are direct and specific.
rBPI21 potentiates the proinflammatory response to pneumococci in a TLR4-independent manner.
The interaction between rBPI21 and pneumococcus led us to examine whether any effect could be observed on viability, hemolytic potential, or the proinflammatory activity of pneumococci. Strain WU2 was grown in THY to which either rBPI21 (10 μg/ml) or the equivalent concentration of diluent was added; no difference in the growth curves was found, indicating that rBPI21 had no inhibitory or killing activity against pneumococcus (data not shown). Moreover, the addition of rBPI21 to pneumolysin had no effect on the hemolytic activity of the toxin (data not shown).
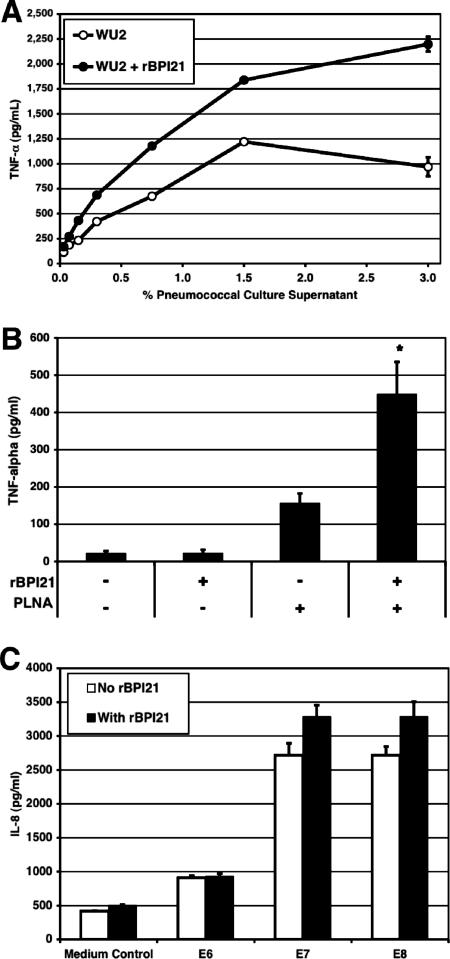
As shown in Fig. 2A, however, the inflammatory response of RAW macrophages stimulated with supernatant from overnight growth of strain WU2 was enhanced when rBPI21 was added (comparison of TNF-α responses in the absence and the presence of rBPI21 by Wilcoxon paired-rank test; P = 0.016). A similar result was obtained when killed whole pneumococcal cells were used to stimulate wild-type macrophages (Fig. 2B); again, the addition of rBPI21 consistently enhanced the inflammatory response (P = 0.016 by Mann-Whitney U test). A modest enhancement of the inflammatory response to pneumococci was also demonstrated using human epithelial cells transfected with TLR2 (Fig. 2C). The response of cells transfected with TLR4 was not significantly increased when rBPI21 was added (data not shown).
FIG. 2.
Effect of rBPI21 on the proinflammatory activity of pneumococci. (A) RAW264.7 macrophages were stimulated with various concentrations of sterile culture supernatants of pneumococcal strain WU2, and TNF-α secretion was measured after 18 h of stimulation. The TNF-α responses in the absence and presence of rBPI21 were compared by the Wilcoxon paired-rank test (P = 0.016). (B) Peritoneal macrophages from TLR4-defective (C3H/HeJ) mice were stimulated with killed PLNA (107 CFU/ml) with or without the addition of rBPI21 (P = 0.016 by the Mann-Whitney U test for comparison of TNF-α secretion in the absence and in the presence of rBPI21). Data shown represent the compilation of five independent experiments. (C) HEK-TLR2 cells were stimulated with increasing concentrations of ethanol-killed pneumococci (corresponding to 106, 107, and 108 CFU/ml or E6, E7, and E8, respectively) with or without prior incubation of bacteria and rBPI21 (10 μg/ml). After 18 h of incubation, cell supernatants were collected and IL-8 concentration was measured by ELISA. Data shown are representative of three experiments.
