Abstract
Early interactions between pathogens and host cells are often decisive for the subsequent course of infection. Here we investigated early events during infection by Listeria monocytogenes, a ubiquitously occurring facultative intracellular microorganism that exhibits severe pathogenicity, mainly in immunocompromised individuals. We show that the inflammatory chemokine CCL2 is highly up-regulated early after Listeria infection in spleens of BALB/c mice. ERTR-9+ macrophages of the marginal zone were identified as the only infected cells and exclusive producers of CCL2 at the early time point. Consequently, clusters of different cell types were formed around infected ERTR-9+ cells. Metallophilic MOMA-1+ marginal zone macrophages were, however, excluded from the clusters and migrated into the B-cell follicles. Depletion of CCL2 during infection resulted in a different composition of cell clusters in the spleen and increased the mortality rate of treated mice. Interestingly, ERTR-9+ macrophages no longer were part of clusters in such mice but remained at their original location in the marginal zone.
Listeria monocytogenes, a gram-positive facultative intracellular bacterium, is associated with serious infections in immunocompromised individuals, newborn children, the elderly, and pregnant women (10, 11, 25, 27). The murine experimental equivalent of listeriosis represents one of the best-understood bacterial infection models to date (30). In addition, various extensively studied tissue culture systems have revealed the Listeria infection cycle and the key components essential for it (for a review, see reference 36).
During this work it became clear that Listeria, when interacting with a particular host cell, induces strong reactions within this cell (23, 29). This is, on the one hand, due to host cell receptors, like Toll-like receptors, scavenger receptors, or uncharacterized intracytosolic receptors that interact with bacterial components (12, 23). On the other hand, virulence factors have been shown to also induce specific signal cascades upon encountering the host cell surface. The spectrum of such events ranges from rearrangement of the cytoskeleton induced by internalins (3, 6, 15) to induction of apoptosis of dendritic cells (DCs) and T cells by listeriolysin (17, 18). In addition, it is well established that Listeria provokes the secretion of several cytokines in macrophages (10, 21, 34) and the up-regulation of cell adhesion molecules on endothelial cells (9).
Despite these extensive studies, little is known about the immediate in vivo effects of Listeria on cells that are encountered first during infection, i.e., the macrophages that are responsible for the removal of the bacteria from circulation. Induction of proliferation and migration within the spleen have been described (5, 26). Similarly, the early production of proinflammatory cytokines has also been ascribed to them (7, 35).
In vitro it was observed that CCL2 is among the inflammatory cytokines and chemokines instantaneously produced by primary macrophages and macrophage lines upon Listeria infection (references 13, 29, and 30 and unpublished data). In agreement, a protective role of CCL2 in murine listeriosis has been observed recently (31). Therefore, we wanted to investigate whether macrophages are responsible for the production of this mediator in the early phase of listeriosis in vivo. Results showed that expression of CCL2 at 4 h postinfection (p.i.) was restricted exclusively to ERTR-9+ macrophages of the marginal zone (MZM), which also was the population exclusively infected at this time point. Interestingly, experiments with mice in which CCL2 was depleted by administration of specific antibodies demonstrated that CCL2 is specifically involved in the activation of the ERTR-9+ macrophage population to migrate into the infectious foci.
MATERIALS AND METHODS
Bacterial strains and culture.
Wild-type L. monocytogenes strain EGD-e serotype 1/2a was used in all experiments. Bacteria were grown in brain heart infusion (BHI) broth at 37°C overnight, and then suspensions were diluted 1/5 in fresh medium and incubated for 2 hours at 37°C, until they reached mid-log phase. Depending on the experiment, bacteria were washed and resuspended in phosphate-buffered saline (PBS).
Mice.
Female BALB/c mice were purchased from Harlan (Borchem, Germany) and used at the age of 10 to 12 weeks.
Antibodies.
Antibodies (Abs) used in this work included rat-anti-mouse ERTR-9-biotin (BMA Biomedicals), rat-anti-mouse MOMA-1-biotin (BMA Biomedicals), MOMA-1-fluorescein isothiocyanate (Pharmingen), rabbit-anti-L. monocytogenes (Dunn Labortechnik), goat-anti-rabbit 7-amino-4-methylcoumarin-3-acetic acid (AMCA; Sigma), goat anti-rabbit Alexa Fluor 488 (Molecular Probes), rat anti-mouse Gr-1-phycoerythrin-Cy7 (eBioscience), rat anti-B220 CyChrome (Pharmingen), hamster anti-murine CD3 (Pharmingen), goat anti-hamster Cy5 and rat anti-mouse DX5 (Pharmingen), CD11b biotinylated (anti-Mac-1; clone M1/70.15.11.5), CD11b-phycoerythrin (eBioscience), CD11c biotinylated (N418; clone CHB 229), CD11c-allophycocyanin (eBioscience), and rabbit anti-mouse laminin 1γ chain (ImmunoDiagnostics). Goat anti-mouse CCL2 affinity-purified polyclonal Abs (R&D Systems) were used to deplete mice of CCL2.
PCR.
Primers for real-time reverse transcription-PCR (RT-PCR) were selected by using DNASTAR Primer Select software (Lasergene) and purchased from MWG (CCL2 forward, GCCCCACTCACCTGCTGCTA; reverse, TTTACGGGTCAACTTCACATTCAA).
