Abstract
Sarcoidosis is an enigmatic disease with a pathology similar to that of tuberculosis. We detected Th-1 immune responses to Mycobacterium tuberculosis ESAT-6 and KatG peptides from peripheral blood mononuclear cells from 15/26 sarcoidosis, 1/24 purified-protein-derivative-negative (PPD−) (P < 0.0001, Fisher's exact test), and 7/8 PPD-positive (PPD+) subjects (P = 0.21). This finding provides immunologic links between mycobacteria and systemic sarcoidosis.
While the antigen(s) responsible for eliciting the sarcoidosis Th-1 immune response has not been identified (9, 13, 14), reviews of sarcoidosis immunology and pathology suggest that mycobacterial antigens could be important (6, 8). Previous studies have reported humoral responses to mycobacterial antigens among sarcoidosis subjects (4, 15) as well as the detection of mycobacterial nucleic acids and proteins in sarcoidosis granulomas (3, 5, 15). We performed enzyme-linked immunospot (ELISPOT) assays to assess for cellular recognition of two Mycobacterium sp. antigens (ESAT-6, an immunodominant T-cell antigen present in some members of the Mycobacterium tuberculosis complex but absent in Mycobacterium bovis BCG [2], and KatG, a catalase-peroxidase [7]) from peripheral blood mononuclear cells (PBMC) from 26 sarcoidosis, 24 purified-protein-derivative-negative (PPD−), and 11 PPD-positive (PPD+) subjects.
This study was approved by the Vanderbilt University Institutional Review Board for human studies, and informed written consent was obtained from the study participants or their surrogates. All sarcoidosis subjects from the available patient database of the Vanderbilt University Pulmonary Clinic were invited to participate in the study. For inclusion in this study, the clinical, histological, and microbiologic criteria used to define sarcoidosis were as previously described (3). Healthy PPD− volunteers were required to have undergone PPD testing by the Vanderbilt employee health services. PPD-positive subjects had written documentation of their PPD statuses and had no evidence of active disease at the time of study enrollment.
The amino acid sequences for the 17 ESAT-6 peptides, 15-mers overlapping by 10 amino acids, were as previously described (11). KatG peptides, 15-mers overlapping by 10 amino acids, were derived from the amino acid sequence of M. tuberculosis (GenBank accession number NP 216424) and are listed in Table 1. Each ESAT-6 and KatG peptide was synthesized by solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) chemistry (Genemed Synthesis, San Diego, CA), to a purity of >70%. Identity was confirmed by mass spectroscopy and purity by high-performance liquid chromatography. PBMC were isolated from blood samples drawn into tubes containing EDTA, separated by Ficoll-Hypaque density gradient separation (Amersham Biosciences), cryopreserved in fetal calf serum with 10% dimethyl sulfoxide, and stored in liquid nitrogen until the time of the analysis.
TABLE 1.
Amino acid sequences for M. tuberculosis KatG peptidesa
| Codons | Peptide sequence |
|---|---|
| 91-105 | WPADYGHYGPLFIRM |
| 95-110 | GHYGPLFIRMAWHAA |
| 101-115 | LFIRMAWHAAGTYRI |
| 106-120 | AWHAAGTYRIHDGRG |
| 111-125 | GTYRIHDGRGGAGGG |
| 116-130 | HDGRGGAGGGMQRFA |
| 121-135 | GAGGGMQRFAPLNSW |
| 126-140 | MQRFAPLNSWPDNAS |
| 301-315 | KSSYGTGTGKDAITS |
| 306-320 | TGTGKDAITSGIEVV |
| 311-525 | DAITSGIEVVWTNTP |
| 316-330 | GIEVVWTNTPTKWDN |
| 321-335 | WTNTPTKWDNSFLEI |
| 326-340 | TKWDNSFLEILYGYE |
| 331-345 | SFLEILYGYEWELTK |
GenBank accession number NP 216424.
ELISPOT assays were performed as previously described (1). The number of specific gamma interferon-secreting T cells was calculated by subtracting the mean negative-control value from the mean spot-forming-cell (SFC) count for duplicate wells inoculated with peptide. Negative controls always had <50 SFC per 106 input cells. A positive response was defined as a concentration of at least 50 SFC/106 PBMC that is at least three times higher than the background level. The research assistants were blind to the clinical diagnoses of the study participants throughout the analysis. Comparisons of distributions of T-cell frequencies were performed using the Kruskal-Wallis test. Categorical comparisons were analyzed using Fisher's exact test. All P values are two sided and were determined using R (version 2.1.1).