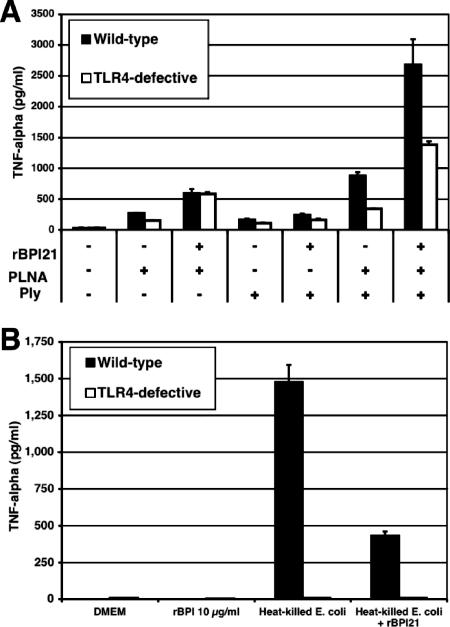
To further evaluate whether any TLR4 dependence could be observed, macrophages from wild-type and TLR4-defective mice were stimulated with purified native pneumolysin, ethanol-killed pneumolysin-deficient pneumococci, or both. As we have shown previously (21), the combination of killed pneumococci and pneumolysin elicited TNF-α secretion in a TLR4-dependent manner. As shown in Fig. 3A, the addition of rBPI21 to this stimulus caused an enhancement of TNF-α secretion in both wild-type and TLR4-defective macrophages (increase of 3.7- and 4.2-fold, respectively); as a control, we also show that rBPI21 reduces the inflammatory response to heat-killed E. coli, as expected (Fig. 3B). Taken together, these results suggest that rBPI21 is able to enhance TNF-α release induced by pneumococci, independently of TLR4, and in contrast to its effects on E. coli-induced inflammatory response.
FIG. 3.
Effect of rBPI21 on the inflammatory response to killed pneumococci and E. coli by peritoneal macrophages from wild-type and TLR4-defective mice. Peritoneal macrophages from wild-type (C3H/HeOuJ) and TLR4-defective (C3H/HeJ) mice were stimulated with ethanol-killed pneumococci (PLNA, pneumolysin-deficient D39 strain, 107 CFU/ml), purified pneumolysin (Ply), and 10 μg/ml rBPI21, singly and in combination (A), or heat-killed E coli (corresponding to a concentration of 104 CFU/ml) with or without preincubation of bacterial with rBPI21 (10 μg/ml) (B). Results shown are representative of three experiments.
rBPI21 potentiates the proapoptotic response to pneumolysin-producing pneumococci in a TNF-α-dependent fashion.
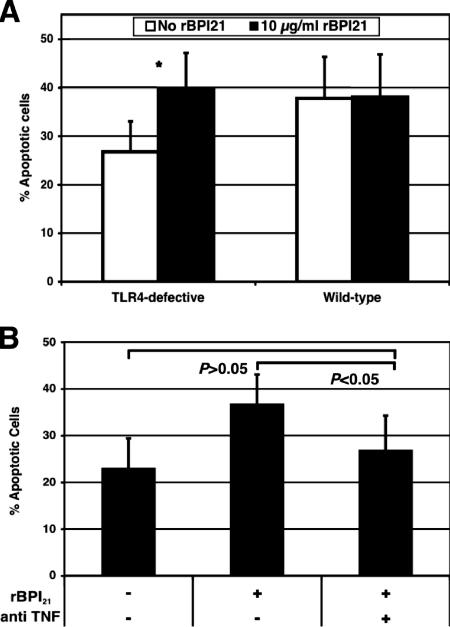
The proapoptotic effects of TNF-α have been well described elsewhere (reviewed in reference 42). We thus examined whether rBPI21 enhanced the apoptotic response to pneumolysin and, if so, whether this effect was indeed dependent on TNF-α. To study this, we evaluated the apoptotic response of TLR4-defective macrophages, known to have a reduced apoptotic response to pneumolysin (39). Peritoneal macrophages from TLR4-defective (C3H/HeJ) mice were stimulated with ethanol-killed preparations of wild-type and pneumolysin-deficient pneumococci, with or without prior incubation of pneumococci with rBPI21; apoptotic cells were enumerated by TUNEL staining and flow cytometry. As shown in Fig. 4A, stimulation of TLR4-defective macrophages with rBPI21 and pneumolysin-positive pneumococci resulted in a significant increase in apoptosis compared to cells stimulated with pneumococci alone (P = 0.04 by Wilcoxon paired-rank test). In wild-type macrophages, rBPI21 did not enhance the apoptotic response to pneumolysin-positive pneumococci. Pneumolysin-deficient pneumococci, as expected, did not induce apoptosis, and preincubation with rBPI21 had no demonstrable effect (data not shown).
