Abstract
Yersinia pseudotuberculosis infects many mammals and birds including humans, livestock, and wild rodents and can be recovered from the lungs of infected animals. To determine the Y. pseudotuberculosis factors important for growth during lung infection, we developed an intranasal model of infection in mice. Following intranasal inoculation, we monitored both bacterial growth in lungs and dissemination to systemic tissues. Intranasal inoculation with as few as 18 CFU of Y. pseudotuberculosis caused a lethal lung infection in some mice. Over the course of 7 days, wild-type Y. pseudotuberculosis replicated to nearly 1 × 108 CFU/g of lung in BALB/c mice, induced histopathology in lungs consistent with pneumonia, but disseminated sporadically to other tissues. In contrast, a ΔyopB deletion strain was attenuated in this model, indicating that translocation of Yersinia outer proteins (Yops) is essential for virulence. Additionally, a ΔyopH null mutant failed to grow to wild-type levels by 4 days postintranasal inoculation, but deletions of any other single effector YOP did not attenuate lung colonization 4 days postinfection. Strains with deletions in yopH and any one of the other known effector yop genes were more attenuated that the ΔyopH strain, indicating a unique role for yopH in lungs. In summary, we have characterized the progression of a lung infection with an enteric Yersinia pathogen and shown that YopB and YopH are important in lung colonization and dissemination. Furthermore, this lung infection model with Y. pseudotuberculosis can be used to test potential therapeutics against Yersinia and other gram-negative infections in lungs.
Yersinia pseudotuberculosis and Yersinia enterocolitica are gram-negative animal and human pathogens most commonly associated with acute gastroenteritis and lymphadenitis (11, 37). Both pathogens are generally transmitted by the fecal-oral route of infection and infect the gastrointestinal tract and associated lymph tissues (37, 65), but both can be isolated from the lungs of infected animals (7, 12, 50, 60, 61). In these cases, multiple organs are usually colonized, indicating that dissemination occurred from gastrointestinal or systemic sites to the lungs. Human lung infections by enteric Yersinia pathogens are rare, although there are reports of such cases (7, 33). In contrast, lung infections by the third pathogenic Yersinia species, Yersinia pestis, is well documented in both human and animal hosts and results in a highly lethal pneumonia (21, 47). While there are cases of experimentally induced lung infection with enteric Yersinia species (14, 20, 59, 80, 82), no systematic study of pneumonic yersiniosis induced by enteric Yersinia in mice has been completed to date.
All three pathogenic Yersinia species carry a 70-kb virulence plasmid, which encodes a type III secretion system (TTSS) and the secreted effector proteins, Yersinia outer proteins (Yops) (19, 67, 78). The TTSS is induced by growth at 37°C (18). Effector Yops—YopH, YpkA/YopO, YopM, YopE, YopT, and YopJ/P—are translocated into host cells where they interfere with normal cellular processes (84). Effector Yops require YopB, LcrV, and YopD for translocation into mammalian cells but not for their secretion into the extracellular milieu (84). Instudies on cultured cells, most effector Yops target and disrupt functions of macrophages and neutrophils (8, 19, 30, 53, 57, 62, 63, 85), cells that play critical roles in the initial defense against invading pathogens. Specifically, YopE, YopT, YopO, and YopH have all been shown to prevent phagocytosis by macrophages and neutrophils (8, 19, 30), while YopJ induces programmed cell death in macrophages (57) and inhibits the mitogen-activate protein kinase pathway (62, 63). YopM interacts with the host protein RSK-1 (54), alters the transcriptional profile of several cytokines in macrophages isolated from Y. pestis-infected spleens, and reduces the number of natural killer cells (45). YopM-β-lactamase fusions are translocated into granulocytes, macrophages, and dendritic cells in spleens following intravenous infection (53), suggesting that YopM also targets phagocytes.
The virulence properties of each of the Yops have been studied in animal models of one or more pathogenic Yersinia species. Deletion of yop genes reduces the ability of Yersinia to colonize host tissues and/or cause death after infection by one or more routes (48, 49, 77, 79, 80). For instance, orogastric infection with a Y. pseudotuberculosis strain lacking the tyrosine phosphatase, YopH, results in diminished colonization of the mesenteric lymph nodes and spleen compared to wild-type Y. pseudotuberculosis (49). Orogastric infection with Y. enterocolitica ΔyopH mutants results in similar attenuation in the small intestine, spleen, and liver (79). ΔyopO mutants of Y. enterocolitica are defective in colonization of the liver during intravenous infections (79) and in colonization of the spleen during orogastric infection of Y. pseudotuberculosis (49). YopM and YopE play a significant role in intravenous infections of mice with Y. pestis (48). In orogastric infections of Y. pseudotuberculosis, a ΔyopE mutant is attenuated in the spleen as well as the Peyer's patches (49), and a ΔyopJ mutant has a 64-fold increase in its 50% lethal dose (LD50) compared to wild-type Y. pseudotuberculosis (56). In addition to their role in gastric and intravenous infection, there is evidence that Yops are important during lung infection (80). All three pathogenic Yersinia organisms were recovered from lungs of intravenously infected mice, and their numbers increased over time (80). In contrast, strains lacking a virulence plasmid were initially recovered in lungs after intravenous inoculation, but their numbers decreased over time. Additionally, mice intranasally inoculated with Y. pestis lacking the virulence plasmid were cleared within 72 h (47). Together, these data suggest that genes encoded on the plasmid are important for persistence in the lungs.
In addition to the Yops and TTSS, other Yersinia virulence factors are known. PhoP, which is the response regulator of the PhoP/Q two-component signal transduction system, plays a crucial role in the ability of Y. pseudotuberculosis to replicate in macrophages (27). Furthermore, Y. pseudotuberculosis ΔphoP mutants have a 75-fold increase in the LD50 following intravenous inoculation of mice (27). Y. pseudotuberculosis also produces the adhesions invasin and YadA, which are important during mouse infection (34, 55). inv is preferentially expressed at 26°C and binds to β1 integrins of eukaryotic cells (39). inv mutants are defective for colonizing the cecum and Peyer's patches following orogastric infection of mice but are not defective in colonizing the spleen following intraperitoneal infection of mice (55). yadA is preferentially expressed at 37°C, and its deletion results in about a 10-fold increase in the LD50 of intraperitoneally infected mice (34).
We investigated the dynamics of Y. pseudotuberculosis infection in lungs of mice after intranasal inoculation with wild-type IP2666pIB1 and an isogenic ΔyopB mutant. Strikingly, we found that mice infected with as little as 18 CFU of wild-type Y. pseudotuberculosis developed a fatal lung infection, whereas mice infected with 3 × 102 CFU of the ΔyopB mutant displayed no visible signs of illness although the bacteria persisted in lungs more than a month and granulomatous lesions were observed.
MATERIALS AND METHODS
Bacterial strains and strain construction.
Y. pseudotuberculosis strains used in this study are listed in Table 1. Yersinia strains were grown at in LB broth for mouse infections and in Luria (L) broth for conjugation and other protocols (see reference 49). The IP2666pIB1yopH(NdeI) strain was the wild-type and/or parental Y. pseudotuberculosis strain (41) used for all experiments, unless otherwise stated. ΔyopH, ΔyopO, ΔyopM, ΔyopJ, and ΔyopE mutants and multiple yop deletion strains were generated in this strain with plasmids and methods described previously (49). ΔyopH, ΔyopO, ΔyopM, and ΔyopE mutants were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of trichloroacetic acid-precipitated supernatants of each mutant grown under YOP secretion conditions (49). ΔyopH, ΔyopM, and ΔyopE mutants were also confirmed by immunoblot analysis of trichloroacetic acid-precipitated supernatants. To generate the ΔyopJ strain, the following primers were used to amplify flanking regions of the yopJ gene: YopJ1C, GAGACAGTCGACGCTGACGACCATCGCCGAG; YopJ2B, CCAAATACATTACTCGAGCATTTATTTATCCTTATTCAGGG; YopJ3B, GGATAAAATAAATGCTCGAGTAATGTATTTTGGAAATCTTGC; and YopJ4A, GAGACAGCATGCCTGGGTATCGGTGCTATGATCGGC. The fragments generated by the primer pair YopJ1C and YopJ2B and the pair YopJ3B and YopJ4A were purified and combined in a second PCR with primers YopJ1C and YopJ4A. The resulting fragment was cloned into the suicide vector pCVD442 using the SalI and Sph1 sites. The deletion was generated as described previously (49). ΔyopJ deletions were verified by PCR with YopJ1C and YopJ4A and confirmed by testing for cytotoxicity in macrophages using a CytoTox 96 Non-Radioactive cytotoxicity assay (Promega) as previously described (57).
TABLE 1.
Yersinia strains
| Strain name | Genotype | Reference or source |
|---|---|---|
| Wild-type IP2666pIB1 | Y. pseudotuberculosis serotype 3; IP2666c pIB1+yopH(NdeI) | 41 |
| LL-89 | Y. pseudotuberculosis strain IP2666c pIB1+yopH(NdeI); Kanr | L. Logsdon and J. Mecsas |
| MLF-13 | LL89 ΔlacZ | This study |
| MLF-5 | ΔyopH | L. Logsdon and J. Mecsas |
| MLF-10 | Δ yopO | This study |
| MLF-3 | Δ yopM | This study |
| MLF-2 | ΔyopE | L. Logsdon and J. Mecsas |
| MLF-36 | ΔyopJ | This study |
| MLF-11 | Δ yopB | This study |
| MLF-20 | ΔyopE yopO | This study |
| MLF-29 | Δ yopE yopO yopM | This study |
| MLF-4 | ΔyopH yopE | L. Logsdon and J. Mecsas |
| MLF-41 | ΔyopH yopO | This study |
| MLF-50 | ΔyopH yopM | This study |
| MLF-34 | ΔyopH yopJ | This study |
| yopH R409A | 41 | |
| yopH Q11R | 41 | |
| yopH V31G | 41 | |
| yopH K342A | 41 | |
| yopH Δ223-6 | 41 | |
| IP2666pIB1 | Serogroup O3, pIB+ | J. Bliska |
| IP2666ΔphoP | IP2666pIB1ΔphoP | J. Bliska; see reference 27 for deletion |
| YPIIIpIB1+ | Y. pseudotuberculosis strain serotype 3 | 55 |
| JMB-111 | Y. pseudotuberculosis strain IP2666pIB1− | 4 |
The IP2666pIB1 ΔlacZ strain was generated by first amplifying flanking fragments of the lacZ gene in Y. pseudotuberculosis by PCR using the following primers: P31, GCATTAGTCGACGGTGCAACTGAGTCTTCC; P32, CGCCACGCATGCCACCTGCGGATCATTGAG; P33, CAGGTGGCATGCGTGGCGGATAAACCGATG; and P34, TGCCGTGAGCTCTTGGTACTGATAGGTTTCAC. The fragments were cloned into pCVD442 as described for yopJ using primers P31, P32, P33, and P34. Clones were verified for a ΔlacZ phenotype on L plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β“-d-galactopyranoside; Sigma). On such medium, the wild type is blue, while the ΔlacZ mutant remains white. The lacZ knockout construct was introduced into a kanamycin-marked IP2666pIB1 strain LL89. To generate LL89, pUTminiTN5Kn2 was introduced into IP2666pIB1 by conjugation as previously described (55). Three kanamycin-resistant exconjugants were tested for virulence in competition experiments with IP2666pIB1 in BALB/c mice. Two exconjugants were as fit as wild type in colonizing the intestinal tract, lymph tissues, and the spleen, and one, named LL-89, was chosen to use as a control in these studies. Sequence analysis of the transposon insertion indicated that the transposon was in the intergenic region between locus numbers YPTB3287 and YTPB3288 in Y. pseudotuberculosis IP32953, which encode a hypothetical protein and a putative GntP gluconate family transporter, respectively. The IP2666pIB1ΔphoP mutant, its parental strain (27), and yopH mutants (41) were provided by James Bliska.