Sixty-one subjects were recruited for participation in the study. Of the 26 sarcoidosis subjects, 46% were African American, 42% were male, and 58% were 50 years of age or younger. Of the 24 PPD− patients, 33% were African American, 4% were Hispanic, 38% were male, and 88% were 50 years of age or younger (Table 2). Of the 11 PPD+ subjects (8 subjects with latent tuberculosis and 3 BCG vaccinees), 18% were African American, 9% Haitian, 18% Asian Indian, 27% male, and 72% 50 years of age or younger (Table 2).
TABLE 2.
Clinical and demographic characterization of study participantsa
| Subject | Age, race, and sex | Disease site(s) | Immunosuppression group(s) | No. of cellsb for indicated protein
|
|
|---|---|---|---|---|---|
| KatG | ESAT-6 | ||||
| Sarcoidosis 1 | 58, AA, F | C, P | Steroids | 580 | 100 |
| Sarcoidosis 2 | 53, AA, M | P | Pentoxifylline | 480 | 320 |
| Sarcoidosis 3 | 50, C, M | P | None | 300 | 550 |
| Sarcoidosis 4 | 55, C, F | P | Steroids | 300 | 280 |
| Sarcoidosis 5 | 52, AA, F | P | None | 110 | 500 |
| Sarcoidosis 6 | 50, AA, M | P | None | 90 | 130 |
| Sarcoidosis 7 | 58, AA, M | P | Steroids | 510 | <50 |
| Sarcoidosis 8 | 52, AA, F | P | Steroids, pentoxifylline | 500 | <50 |
| Sarcoidosis 9 | 40, C, M | P | None | <50 | 590 |
| Sarcoidosis 10 | 33, C, F | P | None | 420 | <50 |
| Sarcoidosis 11 | 51, C, F | P | Steroids | 370 | <50 |
| Sarcoidosis 12 | 31, AA, M | C, P, CNS | Steroids | 240 | <50 |
| Sarcoidosis 13 | 33, C, F | P | None | <50 | 100 |
| Sarcoidosis 14 | 46, AA, M | P | HIV+ | 120 | <50 |
| Sarcoidosis 15 | 62, C, M | P | None | 110 | <50 |
| Sarcoidosis 16 | 47, C, F | P, CNS | Steroids | <50 | <50 |
| Sarcoidosis 17 | 51, AA, F | C | Steroids | <50 | <50 |
| Sarcoidosis 18 | 42, AA, F | P | Steroids | <50 | <50 |
| Sarcoidosis 19 | 49, AA, F | C, P | Steroids | <50 | <50 |
| Sarcoidosis 20 | 22, C, M | P | Steroids | <50 | <50 |
| Sarcoidosis 21 | 41, C, F | C, P | None | <50 | <50 |
| Sarcoidosis 22 | 47, AA, M | P | None | <50 | <50 |
| Sarcoidosis 23 | 47, C, F | P | Steroids | <50 | <50 |
| Sarcoidosis 24 | 51, C, F | P | None | <50 | <50 |
| Sarcoidosis 25 | 37, C, M | P | None | <50 | <50 |
| Sarcoidosis 26 | 61, C, F | P | Pentoxifylline | <50 | <50 |
| Control 1 | 36, AA, F | None | None | <50 | 380 |
| Control 2 | 37, C, M | None | None | <50 | <50 |
| Control 3 | 23, C, M | None | None | <50 | <50 |
| Control 4 | 58, C, F | None | None | <50 | <50 |
| Control 5 | 25, C, F | None | None | <50 | <50 |
| Control 6 | 30, C, M | None | None | <50 | <50 |
| Control 7 | 27, AA, M | None | None | <50 | <50 |
| Control 8 | 33, C, F | None | None | <50 | <50 |
| Control 9 | 31, C, M | None | None | <50 | <50 |
| Control 10 | 31, C, F | None | None | <50 | <50 |
| Control 11 | 25, H, M | None | None | <50 | <50 |
| Control 12 | 32, C, M | None | None | <50 | <50 |
| Control 13 | 24, C, F | None | None | <50 | <50 |
| Control 14 | 37, C, F | None | None | <50 | <50 |
| Control 15 | 27, AA, F | None | None | <50 | <50 |
| Control 16 | 33, AA, F | None | None | <50 | <50 |