FIG. 4.
Effect of rBPI21 on pneumolysin-mediated apoptosis. (A) Macrophages derived from TLR4-defective (C3H/HeJ) and wild-type (C3H/HeOuJ) mice were stimulated with 108 cells/ml of ethanol-killed Ply+ pneumococci in the presence or absence of 10 μg/ml rBPI21. Apoptotic cells were enumerated by TUNEL staining and flow cytometry. Shown is a comparison of percentages of apoptotic cells in the absence and in the presence of BPI (P = 0.04 by Wilcoxon paired-rank test). (B) Stimulations were performed as for panel A in the presence or absence of an anti-TNF-α antibody in macrophages from C3H/HeJ (TLR4-defective) mice. Antibody to TNF-α significantly neutralized the proapoptotic effect of rBPI21; there is no significant difference in apoptosis when rBPI21 is added in the presence of antibodies to TNF-α (P > 0.05 by Kruskal-Wallis test), whereas rBPI21 alone enhances the apoptotic response to pneumococci.
We tested whether this enhancement of apoptosis by rBPI21 was dependent on the increased secretion of TNF-α by these macrophages. Similarly to the experiments described above, TLR4-defective macrophages were stimulated with ethanol-killed pneumolysin-expressing pneumococci and rBPI21 in the absence or presence of neutralizing anti-TNF-α antibodies. Addition of this specific antibody abrogated the rBPI21-mediated enhancement of apoptosis in TLR4-defective cells: no difference in percent apoptotic cells is observed when rBPI21 is added in the presence of antibodies to TNF-α (P > 0.05 by Kruskal-Wallis test) (Fig. 4B); coincubation of pneumolysin-producing pneumococci with anti-TNF-α antibody alone or with an irrelevant isotype-matched antibody had no effect (data not shown). These data indicate that the enhancement of the apoptotic response to pneumolysin by rBPI21 is indeed mediated by TNF-α.
rBPI21 enhances the association of macrophages with live pneumococci.
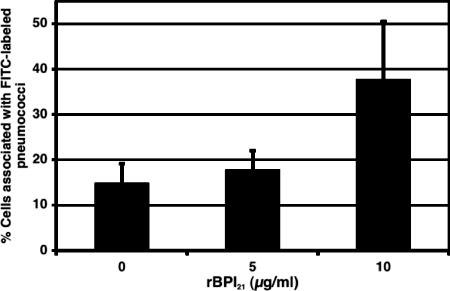
Next we evaluated whether rBPI21 may augment the association with macrophages. To this end, FITC-labeled pneumococci were incubated with rBPI21 at different concentrations and then added to murine macrophages; the association of bacteria with macrophages was then evaluated by flow cytometry. As shown in Fig. 5, prior incubation of bacteria with rBPI21 enhanced the association of live pneumococci with macrophages. Concentrations of rBPI21 between 10 and 20 μg/ml consistently showed enhancement of macrophage association with pneumococci; lower concentrations of rBPI21 had no appreciable effect.
