Abstract
Immune control of the protozoan parasite Trypanosoma cruzi requires the activation of both CD4+ and CD8+ T cells. We recently identified two T. cruzi trans-sialidase peptides that are targets of approximately 30% of all CD8+ T cells during acute T. cruzi infection in mice. To determine whether CD4+ T cells are required for generation of these dominant CD8+ T-cell responses, major histocompatibility complex class II (MHC II)-deficient mice were infected with the Brazil strain of T. cruzi and examined for the generation of antigen-specific CD8+ T cells. Strong trans-sialidase TSKB18- and TSKB20-specific CD8+ T-cell responses were generated in both the presence and the absence of CD4+ help. However, the magnitudes of the immunodominant TSKB20-specific CD8+ T-cell responses detectable using class I MHC-peptide tetramers were consistently lower in the blood and spleens of MHC II-deficient mice. Spleen cells from infected MHC II-deficient mice produced gamma interferon after in vitro stimulation with T. cruzi peptides at levels similar to those in wild-type mice, and MHC II-deficient mice displayed strong T. cruzi peptide-specific cytotoxic T-lymphocyte activity in vivo. Thus, primary CD8+ T-cell responses in experimental T. cruzi infection are generated in the absence of CD4+ T cells, providing further evidence that T. cruzi directly activates and licenses antigen-presenting cells. Nevertheless, unhelped CD8+ T cells in T. cruzi-infected mice fail to reach the frequencies achieved in the presence of CD4 T-cell help and are unable to prevent acute-phase death of these mice.
Trypanosoma cruzi is a kinetoplastid protozoan parasite that causes Chagas' disease in humans, one of the most important public health threats in Latin American countries (33). Both CD8+ and CD4+ T cells have been shown to be essential for the control of T. cruzi infection in experimental models; mice lacking either T-cell compartment, due to gene deletion of major histocompatibility complex (MHC) molecules and components (28, 29) or T-cell coreceptors (21) or to depletion of T cells using anti-CD4 or anti-CD8 antibodies (29), succumb to acute infection and display high systemic and tissue parasite loads (27). However, the role that CD4+ T cells play in the development and regulation of CD8+ T-cell immunity to T. cruzi remains to be determined.
The requirement for CD4+ T-cell help in the induction of CD8+ T-cell responses has been studied in a number of murine models of infection, using MHC II knockout (KO) mice or mice depleted of CD4+ T cells by injection of anti-CD4 antibody. CD4+ T-cell activity is required for priming of the CD8+ T-cell response to peptide-pulsed dendritic cells (DCs) (31) and for the optimal activation of pathogen-specific CD8+ T-cell responses to Listeria monocytogenes (15) and influenza virus (19) infections. Nevertheless, strong primary CD8+ T-cell responses against many infectious agents, such as lymphocytic choriomeningitis virus (12) or vesicular stomatitis virus (15), can be measured readily in animals lacking CD4+ T cells (12, 15, 16, 25, 26, 34). One explanation for this dichotomy is that there is a requirement for CD4+ T cells to “license” antigen-presenting cells in models where nonspecific activation or danger signals are not provided by the antigen (Ag) or pathogen (25). In contrast, when the antigen and/or infection itself provides strong inflammatory signals, antigen-presenting cells may be activated directly, and the primary CD8+ T-cell response develops independently of CD4+ T-cell help (6, 26).
Members of our laboratory recently identified immunodominant and subdominant CD8+ T-cell epitopes from the T. cruzi trans-sialidase gene family. At the peak of the CD8+ T-cell response, nearly 30% of the response is directed against TSKB20, while approximately 8% of CD8+ T cells recognize TSKB18. To determine whether the expansion of these immunodominant T. cruzi-specific CD8+ T cells requires CD4+ T-cell help, we studied the kinetics of TSKB20- and TSKB18-specific T-cell responses in MHC II KO mice infected with the Brazil strain of T. cruzi. Ag-specific responses were examined using TSKB20- and TSKB18-MHC I tetramers and by analysis of functional responses of T. cruzi-specific CD8+ T cells in mice lacking CD4+ T cells.
