Vertebrates, as well as invertebrates and plants, have developed mechanisms to detect and respond to intruders (31, 56). Clearly, inflammation and innate and adaptive immune responses are aimed at destroying the intruders. Gram-negative bacteria contain lipopolysaccharides (LPS) in their outer membranes (82). LPS, which has been studied extensively, is considered the prototypic activator of innate immunity. Picomolar concentrations of LPS are sufficient to stimulate cells of the immune, inflammatory, and vascular systems (81). LPS belongs to the group of molecules produced by pathogens and containing so-called pathogen-associated molecular patterns (PAMPs). PAMPs are recognized by one or more members of a family of transmembrane signaling receptors known as the Toll-like receptor (TLR) family, as well as by intracellular PAMP-detecting molecules, such as nucleotide-binding oligomerization domain 1 (Nod-1), Nod-2, and retinoic acid inducible gene I (RIG-I) (11, 23, 79, 91). To date, 13 different mammalian TLRs have been identified and cloned (77). Activation of TLRs induces intracellular signaling pathways that lead to the production of specific sets of proinflammatory cytokines and chemokines, as well as type I interferons (IFNs) and IFN-inducible gene products (1).
IFN was discovered about 50 years ago as a soluble factor that inhibited viral replication upon induction of specific antiviral genes, such as Oas and Mx, in infected cells. The IFNs were initially classified as classical or type I IFNs and immune or type II IFNs. Type I IFNs consist of multiple alpha IFN (IFN-α) proteins, and single IFN-β, -ɛ, -κ, -ζ (also called limitin), and -ω subtypes, as well as the δ and τ subtypes found in pigs and sheep, respectively (61). IFN-λ1 (interleukin-29 [IL-29]), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B) function somewhat like type I IFN but belong to a new cytokine family (44). Type II IFN consists of a single gene that codes for the cytokine IFN-γ.
The last few years of research have not only led to a much better characterization of the classical antiviral activities of IFN but have also revealed a number of other biologically important immune regulatory functions of type I IFNs. Together, these results led to the conclusion that type I IFNs are essential links between the early innate responses and the subsequent, more specific adaptive immune responses (7, 38). Type I IFNs induce major histocompatibility complex class I expression and have important effects on the maturation and function of dendritic cells (DCs). These IFNs also lead to amplification of their own induction, as well as that of IL-15 and a high-affinity form of the IL-12 receptor, and activate natural killer (NK) cell cytotoxicity (30, 33, 55, 69, 88). Indeed, recent studies have shown that a clear connection exists between type I IFN and antigen-presenting DCs at two levels. First, a specific DC precursor, the plasmacytoid pre-DC (p-preDC), was identified as a cell type that, after stimulation with infectious agents, can secrete very large amounts of type I IFNs. Second, type I IFNs have been shown to act as differentiation and maturation factors for DCs. The signal for the upregulation of costimulatory surface molecules, including CD40, CD80 (B7-1), and CD86 (B7-2), is initiated by LPS, but it is mediated by the IFN-β and the type I interferon receptor (IFNAR) signaling axis (30). Furthermore, type I IFNs are crucial in inducing cytotoxic activity and the proliferation of NK cells (55) and may also play key roles in the induction of effective B-cell responses (10).
In addition, not all cell types express type I IFN, and different cell types also use different molecules to induce the production of type I IFN. Using IRF-7-knockout mice, it was demonstrated that IRF-7 is important in type I IFN induction after virus-activated MyD88-independent signaling, as well as in TLR9-induced MyD88-dependent signaling in DCs (21). Spatiotemporal regulation of MyD88-IRF-7 signaling is critical for type I IFN induction in response to TLR9 activation in pDCs. In cDCs, CpG-A localizes to lysosomes and is unable to induce IFN (32). It also seems that in monocytes no IFN-α is induced after LPS stimulation (8, 26). Also, in contrast to peritoneal macrophages, alveolar macrophages do not produce bioactive IFN-β after TLR3 and TLR4 stimulation (64).
Taken together, the available data suggest that type I IFNs serve as a link between the innate immune response to infection and the adaptive immune response.
In this review, we discuss the importance of type I IFNs in LPS-induced lethal endotoxemia and sepsis, and the rationale for treating endotoxemia in animal models and sepsis in human patients by blocking type I IFN production or activity.
TLR4-MEDIATED TYPE I IFN PRODUCTION
Bacterial LPS is an important structural component of the outer membrane of gram-negative bacteria. It is considered the principal active agent in the pathogenesis resulting from infection with gram-negative bacteria. Indeed, the injection of LPS leads to endotoxemia and endotoxic shock, which closely resemble sepsis and septic shock (6). We briefly review below the major factors involved in the induction of type I IFNs after LPS stimulation and describe the major factors involved in responding to these IFNs.
Beutler and coworkers (63) and Qureshi et al. (65) demonstrated that the genetic defects in two LPS hyporesponsive strains of mice are linked to TLR4. C3H/HeJ mice have a point mutation in the coding region of the Tlr4 gene, resulting in the substitution of a highly conserved proline at codon 712 by histidine, whereas in C57BL/10ScCr mice the Tlr4 gene is deleted. These mutations render these strains resistant to endotoxin (63). Also, C3H/HeJ mice showed enhanced susceptibility to infection by gram-negative bacteria (e.g., Salmonella enterica serovar Typhimurium), indicating that recognition of LPS is essential for clearing the infection (21, 57). It has been demonstrated that the activation of macrophages by LPS results in the release of a variety of inflammatory cytokines, such as tumor necrosis factor (TNF), IL-1, IL-6, IL-8, IL-12, and IFN-β, in addition to smaller mediators such as prostaglandins and nitric oxide (NO) (76). Indeed, it has been shown that peritoneal macrophages of TLR4 knockout mice do not produce any detectable levels of TNF-α and IL-6 (80).