FIG. 5.
Effect of rBPI21 on the association of live pneumococcus with murine macrophages. RAW macrophages were incubated with FITC-labeled pneumococci (strain 0603; see Materials and Methods for details) that had been previously coincubated with different concentrations of rBPI21. Bacterial association with macrophages was subsequently evaluated by flow cytometry. Results shown represent the compilation of three independent experiments.
rBPI21 protects TLR4-defective mice from lethal pneumococcal nasopharyngeal challenge.
Recently, we showed that pneumococcal colonization induces apoptosis in the nasopharyngeal epithelium of mice and enhances host survival: inhibition of nasopharyngeal apoptosis made wild-type mice more prone to invasive disease (39). The in vitro results described here suggested that intranasal administration of rBPI21 might augment apoptosis caused by pneumolysin-producing pneumococci in the nasopharyngeal epithelium of TLR4-defective mice and might thereby confer a survival advantage. To test this hypothesis, TLR4-defective (C3H/HeJ) mice (10 per group) were intranasally inoculated with 108 CFU of pneumococcal strain WU2 (Ply+, type 3). rBPI21 or diluent was administered intranasally in 10-μl (27-μg) doses at time zero and 4, 8, and 16 h postinoculation for a total of four doses. Twenty hours after bacterial inoculation, retrograde tracheal washes were collected from euthanized animals and cells recovered in the tracheal and nasopharyngeal secretions were analyzed for apoptosis by TUNEL staining and flow cytometry (22, 39). Mice that received rBPI21 showed significantly increased nasopharyngeal cell apoptosis compared to mice that received diluent (mean percent of apoptotic cells recovered from 10 mice per group: 43.1% ± 3.4% versus 34.1% ± 2.1%, respectively; P = 0.04). No difference in apoptosis was observed in control mice that received rBPI21 or diluent alone, without pneumococci (data not shown). Thus, results of the in vivo apoptosis experiments are consistent with our in vitro observations that rBPI21 augments the apoptotic response to pneumolysin.
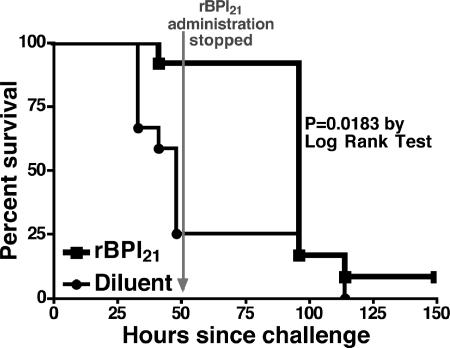
To examine whether rBPI21 would have an impact on pneumococcal disease in vivo, we employed a mouse model in which intranasal exposure to pneumococci leads to sepsis and death (39): TLR4-defective (C3H/HeJ) mice were intranasally inoculated with A66.1 (Ply+, type 3). Inocula consisting of 108 CFU of strain A66.1 were intranasally administered concurrently with 20 μg of rBPI21 or diluent control at time zero and again 4 h later. Subsequently, the same amount of rBPI21 or diluent was administered every 6 to 8 h for 2 days postinoculation. Overall, as shown in Fig. 6, mice that received rBPI21 survived significantly longer than mice that received diluent alone (P = 0.018 by log rank test). Thus, rBPI21 prolonged survival in TLR4-defective (C3H/HeJ) mice that are normally hypersusceptible to intranasal pneumococcal challenge with type 3 pneumococcus.
FIG. 6.
Effect of rBPI21 in an invasive disease model in TLR4-defective mice challenged with a type 3 pneumolysin-positive pneumococcal strain. TLR4-defective (C3H/HeJ) mice (n = 12 per group) received either rBPI21 or the diluent intranasally every 6 to 8 h for a total of seven doses prior to and following intranasal inoculation with strain A66 (Ply+, type 3). Survival was monitored twice daily. Survival time was significantly longer for mice that received BPI than for those that received diluent alone (P = 0.018 by log rank test).