| Control 17 | 33, C, F | None | None | <50 | <50 |
| Control 18 | 31, AA, M | None | None | <50 | <50 |
| Control 19 | 51, C, F | None | None | <50 | <50 |
| Control 20 | 35, C, F | None | None | <50 | <50 |
| Control 21 | 34, AA, F | None | None | <50 | <50 |
| Control 22 | 34, C, M | None | None | <50 | <50 |
| Control 23 | 37, AA, F | None | None | <50 | <50 |
| Control 24 | 32, AA, F | None | None | <50 | <50 |
| PPD+ 1 | 45, C, F | None | None | 400 | 500 |
| PPD+ 2 | 41, AA, F | None | None | 750 | 230 |
| PPD+ 3 | 34, C, F | None | None | 430 | 400 |
| PPD+ 4 | 57, AA, M | None | None | 60 | 500 |
| PPD+ 5 | 49, C, F | None | None | 280 | <50 |
| PPD+ 6 | 63, C, F | None | None | 180 | <50 |
| PPD+ 7 | 64, C, F | None | None | 80 | <50 |
| PPD+ 8 | 49, C, F | None | None | <50 | <50 |
| BCG 1 | 41, A, M | None | None | <50 | <50 |
| BCG 2 | 33, I, M | None | None | <50 | <50 |
| BCG 3 | 42, I, F | None | None | <50 | <50 |
Ages are in years. AA, African American; A, African; C, Caucasian; H, Hispanic; I, Asian Indian; F, female; M, male; C, cutaneous; P, pulmonary; CNS, central nervous system; HIV, human immunodeficiency virus.
Number of gamma interferon-producing spot-forming cells per million PBMC.
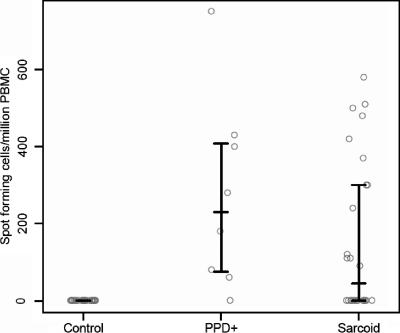
Among the 15 KatG peptides used in the ELISPOT assay, 13 of the 26 sarcoidosis subjects recognized KatG peptide 13 (codons 321 to 335) (Table 2). None of the 24 PPD− or the 3 BCG-vaccinated subjects recognized any of the 15 peptides (Table 2). Seven of the eight subjects with latent tuberculosis infection (Table 2, PPD+ 1 to 8) recognized KatG peptides 13 and 14 (codons 326 to 340). The immune recognition of KatG peptide 13 by 13 of 26 sarcoidosis subjects compared to that by 0 of 24 PPD− control subjects was statistically significant (P < 0.0001) (Table 2). There was no significant difference between the results for sarcoidosis subjects and subjects with latent tuberculosis. We also compared the frequencies of KatG peptide 13-specific T cells in each group. Consistent with the above-described analysis, the T-cell frequencies for sarcoidosis subjects were higher than those for PPD− subjects (P < 0.0001) and lower than those for PPD+ subjects, though the latter difference was not statistically significant (P = 0.17) (Fig. 1).
FIG. 1.
Distribution of T-cell frequencies for PPD− control, PPD+, and sarcoidosis subjects for KatG peptide 13. The bars represent the 25th percentile, median (50th percentile), and 75th percentile for each group; for PPD− controls, these three quantities are all equal to zero. The PPD+ group included the greatest percentage of study participants recognizing KatG peptide 13 as well as the highest median T-cell frequency. Although the sarcoidosis subjects are PPD− and have no culture evidence of infection by mycobacteria, the distribution of the T-cell frequencies for the sarcoidosis subjects was significantly different from that for the PPD− controls (P < 0.0001) and was closer to that for the PPD+ subjects (P = 0.17).