Mouse infections.
Yersinia strains were grown with aeration overnight at 26°C in LB broth as previously described (49) or at 37°C in LB broth supplemented with 5 mM CaCl2 to prevent lysis of bacteria. The following morning, cultures were diluted 1:40 and grown for 8 h at 26°C or 37°C. Cultures were then diluted to an optical density at 600 nm of 0.025 and grown at 26°C or 37°C for approximately 16 h to an optical density at 600 nm of 4 to 6. Bacteria were diluted in sterile phosphate-buffered saline (PBS) at 26°C or 37°C to the appropriate CFU/ml for intranasal infection and maintained at their growth temperature until inoculation into animals. Six- to eight-week-old female BALB/c (Taconic Labs, Charles River Labs, or National Cancer Institute) or Swiss Webster (Taconic Labs) mice were anesthetized with isofluorane and infected intranasally with 40 μl of the bacterial suspension (35). Appropriate dilutions of inocula were plated on L plates to determine the doses administered, which are indicated in figure legends and tables. Each strain was used to infect 2 to 4 mice at a time, and each experiment was repeated two to four times with the exception of the data shown in Fig. 1A and the yopH time to morbidity experiment. At appropriate times after infection, mice were sacrificed by CO2 asphyxiation. Tissues were harvested, weighed, homogenized, and plated on L plates containing 1 μg/mg irgasan (49) to determine the number of CFU. Data are reported as the number of CFU/g of organ for lung, liver, spleen, and trachea; number of CFU/ml of blood; and number of CFU/organ for the lymph nodes and nasopharynx as the small size of the organs and the association of lymph nodes with connective tissue made determination of weights inaccurate. Homogenization of tissues was accomplished using a variable-speed tissue tearor (Biospec Products Inc.) using established methods described previously (2, 13, 49). Samples were blended until the solution appeared homogeneous, which occurred after 10 to 45 s of homogenization, depending on the tissue.
FIG. 1.
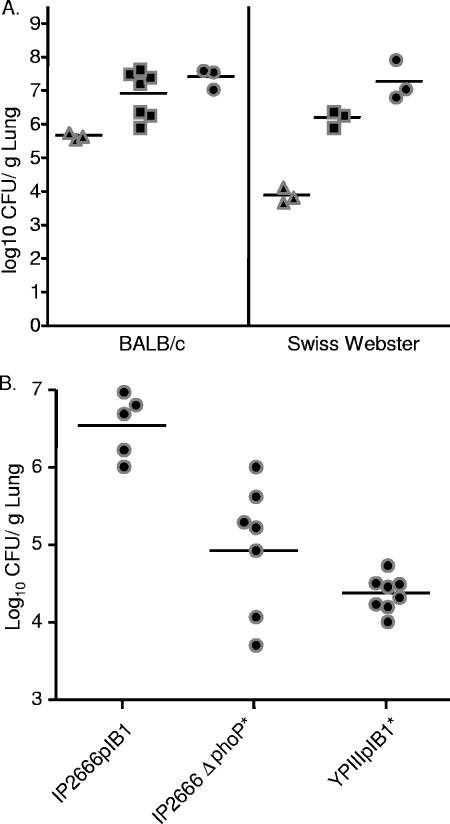
Y. pseudotuberculosis colonization of lungs occurs at low doses and is dependent on phoP. (A) In vivo growth of Y. pseudotuberculosis in BALB/c or Swiss Webster mice. Mice were inoculated intranasally with wild-type Y. pseudotuberculosis with 7.8 × 101 CFU (▴), 8.4 × 102 (▪), or 8.4 × 103 (•). Four days postinoculation lungs were harvested, weighed, homogenized, and plated for CFU. (B) Strains lacking PhoP are attenuated in colonization of lungs. BALB/c mice were intranasally inoculated with 8.0 × 102 CFU of IP2666pIB1, 6.0 × 102 CFU of IP2666pIB1ΔphoP, or 2.5 × 103 CFU of YPIIIpIB1. Four days postinoculation, lungs were weighed, homogenized, and plated for CFU. Each dot represents output from one mouse. Bars represent the geometric mean. P values were determined by a two-tailed Student's t test. *, P < 0.01.
For competition experiments, the IP2666kanrΔlacZ (MLF-13) strain was used as the reference strain. This strain was grown separately and then mixed with the unmarked wild type IP2666pIB1yopH(NdeI) strain or an isogenic Δyop mutant strain generating a mixed inoculum. The mixtures were plated onto L plates containing X-Gal, and blue and white colonies were counted to obtain the ratio of mutant to wild-type Y. pseudotuberculosis in the input inocula. Likewise, after infection appropriate dilutions of tissue homogenates were plated on L plates containing X-Gal to determine the ratio of mutant to wild-type Y. pseudotuberculosis colonies in tissues. A minimum of 100 colonies were counted for each competitive index (CI). The CI was calculated as follows: CI = (number of mutant colonies/number of wild-type colonies)output ratio/(number of mutant colonies/number of wild-type colonies)input ratio.
For time-to-morbidity experiments, 1 week prior to infection, blood from tail veins of some mice was collected into Microtainer serum separator tubes (Becton Dickson) according to the manufacturer's protocols and stored at −20°C for comparison to postinfection bleeds. Mice were inoculated intranasally with various doses of wild-type Y. pseudotuberculosis or ΔyopH mutant and monitored daily for signs of morbidity such as scruffiness, lack of response to touch lethargy, or a hunched appearance. Mice that moved only upon nudging were sacrificed by CO2 asphyxiation. All surviving mice were sacrificed after 28 days, and blood was collected by cardiac puncture and frozen for future analysis.
Histology.
Mice were inoculated with 300 CFU/mouse of wild-type Y. pseudotuberculosis or the ΔyopB strain or with PBS alone. For infections with wild-type Y. pseudotuberculosis, two mice were sacrificed by CO2 asphyxiation at days 1, 2, 4, 6, and 7. For the ΔyopB strain infections, two mice were sacrificed at 1, 2, 4, 6, 7, 14, 21, 28, 35, and 42 days postinoculation. PBS-treated control mice were sacrificed at 1, 4, 7, 14, 28, and 42 days postinoculation for comparison. The lungs were perfused via the trachea with 10% neutral buffered formalin (NBF), harvested, incubated in 10% NBF at 26°C for 24 h, and placed in fresh 10% NBF at 4°C until further processing. Lungs were sliced, transferred to histocassettes (Fisherbrand), incubated at 26°C in 70% ethanol for at least 24 h, and embedded in paraffin. Eight- to 10-micrometer sections were stained with hematoxylin and eosin or prepared for immunohistochemistry (see below). Slides were evaluated in a blind fashion by veterinary pathologist Lauren Richey at Tufts University Department of Laboratory Animal Medicine. Pictures were taken using a color camera (Nikon DS-M5) and an inverted microscope (Nikon TE2000).
For immunohistochemistry staining, the paraffin was extracted from the sections with a 5-min wash in 100% xylene. Samples were treated for 10 min with 70% ethanol, followed by 5-min treatments each with 95% ethanol, 100% ethanol, and distilled water. Immunostaining was carried out using the LSAB 2 System-HRP (DakoCytomation K0673) according to the manufacturer's protocol. Briefly, samples were incubated with a 1:300 dilution of rabbit anti-Y. pseudotuberculosis antibodies (gift of Ralph Isberg) for 30 min, rinsed with PBST (PBS plus 0.5% Tween 20), incubated with a 1:300 dilution of goat anti-rabbit immunoglobulin G (IgG)-biotin (DakoCytomation E0432) for 30 min, rinsed with PBST, and incubated with streptavadin-horseradish peroxidase (HRP). Samples were then incubated for five min with chromatogen solution and rinsed with distilled water. Slides were counterstained with hematoxylin for 3 min, rinsed with distilled water, and incubated in automation buffer (NM30; Biomeda Corp.).
All mice were handled according to protocols approved by the Institutional Animal Care and Use Committee of Tufts University.
Immunodetection assays of anti-Y. pseudotuberculosis IgGs from mouse serum.
A total of 50 μl of heat-killed Yersinia (2.2 μg of protein/ml) cells in PBS was incubated in 96-well flat bottom MaxiSorp Immuno Plates (Nunc) at 4°C overnight. Wells were rinsed six times with PBST, blocked with PBSF (PBS plus 10% fetal bovine serum) for 1 h at room temperature, and washed six times with PBST. Serum from mice was diluted 1:100 in PBSF, and 100 μl was added to the wells and incubated for 1 h at room temperature; wells were then washed six times in PBST. Goat anti-mouse HRP-conjugated antibodies (Sigma) were diluted 1:5,000 in PBSF and incubated at room temperature for 1 h. After six washes with PBST, 100 μl of TMB (3,3′,5,5′-tetramethylbenzidine) substrate (eBioscience) was added for 6 to 12 min, followed by the addition of 50 μl of 1 M phosphoric acid to quench the reaction. Plates were read at 450 nm and 540 nm, and the background (540 nm) was subtracted from the 450-nm reading. All dilutions were performed in duplicate, and the experiment was repeated three times. Postinfection samples were scored as positive if their values were greater than the average plus two times the error of the preinfection samples.