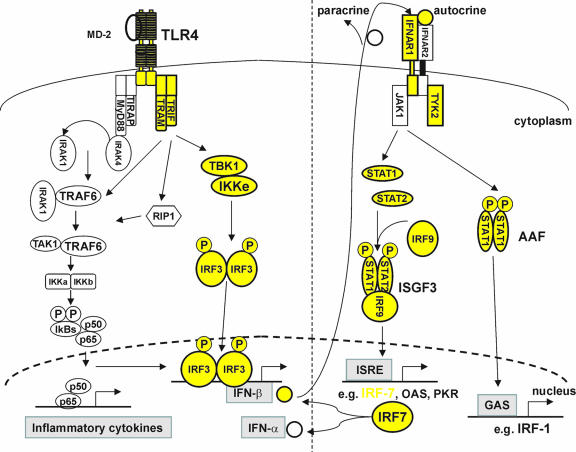
After binding to LPS, TLR4 dimerizes and undergoes a conformational change required for the recruitment of downstream Toll/interleukin-1 receptor (TIR) domain-containing adaptor molecules (Fig. 1). These include the myeloid differentiation primary-response protein 88 (MyD88), the TIR-domain-containing adaptor protein, the TRIF-related adaptor molecule (TRAM), and the TIR-domain-containing adaptor protein inducing IFN-β (TRIF), which together determine the signaling specificity of the response (1). All TLRs, except TLR3, recruit MyD88 in order to activate both NF-κB and the mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK) (1). MyD88 recruits IL-1 receptor-associated kinases through interaction of the death domains. IL-1 receptor-associated kinases are activated by phosphorylation, after which they associate with TRAF6, leading to activation of the IκB kinase (IKK) complex, the degradation of IκB, the nuclear translocation of NF-κB, and the expression of inflammatory cytokines (78, 79).
FIG. 1.
Representation of TLR4-signaling through the MyD88-dependent and MyD88-independent pathways. The MyD88-independent/TRIF-dependent pathway leads to the induction of IFN-β. IFN-β then binds to the IFNAR complex in an autocrine or paracrine way, leading to activation of ISGF3 and AAF. The former binds to IFN-stimulated response elements of IFN-inducible genes, such as those encoding IRF-7, OAS, and PKR. IRF-7 can bind to promoter elements of the genes encoding IFN-β and IFN-α. The molecules highlighted in yellow are those whose deletion in mice leads to resistance to endotoxemia.
Stimulation with LPS also leads to the recruitment of TRIF to the TLR4 receptor complex and consequently to activation of IFN-regulatory factor 3 (IRF-3). This transcription factor induces the expression of the gene encoding IFN-β (1). Hiscott and coworkers identified the kinases responsible for activation of IRF-3. Using two-hybrid screening, they found that IRF-3 was associated with two IKKs (28, 71), namely, TANK-binding kinase 1 (TBK1) and IKK-ɛ/IKK-ι, whose activities are distinct from those of the canonical IKK-α and IKK (79). Mouse embryonic fibroblasts (MEFs) derived from TBK1-deficient (TBK1−/−) mice are impaired in the production of type I IFNs and IFN-inducible gene products in response to LPS but not in the production of proinflammatory cytokines (Table 1) (51). IKK-ɛ−/− mice show normal expression of these genes, but MEFs from IKK-ɛ/TBK1 double-deficient mice, upon specific TLR3 stimulation with poly(I:C), were unable to produce IFN-β and IFN-inducible proteins (note that the TLR3 receptor does not use the MyD88 pathway but only the TRIF pathway). Moreover, in IKK-ɛ/TBK1 double-deficient cells, LPS failed to activate IRF-3 (27). It was also shown that after TLR4 stimulation TRIF can recruit TRAF6-TAK1-TAB2 via its TRAF6-binding site, which is different from its IRF-3 activating site (52). Upon LPS stimulation, phosphorylated IRF-3 dimerizes and translocates to the nucleus. IRF-3 dimers become transcriptionally active after association with p300/CBP coactivators. Activated IRF-3, along with NF-κB, induces the expression of the IFN-β gene (86).
TABLE 1.
Summary of the biological effects, relevant to endotoxemia, observed in mice or cells deficient in genes that are centrally involved in induction of type I IFNs, downstream signaling molecules, or IFNs themselvesa
| Gene KO−/− | LPS stimulation
|
Lethality | Reference | |
|---|---|---|---|---|
| Induction of IFN-β or IFN-dependent genes (mRNA level) | Proinflammatory cytokine production (protein level) or NF-κB activation | |||
| TRIF(m) | ↓↓ | ↓↓ | R | 30 |
| TRIF (c) | ↓↓ | ↓↓ | ND | 89 |
| TRAM (c) | ↓↓ | ↓↓ | ND | 90 |
| TBK1 (c) | ↓↓ | √ (NF-κB) | ND | 60 |
| IKK-ɛ/ι (c) | ↓↓ | ND | ND | 27 |
| IRF-3 | ↓↓ | √ (mRNA level) | R | 67 |
| IFNAR1 | ND | ND | R | 36 |
| TYK2 | ↓↓ | √ (IκB degrad.) | R | 39 |
| STAT1 | ↓↓ | ND | R (±) | 39 |
| STAT2 | ND | ND | ND | 58 |
| IFN-β | ND | √ | R | 39 |
| SOCS-1 | ND | √ (IκB degrad.) | S | 22 |
↓↓, strongly decreased protein/mRNA level; √, inflammatory cytokine production normal; R, resistant to LPS; S, sensitive to LPS; m, mice; c, cells; ND, not done.