Real-time RT-PCR to quantitate chemokine transcripts.
After infection of mice, subpopulations of splenic cells were isolated by cell sorting. Total RNA from cells was prepared with the RNeasy mini kit (QIAGEN) according to the manufacturer's protocol. DNA contamination in the total RNA was eliminated by incubation with DNase I (Amersham Pharmacia Biotech, Freiburg, Germany). cDNA was prepared using Superscript II RNase H− (Invitrogen) according to the manufacturer's instructions and used in either RT-PCR or real-time RT-PCR. The latter reaction was conducted using a SYBR Green PCR master mix kit (Applied Biosystems) with the GeneAmp 5700 sequence detection system (Applied Biosystems). Results were normalized using the housekeeping gene RPS9.
In vivo infection.
BALB/c female mice were infected intravenously (i.v.) with 100 times the 50% lethal dose (5 × 105) of Listeria organisms. After 0, 2, 4, 6, or 24 h, spleens were removed, parts of the organs were used for RNA extraction, aliquots were frozen in liquid nitrogen for histology, and, from part of the spleen, cell suspensions were prepared. The rest of the organ was homogenized in PBS supplemented with 0.2% NP-40 and plated.
Isolation of cells from the spleens of infected mice.
Spleen cell suspensions from infected animals were prepared by washing out the spleens with Iscove's modified Dulbecco's medium supplemented with antibiotics (penicillin, 100 μg/ml; streptavidin, 100 μg/ml; gentamicin, 20 μg/ml), and erythrocytes were lysed for 2 min in ACK buffer and then washed three times in PBS containing EDTA. Cell suspensions were then used for fluorescence-activated cell sorter (FACS) analysis or cell sorting.
Polyclonal Ab treatment.
To neutralize mouse CCL2, affinity-purified polyclonal goat antibodies against murine CCL2 (R&D Systems) were used. Ten micrograms was injected i.v. in 100 μl of sterile PBS 1 h before bacterial infection. Animals were infected i.v. with 5 × 105 CFU of Listeria and then, 4, 6, and 24 h p.i., mice were sacrificed and spleens were removed and prepared for histology and flow cytometry.
Quantification of L. monocytogenes load in organs of infected mice.
Mice were sacrificed at the indicated time points, and fragments of spleens were homogenized in 1 ml of 0.2% NP-40 in PBS. Fifty microliters of these homogenates was plated on BHI agar plates in duplicate, and CFU were estimated after overnight incubation at 37°C.
Quantification of L. monocytogenes load in sorted spleen cell populations.
Cells from infected mice were sorted based on surface markers as described for FACS staining, incubated with Triton X-PBS, and plated on BHI agar plates in duplicate. CFU were estimated after overnight incubation at 37°C.
Flow cytometry and cell sorting.
Single-cell suspensions were prepared in FACS buffer at a density 5 × 105 cells per well. Cells were treated with anti-mouse FcR Abs, followed by staining with appropriate Abs. Flow cytometry was performed using a FACSCalibur or FACSCanto apparatus (Becton Dickinson). Data were analyzed with CellQuestPro software or FACSDiva software (Becton Dickinson).
Described populations of spleen cells were sorted after Ab staining using MOFLO (Cytomation) or FACSVantage DiVa and subsequently reanalyzed to confirm sort quality, which reached 80 to 90%, depending on cell population.
Immunohistochemistry.
Spleens were embedded in Tissue-Tek OCT compound (Sakura), snap-frozen in liquid nitrogen, and stored at −20°C. Cryostat sections of 7 μm were prepared, air dried for 2 h at room temperature, and fixed in acetone (2 min at −20°C). After thawing, slides were blocked with 0.05% bovine serum albumin in PBS and stained with appropriate antibodies. After staining and washing, slides were dried, mounted with Neo-Mount (Merck), and analyzed using a laser scanning confocal microscope.
Confocal microscopy.
Four-color confocal microscopy (AMCA, fluorescein isothiocyanate/Alexa 488, Cy3/Alexa568, and allophycocyanin/Cy5/CyChrome) of cryosections was performed using an LSM 510 META microscope (Zeiss). To avoid overlapping emissions, fluorescent dyes were selectively excited in two series and the fluorescence of single channels was measured by photon counting. Images were processed with Confocal Assistant 4.02, ImagePro 4.5 (Media Cybernetics), and Adobe Photoshop 7.
RESULTS
Clustering of splenic macrophages after infection with L. monocytogenes.