DISCUSSION
A number of recent studies have described the complex interaction between pneumococci and the innate immune response. Pneumococci elicit a range of antimicrobial innate responses (31), involving classical (7) and alternative (28, 36) pathways of complement, C-reactive protein (13, 25), mannose-binding lectin (35), platelet-activating factor receptor (10), and, most recently, LBP (43). Evidence gathered by various groups, including our own, supports the involvement of the TLR pathway as well (6, 11, 14-16, 21, 30, 37, 39, 46). Pneumococcal peptidoglycan and lipoteichoic acid (14, 38, 46) have been shown to be recognized not only by TLR2 but also by cytosolic Nod proteins (27). TLR4, previously thought to recognize only gram-negative bacteria via LPS, also recognizes pneumolysin (21, 39) and other related bacterial cytolysins (29).
The data presented herein further highlight the complexity of the innate immune response to pneumococcus. Here we show that rBPI21, previously thought of as an antimicrobial polypeptide with specificity for gram-negative bacteria and LPS, enhances the innate immune responses to pneumococcus and improves survival of mice challenged with virulent pneumococci. To our knowledge, this is the first report of binding of rBPI21 to a gram-positive bacterial pathogen and of an interaction of an antimicrobial peptide/protein with pneumolysin. rBPI21 interacts with killed or live pneumococci, as well as the CDC pneumolysin. We show here that rBPI21 enhances the inflammatory response to pneumococcus. Furthermore, likely via TNF-α, rBPI21 also augments the apoptotic responses to pneumolysin. rBPI21 also enhanced the association of bacteria with macrophages. Finally, rBPI21 confers a modest but significant survival advantage on immunodeficient mice (TLR4 defective) challenged with pneumolysin-producing pneumococci. Whether rBPI21 would also provide protection to wild-type mice is not presently known.
Until recently, mice were believed to be naturally deficient in BPI; however, more recent reports have shown that mice express BPI-like proteins in various mucosal tissues, including the nasopharynx (19), and that the expression of these proteins can be induced by LPS (12). From our studies, it is apparent that the exogenous addition of rBPI21 to the nasopharyngeal mucosa of mice confers protection against pneumococcal disease. It is noteworthy that neutrophils from premature human infants are deficient in BPI (20, 26). It is thus tempting to speculate that the immaturity of the BPI response in newborns could contribute to their increased susceptibility to pneumococcal infection, among many other potential factors.
It is striking that a molecule that has such a high affinity for LPS could also have a demonstrable role in the pathogenesis of pneumococcal infection. Specifically, the potentiation of pneumolysin-induced apoptosis suggests the possibility of a more complex role of BPI than has previously been considered. Other gram-positive organisms elaborate cholesterol-dependent cytolysins, homologous to pneumolysin—perfringolysin, streptolysin, and listeriolysin (40). It would be of interest to know whether BPI can bind to and perhaps also enhance the effect of these CDC and potentially affect progression of the diseases caused by these bacteria.
In summary, we show that rBPI21, a recombinant antimicrobial protein with specific anti-gram-negative bacterium microbicidal activity and potent LPS-neutralizing effects, confers a survival advantage against invasive pneumococcal disease. This survival advantage appears to be mediated by the enhancement of innate immune responses to pneumococcus and pneumolysin, resulting in increased nasopharyngeal cell apoptosis, which protects against invasive disease.
Acknowledgments
We thank Michael Wessels, Marc Lipsitch, Douglas Golenbock, James Paton, and Bruce Horwitz for helpful suggestions and discussions. Debby Bogaert and Gregory Priebe are thanked for providing FITC-labeled pneumococci and smooth and rough Pseudomonas aeruginosa isolates, respectively.