JAK-STAT PATHWAY
Once produced, type I IFNs bind to a receptor complex consisting of two subunits, IFNAR1 and IFNAR2, termed the IFNAR complex. Stimulation of the IFNAR complex initiates a signaling cascade mediated by the tyrosine kinases janus kinase (JAK) 1 and tyrosine kinase (TYK) 2, which activate the signal transducer and activator of transcription 1 (STAT1) and STAT2 to form a STAT1/STAT2 heterodimer (13). Other pathways, most notably the p38 MAPK and phosphatidylinositol 3-kinase (PI3K) pathways, are also induced (62). STAT1/STAT2 complexes associate with a p48 protein, identified as IRF-9, to form the IFN-stimulated gene factor 3 (ISGF3). This factor recognizes IFN-stimulated response elements in promoter regions of IFN-responsive genes (ISGs) encoding proteins such as PKR (double-stranded RNA-dependent protein kinase), OAS (2′,5′-oligoadenylate synthetase), Mx1 (myxovirus [influenza] resistance 1), and IRF-7 (13). In addition to being part of ISGF3, STAT1 also forms homodimers that bind to a distinct promoter element, the IFN-γ-activated site (GAS). STAT1 homodimers, called the AAF (IFN-α activated factor) complex, induce the IRF-1 gene, another transcriptional activator (41, 42). When IRF-7 is induced by ISGF3, it becomes phosphorylated and is translocated to the nucleus (3), where it activates the IFN-α/β promoters (49, 69). The induction of serum IFN-α/β by viruses is severely impaired in IRF-7 knockout mice, which shows that IRF-7 is essential for the induction of type I IFN after virus infection (34). IFN-α/β gene induction is more severely impaired by blocking IRF-7 expression than by introducing an IRF-3-null mutation (25). Thus, IRF-7 plays a crucial role in the massive IFN-α/β production through a positive feedback loop (see Fig. 1) (3, 69, 70).
The JAK-STAT pathway is negatively regulated by distinct regulatory proteins, including the suppressors of cytokine signaling, SOCS-1 and SOCS-3, which inhibit the kinase activity of JAK1. STAT1 tyrosine phosphorylation after TLR triggering is severely impaired by SOCS-1 and, to a lesser extent, by SOCS-3. Thus, SOCS proteins, which can be induced by cytokines, as well as by TLR ligands such as LPS and CpG, limit the extent of TLR signaling by inhibiting the type I IFN signaling pathway (4). Recent data illustrate that SOCS-1 is also able to inhibit the activity of Mal, one of the four TLR adaptor molecules (48a).
JAK1 phosphorylation is also negatively regulated by protein tyrosine phosphatases (PTPs), such as SRC homology 2 (SH2)-domain-containing PTP1 (SHP1), SHP2, CD45, and T-cell PTP (TCPTP) (72).
STAT1 is not only regulated by the PTPs SHP2 and TCPTP but also by protein inhibitor of activated STAT (PIAS). STAT1-mediated gene activation is regulated by PIAS1 and protein inhibitor of activated STAT y. PIAS1 blocks the DNA-binding activity of STAT dimers and inhibits STAT1-mediated gene activation in response to IFN-γ (47). Protein inhibitor of activated STAT y acts as a transcriptional corepressor of STAT by recruiting corepressor proteins such as histone deacetylase (46).
SH2-containing inositol 5-phosphatase (SHIP) is a negative regulator of the PI3K-pathway. The PI3K pathway stimulates a number of other pathways, including the MyD88-dependent (45) and the TRIF-dependent (87) pathways. Phosphorylation of STAT1 has recently been shown to be PI3K dependent (66).
IFN-β AS A CRITICAL MEDIATOR IN LETHAL ENDOTOXEMIA
Type I IFNs have long been known to be potent antiviral molecules. In the last few years, however, a critical role for these IFNs in LPS-induced endotoxemia has been elucidated. It has become clear that nonviral PAMPs, such as LPS, induce the expression of type I IFN genes. The essential molecules involved in inducing type I IFNs and in responding to them have also become known. The role of these molecules in endotoxemia, as well as that of the IFNs themselves, is now being investigated in knockout mice and in cells. Table 1 lists the phenotypes relevant to this review. Figure 1 depicts molecules whose knockout affects LPS responsiveness.
The most solid evidence that type I IFNs are central mediators in endotoxemia was provided by Karaghiosoff et al., who showed that IFN-β knockout mice are resistant to lethal endotoxemia induced by high doses of LPS and that they have less serum TNF, NO, and IFN-γ after LPS challenge than wild-type animals (39).
IFNAR1−/− mice are highly susceptible to viral infection. In cells from these mice, no signaling in response to type I IFN was detectable, as measured by the induction of OAS. Also, bone marrow macrophages from IFNAR1−/− mice respond abnormally to LPS (36). We indeed found that IFNAR1-deficient mice completely resist LPS-induced lethal endotoxemia (48). In addition, using macrophages from IFNAR1−/− mice, Vadiveloo et al. found that type I IFNs mediate the induction of cyclin D2 by LPS (83).
A mutant mouse, called Lps2 and generated by random mutagenesis with ENU in the laboratory of B. Beutler, appears to contain a distal frameshift error in a TIR adaptor protein, now known as TRIF or TICAM-1. TrifLps2 homozygote mice are markedly resistant to endotoxemia and fail to produce type I IFNs in response to LPS. LPS-induced STAT1 phosphorylation and IRF-3 dimerization are also impaired in these mice (29). TRIF-deficient mice, generated in the laboratory of S. Akira, are also defective in TLR4- and TLR3-induced expression of IFN-β and activation of IRF-3. Furthermore, TRIF-deficient macrophages are impaired in the production of inflammatory cytokines in response to the TLR4 ligand but not in response to ligands of TLR2, TLR7, and TLR9. Also, poly(I:C)-induced NF-κB activation is severely impaired. In contrast, the induction of NF-κB and MAPK JNK by LPS is almost normal. This might be due to an intact MyD88-dependent early NF-κB activation (89).