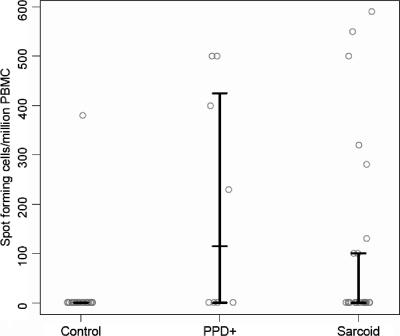
Of the 17 ESAT-6 peptides tested, there was recognition of ESAT-6 peptide 14 (NNALQNLARTISEAG) only. Eight of 26 sarcoidosis subjects, 1 of 24 control subjects (P = 0.024), and 4 of 8 PPD+ subjects (P = 0.41) (Table 2) recognized ESAT-6 peptide 14. While two of the sarcoidosis subjects recognized ESAT-6 peptide 14 only (Table 2, sarcoidosis 9 and 13), six sarcoidosis subjects displayed immune recognition of both ESAT-6 peptide 14 and KatG peptide 13 (Table 2, sarcoidosis 1 to 6). Only one control subject recognized ESAT-6 peptide 14 (Table 2, control 1); the magnitude of this response was similar to that observed in the sarcoidosis and PPD+ participants (Fig. 2). The four PPD+ subjects who recognized ESAT-6 peptide 14 also recognized KatG peptide 13 (Table 2, PPD+ 1 to 4). There was no significant difference in the T-cell frequencies for ESAT-6 peptide 14 between the sarcoidosis and PPD+ subjects (P = 0.27) (Fig. 2). The three subjects with BCG vaccination recognized none of the KatG or ESAT-6 peptides. All study participants exhibited strong responses to phytohemagglutinin. Overall, 15 of 26 (58%) sarcoidosis subjects recognized KatG peptide 13 or ESAT-6 peptide 14, compared to 1 of 24 (4%) PPD− subjects (P < 0.0001) and 7 of 8 PPD+ subjects (P = 0.21).
FIG. 2.
Distribution of T-cell frequencies for the PPD− control, PPD+, and sarcoidosis subjects for ESAT-6 peptide 14. The bars represent the 25th percentile, median (50th percentile), and 75th percentile for each group; for PPD− controls, these three quantities are all equal to zero; for sarcoidosis subjects, the 25th percentiles and medians are both equal to zero. Although all of the sarcoidosis subjects tested were PPD−, there was a significant difference in the distribution of the T-cell frequencies for the sarcoidosis and PPD− subjects (P = 0.024). The lone PPD− subject who recognized ESAT-6 peptide 14 had a T-cell frequency comparable to those for the sarcoidosis and PPD+ subjects. Comparison of the sarcoidosis and PPD+ subjects revealed that there was no significant difference in the distributions of the T-cell frequencies for ESAT-6 peptide 14 (P = 0.27).
This study suggests for the first time that despite the negative culture and histology of sarcoidosis specimens, mycobacterial antigens induce T-cell-specific responses in the blood of sarcoidosis patients at frequencies and magnitudes of response comparable to those for patients who are PPD+. The T-cell frequencies observed in this study are consistent with prior reports of immune recognition of mycobacterial antigens by tuberculosis subjects (Table 2) (10-12). The observation of a cellular immune response to Mycobacterium KatG antigens in 57% of sarcoidosis subjects closely parallels the results for prior PCR analysis (3) as well as the degree of an adaptive immune response to M. tuberculosis KatG proteins among sarcoidosis subjects previously reported in an independent study (15). We did not assess for immune reactivity to the entire KatG protein sequence, so it is possible that other immunogenic peptides exist. The detection of immune recognition of ESAT-6 peptide 14 and KatG peptide 13 is unlikely to be secondary to that of nonspecific reactivity or exposure to routine environmental mycobacteria. First, no significant reactivity was observed among the PPD− control subjects, who live in the same region as the sarcoidosis and PPD+ subjects. Second, ESAT-6 peptide 14 contains the amino acid sequence NNALQNLARTISEAG, which has been previously reported to induce a CD8+ T-cell response from tuberculosis patients (10). The absence of immune reactivity to ESAT-6 peptides by BCG-vaccinated subjects served as a good internal control for the specificity of the ELISPOT assay. M. bovis BCG does not contain esat-6 in its genome; thus, one would not expect to see immune reactivity to 15 ESAT-6 proteins among persons who had undergone BCG vaccination. These results support the hypothesis that mycobacterial antigens may have a role in sarcoidosis pathogenesis by demonstrating that they induce T-cell-antigen-specific Th-1 responses that are known to be important in sarcoidosis granuloma formation. This association does not imply causality but does provide another link between sarcoidosis and mycobacteria, supporting further investigation of the role of mycobacteria in sarcoidosis immunopathogenesis.