RESULTS
Low doses of Y. pseudotuberculosis colonize the lungs of infected mice.
Y. pseudotuberculosis is generally considered an enteric pathogen; however, it has been recovered from the lungs of infected animals (50, 60). Given this observation and that Y. pseudotuberculosis is closely related to Y. pestis (1), we determined whether Y. pseudotuberculosis IP2666pIB1 was capable of colonizing the lungs of mice following intranasal inoculation. In preliminary experiments, BALB/c or Swiss Webster mice were inoculated intranasally with different doses of IP2666pIB1 to determine at which dose, if any, Y. pseudotuberculosis could establish an infection. Mice were monitored daily for signs of illness. Four days postinoculation, mice appeared ill and were sacrificed to determine bacterial loads (Fig. 1A). An initial inoculum of less than 100 CFU grew to almost 1 × 106 CFU/g of lung in BALB/c mice and 1 × 104 CFU/g of lung in Swiss Webster mice (Fig. 1A). Higher levels of colonization were observed when more bacteria were used in the initial inoculum. In mice inoculated with 8.4 × 102 or 8.4 × 103 CFU, Y. pseudotuberculosis levels reached an average of 1 × 107 or 5 × 107 CFU/g of lung in BALB/c mice and 5 × 106 or 5 × 107 CFU/g of lung in Swiss Webster mice, respectively. In additional experiments, CD-1 and C57BL/6 mice were intranasally infected with 500 CFU and showed similar levels of colonization to that of BALB/c (data not shown). Together, these data indicate that Y. pseudotuberculosis can colonize lungs of inbred and outbred strains of mice at low inoculation doses, although the bacteria replicates more efficiently in BALB/c mice than Swiss Webster mice.
To determine if other strains of Y. pseudotuberculosis colonized lungs as proficiently as IP2666pIB1, mice were inoculated with YPIIIpIB1, and infection was allowed to proceed for 4 days. Mice inoculated with the YPIIIpIB1 strain showed fewer and milder symptoms by visual observation and harbored fewer bacteria in lungs than mice infected with IP2666pIB1 (Fig. 1B). Shortly after these experiments were completed, the YPIIIpIB1 strain was shown to carry an inactivating point mutation in phoP (27). PhoP is the response regulator of the PhoP/Q two-component signal transduction system, which is essential for intracellular growth of Y. pseudotuberculosis in macrophages (27, 29). We tested whether PhoP was important for colonizing lungs by inoculating mice intranasally with the IP2666pIB1ΔphoP deletion strain and comparing it to its isogenic IP2666pIB1 parental strain and YPIIIpIB1 (Fig. 1B). The ΔphoP deletion strain was on average 100 times more deficient in colonizing lungs than the isogenic wild-type IP2666pIB1 strain (Fig. 1B), indicating that PhoP-regulated processes are important for lung colonization. The IP2666pIB1 strain background was chosen for use in subsequent experiments because of its ability to highly colonize the lungs.
Growth temperature has little effect on kinetics of growth or dissemination.
Many Yersinia virulence factors including Yops, lipopolysaccharide (LPS), invasin, YadA, and LcrF are differentially regulated at high and low temperatures (3, 40, 44, 46). Expression or repression of any of these factors prior to inoculation could alter the kinetics, dissemination, or outcome of infection. To assess the effect of growth temperature of the inoculum of Y. pseudotuberculosis on these facets of infection, mice were infected with Y. pseudotuberculosis grown at either 26°C or 37°C. Lungs; respiratory-associated tissues, including trachea, nasopharynx, and tracheobronchial lymph nodes; and systemic tissues, including liver, spleen, and blood were harvested at 1, 2, 4, 6, and 7 days postinoculation, and the bacterial load in each tissue was determined.
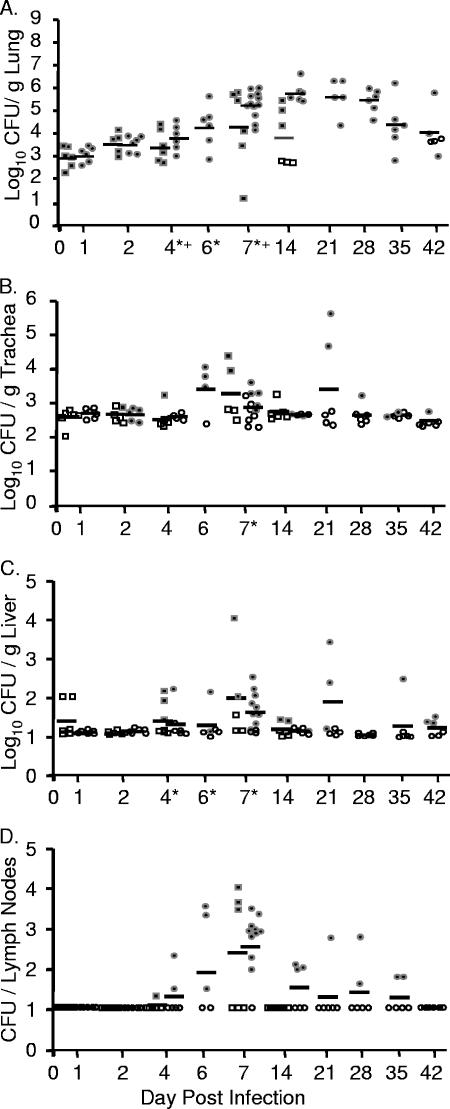
Prior growth at either 26°C or 37°C had little effect on the bacterial burden or dissemination in any tissue. After an initial dose of 3 × 102 to 5 × 102 CFU, bacterial loads from both growth conditions in the lungs increased to an average of 1 × 103 to 2 × 103 CFU/g of lung at 1 day postinoculation and to 1 × 104 at 2 days postinoculation (Fig. 2A). Bacteria continued to replicate in the lungs until day 6, when they reached an average of 1 × 107 to 3 × 107 CFU/g of lung. From day 6 to day 7, bacterial loads remained steady, and most mice were moribund at day 7. No statistically significant differences between bacterial loads in lungs inoculated with bacteria grown at 26°C versus 37°C were observed in the lungs at any day except day 6, when a fourfold defect was statistically significant.
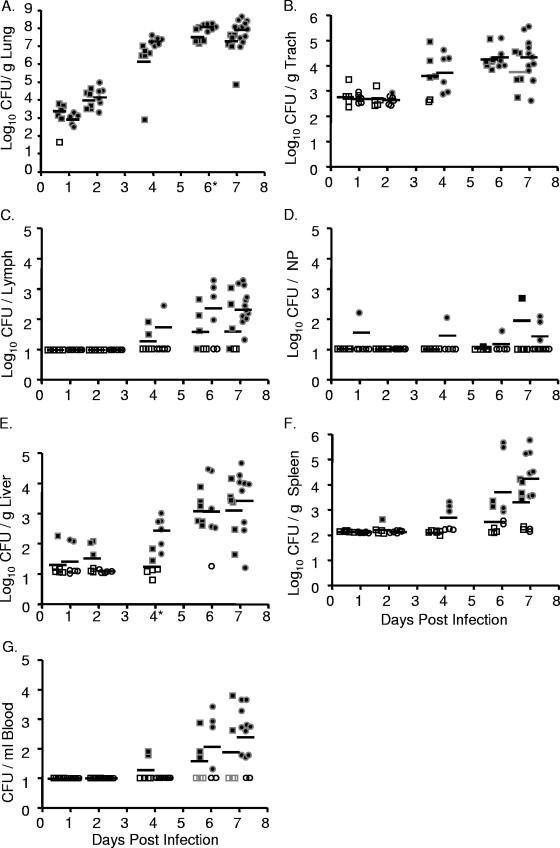
FIG. 2.
Intranasal inoculation with Y. pseudotuberculosis results in a lethal lung infection. BALB/c mice were inoculated with 300 to 500 CFU of Y. pseudotuberculosis grown at 26°C (•) or 37°C (▪). Mice were sacrificed 1, 2, 4, 6, and 7 days postinoculation and lungs (A), trachea (B), lymph nodes (C), nasopharynx (D), liver (E), spleen (F), and blood (G) were harvested, homogenized, and plated for CFU. Each dot represents data from one mouse. Open symbols indicate that bacteria were below the limit of detection (note, the limit of detection per gram of trachea recovered is generally higher than in the lungs, liver, and spleen because the weight of the trachea is smaller). Bars represent the geometric mean. *, P < 0.01 (Student's t test). NP, nasopharynx; Trach, trachea.
Colonization of the trachea, bronchial lymph nodes, and nasopharyngeal tissue was consistently lower than that observed in the lungs, and as in lungs, preinoculation growth temperature had little effect on the levels of Y. pseudotuberculosis in these respiratory tissues (Fig. 2B, C, and D). In the trachea, Y. pseudotuberculosis was not recovered until 4 days postinfection, and bacterial loads reached 2 × 104 CFU/g of trachea by day 6 (Fig. 2B). It should be noted, however, that no evidence of primary tracheal colonization was observed by histology (data not shown). Rather, it appeared that the bacteria recovered in the trachea were due to their high growth in the lungs and subsequent spread into the bronchial tracheal tubes. Bacteria started to disseminate to tracheobronchial lymph nodes by 4 days postinfection, about half the tracheobronchial lymph nodes were colonized by 6 days postinfection, and the majority of lymph nodes were colonized at 7 days postinfection with average levels of 2 × 102 CFU/lymph node (Fig. 2C). Bacteria were only sporadically recovered from the nasopharynx, indicating that this tissue is not a major niche of Y. pseudotuberculosis after intranasal inoculation (Fig. 2D).