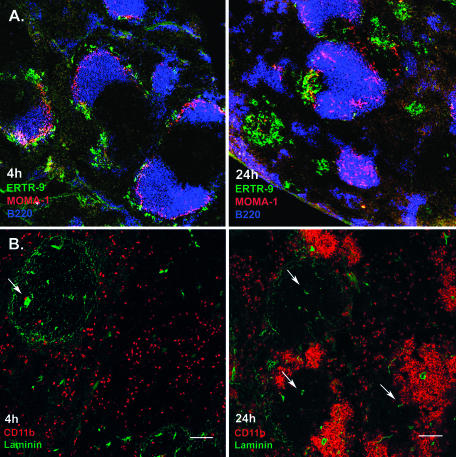
The established regulation of the proinflammatory cytokines and chemokines after Listeria infection should result in a severe repositioning of cells in the spleen due to migration and influx of leukocytes and lymphocytes. Indeed, when immune histology was employed, a severe restructuring of the spleen was observed. At 4 h after intravenous injection of 5 × 105 bacteria, the architecture of the spleen, with white and red pulp separated by the MZ, was still very little affected (Fig. 1A), but these structures were completely altered at the later stage of infection, 24 h p.i. (Fig. 1A). At this time, B-cell follicles in the white pulp remained intact and MOMA-1+ metallophilic marginal zone macrophages (MMZM), usually located at the inner rim of the marginal zone, had migrated into these follicles. ERTR-9+ macrophages that usually are located at the outer rim of the MZ had formed clusters around infection foci in close vicinity to the MZ. To such clusters most of the CD11b+ cells were attracted, consisting of neutrophils and macrophage populations (Fig. 1B). Additionally, many T cells were found in such clusters (data not shown).
FIG. 1.
Migration of macrophage subsets in the spleen after infection with L. monocytogenes. Cryosections from the spleens of infected mice at the indicated time points were prepared, stained with appropriate fluorescent Abs, and analyzed using confocal microscopy. A. Clustering of ERTR-9+ marginal zone macrophages (green) around infection foci and migration of MOMA-1+ metallophilic marginal zone macrophages (red) into B-cell follicles (blue). B. Clustering of CD11b+ cells during infection with L. monocytogenes. CD11b+ cells are stained red, and laminin is green. At 4 h p.i., CD11b+ cells are scattered through the red pulp. At 24 h p.i., these cells form clusters around infection foci in the MZ area. No clusters were observed in the white pulp or surrounding the central arteriole. Arrows indicate central arterioles. Bar, 100 μm. Pictures represent data from at least three independent experiments with at least three mice. More than 20 fields of view were analyzed in each experiment.
Staining of laminin, which detects the endothelial cells of inner membranes and blood vessels, revealed that generally the splenic marginal sinus was in the middle of the cluster (Fig. 1B). Thus, the clusters surrounding infectious foci are formed in the area of the marginal zone.
Importantly, identical clusters were observed when mice were infected with 2 × 103 bacteria. However, their formation required 3 days to be completed (data not shown).
Exclusive infection of ERTR-9+ macrophages by L. monocytogenes.
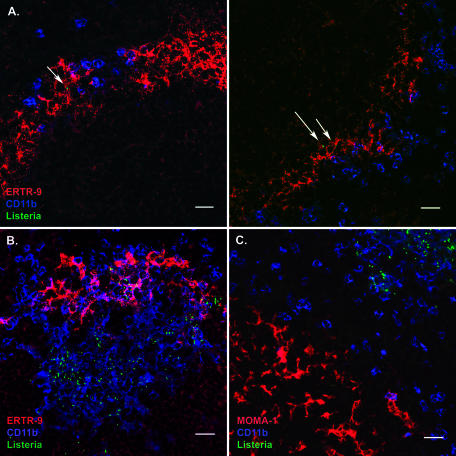
The marginal zone of the spleen is the area where the closed blood circulation opens into the splenic sinus. Therefore, both ERTR-9+ as well as MOMA-1+ macrophages should be exposed to blood-borne pathogens after i.v. infection. However, in the case of Listeria, apparently only one type of macrophage is infected at 4 h p.i. Costaining of splenic cryosections for macrophages and Listeria revealed that bacteria were only found associated with ERTR-9+ macrophages (Fig. 2A), not CD11b+ cells. Even when other cell types appeared to colocalize with Listeria, larger magnifications revealed that the bacteria were in long cellular extensions of ERTR-9+ cells that might be in contact with many other cells in the spleen. This was confirmed by cell sorting and subsequent plating. At 4 h p.i., Listeria organisms were only recovered in significant numbers from the ERTR-9+ macrophages (Table 1).
FIG. 2.
ERTR-9+ macrophages are the sole cell population involved in uptake of L. monocytogenes from the blood. Splenic cryosections of infected mice were prepared as described in the legend for Fig. 1. A. At 4 h p.i., ERTR-9+ macrophages are exclusively associated with bacteria (green) at the early infection stage. Listeria cells are green, CD11b is blue, and ERTR-9 is red. Bar, 20 μm. B. At 24 h p.i., ERTR-9+ macrophages and CD11b macrophages and neutrophils form clusters around infection foci. Listeria cells are green, CD11b is blue, and ERTR-9 is red. Bar, 20 μm. C. MOMA-1+ macrophages do not participate in cluster formation and migrate into the B-cell area of the white pulp. MOMA-1 is red, CD11b is blue, and Listeria cells are green. Bar, 20 μm. Pictures represent data from at least three independent experiments with at least three mice. More than 20 fields of view were analyzed in each experiment.
TABLE 1.