This work was funded in part by NIH grants 1 K08 AI51526-01 and 1R01AI067737-01 and the Pamela and Jack Egan Fund (all to R.M.) and NIH training grant AI07061-26 (to A.S.). O.L. was supported by NIH K08 AI 50583-1 and an unrestricted gift from XOMA (U.S.) LLC.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Beamer, L. J. 2003. Structure of human BPI (bactericidal/permeability-increasing protein) and implications for related proteins. Biochem. Soc. Trans. 31:791-794. [DOI] [PubMed] [Google Scholar]
- 4.Beamer, L. J., S. F. Carroll, and D. Eisenberg. 1997. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 276:1861-1864. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom, C. T., M. Lipsitch, and B. R. Levin. 2000. Natural selection, infectious transfer and the existence conditions for bacterial plasmids. Genetics 155:1505-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branger, J., S. Knapp, S. Weijer, J. C. Leemans, J. M. Pater, P. Speelman, S. Florquin, and T. van der Poll. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canny, G., E. Cario, A. Lennartsson, U. Gullberg, C. Brennan, O. Levy, and S. P. Colgan. 2006. Functional and biochemical characterization of epithelial bactericidal/permeability-increasing protein. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G557-G567. [DOI] [PubMed] [Google Scholar]
- 9.Capodici, C., S. Chen, Z. Sidorczyk, P. Elsbach, and J. Weiss. 1994. Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect. Immun. 62:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. I. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 11.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 12.Eckert, M., I. Wittmann, M. Rollinghoff, A. Gessner, and M. Schnare. 2006. Endotoxin-induced expression of murine bactericidal permeability/increasing protein is mediated exclusively by toll/IL-1 receptor domain-containing adaptor inducing IFN-beta-dependent pathways. J. Immunol. 176:522-528. [DOI] [PubMed] [Google Scholar]
- 13.Gould, J. M., and J. N. Weiser. 2001. Expression of C-reactive protein in the human respiratory tract. Infect. Immun. 69:1747-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71:5541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp, S., C. W. Wieland, C. van 't Veer, O. Takeuchi, S. Akira, S. Florquin, and T. van der Poll. 2004. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 172:3132-3138. [DOI] [PubMed] [Google Scholar]
- 16.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 17.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 18.Latz, E., A. Visintin, E. Lien, K. A. Fitzgerald, B. G. Monks, E. A. Kurt-Jones, D. T. Golenbock, and T. Espevik. 2002. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277:47834-47843. [DOI] [PubMed] [Google Scholar]
- 19.Leclair, E. E. 2003. Four BPI (bactericidal/permeability-increasing protein)-like genes expressed in the mouse nasal, oral, airway and digestive epithelia. Biochem. Soc. Trans. 31:801-805. [DOI] [PubMed] [Google Scholar]
- 20.Levy, O., S. Martin, E. Eichenwald, T. Ganz, E. Valore, S. F. Carroll, K. Lee, D. Goldmann, and G. M. Thorne. 1999. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 104:1327-1333. [DOI] [PubMed] [Google Scholar]
- 21.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. E. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by encapsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannion, B. A., J. Weiss, and P. Elsbach. 1990. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. J. Clin. Investig. 85:853-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra, M. N., C. G. Wilde, J. E. Griffith, J. L. Snable, and R. W. Scott. 1990. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J. Immunol. 144:662-666. [PubMed] [Google Scholar]
- 25.Mold, C., B. Rodic-Polic, and T. W. Du Clos. 2002. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J. Immunol. 168:6375-6381. [DOI] [PubMed] [Google Scholar]