TRAM-deficient mice, also generated by S. Akira and coworkers, are defective in LPS-induced cytokine production (TNF-α and IL-6). In TRAM-deficient macrophages (90) LPS failed to induce IFN-β- and IFN-stimulated genes (Ifit2, Cxcl10, and Ccl5) and to activate STAT1.
TBK1 deficiency resulted in TNF-mediated liver degeneration and consequent embryonic mortality (9). In TBK1−/− macrophages, the LPS-induced activation of IRF-3 and STAT1 was absent or greatly diminished. Also, in response to LPS, TBK1−/− macrophages failed to upregulate the transcription of IFN-β- and IFN-mediated transcription of genes encoding CXCL10, CCL5, IFN-α5, IRF-7, IL-15, and Mx1. However, NF-κB activation and the induction of the NF-κB-regulated genes ICAM-1 and IκBα were normal in response to LPS (60).
Embryonic fibroblasts from IKK-ɛ−/− mice responded normally to LPS with respect to IRF-3 activation and the induction of type I IFN. However, the expression of IRG-1 and CXCL10 mRNA in IKK-ɛ−/−TBK1−/− cells was severely impaired, and the induction of IFN-β, IFN-α, and ISG54 mRNA after stimulation with poly(I:C) was abolished (27).
It was also shown recently, using IRF-3 knockout mice, that IRF-3 is indeed essential for LPS-mediated IFN-β gene induction. Loss of IRF-3 also affects the expression profile of other genes, such as some IFN-α subtypes, CXCL10, and IL-15. As would be expected, IRF-3-deficient mice are resistant to LPS-induced endotoxic shock (67).
As described above, the JAK-STAT pathway is involved in the transduction of the signal induced by the IFNAR1 and leading to expression of IFN-responsive genes, as well as the massive upregulation of IFN-α/β genes. The absence of several molecules of the JAK-STAT pathway causes resistance to LPS-induced endotoxemia. In addition, overexpression of negative regulators of the JAK-STAT pathway (see above) can shut down IFN signaling and hence are expected to protect against endotoxemia.
Tyk2 knockout mice are resistant to shock induced by high doses of LPS. The induction of IL-1β, IL-6, IL-12, TNF, and NO in serum were comparable in wild-type and Tyk2-null mice. LPS-induced MyD88-dependent signaling in vitro was intact, as shown by the normal secretion of TNF, the degradation of IκB, and the phosphorylation of p38, ERK1/2, and JNK. In Tyk2-null macrophages, LPS-induced expression of IFN-β and IFN-α4 mRNA was diminished, and the induction of IFNγ mRNA was low. Moreover, the phosphorylation of IRF-3 was normal, but the induction of IRF-1 and IRF-7 mRNA was reduced (39).
Mice defective in STAT1 are resistant to LPS, but not as much as Tyk2 knockout mice. As would be expected, LPS-induced expression of IFN-β mRNA was reduced in the absence of STAT1. However, in contrast to Tyk2-null macrophages, STAT1-null macrophages were not impaired in IFN-γ expression (39).
STAT2-null mice also showed a loss of the type I IFN autocrine/paracrine loop, which affects several aspects of the immune response (58). Furthermore, it was shown in IRF-7 knockout mice that the transcription factor IRF-7 is essential for the induction of IFNα/β genes after virus infection (34).
Deficiency in SOCS-1 leads to early-onset fatal disease. Experiments on cells from SOCS-1 knockout mice demonstrated that SOCS-1 is necessary for the inhibition of IFN-α/β receptor signaling through effects on Tyk2. As expected, SOCS-1−/− mice did not resist LPS, nor did SOCS1−/− IFN-γ−/− mice (22). On the contrary, SOCS-1 is strongly induced by LPS and is an essential protective molecule, because SOCS-1−/− mice were found to be supersensitive to LPS (43, 54). Moreover, SOCS-1−/− mice develop severe inflammatory disease, which appears to result solely from overactivity of the type I IFN signaling cascade and not from enhanced type II IFN activity (18). The overexpression of SOCS-1 or SOCS-3 downregulated the IFN-induced phosphorylation of STAT1 and STAT3. The overexpression of SOCS-1 in cells abolished the mRNA expression of both OAS and Mx1, and the overexpression of SOCS-3 inhibited mainly OAS mRNA expression. Thus, SOCS-1 and SOCS-3 have important negative regulatory effects on the type I IFN-induced activation of the JAK-STAT pathway (85). For example, in macrophages overexpressing SOCS-1 or SOCS-3, induction of the IFN-β-dependent gene CXCL10 was defective, and LPS-induced STAT1 phosphorylation was abolished (4). Interestingly, recent data indicate that protein therapy using cell-permeable SOCS-3 has a curative effect in mouse models of endotoxemia (37).
Not much is known about the potential role of the other JAK-STAT inhibitory molecules SHP1, SHP2, CD45, and TCPTP; the PIAS proteins; and SHIP in endotoxemia. It was shown that SHIP−/− macrophages are hyper-responsive to LPS, that they are not endotoxin tolerant, and that their STAT1 phosphorylation is not diminished after a second exposure to LPS (73). It is clear, however, that SHIP and PTEN proteins are very potent regulators of the TLR4 signaling pathway (10a, 17a).
