Abstract
In an initial period (≤4 h) Toll-like receptor 4 (TLR4) signaling is required for Yersinia enterocolitica YopP-induced dendritic cell (DC) death. Later (>4 h), DC die independent of TLR4 signaling. In TLR4-deficient DC caspase 8 cleavage is delayed, indicating that TLR4 signaling accelerates caspase 8 activation, leading to DC death.
Yersinia enterocolitica outer protein P (YopP) is encoded by the pYV plasmid of Y. enterocolitica and is injected into the cytosol of host cells by a type III secretion system. By induction of cell death in macrophages and dendritic cells (DC) YopP impairs the immune response of the host (4, 13, 15). For macrophages it was hypothesized that by inhibiting NF-κB activation YopP (YopJ in Y. pseudotuberculosis) potentiates lipopolysaccharide (LPS)-induced apoptosis (7, 15, 16, 21) via Toll-like receptor 4 (TLR4) signaling (6, 20). Alternatively, YopP might directly cause cell death by proteolytic cleavage of Bid, a substrate of caspase 8 (3). Here, we demonstrate that in DC YopP mediates cleavage of caspase 8. Furthermore, we show that DC death is accelerated by TLR4 signaling in the early phase of infection.
The Y. enterocolitica strains used were a wild-type strain (pYV+) (8) and a yopP-negative mutant (YopP−) (16). Overnight cultures grown at 27°C were diluted 1:20 in fresh Luria-Bertani broth and grown for 1.5 h at 37°C. The bacterial concentration was measured densitometrically at 600 nm.
Immature bone marrow-derived DC were obtained from C57BL/6 (Harlan Winkelmann, Borchen, Germany) from C57BL/6 × 129Sv, TLR2−/−, TLR4−/−, and TLR2−/− × TLR4−/− mice (all obtained from the C57BL/6 × 129Sv background and kindly provided by Carsten J Kirschning) and from MyD88−/− (1) and TRIFLps2/Lps2 mice (both obtained from the C57BL/6 background; the latter kindly provided by Bruce Beutler) as previously described (4, 12). Briefly, immature DC were generated from bone marrow-derived cells by cultivating them for 8 to 10 days with medium containing 200 U of granulocyte-macrophage colony-stimulating factor/ml. One hour postinfection at a multiplicity of infection of 10:1 bacteria were killed by addition of gentamicin (100 μg/ml; Sigma, Taufkirchen, Germany). To inhibit caspase activation, DC were incubated with the pancaspase inhibitor zVAD-fmk (100 μM; Bachem, Heidelberg, Germany) 1 h prior to infection.
Cell death was measured by using uptake of propidium iodide (PI) (50 ng/ml; Calbiochem, Bad Soden, Germany). The leakage of DNA from nuclei was assessed by the method of Nicoletti et al. (14). All analyses were performed with a FACSCalibur (BD Biosciences, Heidelberg, Germany) using the WinMDI version 2.8 software (J. Trotter, The Scripps Institute, La Jolla, CA). Student's t test was used to evaluate the difference between means for two groups.
For immunoblotting 40 μg protein was loaded, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electrotransferred to nitrocellulose membranes (Schleicher & Schüll, Dassel, Germany). The membranes were incubated with antibodies to caspase 8 (Alexis, Grünberg, Germany) or caspase 3 (New England Biolabs, Frankfurt, Germany). Immunoreactive bands were visualized with peroxidase-conjugated secondary antibodies using enhanced chemoluminescence reagents (ECL; Amersham Biosciences, Freiburg, Germany). For measurement of caspase 3 activity 4 × 105 DC were lysed in 110 μl buffer containing 0.5% NP-40, 84 mM KCl, 10 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 5 μg/ml aprotinin in 20 mM HEPES (pH 7.4). Caspase 3 activities were determined by incubation of 50 μl of cell lysate with 150 μl reaction buffer containing the fluorogenic substrate N-acetyl-Asp-Glu-Val-Asp-amido-methylcoumarin (Ac-DEVD-AMC) (Bachem, Heidelberg, Germany) at a concentration of 66.66 μM (resulting in a final concentration of Ac-DEVD-AMC of 50 μM), 100 mM NaCl, 10% sucrose, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), and 13.35 mM dithiothreitol in 50 mM HEPES (pH 7.3). The release of aminomethylcoumarin was measured by fluorometry using an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