MATERIALS AND METHODS
Mice and parasites.
Age-matched C57BL/6 (B6) mice were purchased from Jackson Laboratory (Bar Harbor, ME). MHC class II-deficient (B6.129-H2-Ab1) and wild-type B6129F1/Tac mice were purchased from Taconic (Germantown, NY). Mice were infected intraperitoneally with 103 Brazil strain trypomastigotes at 8 weeks of age and were sacrificed by CO2 inhalation at 15, 21, 25, or 28 days postinfection. Tissue culture-derived trypomastigotes of the Brazil strain of T. cruzi were generated by passage in Vero cells, which were maintained in RPMI 1640 with 10% fetal bovine serum and standard supplements.
Peptides.
T. cruzi-derived trans-sialidase peptides TSKB18 (ANYDFTLV) and TSKB20 (ANYKFTLV) (14) were synthesized by Sigma Genosys (St. Louis, MO).
Intracellular cytokine staining.
Splenocytes were isolated from naïve and T. cruzi-infected mice as previously described (13) and were stimulated overnight with 1 μM of peptide. GolgiPlug (BD Pharmingen, San Diego, CA) was added to cultures during the last 4 h of incubation. Intracellular cytokine staining for gamma interferon (IFN-γ) was performed with a Cytofix/Cytoperm kit (BD Pharmingen) in accordance with the manufacturer's instructions and as previously described (13). Cells were labeled with anti-IFN-γ-allophycocyanin (clone XMG1.2) and anti-CD8-phycoerythrin (PE) antibodies (both from BD Pharmingen).
MHC class I tetramer staining.
TSKB18 (ANYDFTLV/Kb) and TSKB20 (ANYKFTLV/Kb) tetramers were synthesized at the Tetramer Core Facility (Emory University, Atlanta, GA). T. cruzi-infected mice were bled serially during acute infection, and whole blood was stained with TSKB20/Kb or TSKB18/Kb tetramers as previously described (14). Cells were also stained with anti-CD8-allophycocyanin, anti-CD11b-Cy5-PE, anti-CD4-Cy5-PE, and anti-B220-Cy5-PE (all from Caltag, Burlingame, CA). For determination of the presence of peptide-specific cells in tissues, mice were perfused with 0.8% sodium citrate solution after being sacrificed. Tissue samples from lungs, hearts, and skeletal muscles from legs were removed, teased apart, and incubated in 2 mg/ml collagenase (Sigma, St. Louis, MO) for 30 to 60 min at 37°C. Samples were passed through a mesh screen, washed with PAB (phosphate-buffered saline with 1% bovine serum albumin and 0.05% sodium azide [both from Sigma]), and stained with TSKB18/Kb, TSKB20/Kb, and antibodies as previously described (14). CD8+ cells were gated in the CD4− CD11b− B220− lymphocyte population. Flow cytometry was carried out on a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA), and data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
In vivo cytotoxicity assay.
Spleen cells from naïve C57BL/6 mice were incubated with 10 μM of TSKB20 or TSKB18 or with no peptide for 1 h at 37°C, washed, and then labeled with 20 μM, 3 μM, or 0.3 μM of 5 (and 6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes, Eugene, OR), respectively. Cells were washed, combined, and transferred (3 × 107 total cells) intravenously to naïve and acutely infected mice at 14, 20, and 27 days postinfection. Sixteen hours after transfer, spleens were harvested, and different CFSE-stained populations were detected using a FACSCalibur instrument (Becton Dickinson). Specific killing of peptide-pulsed target cells was determined using the following formula: specific killing = [1 − (% CFSElow naïve cells/% CFSEhigh naïve cells)/(% CFSElow infected cells/% CFSE-infected cells)] × 100, where CFSElow and CFSEhigh indicate low and high concentrations of CFSE, respectively, used to label the population of target cells pulsed with the peptide.