Number of bacteria in isolated splenocyte populations after Listeria infection in BALB/c mice
| Cellsb | Marker(s) | CFUa at:
|
|
|---|---|---|---|
| 4 h | 24 h | ||
| MZM | ERTR-9+ | 1,201 (13) | 396 (4) |
| mDCs | ERTR-9− CD11c+ CD11bint | 0 | 72 (1) |
| TipDCs | ERTR-9− CD11cint CD11b+ | 0 | 2,093 (93) |
| MMZM | MOMA-1+ | 0 | 269 (3) |
| Granulocytes | MOMA-1− CD11b+ CD11c− Gr-1+ | 0 | 70,329 (85) |
| Macrophages | MOMA-1− CD11b+ CD11c− Gr-1− | 4 (0.04) | 28,426 (94) |
Per population in one spleen. Values in parentheses are CFU per 104 sorted cells. The cells from five sorted spleens were counted, and approximate value for one spleen estimated.
mDCs, myeloid dendritic cells; TipDCs, tumor necrosis factor/inducible nitric oxide synthase-producing DCs.
By 24 h p.i., all bacteria were located within the clusters and were partially associated with ERTR-9+ cells. However, plating of sorted splenic cell populations revealed that now also other phagocytic cells were infected with Listeria (Table 1).
Interestingly, MOMA-1+ macrophages, which appear at the beginning of the infection, were also located in the marginal zone and were poorly infected, although they were able to phagocytose Listeria efficiently ex vivo (data not shown). Nevertheless, despite the lack of direct contact with the bacteria at the early phase of infection, MOMA-1+ macrophages became activated and migrated away from the large clusters into the B-cell areas of the white pulp (Fig. 1A and 2C).
ERTR-9+ macrophages are responsible for early production of CCL2.
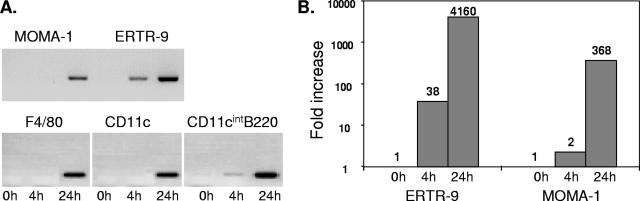
CCL2 was found to be the first chemokine up-regulated in adherent splenocytes isolated from Listeria-infected mice under our conditions (data not shown). Since CCL2 is a chemokine specific for macrophage attraction and the first visible effect of Listeria infection in mice is the migration of macrophages, one might hypothesize that CCL2 is involved in the cluster formation. Consequently, one would expect that the cells infected first, in this case ERTR-9+ macrophages, are the main producers of CCL2 at the early stage of infection. To test this hypothesis, we sorted macrophages and dendritic cells from mice infected with 5 × 105 bacteria at 4 and 24 h p.i. on the basis of the cell markers ERTR-9+, MOMA-1+, F4/80+ (macrophages of the red pulp), CD11c+ (total DCs), and CD11cint/B220+ (plasmacytoid DCs). Analysis of these sorted cells by RT-PCR revealed that ERTR-9+ macrophages indeed were the major producers of CCL2 at 4 h p.i. (Fig. 3A). Only plasmacytoid DCs showed a slight expression of this chemokine at that time point. At later time points, consistent with the presence of bacteria in several phagocytic cell types, all the cell populations tested were able to produce CCL2. Real-time RT-PCR confirmed these results with isolated ERTR-9+ and MOMA-1+ macrophages from infected mice. Only ERTR-9+ cells exhibited a significant up-regulation of CCL2 mRNA at 4 h p.i. (Fig. 3B).
FIG. 3.
CCL2 expression in macrophage and DC populations of the spleen after infection by L. monocytogenes. Mice were infected with 5 × 105 CFU of L. monocytogenes. Spleens of mice at 4 and 24 h p.i. or of uninfected controls were removed, macrophage and DC populations were sorted using the indicated markers, and RNA was isolated. RT-PCR or real-time RT-PCR from cDNA was used to study gene expression of CCL2. A. Induction of CCL2 in different macrophage and DC populations as revealed by RT-PCR. B. Quantitation of CCL2 mRNA induction in ERTR-9+ and MOMA-1+ macrophages using real-time RT-PCR. Cells were sorted from five pooled spleens, and all experiments were repeated at least three times.
Importantly, when the same cells were isolated from mice that were infected with 2 × 103 Listeria cells, expression of CCL2 mRNA at early time points could be detected exclusively in ERTR-9+ cells, although the RT-PCR signal was low compared to the signal obtained with cells from mice infected with a high dose (data not shown).
Higher susceptibility of CCL2-depleted mice to infection by L. monocytogenes.
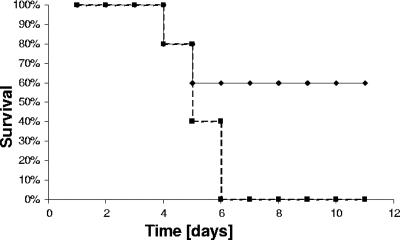
Since the chemokine CCL2, which attracts macrophages, is apparently up-regulated already during the early phase of Listeria infection in the spleen of BALB/c mice, we decided to investigate the importance of CCL2 in the host defense against Listeria. To this end, CCL2 was depleted by injecting specific antibodies 1 h before infection. Subsequently, mice were infected with 5 × 105 bacteria. An antibody dose of 10 μg/mouse was chosen, although already lower amounts had been shown to neutralize completely the in vivo activity of CCL2 (22). When 104 Listeria were injected into CCL2-depleted mice, a higher susceptibility compared to controls was evident, although the numbers of CFU were not dramatically different in either type of animal (Fig. 4 and data not shown). This is consistent with the findings of Serbina et al. (31, 33). An unexpected discrepancy between bacterial CFU in the spleen and mortality of infected mice was similarly found by Depaolo (8) in the case of Salmonella infection. The reason for this phenomenon is not clear so far.