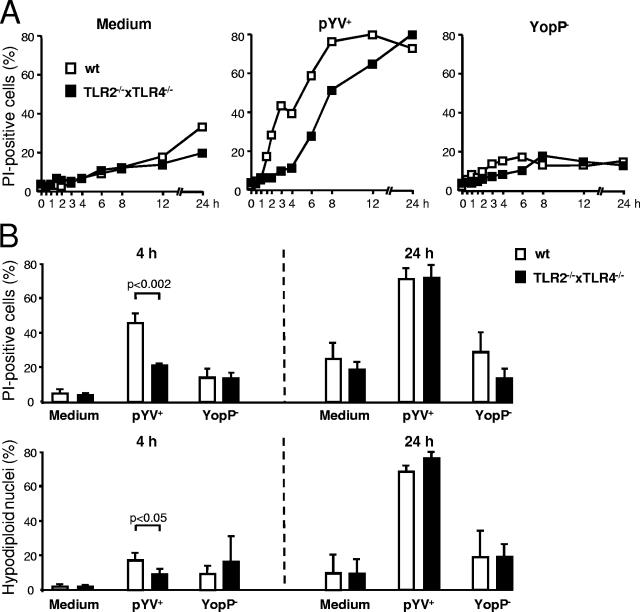
In the present study the impact of TLR signaling on YopP-mediated DC death was analyzed. Death rates were assessed by measuring the percentage of PI-positive DC, which indicated the lack of cellular membrane integrity, and by the Nicoletti procedure, which demonstrated the amount of hypodiploid nuclei. As shown in Fig. 1A, death of DC was observed after infection with YopP-secreting Yersinia (pYV+) but not after infection with YopP-deficient Yersinia (YopP−), confirming the finding that Yersinia-induced DC death is due to YopP (4, 5). Compared to the death of DC from wild-type mice, the death of DC from TLR2−/− × TLR4−/− mice was delayed by about 2 to 3 h, whereas the percentage of dead DC was not reduced after 24 h. Four hours postinfection this delay led to a significant reduction in the portion of PI-positive cells in TLR double-knockout DC (Fig. 1B). At the same time the percentage of hypodiploid nuclei was low in wild-type DC and also in TLR2−/− × TLR4−/− knockout DC. This finding is not surprising because hypodiploid nucleus formation occurs in late apoptosis. Consequently, the percentage of PI-positive cells and also the percentage of hypodiploid nuclei were high in DC from wild-type and TLR-deficient mice 24 h after infection. This finding indicates that in the early phase (≤4 h postinfection) TLR signaling is required for YopP-induced cell death, whereas at later times (>4 h postinfection) YopP-induced cell death occurs independent of TLR2/4 signaling.
FIG. 1.
TLR signaling accelerates YopP-induced cell death. DC from TLR2/4 double-knockout mice (TLR2−/−×TLR4−/−) and wild-type mice (wt) were infected with the Yersinia wild-type strain (pYV+) or the YopP-deficient mutant (YopP−). (A) Flow cytometric analysis of DC stained with PI. The data are representative of three independent experiments. (B) Flow cytometric analysis of DC stained with PI (upper panel) and of nuclei (lower panel) 4 h and 24 h after Yersinia infection. The data are the means ± standard errors of the means for three individual experiments. The data for medium are data for untreated cells.
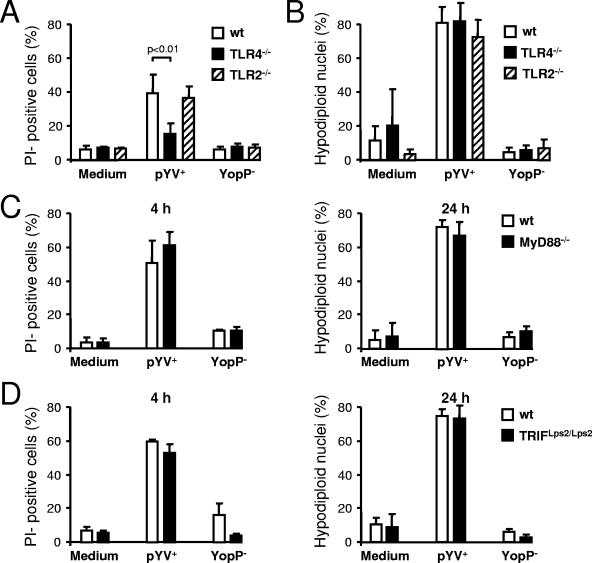
To analyze whether the acceleration of YopP-induced DC death was mediated by TLR2 or by TLR4, DC from single-knockout mice (TLR4−/− and TLR2−/−) were infected, and the DC death rates were assessed. Whereas 4 h after infection the cell death rate was lower for TLR4-deficient DC than for wild-type or TLR2 knockout DC (Fig. 2A), 24 h after infection the levels of hypodiploid nucleus formation were similar in all DC populations (Fig. 2B), indicating that acceleration of DC death was mediated exclusively by TLR4 signaling. Therefore, the results obtained up to 4 h postinfection agree with findings of previous studies performed with macrophages (6, 20), and it seems likely that acceleration of cell death is due to the well-established synergistic effects of LPS via TLR4 signaling and the inhibition of NF-κB activation by YopP/J (16, 21). However, the mechanisms by which this is achieved remain unknown. Recently, it was reported that the TLR adaptors MyD88 and TRIF contribute to the LPS/YopP-dependent apoptotic signaling in macrophages (17, 18). However, 4 and 24 h after infection the death rates of wild-type DC and DC from MyD88 and TRIF knockout mice were similar (Fig. 2C and D) suggesting that acceleration of DC death can be mediated either by MyD88 or TRIF or by other TLR4 adaptor molecules (e.g., TIRAP/Mal) (9).