Statistical analysis.
The results in the figures are expressed as means ± standard deviations (SD). Differences between experimental groups were tested for statistical significance by Student's t test for unpaired samples (two-tailed).
RESULTS AND DISCUSSION
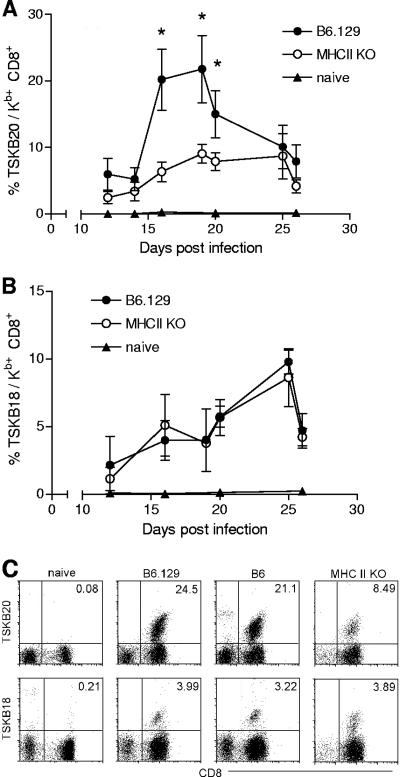
To determine the requirement of CD4+ T cells in the development of peptide-specific CD8+ T-cell responses to T. cruzi infection, MHC II KO mice were infected with 103 Brazil strain parasites, and the kinetics of Ag-specific CD8+ T-cell responses was followed by serially bleeding mice and staining whole blood with peptide-MHC I tetramers. CD8+ T cells specific for the dominant TSKB20 epitope and the subdominant TSKB18 epitope were detected in both wild-type and MHC II KO mice during acute T. cruzi infection (Fig. 1). As previously reported, the TSKB20-specific response in B6 mice infected with the Brazil strain of T. cruzi peaked at approximately 19 to 20 days postinfection, with nearly 20% of all CD8+ T cells recognizing TSKB20-bearing MHC I tetramers (14). The TSKB20-specific CD8+ T-cell response also peaked during this time period in MHC II KO mice, but at <10% of all CD8+ T cells (Fig. 1A). In contrast, the frequencies of TSKB18-specific CD8+ T cells were comparable between B6 and MHC II KO mice (Fig. 1B). Similar frequencies of CD8+ T cells recognizing TSKB20/Kb and TSKB18/Kb tetramers were observed in B6 and B6129F1/Tac mice (the strain used as the wild-type control for MHC II KO mice [11]) (Fig. 1C). In the only previous report of CD8+ T-cell responses to multiple epitopes in the absence of CD4+ T-cell help, no differences were observed in the frequencies of CD8+ T cells specific for the dominant DbNP396 and subdominant DbGP33 epitopes following infection of B6 or CD4−/− mice with lymphocytic choriomeningitis virus (10). Interestingly, in experimental T. cruzi infection, the lack of CD4+ T cells selectively impaired the dominant TSKB20-specific CD8+ T-cell response while leaving the subdominant response to TSKB18 intact. This result raises the possibility that immunodominant CD8+ T-cell responses may become dominant by virtue of their ability to benefit from CD4+ T-cell help.
FIG. 1.
Antigen-specific CD8+ T cells develop in the absence of CD4+ T-cell help. Blood from uninfected naïve mice (filled triangles), infected B6.129 wild-type mice (filled circles), and infected MHC II KO mice (empty circles) was sampled at the indicated time points after infection and stained with H-2Kb tetramers bearing the TSKB20 (A) or TSKB18 (B) T. cruzi peptide. Values represent the means ± SD of tetramer-positive cells among CD8+ T cells. Statistically significant differences (P < 0.05) in the frequencies of peptide-specific CD8+ T cells between MHC II KO and wild-type mice are indicated by asterisks. (C) TSKB20/Kb and TSKB18/Kb staining of blood from naïve mice, infected B6.129 mice, infected B6 mice, and infected MHC II KO mice at 19 days postinfection. Cells shown are gated on CD4− CD11b− B220− lymphocytes. Data are representative of two experiments (n = 3 to 5 mice per group).