IFN-α/β AS A THERAPEUTIC TOOL AND TARGET
Almost 50 years of intense research have made it clear that type I IFNs are absolutely essential in the defense of vertebrates against many viruses. First, the IFN genes are strongly conserved, and their orthologues have been found in different species, e.g., fish and birds. Second, deficiency of IFN receptor genes leads to dramatically increased sensitivity for many viruses (36, 53). Third, exogenously added IFNs have antiviral effects, such as the curative effects on hepatitis C-infected patients (59). However, type I IFNs are also important in controlling other diseases and pathologies. In leishmaniasis, low doses of IFN-β protect mice from progressive cutaneous and fatal visceral disease after infection with Leishmania major parasites (50). Also, IFN-β is the most commonly used therapy for relapsing multiple sclerosis (24), and it can also inhibit collagen-induced arthritis in mice (84). Finally, type I IFNs inhibit the proliferation of several human cancers, a therapy that has been evaluated in clinical trials, e.g., for renal cancer (14, 40, 68). Based on these findings, one can conclude that type I IFNs are essential and that they should be given to patients under certain pathological conditions.
It is now also clear that resistance to endotoxemia can be induced by deletion of genes encoding IFN-β, IFNAR1, Tyk2, or other genes involved in the induction of, or in the response to, type I IFNs. The data support the idea that type I IFNs should be considered as mediators in endotoxemia, although the mechanism by which they mediate the toxic effects of LPS is still an open question. However, several studies have clearly shown that type I IFNs can induce the expression of genes encoding other inflammatory molecules, such as NF-IL-6 and many chemokines (12, 15, 48). Chemokines may orchestrate cell migration, which, in collaboration with cytokines and other inflammatory products, can lead to organ damage. Thus, excessive expression of IFN-β could probably lead to organ damage (Fig. 2), and blocking type I IFNs or their upstream or downstream mediators could be an option. A recent study clearly shows that this concept could be used in the treatment of endotoxemia in experimental animals (37). Two central questions, however, need to be addressed.
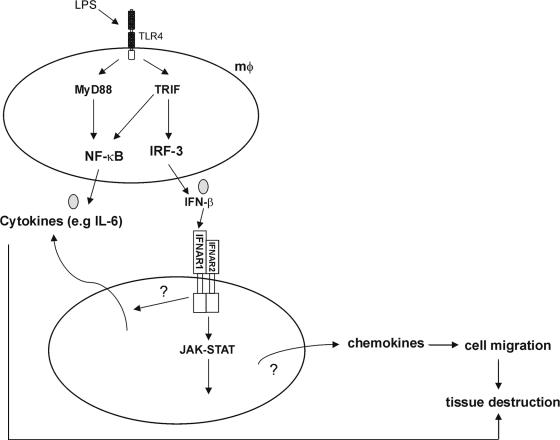
FIG. 2.
Contribution of IFN-β in endotoxemia. IFN-β is produced by macrophages and induces the expression of several genes, especially chemokines, but also other cytokines. Excessive production of IFN-β may lead to organ damage by overstimulating the production of chemokines that may orchestrate cell migration, which, in collaboration with cytokines and other inflammatory products, can lead to organ damage.
First, to what extent will an inhibitory molecule, such as a neutralizing antibody to IFN-β or an IFNAR1 antagonist, compromise the organism's ability to combat viruses and/or bacteria? Studies using IFNAR1-knockout mice indeed indicate that these mice may be resistant to endotoxemia (48) but supersensitive to viral infections (53). Further studies with experimental animals or clinical trials are needed to answer this first question. Indeed, we have to keep in mind that various clinical trials aimed at blocking proinflammatory cytokines such as IL-1 or TNF, which are elevated in sepsis, have failed. For example, studies using TNF- or TNF receptor-deficient mice have confirmed that TNF is a good marker but that it is functionally inert during endotoxemia in mice, since these knockout mice were neither protected nor sensitized during endotoxemia (1a). Furthermore, in the absence of these cytokines, animals may become unable to clear live bacteria and more susceptible to infection. The finding that a certain cytokine knockout mouse strain is protected against LPS may mean that this cytokine is a mediator in endotoxemia but that it may very well form part of a protective network against infection with gram-negative bacteria. For example, IFN-γ receptor knockout mice have proven to be extremely susceptible to BCG, and treatment with infliximab sensitizes for Mycobacterium tuberculosis (17). However, some possibilities can be contemplated. First, e.g., in the case of the IFNAR1, it would be interesting to investigate whether inhibition of the receptor by a certain degree, e.g., 90% could be sufficient to protect experimental animals against sepsis, while the remaining degree, e.g., 10% of receptor activity, could be enough to mount adequate antiviral and antibacterial responses. Whether a sufficiently strong inhibition of the IFNAR1 will be possible has to be evaluated first. Inhibition of ligand could be problematic too, since many type I IFNs may play a role in endotoxemia. Luckily, the resistance of IFN-β deficient mice to endotoxemia resembles that of IFNAR1 knockouts, indicating that IFN-β is the type I IFN playing the predominant role in endotoxemia. Moreover, IFN-β is the major type I IFN induced by LPS (36), although IFN-α4 was also induced by LPS in macrophages (39). Furthermore, septic patients may be treated with IFN-blocking agents over a short time in strictly contained conditions to prevent viral infection.
The second question that needs to be addressed is whether type I IFNs also play a mediating role in real sepsis and not just in endotoxemia. To our knowledge, there are no published studies endorsing this hypothesis in the case of gram-negative sepsis. However, it was clearly demonstrated that type I IFNs are essential in the lethal response of mice to gram-positive Listeria monocytogenes infection, probably because macrophages are sensitized to cell death by the production of type I IFN induced by this organism (75). Although our knowledge of the role of type I IFNs in endotoxemia indicates that they also play a mediating role in sepsis, experimental evidence is needed to confirm this hypothesis. Thus, we should exercise great caution when depicting IFN-β as a possible drug target for sepsis.