FIG. 2.
TLR4 but not TLR2 contributes to DC death. DC from TLR single-knockout (TLR2−/− and TLR4−/−), MyD88−/−, TRIFLps2/Lps2, and wild-type (wt) mice were infected with Yersinia (pYV+ or YopP−). (A and B) Flow cytometric analysis of DC stained with PI 4 h postinfection (A) and of nuclei 24 h postinfection (B). (C and D) Analysis of DC stained with PI and of nuclei at different times postinfection. The data are the means ± standard errors of the means for three individual experiments. The data for medium are data for untreated cells.
Cleavage of caspase 8 was postulated previously to cause LPS-triggered YopP-mediated death of macrophages (3, 6, 17, 18). However, as cleavage of caspase 8 has not been observed for infected macrophages, this conclusion was drawn from the results of transfection experiments (13, 14, 17) and experiments using TRIF-deficient macrophages treated with LPS and MG-132 (18).
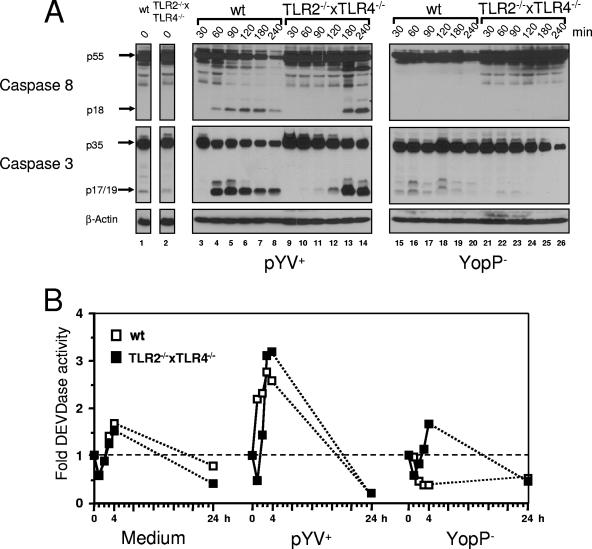
Therefore, immunoblotting for caspase 8 and its substrate, caspase 3, was performed to analyze whether caspase 8 is involved in DC death (Fig. 3A). In wild-type DC caspase 8 and caspase 3 were activated as soon as 60 min after infection with pYV+, as indicated by the detection of cleaved caspase 8 (p18) and caspase 3 (p17/19) fragments (Fig. 3, lane 4). Interestingly, the activation of caspases was delayed by about 120 min in TLR2−/− × TLR4−/− cells (lane 13). This delay was confirmed by measurement of caspase 3 activity by use of the fluorogenic caspase 3 substrate Ac-DEVD-AMC in extracts of infected DC. As shown in Fig. 3B, as soon as 1 and 2 h after Yersinia pYV+ infection the caspase 3 activity, as measured by DEVDase activity of wild-type DC lysates, was markedly increased, while this activity was still low in lysates from TLR2−/− × TLR4−/− DC. The maximum levels of DEVDase activity were reached 3 to 4 h postinfection in both cell types, confirming that there was a delay in caspase 3 activation of about 2 h in TLR2−/− × TLR4−/− DC. These experiments also revealed that 24 h after infection with pYV+ the caspase 3 activity was reduced to background levels in DC, indicating that at this late time apoptotic cell death was already promoted by downstream substrates of caspase 3.