Dissemination to the spleen, liver, and blood occurred sporadically, and levels of colonization were much lower than levels observed in the lungs. Y. pseudotuberculosis was detected at very low levels in some of the livers at 1 and 2 days postinfection but not in the blood and in only one spleen (Fig. 2E, F, and G). By 6 days postinfection, all but one liver harbored Y. pseudotuberculosis although levels ranged from 1×101 to 7 × 104 CFU/g of liver (Fig. 2E). No substantial colonization of either the spleen or blood was observed before 4 days postinoculation, after which it occurred sporadically (Fig. 2F and G). No significant differences were observed in any systemic tissue between bacteria grown at either 26°C or 37°C, except in the liver at day 4, when a defect in bacteria grown at 37°C was observed. These data demonstrate that following intranasal inoculation with Y. pseudotuberculosis, dissemination starts to occur from the lungs to other respiratory and systemic tissues by 4 days postinoculation and that bacterial growth at 26°C versus 37°C prior to inoculation has little effect on the outcome of infection. The growth within all other tissues is lower than that observed in the lungs, and the range in numbers of CFU of Y. pseudotuberculosis recovered in those tissues is generally much greater. Combined, these data indicate that the lungs are the primary site of infection and suggest that the morbidity reached at day 7 is most likely due to bacterial loads in the lungs.
Histopathology of lung infection with Y. pseudotuberculosis is consistent with pneumonia.
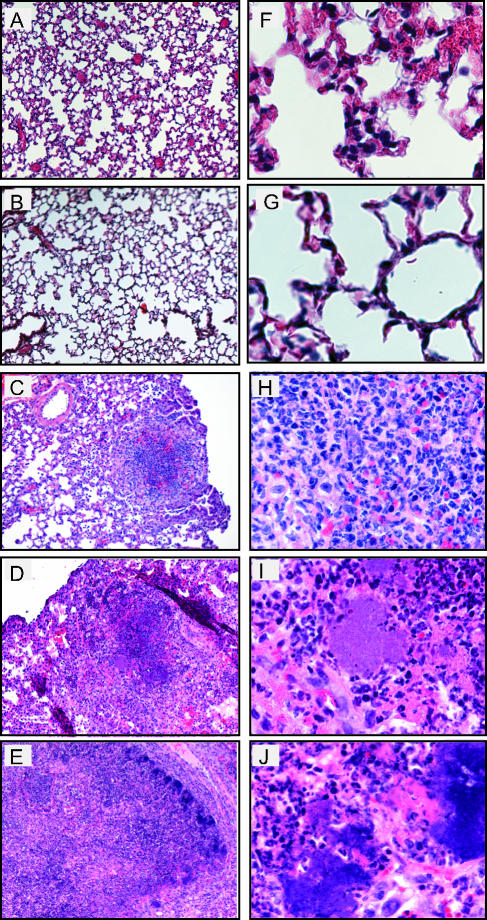
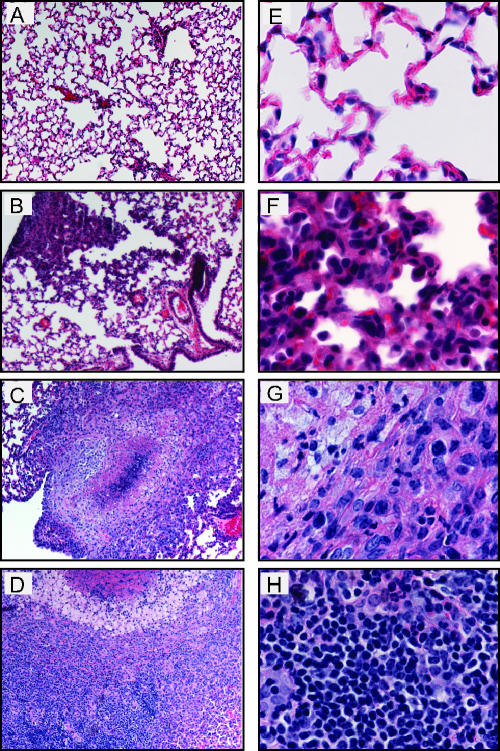
Histological analysis was performed on lungs of mice inoculated with Y. pseudotuberculosis to determine the location and types of immune cells recruited to lungs and damage caused by infection over time. Lungs were harvested 1, 2, 4, 6, and 7 days postinoculation, and sections were stained with hematoxylin and eosin to visualize cells (Fig. 3). One- and two-day postinoculation lungs infected with Y. pseudotuberculosis appeared similar to PBS-inoculated lungs (Fig. 3A, B, F, and G, and data not shown). The alveoli were open and contained no large areas of inflammation, although occasionally small areas of congestion were observed in both PBS-treated and infected lungs (data not shown). In contrast, by 4 days postinoculation, the normal architecture of the lung was disrupted in many areas as lungs contained multifocal, suppurative, necrotic lesions, and fibrination in alveolar spaces (Fig. 3C and H). In addition, large colonies of blue-purple staining rods were observed at high magnification in regions of inflammation and necrosis. The size of the inflammatory lesions and size of the colonies increased over the course of the infection (Fig. 3D, E, I, and J), consistent with the increased bacterial loads observed during the 7-day infection. The presence of Y. pseudotuberculosis in these disruptive lesions was confirmed by immunohistochemistry using serum against Y. pseudotuberculosis (Fig. 4).
FIG. 3.
Histological analysis of lungs from BALB/c mice inoculated with wild-type Y. pseudotuberculosis. Mice were inoculated with 300 to 500 CFU of Y. pseudotuberculosis, and lungs were harvested 1 (A and F), 2 (B and G), 4 (C and H), 6 (D and I), and 7 (E and J) days postinoculation, sectioned, and stained with hematoxylin and eosin. Slides are shown at a magnification of ×10 (A to E) or ×60 (F to J).
FIG. 4.
Immunohistochemical analysis of lung sections. Sections from mice 4 days postinoculation with PBS (A and C) or with 300 to 500 CFU of Y. pseudotuberculosis grown at 26°C (B and D) were stained with anti-Y. pseudotuberculosis antibodies. Goat anti-mouse HRP-conjugated antibody was used as a secondary stain. Brown areas represent areas of HRP activity; lighter brown/gray areas are red blood cells. Samples shown are at a magnification of ×10 (A and B) and ×60 (C and D). Arrow (D) indicates an area of colocalization of antibody with bacterial colonies. Boxed sections in panels A and B indicate the areas magnified in panels C and D, respectively.
Time and dose to morbidity after Y. pseudotuberculosis intranasal inoculation.
To determine at what dose Y. pseudotuberculosis infections resulted in 50% of the mice reaching morbidity, groups of 3 or 4 BALB/c mice were inoculated with different doses of IP2666pIB1 grown at 26°C or 37°C (Table 2).Mice were monitored daily for signs of illness, and those that appeared moribund were sacrificed. Fifty percent of mice inoculated with a dose of 18 CFU of Y. pseudotuberculosis grown at 26°C became moribund between 16 to 18 days postinfection, and 2 out of 3 mice inoculated with 48 CFU of Y. pseudotuberculosis grown at 37°C became moribund (Table 2). These data indicate that the dose for 50% of mice to reach morbidity is below 50 CFU. Higher doses of Y. pseudotuberculosis resulted in a faster time to morbidity as mice inoculated with greater than 2 × 103 CFU of Y. pseudotuberculosis were moribund between 5 to 7 days postinfection whereas mice inoculated with less than 100 CFU were moribund between 11 to 18 days postinfection (Table 2).
TABLE 2.
LD50 of Y. pseudotuberculosis in intranasal infection of BALB/c mice
| Strain (culture condition) | Dose (no. of CFU/mouse)
|
No. of dead mice/ total no. of mice | Day of death(s) | IgG+a | ||
|---|---|---|---|---|---|---|
| Expt. 1 | Expt. 2 | Expt. 3 | ||||
| IP2666pIB1 (26°C) | 0.8 | 0.8 | 0/7 | 1/4 | ||
| 1.6 | 0/3 | 0/3 | ||||
| 3 | 0/3 | 1/4 | ||||
| 4 | 4 | 2/6 | 15, 17 | 0/3 | ||
| 6 | 0/4 | 0/4 | ||||
| 18 | 2/4 | 16, 18 | 2/2 | |||
| 26 | 26 | 5/6 | 12, 13, 14, 15, 17 | |||
| 61 | 3/3 | 11, 11, 16 | ||||
| 93 | 4/4 | 11, 11, 11, 13 | ||||
| 182 | 3/3 | 11, 12, 13 | ||||
| 460 | 3/3 | 9, 9, 10 | ||||
| 496 | 3/3 | 8, 8,8 | ||||
| 9,280 | 4/4 | 5, 5, 5, 7 | ||||
| IP2666pIB1 (37°C) | 1.3 | 0/3 | ||||
| 1.7 | 0/3 | 0/3 | ||||
| 2.2 | 1/3 | 18 | ||||
| 4.0 | 0/3 | 0/3 | ||||
| 33 | 1/3 | 11 | 2/2 | |||
| 48 | 2/3 | 11, 12 | ||||
| 140 | 3/3 | 9, 9, 10 | ||||
| 2,360 | 3/3 | 5, 5, 6 | ||||
| 3,800 | 3/3 | 5, 5 ,5 | ||||
| IP2666ΔyopH (26°C) | 3 | 0/3 | 0/3 | |||
| 16 | 0/3 | 1/1 | ||||
| 182 | 1/3 | 16 | 2/2 | |||
| 1,192 | 2/3 | 10, 12 | 1/1 | |||
| 9,900 | 3/3 | 9, 10, 10 | ||||
No. of seropositive mice/total no. of mice. The number of seropositive mice was determined by enzyme-linked immunosorbent assay against heat-killed Yersinia.
Since all surviving mice received very low doses of Y. pseudotuberculosis, it was possible that they did not receive any CFU. Some surviving mice were tested for the presence of anti-Yersinia antibodies by enzyme-linked immunosorbent assay (Table 2). Surviving mice that received a dose of 18 CFU or 33 CFU developed IgG antibodies to Y. pseudotuberculosis, indicating that the inoculation of these mice successfully delivered bacteria to the nasal cavities and/or lungs and that the mice were able to control the infection. Likewise, several mice receiving a lower inoculation dose also mounted an immune response. However, IgG antibodies to Y. pseudotuberculosis were detected in only ∼10% of the tested mice inoculated at doses below 18 CFU (Table 2), indicating that the bacteria either never reached the nasal cavities or lungs or were cleared before a significant infection and/or immune response occurred.
Translocation of Yops is necessary for wild-type lung colonization.