Despite the decreased frequencies of TSKB20-specific CD8+ T cells, over 20% of CD8+ T cells in acutely infected MHC II KO mice were clearly T. cruzi specific (Fig. 1A and B). This potent activation of CD8+ T cells in the absence of CD4+ T-cell help provides in vivo evidence that T. cruzi infection activates rather than inhibits DC antigen-presenting functions. T. cruzi-infected DCs have been reported to express low levels of lipopolysaccharide-induced MHC II and costimulatory molecules (30) and to less efficiently present alloantigen (2). However, T. cruzi-infected murine (13) and human (1) DCs stimulate T cells in infected mice and humans, respectively, and DCs isolated from acutely T. cruzi-infected mice induce proliferation of T. cruzi-specific CD4+ T cells (2). The ability of T. cruzi-infected DCs to stimulate parasite-specific T-cell responses, even in the absence of CD4+ T-cell help, coupled with evidence that T. cruzi-derived products, such as glycosylphosphatidylinositol anchors (8) and the thiol-disulfide oxidase-related molecule Tc52 (17a), signal through TLR2, clearly demonstrates that T. cruzi activates DCs to competently process and present antigen.
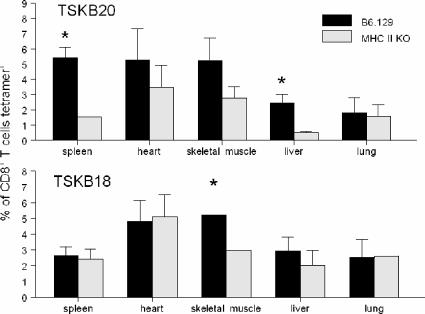
Previous studies have reported that peripheral tissues are differentially dependent on CD4+ T-cell help for maintenance of pathogen-specific CD8+ T cells (18). We therefore examined various peripheral tissues of T. cruzi-infected MHC II KO mice for the presence of parasite-specific CD8+ T cells. CD8+ T cells recovered from spleens and livers had lower frequencies of TSKB20-specific CD8+ cells in MHC II KO mice than in wild-type mice (Fig. 2, top panel), possibly due to the fact that these tissues receive a steady supply of arteriole blood (9) and therefore reflect the situation in the blood. However, no differences in the percentages of TSKB20 tetramer-positive cells between class II MHC-deficient and wild-type mice were detected in lungs, skeletal muscles, or hearts (Fig. 2), suggesting that there is no defect in the tissue migratory capacity of TSKB20-specific T cells. Similar frequencies of TSKB18-specific CD8+ T cells were recovered from tissues of B6 and MHC II KO mice, although there were slightly lower frequencies of these cells in the skeletal muscles of MHC II KO mice than in those of B6 mice (Fig. 2). Altogether, these data indicate that T. cruzi-specific CD8+ T cells generated in the absence of CD4+ T-cell help have little impairment in the ability to migrate to peripheral tissues.
FIG. 2.
Antigen-specific CD8+ T cells are present in peripheral tissues of wild-type and MHC II KO mice. Lymphocytes were recovered from tissues of B6.129 wild-type mice (black bars) and MHC II KO mice (gray bars) 28 days after T. cruzi infection and were stained with TSKB20 (top)- and TSKB18 (bottom)-MHC I tetramers. Bars represent the mean frequencies of CD8+ tetramer-positive lymphocytes for three mice per group; error bars represent SD. Asterisks denote significant differences in the frequencies of CD8+ tetramer-positive lymphocytes between B6/129 wild-type mice and MHC II KO mice (P < 0.05).