Finally, endotoxemia (and probably sepsis) are not the only disorders in which type I IFNs play a detrimental role and in which the type I IFN system should be blocked. Given the diverse and potent effects of type I IFNs in the innate and adaptive immune systems, it is not surprising that they play a pivotal pathogenic role in several autoimmune diseases. Increased levels of IFN-α in serum were found to correlate with exacerbation of systemic lupus erythematosus (5, 35) and insulin-dependent diabetes mellitus (16, 74) in humans and rodents, and IFN-α overexpression in beta cells at the onset of diabetes has been reported in human patients (19).
CONCLUSION
Many recent data suggest that type I IFNs and several molecules involved in inducing them and in responding to them play essential mediating roles in endotoxemia induced by gram-negative cell wall components and in several autoimmune disorders. These data indicate that key molecules, such as IFN-β and IFNAR1, may be considered as new therapeutic targets in endotoxemia in experimental animals and sepsis in human patients, provided that their essential role in antiviral defense and in activation of the immune system are not compromised.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 25 September 2006.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 1a.Amiot, F., C. Fitting, K. J. Tracey, J. M. Cavaillon, and F. Dautry. 1997. Lipopolysaccharide-induced cytokine cascade and lethality in LT alpha/TNF alpha-deficient mice. Mol. Med. 3:864-875. [PMC free article] [PubMed] [Google Scholar]
- 2.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 3.Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. [DOI] [PubMed] [Google Scholar]
- 4.Baetz, A., M. Frey, K. Heeg, and A. H. Dalpke. 2004. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J. Biol. Chem. 279:54708-54715. [DOI] [PubMed] [Google Scholar]
- 5.Bengtsson, A. A., G. Sturfelt, L. Truedsson, J. Blomberg, G. Alm, H. Vallin, and L. Ronnblom. 2000. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9:664-671. [DOI] [PubMed] [Google Scholar]
- 6.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A. 2001. Interferons alpha and beta as immune regulators: a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan, C., J. Mattner, and U. Schleicher. 2004. The role of type I interferons in non-viral infections. Immunol. Rev. 202:33-48. [DOI] [PubMed] [Google Scholar]
- 9.Bonnard, M., C. Mirtsos, S. Suzuki, K. Graham, J. Huang, M. Ng, A. Itie, A. Wakeham, A. Shahinian, W. J. Henzel, A. J. Elia, W. Shillinglaw, T. W. Mak, Z. Cao, and W. C. Yeh. 2000. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 19:4976-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, D., I. Caramalho, and J. Demengeot. 2002. IFN-alpha/beta enhances BCR-dependent B-cell responses. Int. Immunol. 14:411-419. [DOI] [PubMed] [Google Scholar]
- 10a.Cao, X. G. Wei, H. Fang, J. Guo, M. Weinstein, C. B. Marsh, M. C. Ostrowski, and S. Tridandapani. 2004. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J. Immunol. 172:4851-4857. [DOI] [PubMed] [Google Scholar]
- 11.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 12.Coelho, L. F., G. Magno de Freitas Almeidaqq, F. J. Mennechet, A. Blangy, and G. Uze. 2005. Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proc. Natl. Acad. Sci. USA 102:11917-11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 14.Decatris, M., S. Santhanam, and K. O'Byrne. 2002. Potential of interferon-alpha in solid tumours: part 1. BioDrugs 16:261-281. [DOI] [PubMed] [Google Scholar]
- 15.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devendra, D., and G. S. Eisenbarth. 2004. Interferon alpha-a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin. Immunol. 111:225-233. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello, C. 2004. Anti-cytokine therapeutics and infections. Vaccine 21(Suppl. 2):S24-S34. [DOI] [PubMed] [Google Scholar]
- 17a.Fang, H., R. A. Pengal, X. Cao, L. P. Ganesan, M. D. Wewers, C. B. Marsh, and S. Tridandapani. 2004. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J. Immunol. 173:360-366. [DOI] [PubMed] [Google Scholar]
- 18.Fenner, J. E., R. Starr, A. L. Cornish, J. G. Zhang, D. Metcalf, R. D. Schreiber, K. Sheehan, D. J. Hilton, W. S. Alexander, and P. J. Hertzog. 2006. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat. Immunol. 7:33-39. [DOI] [PubMed] [Google Scholar]
- 19.Foulis, A. K., M. A. Farquharson, and A. Meager. 1987. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet ii:1423-1427. [DOI] [PubMed] [Google Scholar]
- 20.Friedman, G., E. Silva, and J. L. Vincent. 1998. Has the mortality of septic shock changed with time. Crit. Care Med. 26:2078-2086. [DOI] [PubMed] [Google Scholar]
- 21.Gautier, G., M. Humbert, F. Deauvieau, M. Scuiller, J. Hiscott, E. E. Bates, G. Trinchieri, C. Caux, and P. Garrone. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201:1435-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gingras, S., E. Parganas, A. de Pauw, J. N. Ihle, and P. J. Murray. 2004. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of Toll-like receptor signaling. J. Biol. Chem. 279:54702-54707. [DOI] [PubMed] [Google Scholar]
- 23.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 24.Hafler, D. A. 2004. Multiple sclerosis. J. Clin. Investig. 113:788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hata, N., M. Sato, A. Takaoka, M. Asagiri, N. Tanaka, and T. Taniguchi. 2001. Constitutive IFN-alpha/beta signal for efficient IFN-alpha/beta gene induction by virus. Biochem. Biophys. Res. Commun. 285:518-525. [DOI] [PubMed] [Google Scholar]