In other routes of infection, translocation of Yops is important for full virulence of Y. pseudotuberculosis (49). Translocation, but not secretion, of effector Yops is dependent on YopB, a component of the translocon of the TTSS. To assess the importance of translocation of Yops during lung infection, mice were infected with Y. pseudotuberculosis ΔyopB grown at 26°C and monitored for 42 days or infected with bacteria grown at 37°C and monitored for 14 days. Bacterial loads were determined in the lungs, trachea, bronchial tracheal lymph nodes, nasopharynx, spleen, liver, and blood. Throughout the course of infection, none of the mice displayed visible symptoms of illness. At days 1 and 2 postinfection, Y. pseudotuberculosis ΔyopB colonization levels were similar to wild type in the lungs; however, by day 4 four little growth had occurred in the yopB mutant (Fig. 5A), and there was a significant difference between wild-type and ΔyopB strains. Colonization peaked at 5 × 105 CFU/g of lung 14 days postinoculation of bacteria, which was approximately 100-fold lower than peak wild-type levels when mice were moribund at day 7 (Fig. 2A). Y. pseudotuberculosis ΔyopB bacteria grown at 37°C were cleared from some lungs by 14 days and by 42 days for bacteria grown at 26°C. Since mice were not monitored beyond 14 days when infected with the ΔyopB mutant grown at 37°C, it is unclear if initial growth temperature significantly affects the time of persistence in the lungs. Nonetheless, these observations indicated that the ΔyopB mutant induces a less virulent infection of lungs, which can persist for several weeks, before bacteria are cleared from infection.
FIG. 5.
YopB is essential for high levels of colonization and dissemination. BALB/c mice were infected with 300 to 500 CFU of Y. pseudotuberculosis ΔyopB grown at 26°C (•) or 37°C (▪). Mice inoculated with the ΔyopB mutant grown at 26°C were sacrificed at 1, 2, 4, 6, 7, 14, 21, 28, 35, and 42 days postinoculation, and mice inoculated with ΔyopB strain grown at 37°C were sacrificed at 1, 2, 4, 7, and 14 days postinoculation. Lungs (A), trachea (B), liver (C), and lymph nodes (D) were harvested, homogenized, and plated for CFU. Each circle represents the CFU from a mouse inoculated with the ΔyopB strain grown at 26°C, and each square represents CFU from a mouse inoculated with the ΔyopB strain grown at 37°C. Open symbols indicate that no bacteria were recovered at that limit of detection. Bars represent the geometric mean. P values were determined between the levels of the ΔyopB strain recovered as shown here and levels of wild-type Y. pseudotuberculosis shown in Fig. 2 using a two-tailed Student's t test. *, P < 0.01 between wild type and yopB mutant grown at 26°C; +, P < 0.01 between wild type and yopB mutant grown at 37°C.
The ΔyopB strain was also deficient in colonizing the trachea (Fig. 5B) and liver (Fig. 5C), and no bacteria were detected in the spleen, blood, and nasopharynx at any time point (data not shown). The ΔyopB strain reached similar, albeit low levels, as wild type in the lymph tissues at 7 days postinoculation (Fig. 5D). Together, these data indicate that translocation of Yops is critical for virulence, for efficient replication in lungs, and for dissemination to and/or colonization of systemic tissues after intranasal inoculation.
Lungs of mice infected with the ΔyopB strain were examined by histology and showed several marked differences in their histopathology compared to those infected with the wild type. Notably, the ΔyopB mutant-infected lungs showed only mild pathology by 7 days postinoculation (Fig. 6B and F). Inflammatory lesions had no significant necrosis or cell debris at that time. By 28 days postinoculation, two types of lesions were found in the lungs of the yopB strain-infected mice. Large granulomatous lesions were found that contained foamy macrophages surrounding a necrotic center (Fig. 6C) which increased in size through 42 days postinoculation (Fig. 6D). In addition, several smaller lesions, similar to those observed at day 7, were found scattered throughout the lungs. Combined, these data indicate that the yopB mutant induces a slower and more controlled, though chronic, state of inflammation compared to that observed with wild-type Y. pseudotuberculosis infection in lungs.
FIG. 6.
Histological analysis of lungs from BALB/c mice inoculated with ΔyopB Y. pseudotuberculosis. Mice were inoculated with 300 to 500 CFU of Y. pseudotuberculosis, and lungs were harvested 1 (A and E), 7 (B and F), 28 (C and G), and 42 (D and H) days postinoculation. Lungs were sectioned and stained with hematoxylin and eosin. Slides show lungs at a magnification of ×10 (A to D) or ×60 (E to H).
A yopH mutant strain is attenuated in intranasal infections.
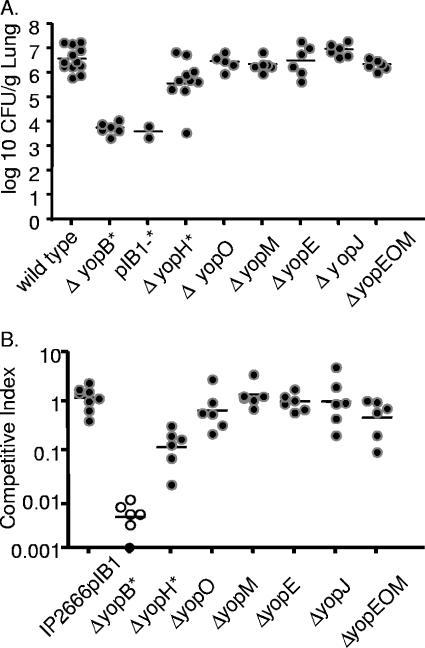
To investigate which of the translocated, effector Yops are important for lung colonization, mice were intranasally inoculated with Y. pseudotuberculosis IP2666pIB1 strains containing deletions of one or more yop genes (Fig. 7). Mice were inoculated with 8 × 102 CFU of either wild-type IP2666pIB1, ΔyopB, IP2666pIB1− (lacking the virulence plasmid), ΔyopH, ΔyopO, ΔyopM, ΔyopE, ΔyopJ, or the ΔyopEOM triple mutant strain. Mice were sacrificed 4 days postinoculation, a time point chosen because colonization levels were high with wild-type infection and the ΔyopB strain was severely attenuated. The IP2666pIB1− strain, like the ΔyopB mutant, grew approximately 1,000-fold less than wild-type Y. pseudotuberculosis mice and showed no signs of illness over the course of the 4-day infection (Fig. 7A). These results suggest that no other factors encoded on pIB1 play additional, significant roles in the absence of Yop translocation in infected lungs. Although mice infected with the yopH strain became ill, this strain had a 10-fold defect in colonization relative to wild-type Y. pseudotuberculosis, indicating that YopH enhances replication in lungs. Single yopJ, yopE, yopO, or yopM deletion strains were not attenuated 4 days postinoculation. Furthermore, the yopEOM triple mutant showed no observable attenuation in a single-strain infection (Fig. 7A), indicating that these three Yops do not function redundantly in this model of infection.
FIG. 7.
Colonization of lungs of BALB/c mice with yop mutant strains 4 days after intranasal inoculation in single-strain and competition infections. (A) Mice were inoculated with ∼8 × 102 CFU wild-type Y. pseudotuberculosis or an isogenic mutant of yopB, yopH, yopO, yopM, yopE, yopJ, or a yopEOM triple mutant or a strain of IP2666 lacking the virulence plasmid (pIB1−). Four days postinoculation lungs were harvested, homogenized, and plated for CFU. (B) BALB/c mice were inoculated with ∼8 × 102 CFU of an equal mixture of MLF-13 and either IP2666pIB1 or a yopB, yopH, yopO, yopM, yopE, or yopJ mutant or a yopEOM triple mutant. Four days postinoculation lungs were harvested, homogenized, and plated on L plates containing X-Gal, and the ratios of wild type to mutants were determined. Each dot represents data from one mouse. Open circles indicate that no bacteria were recovered at the limit of detection. Bars represent the geometric mean. P values were calculated by a two-tailed Student's t test. *, P < 0.05 for the number of CFU of wild-type Y. pseudotuberculosis recovered versus the number of CFU of a mutant strain.
To assess whether the single yop mutant strains were more attenuated in the presence of wild-type Y. pseudotuberculosis, a phenomenon which has been observed in other routes of infection (49), coinfection experiments were performed. Mice were intranasally inoculated with an equal mixture of the reference strain, IP2666pIB1ΔlacZ and either wild-type IP2666pIB1 or the ΔyopB, ΔyopH, ΔyopO, ΔyopM, ΔyopE, ΔyopJ, or ΔyopEOM mutant. As expected, the reference strain was as fit as the unmarked wild-type Y. pseudotuberculosis strain, with an average CI of 1 (Fig. 7B). As in single-strain infections, the yopB mutant was severely attenuated, and the ΔyopH strain was attenuated 10-fold relative to wild-type Y. pseudotuberculosis (Fig. 7B). Furthermore, the Δ yopO, ΔyopM, ΔyopE, ΔyopJ, or ΔyopEOM strain competed as well as wild-type Y. pseudotuberculosis (Fig. 7B). Thus, in contrast to competition studies after orogastric inoculation, none of the single yop mutants was more attenuated in the presence of wild-type Y. pseudotuberculosis than in the absence of wild-type Y. pseudotuberculosis (Fig. 7A) at this time point.
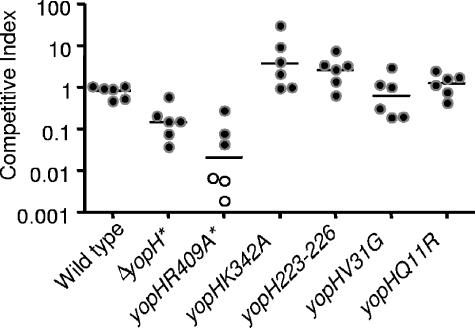
To further investigate the role of YopH in lung colonization, two additional types of infections were carried out. First, coinfection studies were performed with a series of yopH point mutations or small deletions with wild-type Y. pseudotuberculosis (Fig. 8). The yopH mutations studied were yopHR409A, a catalytically inactive mutant (41, 89); yopHQ11R and yopHV31G, which are deficient in binding to the focal adhesion protein p130CAS (58); yopHK342A which participates in binding of phosphopeptides to a second site in YopH (41); and yopHΔ223-226 which is defective in binding to focal adhesion complexes in cultured cells (68). The catalytically inactive mutant was 100-fold attenuated compared to wild-type Y. pseudotuberculosis (Fig. 8). In contrast, none of the other mutations showed significant colonization defects in this model of infection, indicating that these functions of YopH are not required for lung colonization at day 4. Second, the estimated LD50 of a yopH mutant was determined to be approximately 575 CFU (Table 2) by the method of Reed and Muench (72), which is ∼20- to 30-fold higher than that of wild-type Y. pseudotuberculosis. This indicates that YopH is involved in causing morbidity as well as in colonization of the lungs.