FIG. 4.
Increased mortality of BALB/c mice depleted of CCL2 after L. monocytogenes infection. BALB/c mice (treated previously with anti-CCL2 Abs or control Abs) were infected with 104 CFU of bacteria. The mortality rate of infected animals was examined. Solid line, controls; dotted line, CCL2-depleted animals. The experiment was done twice with at least five animals per group.
Altered splenic cell composition in CCL2-depleted mice infected with L. monocytogenes.
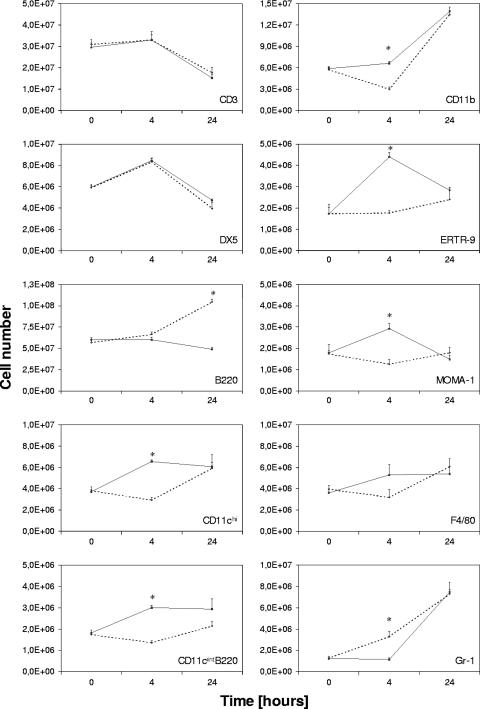
In view of the fact that depletion of the CCL2 chemokine alters the defense against Listeria, it was important to verify the influence of anti-CCL2 treatment on cell composition kinetics in the infected spleen. Therefore, single-cell suspensions were stained for cell surface markers of the major splenic cell populations. Consistent with a role of CCL2 at the early phase of infection, the most significant differences in cell composition were visible at 4 h p.i. (Fig. 5). As expected, mainly macrophages and dendritic cells were affected by CCL2 depletion. Antibody-treated animals showed no increase in the frequency of ERTR-9+ and MOMA-1+ macrophages and CD11cint B220+ plasmacytoid dendritic cells at 4 h p.i. In contrast, in untreated mice, the percentage of MOMA-1+ and ERTR-9+ cells initially increased (Fig. 5). Numbers of T cells, NK cells, and B cells, as well as granulocytes, appeared to be not affected by the depletion. The same frequencies were observed in both types of mice, with the fraction of T and NK cells dramatically decreasing during this period. This is most likely due to the apoptosis induced by Listeria described before (18). CD11b+ and Gr-1+ cells increased significantly in frequency during the observation time, and this increase was unaffected by CCL2 depletion. Thus, CCL2 appears to be involved in the recruitment and possibly the survival of splenic subpopulations of macrophages and to some extent dendritic cells after Listeria infection.
FIG. 5.
Alteration in cellular composition of the spleen in anti-CCL2 Ab-treated mice after L. monocytogenes infection. BALB/c mice treated with anti-CCL2 or goat immunoglobulin G (control mice) were infected with Listeria, and at the indicated time points single-cell suspensions were prepared from spleens of infected animals. Cells were stained using Abs recognizing surface markers characteristic for the major splenic cell populations and analyzed using a FACSCalibur. Control mice (solid line) and anti-CCL2-treated mice (dotted line) are shown. All staining was repeated twice with at least three animals per group, and results are expressed as means ± standard deviations. *, P ≤ 0.01.
Effect of CCL2 depletion on clustering of macrophages.
Based on the differences described above, we decided to compare the architecture of the spleen after infection of control versus CCL2-depleted mice. Interestingly, in CCL2-deficient mice, ERTR-9+ macrophages neither migrated nor formed clusters during Listeria infection (Fig. 6A). Less than 8% of ERTR-9+ cells were associated with clusters (Fig. 6C), and even at 24 h p.i. the original marginal zone was still apparent. Nevertheless, cell clusters were formed independently of ERTR-9+ cells in the marginal zone area and contained, as in untreated mice, CD11b+ macrophages, Gr-1+ neutrophils, and some T cells (data not shown) as well as bacteria. We observed no difference in migration of MOMA-1+ macrophages in both types of mice. These cells migrate to the B-cell follicles in anti-CCL2 Ab-treated mice as well as in untreated mice (Fig. 6B). These results suggest a strong influence of CCL2 chemokine on ERTR-9+ marginal zone macrophage activation and migratory capacities.