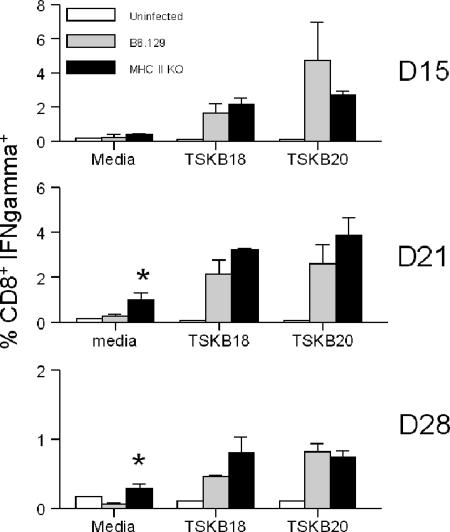
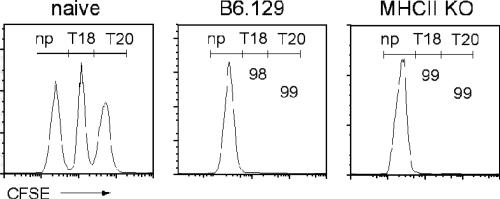
To determine the effector activity of peptide-specific CD8+ T cells generated in MHC II KO mice infected with T. cruzi, IFN-γ production and cytotoxic T-lymphocyte (CTL) activity were measured following exposure of cells to T. cruzi peptides. Splenic CD8+ T cells from T. cruzi-infected MHC II KO mice produced IFN-γ in response to T. cruzi peptides at frequencies similar to those of CD8+ T cells from wild-type mice at 15, 21, and 28 days postinfection (Fig. 3). Spontaneous IFN-γ production by CD8+ T cells from infected MHC II KO mice was increased in comparison to that by infected B6.129 CD8+ T cells at 21 and 28 days postinfection (Fig. 3), possibly due to the higher activation of these cells induced by the high level of parasite antigen in the MHC II KO mice. Class II MHC-deficient mice also exhibited highly efficient killing of TSKB18 or TSKB20 peptide-pulsed target cells (Fig. 4), similar to wild-type B6 mice. These data demonstrate that although mice lacking CD4+ T cells developed decreased frequencies of TSKB20-specific CD8+ T cells, the overall CD8 T-cell-dependent IFN-γ production and cytotoxic activity remained similar to those of wild-type mice.
FIG. 3.
Antigen-specific IFN-γ-producing cells are generated in the absence of CD4+ T-cell help following T. cruzi infection. Spleen cells were harvested from MHC II KO mice (black bars), wild-type mice (gray bars), and uninfected naïve mice (white bars) at days 15, 21, and 28 (D15, D21, and D28) postinfection. Frequencies of IFN-γ-producing CD8+ T cells were determined by intracellular cytokine staining after overnight in vitro stimulation with T. cruzi peptides, as described in Materials and Methods. Values represent the means ± SD of IFN-γ+ cells among CD8+ T cells (n = three mice per group) and are representative of two experiments. Statistically significant differences in IFN-γ production between B6.129 and MHC II KO mice are denoted by asterisks.
FIG. 4.
T. cruzi-infected MHC II KO mice maintain peptide-specific CTL activity. Spleen cells pulsed with T. cruzi peptides (no peptide, TSKB18, or TSKB20) were labeled with CFSE (low, medium, and high concentrations, respectively) and transferred to naïve mice, infected MHC II KO mice, and infected B6.129 wild-type mice as described in Materials and Methods. Numbers represent the percent specific killing of the target cells loaded with the indicated peptide at day 15 postinfection and were calculated as described in Materials and Methods. Histograms are gated on CFSE+ lymphocytes. Data are representative of two experiments (n = three mice per group). np, no peptide pulse; T20, TSKB20; T18, TSKB18.