FIG. 8.
Colonization of Y. pseudotuberculosis yopH mutants in competition with wild-type Y. pseudotuberculosis 4 days postinoculation. BALB/c mice were inoculated with ∼8 × 102 CFU of an equal mixture of MLF-13 and either the MLF-9, yopH(R409A), yopH(K342A), yopH(V31G), yopH(Q11R), or yopHΔ223-226 strain. Bacteria from lungs were grown on L plates containing X-Gal, and the competitive index was calculated as described in Materials and Methods. Black circles represent the CI from individual mice, open circles indicate that no mutants were detected, and bars represent the geometric mean. P values were determined by a two-tailed Student's t test. *, P < 0.05.
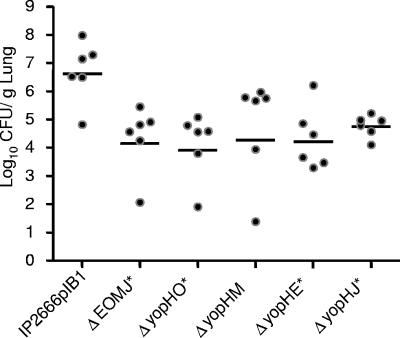
The observation that the ΔyopB strain was more defective in lung colonization than the ΔyopH strain (P < 0.01), suggested that one or more additional effector Yops may play a significant role in lung colonization in the absence of YopH. Thus, a series of double yop mutants was tested for their ability to colonize lungs after intranasal inoculation. All four double mutants, ΔyopHO, ΔyopHE, ΔyopHM, and ΔyopHJ, were more attenuated in their ability to colonize lungs than the ΔyopH strain (Fig. 9 and 7A), indicating that each of these Yops contributes to colonization in the absence of YopH. Moreover, the ΔyopEOMJ strain was significantly more attenuated than the ΔyopEOM strain (Fig. 7A), suggesting that yopJ plays an important role in lung colonization in the absence of yopE, yopO, and yopM.
FIG. 9.
Colonization of Y. pseudotuberculosis yopH double mutants. Mice were intranasally inoculated with ∼8 × 102 CFU of wild-type Y. pseudotuberculosis or mutants of the yopHE, yopHO, yopHM, yopHJ, or yopEOMJ strains. Four days postinoculation lungs were weighed, homogenized, and plated for CFU. Black circles represent the CFU recovered from individual mice, open circles indicate that the number of CFU was below the limit of detection, and bars represent the geometric mean. P values were determined by a two-tailed Student's t test. *, P < 0.05.
DISCUSSION
Here, we demonstrate that intranasal inoculation with Y. pseudotuberculosis causes a fulminant lung infection in mice. Bacterial growth at 26°C or 37°C prior to inoculation had no effect on initial colonization levels, dissemination, or time to morbidity, indicating that virulence factors upregulated at either temperature are equally capable of initiating infection. For instance, Yops are expressed at 37°C, but our data indicate that bacteria not expressing yop genes immediately prior to inoculation are as fit as those that do. It is clear, however, that translocation of Yops is essential for virulence by 96 h postinoculation as the ΔyopB mutant was significantly attenuated at this time. Expression of yadA and inv is also regulated by temperature; yadA is expressed at 37°C but not 26°C (10, 43), and inv is inversely regulated (10, 40). Our data indicate that bacteria expressing either of these factors immediately prior to inoculation are equally fit to cause lung infection. It is of interest to determine whether removing these factors from Y. pseudotuberculosis altered the course of intranasal infection of mice. Finally, the LPS of Y. pseudotuberculosis has different modifications depending on growth temperature (6, 70), but these changes did not alter initial colonization levels or dissemination to other tissues.
Low doses of Y. pseudotuberculosis resulted in robust lung colonization, illness, and lethality, as 50% of the mice infected with 18 CFU and 100% of mice infected with 61 CFU of Y. pseudotuberculosis became moribund within 17 days. Interestingly, the LD50 of Y. pestis in C57BL/6 mice after intranasal inoculation is ∼3.0 × 102 CFU, which is about 10-fold higher than what we observed with Y. pseudotuberculosis (47, 86). However, the course of infection with Y. pestis is much faster as mice succumb to infection by day 3 or 4 postinoculation. Furthermore, Y. pestis levels in the lungs were higher, and dissemination to other tissues occurred sooner (by day 3) and more uniformly (36, 47). The lower levels of colonization of Y. pseudotuberculosis and slower time to morbidity relative to Y. pestis are unlikely due to the difference in mouse background as Y. pseudotuberculosis colonization of C57BL/6 mice is similar to that of BALB/c mice (data not shown). Furthermore, both Swiss Webster and BALB/c mice succumb to aerosolized or intranasal inoculation of lethal doses of Y. pestis within 4 days (36, 42, 71, 86). Combined, these data indicate that the more rapid morbidity associated with Y. pestis infection is likely a result of a physiological difference between Y. pseudotuberculosis and Y. pestis. For example, Y. pestis has a shorter LPS (66) and a different lipid A composition that is modified during growth at 37°C (70), elaborates a peptide capsule (16), and lacks functional copies of inv (75) and yadA (76). Furthermore, while Y. pestis has a functional copy of yopT, some infectious Y. pseudotuberculosis serotypes, including the serotype tested here, do not (83). One or more of these differences could affect the host response to infection, which, in turn, may alter the course of infection.
Our histopathological analysis indicated that Y. pseudotuberculosis-induced pneumonia had features resembling those caused by Y. pestis, although inflammation arose at later time points after infection. The lungs of mice inoculated with low doses of both Yersinia species were quiescent early in infection (47), whereas later—day 2 for Y. pestis and day 4 for Y. pseudotuberculosis—pathology became evident. Specifically, lungs of mice intranasally inoculated with Y. pestis had an influx of neutrophils, extracellular bacteria, and hemorrhaging at foci of infection by 2 days postinoculation (47, 71), similar to effects observed with Y. pseudotuberculosis by 4 days postinoculation. In human cases, pneumonic plague is associated with high bacterial loads, multifocal pneumonia, increased abundance of neutrophils, fibrination of alveolar spaces, and pulmonary edema (31). The lungs of cats and African green monkeys infected with Y. pestis demonstrate nearly identical pathologies (23, 87). One difference between the Y. pestis and Y. pseudotuberculosis histopathology is that in Y. pseudotuberculosis infections, we often observe large colonies of bacteria growing in tight masses such as those depicted in Fig. 3I as well as smaller, more dispersed microcolonies (Fig. 4D). These large, tightly packed colonies are reminiscent of what we observe with Y. pseudotuberculosis infections in other organs, such as the mesenteric lymph nodes after oral infection (4) and spleen after intravenous infection (M. McCoy and J. Mecsas, unpublished data).
The low infectious dose of Y. pseudotuberculosis in mice is particularly striking compared to the LD50 of other respiratory pathogens. For instance, Streptococcus pneumoniae has an LD50 of 1 × 105 CFU/mouse in intranasally infected MF-1 mice (17), but the same dose is ineffective in causing disease in the Swiss Webster mouse line, indicating an even higher lethal dose in this background (35). Similarly, the LD50 of Pseudomonas aeruginosa is approximately 1 × 107 CFU/mouse in the C3H/HeJ background (25). Sublethal doses of P. aeruginosa fail to grow in the lungs of mice and are cleared within 14 days (25, 26), whereas high doses result in death within 48 h (26). Murine lung models have also been used to study other gram-negative pathogens such as Vibrio cholerae and Shigella spp. (24, 51, 69, 73). As with non-Yersinia respiratory pathogens, these organisms generally require high intranasal doses between 1 × 106 and 2 × 108 CFU/mouse. Despite the fact that mice become ill following exposure to these organisms, the bacteria decrease in number within hours to days following inoculation. Generally, lungs from these mice demonstrate mild to moderate pathology between 6 and 24 h postinoculation. In the V. cholerae model, half the mice do not survive 24 h postinoculation, and those that do survive have moderate pneumonia and fibrination in the lungs (24). Mice intranasally infected with Shigella flexneri develop moderate inflammation within 6 h but then resolve it within 48 h postinoculation (73). In the model presented here, low inoculation doses of Y. pseudotuberculosis grow to high levels in the lung and induce a pronounced pneumonia over the course of a week. These aspects of Y. pseudotuberculosis lung infection will aid in the study of both bacterial virulence factors involved in the development of pneumonia, in the development of host defenses in lungs, and in the testing of prophylactic therapeutics and therapeutics delivered after infection has occurred.
Mice infected with the ΔyopB strain showed no visible signs of illness up to 42 days postinoculation, but bacteria were recovered in some lungs 42 days after inoculation. Consistently, the histopathology of wild-type Y. pseudotuberculosis-infected mouse is vastly different from that of infection with the ΔyopB strain. Lungs infected with wild type showed severe pathology, while lungs infected with the ΔyopB strain appeared healthy up to 6 days postinfection. Several weeks postinoculation with the ΔyopB strain, infected lungs presented large, structured granulomas containing lymphocytes and foamy macrophages not observed in a wild-type infection. The histopathology of the ΔyopB strain infection demonstrates chronic elements, such as collagen deposits and lymphocytic, granulomatous formations reminiscent of structures formed in the intragranulomatous necrosis in a pulmonary granulomas model (32). Although the ΔyopB strain has not been tested in a Y. pestis background in a mouse model of pneumonic plague, a strain lacking the virulence plasmid was cleared after 3 days postintranasal inoculation (47). A Y. pestis ΔyopB strain might induce a similar inflammatory state as its Y. pseudotuberculosis counterpart. On the other hand, Y. pseudotuberculosis may have other factors that allow for its persistence, as a plasmid-minus strain of Y. pseudotuberculosis is cleared from lungs less rapidly than either Y. pestis or Y. enterocolitica following intravenous inoculation (80).
Fewer Yops appeared to be required for colonization of lungs after intranasal inoculation than with other routes of infection as single mutants of ΔyopE, ΔyopO, ΔyopM, and ΔyopJ did not show any significant attenuation in lungs 4 days after intranasal inoculation. However, all are attenuated in at least one other route of infection with various Yersinia species (48, 49, 56, 79). Although deletions of these yop genes had little effect on lung colonization at day 4 postinoculation, a role for these Yops might be observed in colonization or morbidity studies if the infection proceeded longer or if lower inoculation doses were given to mice. Our data suggest that YopE, YopO, or YopM enhances or augments the role of YopJ in lung colonization since in the absence of all four Yops, the ΔyopEOMJ strain colonized the lungs poorly, whereas a ΔyopEOM strain colonized lungs efficiently. While YopJ, YopO, YopM, and YopE have different biochemical activities and bind to different host proteins (5, 8, 54, 63), most of these Yops alter the ability of macrophages and/or neutrophils to respond normally to bacteria (30, 45, 57), supporting the idea that these Yops may all inactivate the same cell type(s) during lung infection, albeit by different mechanisms. In fact, our data with double mutants consisting of YopH and these other effector Yops suggest that these other Yops play redundant roles in infection.