FIG. 3.
TLR4 accelerates YopP-dependent activation of caspases. DC from TLR2/4 double-knockout mice (TLR2−/− × TLR4−/−) and wild-type mice (wt) were infected with the Yersinia wild-type strain (pYV+) or the YopP-deficient mutant (YopP−). (A) Immunoblotting was performed for caspases 8 and 3. The arrows indicate the positions of uncleaved procaspases and the cleaved fragments. (B) DC lysates were incubated with the fluorogenic caspase 3 substrate Ac-DEVD-AMC, and the means of fluorescence units/min (determined in duplicate) were analyzed by fluorometry. The DEVDase activity at zero time was defined as 1. The results are expressed as fold increases in DEVDase activity compared to the activity at zero time. The data are the means for two independent experiments. The data for medium are data for untreated cells.
As the delay in caspase activation paralleled the delay in DC death, we obtained evidence that upstream caspase 8 activation seems to be a key step in YopP-induced apoptosis of DC. On the other hand, as demonstrated recently, YopP-mediated DC death is not completely blocked by the pancaspase inhibitor zVAD-fmk (5). In this study, zVAD-sensitive DC death was accompanied by activation of caspases 3, 8, and 9 and had typical features of apoptosis, while zVAD-insensitive DC death was independent of caspase action and exhibited necrosis-like features. This observation implies that TLR4 signaling might induce early DC death via activation of caspase 8 only under “physiological” conditions and not in the presence of caspase inhibitors.
To examine whether TLR2−/− × TLR4−/− DC also exhibited caspase-independent death, DC were incubated with the pancaspase inhibitor zVAD-fmk 1 h prior to infection. These experiments revealed that after 6 h after infection with wild-type Yersinia the percentage of PI-positive DC treated with zVAD-fmk was only partially reduced, from 61% to 32% (wild-type DC) and from 28% to 15% (TLR2−/− × TLR4−/− DC), indicating that YopP-induced DC death is partially independent of caspases in wild-type DC, as well as in TLR2−/− × TLR4−/− DC (data not shown). Interestingly, cell death in zVAD-fmk-treated DC and therefore caspase-independent cell death were delayed by about 2 h in TLR2−/− × TLR4−/− DC, as indicated by comparable rates of death of wild-type DC 6 h postinfection (32% PI positive) and TLR2−/− × TLR4−/− DC 8 h postinfection (28% PI positive) (data not shown). As the delay was also obvious when DC had not been treated with zVAD-fmk (Fig. 1A), these findings suggest that similar mechanisms are involved in both caspase-dependent and caspase-independent DC death in wild type DC, as well as in TLR2−/− × TLR4−/− DC. Furthermore, these data indicate that there must be a link between the caspase-dependent pathway that is probably initiated by activation of caspase 8 and the caspase-independent DC death pathway. Although we do not have experimental data for this idea, this link might be the Fas-associated death domain (FADD).
Previous data revealed that FADD, which mediates caspase 8 activation, plays an important role in LPS-triggered YopP-induced cell death of macrophages (6, 17). As YopP-induced caspase 8 activation is triggered by TLR4 signaling in DC, we assume that there is an unknown link between the TLR4 receptor and FADD causing early, TLR4-dependent DC death. Besides activation of caspase 8, the death receptor-associated molecule FADD serves as a platform for the recruitment of other adaptor proteins, such as TNFR-associated factor 2 (TRAF2) and receptor-interacting protein (RIP), to build a receptor complex. It has been suggested that RIP and/or TRAF2 are the key mediators of caspase-independent, necrosis-like cell death induced by death receptors (2, 10, 19). Therefore, the early, caspase-independent, TLR4-dependent cell death induced by YopP in DC could be attributed to RIP and/or TRAF2. However, blockade of caspases can increase the sensitivity of the necrotic cell death response to death receptors (11), an effect which could not be excluded in pYV+-infected DC when caspases were blocked by zVAD-fmk.
There are two possibilities to explain TLR4-independent, delayed DC death characterized by delayed caspase activation. First, due to TLR4 knockout LPS signaling is blocked and YopP alone causes death of DC. The recent studies of YopP/J-induced cell death in macrophages and DC suggest that YopP directly or indirectly might cause the formation of the death-inducing signaling complex and thereby caspase 8 activation (3, 6, 17) and the recruitment of RIP and/or TRAF2, causing caspase-independent cell death when caspases are blocked. Second, LPS still contributes to DC death by bypassing TLR4 signaling via an unknown pathway. The latter conclusion might also be drawn from the results of experiments with TLR4-deficient macrophages. In these macrophages IκBα degradation and JNK activation were delayed but not eliminated upon infection with a YopJ-deficient mutant of Y. pseudotuberculosis (20).