The exact role of CD4+ T-cell help in the activation and maintenance of Ag-specific CD8+ T cells remains unclear. Certainly, CD8+ T cells expand in response to infection of CD4+ T-cell-deficient mice, although generally not to the same extent as in wild-type mice (10, 12, 15, 16, 18, 24-26, 34). It is possible that NK or γ/δ T cells, both of which are activated by T. cruzi infection, partially compensate for the absence of CD4+ T-cell help (17, 22). However, a lack of CD4+ T-cell help during priming typically results in defective recall responses, poor protective capacity, and a loss of Ag-specific CD8+ T cells over time (3, 6, 10, 24, 26). Several mechanisms have been proposed by which CD4+ T cells promote CD8+ T-cell development and survival. CD4+ T cells engage CD40 on DCs, resulting in maturation of the DCs (4, 20, 23) and promoting the recruitment of CD8+ T cells (5). CD4+ T-cell help may also come in a more direct form to CD8+ T cells, via contact-dependent interactions (7) or soluble mediators (32). Our data support the model that priming of a fully potent CD8+ T-cell response requires T-cell help even in cases where DCs are activated by infection. Continued CD4+ T-cell help has also been implicated in maintaining the memory CD8+ T-cell pool after contraction of the primary response (reviewed in reference 6). Unfortunately, the high susceptibility of MHC II KO mice to T. cruzi infection (100% mortality by D35 after infection [27]) makes it difficult to assess the role of CD4+ T-cell help in the development and maintenance of memory CD8+ T cells in this model.
The data shown herein demonstrate that while CD4+ T-cell help may facilitate development of maximal CD8+ T-cell responses to dominant T. cruzi epitopes, CD4+ T-cell help is generally dispensable for development of functional Ag-specific CD8+ T cells following infection with the protozoan parasite Trypanosoma cruzi. These data are consistent with most studies of pathogen-driven CD8+ T-cell responses in CD4+ T-cell-deficient mice, with the novel observation that dominant but not subdominant CD8+ T-cell responses rely on CD4+ T-cell help to reach peak frequencies. Additionally, these studies suggest that T. cruzi directly licenses antigen-presenting cells in vivo, leading to the generation of strong primary anti-parasitic CD8+ T-cell responses.
Acknowledgments
We thank Julie Nelson of the Center for Tropical and Emerging Global Diseases flow cytometry facility at the University of Georgia for technical assistance and Kim Klonowski for advice on the manuscript.
Grant support was provided to R.L.T. (National Institutes of Health grants AI-022070, AI-044979, and AI-033106).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Albareda, M. C., S. A. Laucella, M. G. Alvarez, A. H. Armenti, G. Bertochi, R. L. Tarleton, and M. Postan. 2006. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int. Immunol. 18:465-471. [DOI] [PubMed] [Google Scholar]
- 2.Alba Soto, C. D., G. A. Mirkin, M. E. Solana, and S. M. Gonzalez Cappa. 2003. Trypanosoma cruzi infection modulates in vivo expression of major histocompatibility complex class II molecules on antigen-presenting cells and T-cell stimulatory activity of dendritic cells in a strain-dependent manner. Infect. Immun. 71:1194-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens, G., M. Li, C. M. Smith, G. T. Belz, J. Mintern, F. R. Carbone, and W. R. Heath. 2004. Helper T cells, dendritic cells and CTL immunity. Immunol. Cell Biol. 82:84-90. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, S. R. M., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. A. P. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 5.Beuneu, H., Z. Garcia, and P. Bousso. 2006. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J. Immunol. 177:1406-1410. [DOI] [PubMed] [Google Scholar]
- 6.Bevan, M. J. 2004. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4:595-602. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297:2060-2063. [DOI] [PubMed] [Google Scholar]
- 8.Campos, M. A., and R. T. Gazzinelli. 2004. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Mediators Inflamm. 13:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crispe, I. N., M. Giannandrea, I. Klein, B. John, B. Sampson, and S. Wuensch. 2006. Cellular and molecular mechanisms of liver tolerance. Immunol. Rev. 213:101-118. [DOI] [PubMed] [Google Scholar]