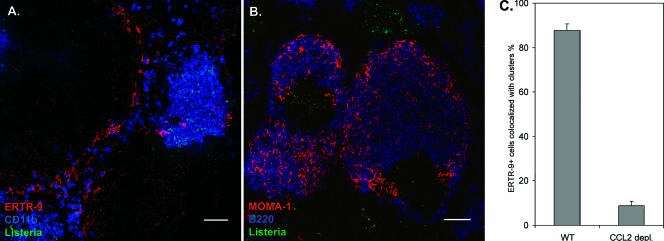
FIG. 6.
Clustering of phagocytic cells by L. monocytogenes is changed after CCL2 depletion. Spleens were isolated from infected animals treated or untreated with anti-CCL2 24 h after infection. Cryosections were prepared and stained with appropriate fluorescent Abs. A. Migration of ERTR-9+ macrophages is no longer observed in CCL2-depleted mice, and bacteria are no longer associated with these macrophages. CD11b cells are stained blue, ERTR-9 is red, and Listeria cells are green. B. Migration of MOMA-1+ cells into the B-cell follicle is unaltered in CCL2-depleted mice compared to control BALB/c mice. MOMA-1 cells are stained red, B220 is blue, and Listeria cells are green. Bars, 50 μm. These experiments were done twice using three mice. More than 20 fields of view were analyzed in each experiment. The control staining of spleens from infected but untreated mice is depicted in Fig. 2, since these two experiments were done in parallel. C. Percentage of ERTR-9+ cells associated with infectious foci at 24 h p.i. in BALB/c mice treated with anti-CCL2 compared to untreated control mice. ERTR-9+ cells were counted in fields of view of immunohistological sections from spleens of at least three treated and untreated mice, and means and standard errors were calculated. More than 10 fields of view were analyzed for each condition.
DISCUSSION
The marginal zone of the spleen is a unique region where the closed blood circulation opens into the splenic sinus. It is a transit area for the cells leaving the bloodstream and entering the white pulp (24). Due to its strategic function, the marginal zone contains a large number of resident macrophages, B cells, dendritic cells, and fibroblasts. All these populations interact and are dependent upon each other. An especially important role in this location is played by macrophages, since they sample blood-borne antigens and pathogens. These cells could be divided into at least two populations: MZM and MMZM. MZM are recognized by ERTR-9, and MMZM are recognized by MOMA-1 antibodies, respectively. Both populations have virtually no CD11b, CD11c, or F4/80, but subpopulations of them are positive for B220 (data not shown).
It was previously shown that macrophages of the marginal zone are able to ingest Listeria (1, 5), but it was postulated that both populations are equally contributing to this process. We have demonstrated that only one macrophage type, ERTR-9+, colocalizes with bacteria at the early stage of infection. Already at 2 h p.i. bacteria were observed inside ERTR-9+ cells (>90%) (data not shown), while some still resided in the marginal sinus (<10%). At 4 h p.i. all detectable bacteria were found in the ERTR-9+ population.
The reason for such an exquisite cell specificity of Listeria is not clear, but the anatomical location certainly contributes to this, since cells of the marginal zone are preferentially exposed to blood-borne pathogens. In addition, ex vivo experiments have shown that MOMA-1+ cells are able to internalize bacteria almost as well as ERTR-9+ macrophages (data not shown). This raises the question why MOMA1+ macrophages, occupying the same location, are not infected and do not take part in cluster formation. One explanation could be that ERTR-9+ macrophages express specific receptors, like the C-type lectin characterized by the ERTR-9 antibody (SIGNR1), or additional adhesion molecules (16, 20).
Migration of cells in and out of lymphoid organs during infection with bacteria is an intensively studied phenomenon presently. Chemokines and chemokine receptors are most important in this process. In particular, the proinflammatory chemokine CCL2, previously called monocyte chemotactic protein 1, is immediately up-regulated upon infections with many bacterial pathogens. In general, it attracts monocytes and macrophages towards infectious foci.
Infection with Listeria monocytogenes results in up-regulation of many proinflammatory cytokines and chemokines in the spleen (13, 33). Here we have shown that CCL2 is one of the first chemokines up-regulated in ERTR-9+ macrophages, which are the only population infected by Listeria during the early phase of infection. Up-regulation of CCL2 in macrophages infected with Listeria was also observed in vitro (unpublished data) (13, 31) and ex vivo using a splenic adherent cell population isolated from infected BALB/c mice (unpublished data). Immediate up-regulation of CCL2 was independent of whether a lethal or sublethal dose was employed for infection. Thus, our data also show that ERTR-9+ macrophages of the marginal zone are the major producers of CCL2 at the early stage of infection. This implies that direct infection with Listeria is required for initiation of CCL2 production.
The early expression of the proinflammatory chemokine CCL2 by infected ERTR-9+ marginal zone macrophages suggests its important role in the innate host defense. Indeed, antibody depletion of CCL2 strongly enhanced the mortality of BALB/c mice infected with Listeria. Consistently, a strong effect has been observed by others in recombinant C57BL/6 mice lacking CCL2 expression (31, 33).