- 26.Hayes, M. P., J. C. Enterline, T. L. Gerrard, and K. C. Zoon. 1991. Regulation of interferon production by human monocytes: requirements for priming for lipopolysaccharide-induced production. J. Leukoc. Biol. 50:176-181. [DOI] [PubMed] [Google Scholar]
- 27.Hemmi, H., O. Takeuchi, S. Sato, M. Yamamoto, T. Kaisho, H. Sanjo, T. Kawai, K. Hoshino, K. Takeda, and S. Akira. 2004. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiscott, J., N. Grandvaux, S. Sharma, B. R. Tenoever, M. J. Servant, and R. Lin. 2003. Convergence of the NF-κB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. N. Y. Acad. Sci. 1010:237-248. [DOI] [PubMed] [Google Scholar]
- 29.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 30.Hoebe, K., E. M. Janssen, S. O. Kim, L. Alexopoulou, R. A. Flavell, J. Han, and B. Beutler. 2003. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat. Immunol. 4:1223-1229. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann, J. A., and J. M. Reichhart. 2002. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 3:121-126. [DOI] [PubMed] [Google Scholar]
- 32.Honda, K., Y. Ohba, H. Yanai, H. Negishi, T. Mizutani, A. Takaoka, C. Taya, and T. Taniguchi. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434:1035-1040. [DOI] [PubMed] [Google Scholar]
- 33.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 35.Hooks, J. J., H. M. Moutsopoulos, S. A. Geis, N. I. Stahl, J. L. Decker, and A. L. Notkins. 1979. Immune interferon in the circulation of patients with autoimmune disease. N. Engl. J. Med. 301:5-8. [DOI] [PubMed] [Google Scholar]
- 36.Hwang, S. Y., P. J. Hertzog, K. A. Holland, S. H. Sumarsono, M. J. Tymms, J. A. Hamilton, G. Whitty, I. Bertoncello, and I. Kola. 1995. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. USA 92:11284-11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo, D., D. Liu, S. Yao, R. D. Collins, and J. Hawiger. 2005. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat. Med. 11:892-898. [DOI] [PubMed] [Google Scholar]
- 38.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karaghiosoff, M., R. Steinborn, P. Kovarik, G. Kriegshauser, M. Baccarini, B. Donabauer, U. Reichart, T. Kolbe, C. Bogdan, T. Leanderson, D. Levy, T. Decker, and M. Muller. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 4:471-477. [DOI] [PubMed] [Google Scholar]
- 40.Kaynor, C., M. Xin, J. Wakefield, J. Barsoum, and X. Q. Qin. 2002. Direct evidence that IFN-beta functions as a tumor-suppressor protein. J. Interferon Cytokine Res. 22:1089-1098. [DOI] [PubMed] [Google Scholar]
- 41.Kimura, T., Y. Kadokawa, H. Harada, M. Matsumoto, M. Sato, Y. Kashiwazaki, M. Tarutani, R. S. Tan, T. Takasugi, T. Matsuyama, T. W. Mak, S. Noguchi, and T. Taniguchi. 1996. Essential and non-redundant roles of p48 (ISGF3 gamma) and IRF-1 in both type I and type II interferon responses, as revealed by gene targeting studies. Genes Cells 1:115-124. [DOI] [PubMed] [Google Scholar]
- 42.Kimura, T., K. Nakayama, J. Penninger, M. Kitagawa, H. Harada, T. Matsuyama, N. Tanaka, R. Kamijo, J. Vilcek, T. W. Mak, et al. 1994. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science 264:1921-1924. [DOI] [PubMed] [Google Scholar]
- 43.Kinjyo, I., T. Hanada, K. Inagaki-Ohara, H. Mori, D. Aki, M. Ohishi, H. Yoshida, M. Kubo, and A. Yoshimura. 2002. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583-591. [DOI] [PubMed] [Google Scholar]
- 44.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 45.Li, X., J. C. Tupper, D. D. Bannerman, R. K. Winn, C. J. Rhodes, and J. M. Harlan. 2003. Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-κB in endothelial cells. Infect. Immun. 71:4414-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, B., M. Gross, J. ten Hoeve, and K. Shuai. 2001. A transcriptional corepressor of Stat1 with an essential LXXLL signature motif. Proc. Natl. Acad. Sci. USA 98:3203-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu, B., J. Liao, X. Rao, S. A. Kushner, C. D. Chung, D. D. Chang, and K. Shuai. 1998. Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. USA 95:10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahieu, T., J. Park, H. Revets, B. Pasche, A. Lengeling, J. Staelens, A. Wullaert, I. Vanlaere, T. Hochepied, F. van Roy, K. M., and C. Libert. 2006. The wild-derived inbred mouse strain SPRET/Ei is resistant to LPS and defective in IFN-b production. Proc. Natl. Acad. Sci. USA 103:2292-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Mansell, A., R. Smith, S. L. Doyle, P. Gray, J. E. Fenner, P. J. Crack, S. E. Nicholson, D. J. Hilton, L. A. O'Neill, and P. J. Hertzog. 2006. Suppressor of cytokine signaling 1 negatively regulated Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7:148-155. [DOI] [PubMed] [Google Scholar]
- 49.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattner, J., A. Wandersee-Steinhauser, A. Pahl, M. Rollinghoff, G. R. Majeau, P. S. Hochman, and C. Bogdan. 2004. Protection against progressive leishmaniasis by IFN-β. J. Immunol. 172:7574-7582. [DOI] [PubMed] [Google Scholar]
- 51.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moynagh, P. N. 2005. TLR signalling and activation of IRFs: revisiting old friends from the NF-κB pathway. Trends Immunol. 26:469-476. [DOI] [PubMed] [Google Scholar]
- 53.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa, R., T. Naka, H. Tsutsui, M. Fujimoto, A. Kimura, T. Abe, E. Seki, S. Sato, O. Takeuchi, K. Takeda, S. Akira, K. Yamanishi, I. Kawase, K. Nakanishi, and T. Kishimoto. 2002. SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677-687. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen, K. B., T. P. Salazar-Mather, M. Y. Dalod, J. B. Van Deusen, X. Q. Wei, F. Y. Liew, M. A. Caligiuri, J. E. Durbin, and C. A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279-4287. [DOI] [PubMed] [Google Scholar]