- 26.Nupponen, I., R. Turunen, T. Nevalainen, H. Peuravuori, M. Pohjavuori, H. Repo, and S. Andersson. 2002. Extracellular release of bactericidal/permeability-increasing protein in newborn infants. Pediatr. Res. 51:670-674. [DOI] [PubMed] [Google Scholar]
- 27.Opitz, B., A. Puschel, B. Schmeck, A. C. Hocke, S. Rosseau, S. Hammerschmidt, R. R. Schumann, N. Suttorp, and S. Hippenstiel. 2004. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J. Biol. Chem. 279:36426- 36432. [DOI] [PubMed] [Google Scholar]
- 28.Pangburn, M. K. 1989. Analysis of recognition in the alternative pathway of complement. Effect of polysaccharide size. J. Immunol. 142:2766-2770. [PubMed] [Google Scholar]
- 29.Park, J. M., V. H. Ng, S. Maeda, R. F. Rest, and M. Karin. 2004. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J. Exp. Med. 200:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picard, C., A. Puel, M. Bonnet, C. L. Ku, J. Bustamante, K. Yang, C. Soudais, S. Dupuis, J. Feinberg, C. Fieschi, C. Elbim, R. Hitchcock, D. Lammas, G. Davies, A. Al-Ghonaium, H. Al-Rayes, S. Al-Jumaah, S. Al-Hajjar, I. Z. Al-Mohsen, H. H. Frayha, R. Rucker, T. R. Hawn, A. Aderem, H. Tufenkeji, S. Haraguchi, N. K. Day, R. A. Good, M. A. Gougerot-Pocidalo, A. Ozinsky, and J. L. Casanova. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299:2076-2079. [DOI] [PubMed] [Google Scholar]
- 31.Picard, C., A. Puel, J. Bustamante, C. L. Ku, and J. L. Casanova. 2003. Primary immunodeficiencies associated with pneumococcal disease. Curr. Opin. Allergy Clin. Immunol. 3:451-459. [DOI] [PubMed] [Google Scholar]
- 32.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4. Gene Sci. 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 33.Priebe, G. P., M. M. Brinig, K. Hatano, M. Grout, F. T. Coleman, G. B. Pier, and J. B. Goldberg. 2002. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine. Infect. Immun. 70:1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priebe, G. P., C. R. Dean, T. Zaidi, G. J. Meluleni, F. T. Coleman, Y. S. Coutinho, M. J. Noto, T. A. Urban, G. B. Pier, and J. B. Goldberg. 2004. The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect. Immun. 72:4224-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy, S., K. Knox, S. Segal, D. Griffiths, C. E. Moore, K. I. Welsh, A. Smarason, N. P. Day, W. L. McPheat, D. W. Crook, and A. V. Hill. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 359:1569-1573. [DOI] [PubMed] [Google Scholar]
- 36.Sahu, A., T. R. Kozel, and M. K. Pangburn. 1994. Specificity of the thioester-containing reactive site of human C3 and its significance to complement activation. Biochem. J. 302:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 38.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 73:6479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tweten, R. K. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visintin, A., E. Latz, B. G. Monks, T. Espevik, and D. T. Golenbock. 2003. Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J. Biol. Chem. 278:48313-48320. [DOI] [PubMed] [Google Scholar]
- 42.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 43.Weber, J. R., D. Freyer, C. Alexander, N. W. Schroder, A. Reiss, C. Kuster, D. Pfeil, E. I. Tuomanen, and R. R. Schumann. 2003. Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity 19:269-279. [DOI] [PubMed] [Google Scholar]
- 44.Weiss, J., P. Elsbach, I. Olsson, and H. Odeberg. 1978. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J. Biol. Chem. 253:2664-2672. [PubMed] [Google Scholar]
- 45.Weiss, J., M. Hutzler, and L. Kao. 1986. Environmental modulation of lipopolysaccharide chain length alters the sensitivity of Escherichia coli to the neutrophil bactericidal/permeability-increasing protein. Infect. Immun. 51:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 47.Zweigner, J., R. R. Schumann, and J. R. Weber. 2006. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 8:946-952. [DOI] [PubMed] [Google Scholar]