However, the finding that upon CCL2 depletion ERTR-9+ macrophages do not migrate towards infectious foci of Listeria was unexpected. Normally, at 24 h after infection inflammatory cell clusters were formed by ERTR-9+ cells together with CD11b+ macrophages, Gr-1+ granulocytes, and CD3+ T cells around Listeria-infected cells. In contrast, in CCL2-depleted mice no migration of ERTR-9+ cells was observed, but the migration of all other cell subsets was unaltered. Similarly, colonization of the spleen by Listeria was very little influenced (data not shown). This appears contradictory to data of Sebrina et al. (31) that demonstrated a strong increase of Listeria in the spleens of mice with the CCL2 gene deleted. However, the same authors demonstrated that lack of CCL2 responsiveness did not influence the immigration of CD11b+ into the spleen after Listeria infection (32). Rather, it results in a block of emigration of such cells from the bone marrow and their absence in the circulation.
The reason for the different behaviors of ERTR-9+ cells is unclear. Additional chemokines might be produced to which cells other than ERTR-9 might have receptors. The same reasoning might explain the differential survival of monocyte/granulocyte populations with or without CCL2 depletion. On the other hand, CCL2 might signal in ERTR-9+ macrophages via additional receptors, as described for astrocytes (4, 37). Thus, depletion of CCL2 might influence ERTR-9+ macrophages more severely than other cells.
Taken together, our data allow the following interpretation of the sequence of events during Listeria infection. Infection of ERTR-9+ macrophages, the primary target cell for Listeria, induces CCL2 production in these cells, resulting in the attraction of other phagocytic cells, including noninfected ERTR-9+ macrophages, CD11b+ macrophages, and Gr-1+ granulocytes as well as some CD3+ T cells. Thus, infected ERTR-9+ marginal zone macrophages represent the condensation nucleus of the cell clusters formed in the spleen. When CCL2 is depleted, clusters are formed nevertheless due to additional mediators, but uninfected ERTR-9+ macrophages no longer take part in cell migration. Obviously, migration of this population depends exclusively on an early CCL2 signal. Moreover, it is possible that due to the CCL2 dysfunction, infected ERTR-9+ macrophages are killed by the bacterium and therefore are no longer detectable in the clusters at 24 h p.i. Such a scenario is suggested by the severe decrease in the number of such cells at 24 h p.i.
Our interpretation of the sequence of events is in apparent contrast to that suggested by Muraille et al. (5, 26). Cluster formation was observed by them but was interpreted as a migration of infected macrophages into the T-cell areas of the spleen. Laminin staining of cryosections allowed us to clarify this controversy. T cells and most other cells are in fact migrating towards the infectious foci formed in the marginal zone. The complex clusters of cells sometimes reach from the marginal zone into the white and/or the red pulp. However, the majority of such clusters do not surround the central arteriole, a hallmark of the T-cell zone, although occasionally clusters reach up to this vessel.
Intriguingly, the second population of macrophages found in the marginal zone, recognized by MOMA-1 antibodies, is not infected by Listeria. This was unexpected, considering the anatomical localization of these macrophages in the marginal zone and their verified ability to efficiently phagocytose Listeria ex vivo. Evidently, these macrophages are not attracted to the clusters of phagocytic cells formed around the infectious foci. Rather, they migrate to the B-cell follicles independently of CCL2. It is tempting to speculate that these macrophages up-regulate the chemokine receptor CXCR5, which is responsible for homeostatic recruitment of B cells to the follicles (2, 14, 19, 25, 28). Clustering of MOMA-1+ macrophages in the B-cell follicles might then be due to the location of a cellular source of the chemokine CCL13, the ligand of CXCR5.
Taken together, our results imply an important function for CCL2 during the initial phase of the innate immune response against L. monocytogenes. On the other hand, our data also extend the complexity of host reactions that are encountered during Listeria infection.
Acknowledgments
This work was supported in part by grants of the DFG to S.W. and J.B. and of the BMBF to S.W.
We thank Lothar Gröbe for help with cell sorting, Patricia Gezlaff and Katarina Raba for expert technical help, and Christofer Samuelsson for critical reading of the manuscript. We also thank Reinhold Förster for helpful discussions and Stefan Kaufmann and Hans-Willi Mittrücker for providing the possibility for some of the sorting experiments.