- 56.Nurnberger, T., F. Brunner, B. Kemmerling, and L. Piater. 2004. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198:249-266. [DOI] [PubMed] [Google Scholar]
- 57.O'Brien, A. D., D. L. Rosenstreich, I. Scher, G. H. Campbell, R. P. MacDermott, and S. B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 124:20-24. [PubMed] [Google Scholar]
- 58.Park, C., S. Li, E. Cha, and C. Schindler. 2000. Immune response in Stat2 knockout mice. Immunity 13:795-804. [DOI] [PubMed] [Google Scholar]
- 59.Pearlman, B. L. 2004. Hepatitis C treatment update. Am. J. Med. 117:344-352. [DOI] [PubMed] [Google Scholar]
- 60.Perry, A. K., E. K. Chow, J. B. Goodnough, W. C. Yeh, and G. Cheng. 2004. Differential requirement for TANK-binding kinase-1 in type I interferon responses to Toll-like receptor activation and viral infection. J. Exp. Med. 199:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pestka, S., C. D. Krause, and M. R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8-32. [DOI] [PubMed] [Google Scholar]
- 62.Platanias, L. C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375-386. [DOI] [PubMed] [Google Scholar]
- 63.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 64.Punturieri, A., R. S. Alviani, T. Polak, P. Copper, J. Sonstein, and J. L. Curtis. 2004. Specific engagement of TLR4 or TLR3 does not lead to IFN-β-mediated innate signal amplification and STAT1 phosphorylation in resident murine alveolar macrophages. J. Immunol. 173:1033-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhee, S. H., B. W. Jones, V. Toshchakov, S. N. Vogel, and M. J. Fenton. 2003. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J. Biol. Chem. 278:22506-22512. [DOI] [PubMed] [Google Scholar]
- 67.Sakaguchi, S., H. Negishi, M. Asagiri, C. Nakajima, T. Mizutani, A. Takaoka, K. Honda, and T. Taniguchi. 2003. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem. Biophys. Res. Commun. 306:860-866. [DOI] [PubMed] [Google Scholar]
- 68.Santhanam, S., M. Decatris, and K. O'Byrne. 2002. Potential of interferon-alpha in solid tumours: part 2. BioDrugs 16:349-372. [DOI] [PubMed] [Google Scholar]
- 69.Sato, M., N. Hata, M. Asagiri, T. Nakaya, T. Taniguchi, and N. Tanaka. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106-110. [DOI] [PubMed] [Google Scholar]
- 70.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 71.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 72.Shuai, K., and B. Liu. 2003. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3:900-911. [DOI] [PubMed] [Google Scholar]
- 73.Sly, L. M., M. J. Rauh, J. Kalesnikoff, C. H. Song, and G. Krystal. 2004. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity 21:227-239. [DOI] [PubMed] [Google Scholar]
- 74.Stewart, T. A. 2003. Neutralizing interferon alpha as a therapeutic approach to autoimmune diseases. Cytokine Growth Factor Rev. 14:139-154. [DOI] [PubMed] [Google Scholar]
- 75.Stockinger, S., T. Materna, D. Stoiber, L. Bayr, R. Steinborn, T. Kolbe, H. Unger, T. Chakraborty, D. E. Levy, M. Muller, and T. Decker. 2002. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J. Immunol. 169:6522-6529. [DOI] [PubMed] [Google Scholar]
- 76.Sweet, M. J., and D. A. Hume. 1996. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 60:8-26. [DOI] [PubMed] [Google Scholar]
- 77.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takeda, K., and S. Akira. 2004. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34:73-82. [DOI] [PubMed] [Google Scholar]
- 79.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 80.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 81.Ulevitch, R. J., and P. S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437-457. [DOI] [PubMed] [Google Scholar]
- 82.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 83.Vadiveloo, P. K., H. Christopoulos, U. Novak, I. Kola, P. J. Hertzog, and J. A. Hamilton. 2000. Type I interferons mediate the lipopolysaccharide induction of macrophage cyclin D2. J. Interferon Cytokine Res. 20:355-359. [DOI] [PubMed] [Google Scholar]
- 84.van Holten, J., K. Reedquist, P. Sattonet-Roche, T. J. Smeets, C. Plater-Zyberk, M. J. Vervoordeldonk, and P. P. Tak. 2004. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res. Ther. 6:R239-R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vlotides, G., A. S. Sorensen, F. Kopp, K. Zitzmann, N. Cengic, S. Brand, R. Zachoval, and C. J. Auernhammer. 2004. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem. Biophys. Res. Commun. 320:1007-1014. [DOI] [PubMed] [Google Scholar]
- 86.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 87.Weinstein, S. L., A. J. Finn, S. H. Dave, F. Meng, C. A. Lowell, J. S. Sanghera, and A. L. DeFranco. 2000. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J. Leukoc. Biol. 67:405-414. [DOI] [PubMed] [Google Scholar]
- 88.Wu, C. Y., M. Gadina, K. Wang, J. O'Shea, and R. A. Seder. 2000. Cytokine regulation of IL-12 receptor beta2 expression: differential effects on human T and NK cells. Eur. J. Immunol. 30:1364-1374. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto, M., S. Sato, H. Hemmi, S. Uematsu, K. Hoshino, T. Kaisho, O. Takeuchi, K. Takeda, and S. Akira. 2003. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4:1144-1150. [DOI] [PubMed] [Google Scholar]
- 91.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]