Acknowledgments
We thank Wilfred Ajayi and the Vanderbilt Pediatric Immunology Core for technical assistance with this project. We thank Ron du Bois for reviewing the manuscript.
This work was supported by grants from the National Institutes of Health (HL R21077460-01, R01 HL83839-01, and 5M01 RR 00095) and the Robert Wood Johnson Foundation (041300).
Editor: J. L. Flynn
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Altfeld M. A., B. Livingston, and N. Reshamwala. 2001. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 75:1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520. [DOI] [PubMed] [Google Scholar]
- 3.Drake, W. P., Z. Pei, R. D. Collins, D. Pride, T. L. Cover, and M. J. Blaser. 2002. Molecular analysis of sarcoidosis specimens for Mycobacterium nucleic acids. Emerg. Infect. Dis. 8:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubaniewicz, A., S. Kampfer, and M. Singh. 2006. Serum anti-mycobacterial heat shock proteins antibodies in sarcoidosis and tuberculosis. Tuberculosis 86:60-67. [DOI] [PubMed] [Google Scholar]
- 5.Dubaniewicz, A., M. Dubaniewicz-Wybieralska, A. Sternau, Z. Zwolska, E. Izycka-Swieszewska, E. Augustynowicz-Kopec, J. Skokowski, M. Singh, and L. Zimnoch. 2006. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J. Clin. Microbiol. 44:3448-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley, N., C. Lambert, M. McNicol, N. Johnson, and G. A. Rook. 1990. An inhibitor of tumour necrosis factor in the serum of subjects with sarcoidosis, tuberculosis and Crohn's disease. Clin. Exp. Immunol. 80:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heym, B., Y. Zhang, S. Poulet, D. Young, and S. T. Cole. 1993. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J. Bacteriol. 175:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell D. N., J. G. Scadding, B. E. Heard, and K. F. Hinson. 1977. Sarcoidosis: histopathological definition and clinical diagnosis. J. Clin. Pathol. 30:395-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman L. S., C. S. Rose, and L. A. Maier.1997. Sarcoidosis. N. Engl. J. Med. 336:1224-1234. [DOI] [PubMed] [Google Scholar]
- 10.Pathan, A. A., K. A. Wilkinson, R. J. Wilkinson, M. Latif, H. McShane, G. Pasvol, A. V. Hill, and A. Lalvani. 2000. High frequencies of circulating IFN-gamma-secreting CD8+ cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur. J. Immunol. 30:2713-2721. [DOI] [PubMed] [Google Scholar]
- 11.Pathan, A. A., K. A. Wilkinson, P. Klenerman, H. McShane, R. N. Davidson, G. Pasvol, A. V. Hill, and A. Lalvani. 2001. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J. Immunol. 167:5217-5225. [DOI] [PubMed] [Google Scholar]
- 12.Ravn, P., M. E. Munk, A. B. Andersen, B. Lundgren, J. D. Lundgren, L. N. Nielsen, A. Kok-Jensen, P. Andersen, and K. Weldingh. 2005. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 12:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson, B. W., T. L. McLemore, and R. G. Crystal. 1985. Gamma interferon is spontaneously released by alveolar macrophages and lung T-lymphocytes in subjects with pulmonary Sarcoidosis. J. Clin. Investig. 75:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saltini, C., J. R. Spurzem, J. J. Lee, P. Pinkston, and R. G. Crystal. 1986. Spontaneous release of IL-2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+T cell subset. J. Clin. Investig. 77:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song, Z., L. Marzilli, B. M. Greenlee, E. S. Chen, R. F. Silver, F. B. Askin, A. S. Teirstein, Y. Zhang, R. J. Cotter, and D. R. Moller. 2005. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic Sarcoidosis. J. Exp. Med. 201:755-767. [DOI] [PMC free article] [PubMed] [Google Scholar]