- 10.Fuller, M. J., D. A. Hildeman, S. Sabbaj, D. E. Gaddis, A. E. Tebo, L. Shang, P. A. Goepfert, and A. J. Zajac. 2005. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174:5926-5930. [DOI] [PubMed] [Google Scholar]
- 11.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Proc. Natl. Acad. Sci. USA 253:1417-1420. [DOI] [PubMed] [Google Scholar]
- 12.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 13.Martin, D. L., and R. L. Tarleton. 2005. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J. Immunol. 174:1594-1601. [DOI] [PubMed] [Google Scholar]
- 14.Martin, D. L., D. B. Weatherley, S. A. Laucella, M. A. Cabinian, M. T. Crim, et al. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzo, A. L., V. Vezys, K. D. Klonowski, S. J. Lee, G. Muralimohan, M. Moore, D. F. Tough, and L. Lefrancois. 2004. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 173:969-975. [DOI] [PubMed] [Google Scholar]
- 16.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomizo, A., F. Cardillo, E. Postol, L. P. de Carvalho, and J. Mengel. 2006. V gamma 1 gammadelta T cells regulate type-1/type-2 immune responses and participate in the resistance to infection and development of heart inflammation in Trypanosoma cruzi-infected BALB/c mice. Microbes Infect. 8:880-888. [DOI] [PubMed] [Google Scholar]
- 17a.Ouaissi, A., E. Guilvard, Y. Delneste, G. Caron, G. Magistrelli, N. Herbault, N. Thieblemont, and P. Jeannin. 2002. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J. Immunol. 168:6366-6374. [DOI] [PubMed] [Google Scholar]
- 18.Pope, C., S. K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402-3409. [DOI] [PubMed] [Google Scholar]
- 19.Riberdy, J. M., J. P. Christensen, K. Branum, and P. C. Doherty. 2000. Diminished primary and secondary influenza virus-specific CD8+ T-cell responses in CD4-depleted Ig−/− mice. J. Virol. 74:9762-9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 21.Rottenberg, M. E., M. Bakhiet, T. Olsson, K. Kristensson, T. Mak, H. Wigzell, and A. Orn. 1993. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect. Immun. 61:5129-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sardinha, L. R., R. M. Elias, T. Mosca, K. R. Bastos, C. R. Marinho, M. R. D'Imperio Lima, and J. M. Alvarez. 2006. Contribution of NK, NK T, gamma delta T, and alpha beta T cells to the gamma interferon response required for liver protection against Trypanosoma cruzi. Infect. Immun. 74:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenberger, S. P., R. E. M. Toes, E. I. H. van der Voort, R. Offringa, and C. J. M. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 24.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 25.Shedlock, D. J., J. K. Whitmire, J. Tan, A. S. MacDonald, R. Ahmed, and H. Shen. 2003. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J. Immunol. 170:2053-2063. [DOI] [PubMed] [Google Scholar]
- 26.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 28.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of [beta]2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 29.Tarleton, R. L., J. Sun, L. Zhang, and M. Postan. 1994. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas' disease. Infect. Immun. 62:1820-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Overtvelt, L., M. Andrieu, V. Verhasselt, F. Connan, J. Choppin, V. Vercruysse, M. Goldman, A. Hosmalin, and B. Vray. 2002. Trypanosoma cruzi down-regulates lipopolysaccharide-induced MHC class I on human dendritic cells and impairs antigen presentation to specific CD8+ T lymphocytes. Int. Immunol. 14:1135-1144. [DOI] [PubMed] [Google Scholar]
- 31.Wang, J. C., and A. M. Livingstone. 2003. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J. Immunol. 171:6339-6343. [DOI] [PubMed] [Google Scholar]
- 32.Williams, M. A., A. J. Tyznik, and M. J. Bevan. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441:890-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 2002. Control of Chagas disease. WHO Tech. Rep. Ser. 905:i-vi, 1-109, back cover. [PubMed] [Google Scholar]
- 34.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 102:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]