The observation that YopH is required for productive infection after many different routes of inoculation (9, 41, 45, 49, 79) implies that YopH has multiple roles in infection and/or that its function is required for survival in many different tissues. Curiously, the catalytically inactive yopH mutant, yopH(R409A), was even more attenuated than the null mutant. Translocation of this mutant could reduce the translocation levels of the other effector Yops in vivo, and thus there are fewer Yops in key cells to counteract host responses, rendering the bacteria more sensitive to host defenses. Alternatively, the presence of the mutant protein may sequester intracellular host targets, leading to an unfavorable environment for Yersinia. When other effector yop deletions were introduced into the ΔyopH strain background, colonization was attenuated by approximately 10-fold compared to the ΔyopH strain, indicating that in the absence of YopH, the lack of these Yops renders the bacteria more susceptible to host defenses and/or less capable of establishing an infection. One possible explanation for this result is that in the absence of YopH, the host can mount a more aggressive response to Yersinia (74), and a concomitant loss of other Yops leaves Yersinia more vulnerable to these host defenses than when the bacteria express YopH.
During our analysis it became apparent that PhoP is important in lung colonization as IP2666 ΔphoP was attenuated for growth in lungs by 100-fold compared to wild type. PhoP is important for virulence of Y. pseudotuberculosis and Y. pestis when infection is initiated by other routes of inoculation. Subcutaneous inoculation of a Y. pestis ΔphoP mutant demonstrated that there was a 75-fold increase in the LD50 compared to wild-type Y. pestis (64). Similarly, oral inoculation of a Y. pseudotuberculosis ΔphoP mutant showed that the LD50 was higher than that of an isogenic wild-type Y. pseudotuberculosis strain (27). Both Y. pseudotuberculosis and Y. pestis require PhoP to replicate in macrophages (27, 64). Although histological analysis showed that Y. pseudotuberculosis was amassed in large extracellular colonies at 4 days postinoculation, it is possible that replication in macrophages early in infection is necessary for the establishment of this fulminant infection (15). Alternatively, since PhoP regulates a number of genes (28, 52, 64, 88, 90), a ΔphoP strain may be deficient in lung colonization due to its inability to appropriately regulate genes involved in other facets of Yersinia physiology.
Given the low infectious dose of Y. pseudotuberculosis in mice, it is remarkable that we could find only one case in the literature of pneumonia in humans that may have been triggered by aerosol-borne Y. pseudotuberculosis (33). The infrequency of documented human cases of pneumonia triggered by enteric Yersinia pathogens (7, 33, 81) may reflect differences in lung anatomy between mice and humans (38), differences in response to infection with enteric Yersinia or gram-negative pathogens between mice and humans (22), or the infrequency of encountering aerosolized enteric Yersinia. Alternatively, humans may be more resistant to infection because humans are outbred, and the mice used for most of the studies are inbred. Indeed, the outbred strain of mice used here was more resistant to Y. pseudotuberculosis replication. It is noteworthy that experimentally induced pneumonia with aerosolized enteric Yersinia has been documented in several other mammals (20, 82), and there are cases of the recovery of enteric Yersinia from the lungs of infected animals (7, 12, 50, 60, 61), although in such cases spread to the lungs most likely occurred after oral ingestion and subsequent systemic spread.
In conclusion, we have described an intranasal model system of lung infection in mice using Y. pseudotuberculosis. There are several noteworthy differences between Y. pseudotuberculosis and Y. pestis which may account for the differences in the rates of growth in the lungs and kinetics of dissemination in mice, including the LPS structure, the absence of YadA and Inv in Y. pestis, and the presence of two additional virulence factors. Determining whether these or other differences between Y. pestis and Y. pseudotuberculosis affect dissemination and/or alter the need for different Yops during lung infection will give insights into host defenses in lungs, as well as effective and/or redundant bacterial mechanisms to counteract host defenses during infection. In addition, it is of interest to determine whether virulence factors such as PhoP, YopB, and YopH are also important in Y. pestis infection of lungs. PhoP is important for virulence of both Y. pestis and Y. pseudotuberculosis when infection is initiated by other routes of inoculation (27, 64). Given the similarities and differences between the course of infection of Y. pestis and Y. pseudotuberculosis, it is a reasonable hypothesis that some of the virulence factors found to be important in Y. pseudotuberculosis infection will also be involved in lung infection with Y. pestis, while others may be important only in one species. By understanding the infection of lungs with Y. pseudotuberculosis, we can begin identifying common virulence factors between these two species of Yersinia and test potential therapeutics and/or vaccines directed against features common to both.
Acknowledgments
We thank members of the Mecsas Laboratory for strains, advice, and critical reading of the manuscript; Andrew Camilli and Julianne LeMieux for demonstrating intranasal inoculations, helpful discussions, and critical reading of the manuscript; Lauren Richey for professional analysis of histological samples; James Bliska for providing the phoP mutant and yopH point mutants and their isogenic parental strains prior to publication; and Molly Bergman for the enzyme-linked immunosorbent assay protocol.
This work was funded by NIH grant R01-AI056068 to J.M. M.L.F. was funded by the NIH Molecular Analysis of Microbial Pathogens 5T32GM07310 and Molecular Genetics of Basic Cell Function 5T32GM007310 training grants. C.C. was funded by training grant GM66567. We are grateful to the Center for Gastroenterology Research on Absorptive and Secretory Processes funded by NIDDK P30-34928 and their help in preparing histological samples and reagents.
Editor: D. L. Burns
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelichio, M. J., D. S. Merrell, and A. Camilli. 2004. Spatiotemporal analysis of acid adaptation-mediated Vibrio cholerae hyperinfectivity. Infect. Immun. 72:2405-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov, A. P., S. V. Dentovskaya, G. M. Titareva, I. V. Bakhteeva, R. Z. Shaikhutdinova, S. V. Balakhonov, B. Lindner, N. A. Kocharova, S. y. N. Senchenkova, O. Holst, G. B. Pier, and Y. A. Knirel. 2005. Intraspecies and temperature-dependent variations in susceptibility of Yersinia pestis to the bactericidal action of serum and to polymyxin B. Infect. Immun. 73:7324-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balada, J. M., and J. Mecsas. 2006. Yersinia has a tropism for B and T cell zones of lymph node that is independent of the type III secretion system. PLOS Pathog, in press. [DOI] [PMC free article] [PubMed]
- 5.Barz, C., T. N. Abahji, K. Trulzsch, and J. Heesemann. 2000. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482:139-143. [DOI] [PubMed] [Google Scholar]
- 6.Bengoechea, J., B. Lindner, U. Seydel, R. Diaz, and I. Moriyon. 1998. Yersinia pseudotuberculosis and Yersinia pestis are more resistant to bactericidal cationic peptides than Yersinia enterocolitica. Microbiology 144:1509-1515. [DOI] [PubMed] [Google Scholar]
- 7.Bigler, R. D., R. R. Atkins, and E. J. Wing. 1981. Yersinia enterocolitica lung infection. Arch. Intern. Med. 141:1529-1530. [PubMed] [Google Scholar]
- 8.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 9.Bliska, J., K. Guan, J. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA 88:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bresnahan, J. F., U. G. Whitworth, Y. Hayes, E. Summers, and J. Pollock. 1984. Yersinia enterocolitica infection in breeding colonies of ruffed lemurs. J. Am. Vet. Med. Assoc. 185:1354-1356. [PubMed] [Google Scholar]
- 13.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 101:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnoy, C., C. Mullet, H. Muller-Alouf, E. Leteurtre, and M. Simonet. 2000. Superantigen YPMa exacerbates the virulence of Yersinia pseudotuberculosis in mice. Infect. Immun. 68:2553-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 83:348-363. [PubMed] [Google Scholar]
- 16.Chen, T. H., and S. S. Elberg. 1977. Scanning electron microscopic study of virulent Yersinia pestis and Yersinia pseudotuberculosis type 1. Infect. Immun. 15:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiavolini, D., G. Memmi, T. Maggi, F. Iannelli, G. Pozzi, and M. Oggioni. 2003. The three extra-cellular zinc metalloproteinases of Streptococcus pneumoniae have a different impact on virulence in mice. BMC Microbiol. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelis, G., J.-C. Vanootegem, and C. Sluiters. 1987. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb. Pathog. 2:367-379. [DOI] [PubMed] [Google Scholar]
- 19.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Genaro, M. S., M. E. Escudero, C. Aguilera, L. Scardapane, and A. M. de Guzman. 1998. Intranasal immunization with Yersinia enterocolitica O:8 cellular extract protects against local challenge infection. Microbiol. Immunol. 42:781-788. [DOI] [PubMed] [Google Scholar]
- 21.Doll, J. M., P. S. Zeitz, P. Ettestad, A. L. Bucholtz, T. Davis, and K. Gage. 1994. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51:109-114. [DOI] [PubMed] [Google Scholar]
- 22.Eskay, R. L., M. Grino, and H. T. Chen. 1990. Interleukins, signal transduction, and the immune system-mediated stress response. Adv. Exp. Med. Biol. 274:331-343. [DOI] [PubMed] [Google Scholar]
- 23.Fingold, J. M. 1969. Pneumonic plague in monkeys. An electron microscopic study. Am. J. Pathol. 54:167-183. [PMC free article] [PubMed] [Google Scholar]