We do not have a commercial or other association that might pose a conflict of interest.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Aichele, P., J. Zinke, L. Grode, R. A. Schwendener, S. H. Kaufmann, and P. Seiler. 2003. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 171:1148-1155. [DOI] [PubMed] [Google Scholar]
- 2.Ansel, K. M., V. N. Ngo, P. L. Hyman, S. A. Luther, R. Forster, J. D. Sedgwick, J. L. Browning, M. Lipp, and J. G. Cyster. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406:309-314. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, B., D. Raffelsbauer, M. Kuhn, M. Goetz, S. Hom, and W. Goebel. 2002. InlA- but not InlB-mediated internalization of Listeria monocytogenes by non-phagocytic mammalian cells needs the support of other internalins. Mol. Microbiol. 43:557-570. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer, N., M. W. Zuurman, T. Wei, R. M. Ransohoff, H. W. Boddeke, and K. Biber. 2004. Induction of glial L-CCR mRNA expression in spinal cord and brain in experimental autoimmune encephalomyelitis. Glia 46:84-94. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, J. W. 1996. Early pathogenesis of Listeria monocytogenes infection in the mouse spleen. J. Med. Microbiol. 44:295-302. [DOI] [PubMed] [Google Scholar]
- 6.Cossart, P., J. Pizarro-Cerda, and M. Lecuit. 2003. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol. 13:23-31. [DOI] [PubMed] [Google Scholar]
- 7.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 158:5297-5304. [PubMed] [Google Scholar]
- 8.Depaolo, R. W., R. Lathan, B. J. Rollins, and W. J. Karpus. 2005. The chemokine CCL2 is required for control of murine gastric Salmonella enterica infection. Infect. Immun. 73:6514-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drevets, D. A. 1997. Listeria monocytogenes infection of cultured endothelial cells stimulates neutrophil adhesion and adhesion molecule expression. J. Immunol. 158:5305-5313. [PubMed] [Google Scholar]
- 10.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12:425-431. [DOI] [PubMed] [Google Scholar]
- 11.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes Listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 12.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 13.Flesch, I. E., J. Barsig, and S. H. Kaufmann. 1998. Differential chemokine response of murine macrophages stimulated with cytokines and infected with Listeria monocytogenes. Int. Immunol. 10:757-765. [DOI] [PubMed] [Google Scholar]
- 14.Forster, R., A. E. Mattis, E. Kremmer, E. Wolf, G. Brem, and M. Lipp. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87:1037-1047. [DOI] [PubMed] [Google Scholar]
- 15.Frischknecht, F., and M. Way. 2001. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 11:30-38. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., P. C. Groot, M. A. Nolte, S. J. van Vliet, S. T. Gangaram-Panday, G. C. van Duijnhoven, G. Kraal, A. J. van Oosterhout, and Y. van Kooyk. 2002. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood 100:2908-2916. [DOI] [PubMed] [Google Scholar]
- 17.Guzman, C. A., E. Domann, M. Rohde, D. Bruder, A. Darji, S. Weiss, J. Wehland, T. Chakraborty, and K. N. Timmis. 1996. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol. Microbiol. 20:119-126. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, J., L. L. Lau, and H. Shen. 2003. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J. Immunol. 171:4352-4358. [DOI] [PubMed] [Google Scholar]
- 19.Junt, T., K. Fink, R. Forster, B. Senn, M. Lipp, M. Muramatsu, R. M. Zinkernagel, B. Ludewig, and H. Hengartner. 2005. CXCR5-dependent seeding of follicular niches by B and Th cells augments antiviral B cell responses. J. Immunol. 175:7109-7116. [DOI] [PubMed] [Google Scholar]
- 20.Kang, Y. S., S. Yamazaki, T. Iyoda, M. Pack, S. A. Bruening, J. Y. Kim, K. Takahara, K. Inaba, R. M. Steinman, and C. G. Park. 2003. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15:177-186. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann, S. H., and G. Kaplan. 1996. Immunity to intracellular bacteria. Res. Immunol. 147:487-489. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd, C. M., A. W. Minto, M. E. Dorf, A. Proudfoot, T. N. Wells, D. J. Salant, and J. C. Gutierrez-Ramos. 1997. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 185:1371-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaffrey, R. L., P. Fawcett, M. O'Riordan, K. D. Lee, E. A. Havell, P. O. Brown, and D. A. Portnoy. 2004. A specific gene expression program triggered by gram-positive bacteria in the cytosol. Proc. Natl. Acad. Sci. USA 101:11386-11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mebius, R. E., and G. Kraal. 2005. Structure and function of the spleen. Nat. Rev. Immunol. 5:606-616. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, N. J., R. A. Wilkinson, and J. A. Fishman. 2002. Listeria monocytogenes infection in caspase-11-deficient mice. Infect. Immun. 70:2657-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muraille, E., R. Giannino, P. Guirnalda, I. Leiner, S. Jung, E. G. Pamer, and G. Lauvau. 2005. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. Eur. J. Immunol. 35:1463-1471. [DOI] [PubMed] [Google Scholar]
- 27.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohl, L., G. Bernhardt, O. Pabst, and R. Forster. 2003. Chemokines as organizers of primary and secondary lymphoid organs. Semin. Immunol. 15:249-255. [DOI] [PubMed] [Google Scholar]
- 29.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812-823. [DOI] [PubMed] [Google Scholar]
- 30.Portnoy, D. A., V. Auerbuch, and I. J. Glomski. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serbina, N. V., W. Kuziel, R. Flavell, S. Akira, B. Rollins, and E. G. Pamer. 2003. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity 19:891-901. [DOI] [PubMed] [Google Scholar]
- 32.Serbina, N. V., and E. G. Pamer. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7:311-317. [DOI] [PubMed] [Google Scholar]
- 33.Serbina, N. V., T. P. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 34.Unanue, E. R. 1996. Macrophages, NK cells and neutrophils in the cytokine loop of Listeria resistance. Res. Immunol. 147:499-505. [DOI] [PubMed] [Google Scholar]
- 35.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11-25. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuurman, M. W., J. Heeroma, N. Brouwer, H. W. Boddeke, and K. Biber. 2003. LPS-induced expression of a novel chemokine receptor (L-CCR) in mouse glial cells in vitro and in vivo. Glia 41:327-336. [DOI] [PubMed] [Google Scholar]