In summary, we demonstrated that death of DC is delayed when TLR4 signaling is absent and that this delay is paralleled by a delay in caspase 8 cleavage.
Acknowledgments
This work was supported by grants from the Eberhard Karls University of Tübingen (grants fortüne 1109-0-0, fortüne 1109-0-1, and IZKF 1459-0-0 [Medizinische Fakultät]) and by the DFG (grants AU 102/10-3, AU 102/9-2, and AU 102/9-3).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Chan, F. K., J. Shisler, J. G. Bixby, M. Felices, L. Zheng, M. Appel, J. Orenstein, B. Moss, and M. J. Lenardo. 2003. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 278:51613-51621. [DOI] [PubMed] [Google Scholar]
- 3.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, G. M. van, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 4.Erfurth, S. E., S. Grobner, U. Kramer, D. S. Gunst, I. Soldanova, M. Schaller, I. B. Autenrieth, and S. Borgmann. 2004. Yersinia enterocolitica induces apoptosis and inhibits surface molecule expression and cytokine production in murine dendritic cells. Infect. Immun. 72:7045-7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gröbner, S., S. E. Autenrieth, I. Soldanova, D. S. J. Gunst, M. Schaller, E. Bohn, S. Müller, M. Leverkus, S. Wesselborg, I. B. Autenrieth, and S. Borgmann. 2006. Yersinia YopP-induced apoptotic cell death in murine dendritic cells is partially independent from action of caspases and exhibits necrosis-like features. Apoptosis 11:1959-1968. [DOI] [PubMed] [Google Scholar]
- 6.Haase, R., C. J. Kirschning, A. Sing, P. Schrottner, K. Fukase, S. Kusumoto, H. Wagner, J. Heesemann, and K. Ruckdeschel. 2003. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J. Immunol. 171:4294-4303. [DOI] [PubMed] [Google Scholar]
- 7.Haase, R., K. Richter, G. Pfaffinger, G. Courtois, and K. Ruckdeschel. 2005. Yersinia outer protein P suppresses TGF-beta-activated kinase-1 activity to impair innate immune signaling in Yersinia enterocolitica-infected cells. J. Immunol. 175:8209-8217. [DOI] [PubMed] [Google Scholar]
- 8.Heesemann, J., and R. Laufs. 1983. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J. Bacteriol. 155:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai, T., and S. Akira. 2006. TLR signaling. Cell Death Differ. 13:816-825. [DOI] [PubMed] [Google Scholar]
- 10.Lin, Y., S. Choksi, H. M. Shen, Q. F. Yang, G. M. Hur, Y. S. Kim, J. H. Tran, S. A. Nedospasov, and Z. G. Liu. 2004. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 279:10822-10828. [DOI] [PubMed] [Google Scholar]
- 11.Los, M., M. Mozoluk, D. Ferrari, A. Stepczynska, C. Stroh, A. Renz, Z. Herceg, Z. Q. Wang, and K. Schulze-Osthoff. 2002. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 13:978-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 13.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 15.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 17.Ruckdeschel, K., O. Mannel, and P. Schrottner. 2002. Divergence of apoptosis-inducing and -preventing signals in bacteria-faced macrophages through myeloid differentiation factor 88 and IL-1 receptor-associated kinase members. J. Immunol. 168:4601-4611. [DOI] [PubMed] [Google Scholar]
- 18.Ruckdeschel, K., G. Pfaffinger, R. Haase, A. Sing, H. Weighardt, G. Hacker, B. Holzmann, and J. Heesemann. 2004. Signaling of apoptosis through TLRs critically involves Toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J. Immunol. 173:3320-3328. [DOI] [PubMed] [Google Scholar]
- 19.Shen, H. M., Y. Lin, S. Choksi, J. Tran, T. Jin, L. Chang, M. Karin, J. Zhang, and Z. G. Liu. 2004. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol. Cell. Biol. 24:5914-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Y., and J. B. Bliska. 2003. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect. Immun. 71:1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou, H., D. M. Monack, N. Kayagaki, I. Wertz, J. Yin, B. Wolf, and V. M. Dixit. 2005. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J. Exp. Med. 202:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]