- 24.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines, III, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalanos. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455-1462. doi: 10.1084/jem.20020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George, S. E., M. J. Kohan, M. I. Gilmour, M. S. Taylor, H. G. Brooks, J. P. Creason, and L. D. Claxton. 1993. Pulmonary clearance and inflammatory response in C3H/HeJ mice after intranasal exposure to Pseudomonas spp. Appl. Environ. Microbiol. 59:3585-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George, S. E., M. J. Kohan, D. A. Whitehouse, J. P. Creason, C. Y. Kawanishi, R. L. Sherwood, and L. D. Claxton. 1991. Distribution, clearance, and mortality of environmental pseudomonads in mice upon intranasal exposure. Appl. Environ. Microbiol. 57:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabenstein, Jens, P., M. Marceau, C. Pujol, M. Simonet, and J. B. Bliska. 2004. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect. Immun. 72:4973-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabenstein, Jens P., H. S. Fukuto, L. E. Palmer, and J. B. Bliska. 2006. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect. Immun. 74:3727-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guarner, J., W. Shieh, P. Greer, J. Gabastou, M. Chu, E. Hayes, K. Nolte, and S. Zaki. 2002. Immunohistochemical detection of Yersinia pestis in formalin-fixed, paraffin-embedded tissue. Anat. Pathol. 117:205-209. [DOI] [PubMed] [Google Scholar]
- 32.Guirado, E., S. Gordillo, O. Gil, J. Diaz, G. Tapia, C. Vilaplana, V. Ausina, and P.-J. Cardona. 2006. Intragranulomatous necrosis in pulmonary granulomas is not related to resistance against Mycobacterium tuberculosis infection in experimental murine models induced by aerosol. Int. J. Exp. Pathol. 87:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagiwara, S., Y. Ishii, Y. Sugiyama, and S. Kitamura. 1995. Hypersensitivity pneumonitis caused by a home humidifier. Nihon Kyobu Shikkan Gakkai Zasshi 33:1024-1029. [In Japanese.] [PubMed] [Google Scholar]
- 34.Han, Y., and V. Miller. 1997. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect. Immun. 65:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 36.Honko, A. N., N. Sriranganathan, C. J. Lees, and S. B. Mizel. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 74:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubbert, W. T., C. W. Petenyi, L. A. Glasgow, C. T. Uyeda, and S. A. Creighton. 1971. Yersinia pseudotuberculosis infection in the United States. Septicemia, appendicitis, and mesenteric lymphadenitis. Am. J. Trop. Med. Hyg. 20:679-684. [DOI] [PubMed] [Google Scholar]
- 38.Irvin, C. G., and J. H. Bates. 15 May 2003. Measuring the lung function in the mouse: the challenge of size. Respir. Res. 4:4. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 40.Isberg, R. R., A. Swain, and S. Falkow. 1988. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect. Immun. 56:2133-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov, M. I., J. A. Stuckey, H. L. Schubert, M. A. Saper, and J. B. Bliska. 2005. Two substrate-targeting sites in the Yersinia protein tyrosine phosphatase co-operate to promote bacterial virulence. Mol. Microbiol. 55:1346-1356. [DOI] [PubMed] [Google Scholar]
- 42.Jones, T., J. J. Adamovicz, S. L. Cyr, C. R. Bolt, N. Bellerose, L. M. Pitt, G. H. Lowell, and D. S. Burt. 2006. Intranasal protollin (TM)/F1-V vaccine elicits respiratory and serum antibody responses and protects mice against lethal aerosolized plague infection. Vaccine 24:1625-1632. [DOI] [PubMed] [Google Scholar]
- 43.Kapperud, G., E. Namork, and H. J. Skarpeid. 1985. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect. Immun. 47:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawahara, K., H. Tsukano, H. Watanabe, B. Lindner, and M. Matsuura. 2002. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infect. Immun. 70:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerschen, E. J., D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72:4589-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6:395-409. [PubMed] [Google Scholar]
- 47.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 102:17786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung, K. Y., B. S. Reisner, and S. C. Straley. 1990. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58:3262-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Logsdon, L. K., and J. Mecsas. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 71:4595-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mair, N. S., and G. S. Ziffo. 1974. Isolation of Y. pseudotuberculosis from a foal. Vet. Rec. 94:152-153. [DOI] [PubMed] [Google Scholar]
- 51.Mallett, C. P., T. L. Hale, R. W. Kaminski, T. Larsen, N. Orr, D. Cohen, and G. H. Lowell. 1995. Intransal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect. Immun. 63:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marceau, M., F. Sebbane, F. Ewann, F. Collyn, B. Lindner, M. A. Campos, J.-A. Bengoechea, and M. Simonet. 2004. The pmrF polymyxin-resistance operon of Yersinia pseudotuberculosis is upregulated by the PhoP-PhoQ two-component system but not by PmrA-PmrB, and is not required for virulence. Microbiology 150:3947-3957. [DOI] [PubMed] [Google Scholar]
- 53.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald, C., P. O. Vacratsis, J. B. Bliska, and J. E. Dixon. 2003. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 278:18514-18523. [DOI] [PubMed] [Google Scholar]
- 55.Mecsas, J., I. Bilis, and S. Falkow. 2001. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infect. Immun. 69:2779-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montagna, L. G., M. I. Ivanov, and J. B. Bliska. 2001. Identification of residues in the N-terminal domain of the Yersinia tyrosine phosphatase that are critical for substrate recognition. J. Biol. Chem. 276:5005-5011. [DOI] [PubMed] [Google Scholar]
- 59.Najdenski, H., A. Vesselinova, E. Golkocheva, S. Garbom, and H. Wolf-Watz. 2003. Experimental infections with wild and mutant Yersinia pseudotuberculosis strains in rabbits. J. Vet. Med. Series B 50:280-288. [DOI] [PubMed] [Google Scholar]
- 60.Nikolova, S., Y. Tzvetkov, H. Najdenski, and A. Vesselinova. 2001. Isolation of pathogenic yersiniae from wild animals in Bulgaria. J. Vet. Med. Series B 48:203-209. [DOI] [PubMed] [Google Scholar]
- 61.Obwolo, M. J., and T. J. Gruffydd-Jones. 1977. Yersinia pseudotuberculosis in the cat. Vet. Rec. 100:424-425. [DOI] [PubMed] [Google Scholar]
- 62.Orth, K. 2002. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 5:38-43. [DOI] [PubMed] [Google Scholar]
- 63.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290:1594-1597. [DOI] [PubMed] [Google Scholar]
- 64.Oyston, P. C. F., N. Dorrell, K. Williams, S.-R. Li, M. Green, R. W. Titball, and B. W. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paff, J. R., D. A. Triplett, and T. N. Saari. 1976. Clinical and laboratory aspects of Yersinia pseudotuberculosis infections, with a report of two cases. Am. J. Clin. Pathol. 66:101-110. [DOI] [PubMed] [Google Scholar]
- 66.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. G. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. F. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 67.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Persson, C., R. Nordfelth, K. Andersson, A. Forsberg, H. Wolf-Watz, and M. Fallman. 1999. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33:828-838. [DOI] [PubMed] [Google Scholar]
- 69.Phalipon, A., M. Kaufmann, P. Michetti, J. Cavaillon, M. Huerre, P.Sansonetti, and J. Kraehenbuhl. 1995. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 52:1363-1373. [DOI] [PubMed] [Google Scholar]
- 71.Reed, D. S., and M. J. Martinez. 2006. Respiratory immunity is an important component of protection elicited by subunit vaccination against pneumonic plague. Vaccine 24:2283-2289. [DOI] [PubMed] [Google Scholar]
- 72.Reed, L. J., and H. A. Muench. 1938. A simple method for estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 73.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 74.Sauvonnet, N., I. Lambermont, P. v. d. Bruggen, and G. R. Cornelis. 2002. YopH prevents monocyte chemoattractant protein 1 expression in macrophages and T-cell proliferation through inactivation of the phosphatidylinositoI 3-kinase pathway. Mol. Microbiol. 45:805-815. [DOI] [PubMed] [Google Scholar]
- 75.Simonet, M., B. Riot, N. Fortineau, and P. Berche. 1996. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect. Immun. 64:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skurnik, M., and H. Wolf-Watz. 1989. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol. Microbiol. 3:517-529. [DOI] [PubMed] [Google Scholar]
- 77.Straley, S. C., and M. L. Cibull. 1989. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL− Yersinia pestis in BALB/c mice. Infect. Immun. 57:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Titball, R. W., J. Hill, D. G. Lawton, and K. A. Brown. 2003. Yersinia pestis and plague. Biochem. Soc. Trans. 31:104-107. [DOI] [PubMed] [Google Scholar]
- 79.Trulzsch, K., T. Sporleder, E. I. Igwe, H. Russmann, and J. Heesemann. 2004. Contribution of the major secreted Yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect. Immun. 72:5227-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Zonneveld, M., J. M. Droogh, M. W. J. A. Fieren, I. C. Gyssens, T. van Gelder, and W. Weimar. 2002. Yersinia pseudotuberculosis bacteraemia in a kidney transplant patient. Nephrol. Dial. Transplant. 17:2252-2254. [DOI] [PubMed] [Google Scholar]
- 82.Velianov, D., S. Nikolova, K. Naidenski, T. Raducheva, and V. Kusovski. 1993. Studies on aerosol Yersinia pseudotuberculosis infection of guinea-pigs. Acta Microbiol. Bulg. 30:3-10. [PubMed] [Google Scholar]
- 83.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signalling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 85.Visser L. G., A. Annema, and R. van Furth. 1995. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect. Immun. 63:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, S., D. Heilman, F. Liu, T. Giehl, S. Joshi, X. Huang, T.-h. Chou, J. Goguen, and S. Lu. 2004. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 22:3348-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watson, R. P., T. W. Blanchard, M. G. Mense, and P. W. Gasper. 2001. Histopathology of experimental plague in cats. Vet. Pathol. 38:165-172. [DOI] [PubMed] [Google Scholar]
- 88.Winfield, M. D., T. Latifi, and E. A. Groisman. 2005. Transcriptional regulation of the 4-amino-4-deoxy-l-arabinose biosynthetic genes in Yersinia pestis. J. Biol. Chem. 280:14765-14772. [DOI] [PubMed] [Google Scholar]
- 89.Zhang, Z. Y., Y. Wang, L. Wu, E. B. Fauman, J. A. Stuckey, H. L. Schubert, M. A. Saper, and J. E. Dixon. 1994. The Cys(X)5Arg catalytic motif in phosphoester hydrolysis. Biochemistry 33:15266-15270. [DOI] [PubMed] [Google Scholar]
- 90.Zhou, D., Y. Han, L. Qin, Z. Chen, J. Qiu, Y. Song, B. Li, J. Wang, Z. Guo, and Z. Du. 2005. Transcriptome analysis of the Mg2+-responsive PhoP regulator in Yersinia pestis. FEMS Microbiol. Lett. 250:85-95. [DOI] [PubMed] [Google Scholar]