Abstract
In order to test vaccines against enterotoxigenic Escherichia coli (ETEC)-induced diarrhea, challenge models are needed. In this study we compared clinical and immunological responses after North American volunteers were orally challenged by two ETEC strains. Groups of approximately eight volunteers received 109 or 1010 CFU of E. coli B7A (LT+ ST+ CS6+) or 108 or 109 CFU of E. coli H10407 (LT+ ST+ CFA/I+). About 75% of the volunteers developed diarrhea after challenge with 1010 CFU B7A or either dose of H10407. B7A had a shorter incubation period than H10407 (P = 0.001) and caused milder illness; the mean diarrheal output after H10407 challenge was nearly twice that after B7A challenge (P = 0.01). Females had more abdominal complaints, and males had a higher incidence of fever. Ciprofloxacin generally diminished or stopped symptoms and shedding by the second day of antibiotic treatment, but four subjects shed for one to four additional days. The immune responses to colonization factors CS6 and colonization factor antigen I (CFA/I) and to heat-labile toxin (LT) were measured. The responses to CFA/I were the most robust responses; all volunteers who received H10407 had serum immunoglobulin A (IgA) and IgG responses, and all but one volunteer had antibody-secreting cell (ASC) responses. One-half the volunteers who received B7A had an ASC response to CS6, and about one-third had serum IgA or IgG responses. Despite the differences in clinical illness and immune responses to colonization factors, the immune responses to LT were similar in all groups and were intermediate between the CFA/I and CS6 responses. These results provide standards for immune responses after ETEC vaccination.
Enterotoxigenic Escherichia coli (ETEC)-induced diarrhea is recognized frequently in individuals who travel in developing countries around the world (4, 7, 8, 26, 29, 47) and is a major medical problem for military personnel deployed in these countries (26, 44, 45). Since strict personal hygiene and avoidance of local water and fresh and undercooked foods are recommendations with which travelers have difficulty complying, other means to reduce the ETEC attack rate must be considered. The use of short-term chemoprophylaxis and self-treatment for diarrhea are effective for travelers who are unwilling to accept even a short period of illness because of the serious impact it would have on their overall mission. However, the routine use of antimicrobial prophylaxis for the general traveler is not recommended because of the potential for associated adverse drug reactions and the potential to worsen the problem of antibiotic resistance of enteric bacteria (8, 25, 29, 43, 47). These factors make development of vaccines against ETEC a priority.
ETEC strains produce a heat-labile toxin (LT) and/or a heat-stable toxin (ST) that are largely responsible for the symptoms of diarrhea. LT is immunogenic, but ST is a peptide and nonimmunogenic. ETEC strains also express antigenically distinct fimbriae collectively termed colonization factors (CF) (5). The most common CF include colonization factor antigen I (CFA/I), CFA/II, and CS6 (59). The protective role of the host immune response to specific antigens, such as CF or toxins, or of specific components of the immune system, such as secretory immunoglobulin A (IgA) in the intestine, is not well defined. Previous studies showed that CF were protective when volunteers were challenged with homologous strains of ETEC after they were either passively immunized with immunoglobulin with specific activity against CF (18, 54) or actively immunized or infected (9, 12, 14, 34, 36, 37, 55). Antitoxic protection based on an immune response to the heat-labile toxin was suggested based on field trials by Clemens et al. (6), who used the B subunit of whole-cell cholera vaccine and induced cross-protection against severe diarrhea caused by ETEC LT in immunized volunteers.
The data described above suggest that an ETEC vaccine would have to include the most prevalent colonization factors and have antitoxin activity. Thus, vaccine development is complicated because a vaccine would require several CF, as well as LT, and would require tests of a number of vaccine prototypes. Because there is no established immunological correlate of protection against ETEC diarrhea, the efficacy of vaccine candidates that are developed is likely to be evaluated after a direct challenge to humans with ETEC.
The use of human volunteers for studying immunity and the pathogenesis of ETEC infection after oral ingestion of strain B7A or H10407 has been described previously. ETEC strain B7A has been given to nearly 50 volunteers (9, 35, 36). B7A is a serotype O148:H28 strain and expresses LT, ST, CS6 (40), longus (19), and an unidentified adhesin (33). The most common symptoms in volunteers challenged with B7A were diarrhea, abdominal cramps, and malaise. Previous exposure to B7A protected volunteers from illness but not shedding (36). The level of serum antibody response to lipopolysaccharide varied from 20 to 90% (9, 36), but the immune response to other antigens has not been reported.
E. coli strain H10407 is the most commonly used ETEC challenge strain and has been given to over 200 volunteers (2, 11-14, 18, 20, 35, 37, 54). It is a serotype O78:K80:H11 strain and was isolated from an adult with severe diarrhea in Dacca, Bangladesh (13). H10407 expresses the enterotoxins LT (50), ST (both STIa and STIb) (41), and CFA/I (13). It also expresses EAST (60), Tia (15), Tib (38), Leo (16), and EatA (46), which may play roles in virulence. The symptoms in volunteers challenged with H10407 were (from most frequent to least frequent) gurgling, abdominal cramps, diarrhea, anorexia, malaise, nausea or vomiting, headache, and fever. The diarrheal stool volumes ranged from 0.1 to 11 liters, and the time until onset of symptoms after challenge was typically 2 days. Nearly all challenged volunteers shed H10407, and shedding lasted approximately 1 week unless the volunteers were treated with antibiotics. Previous exposure to H10407 protected volunteers from illness but not shedding (37). Serum IgG responses to CFA/I, LT, and O78 were detected after challenge (13, 20, 35, 37, 49, 54) in many volunteers, but secretory IgA was assayed in only one study (35).
Here we describe the first direct comparison of two well-established ETEC challenge models, B7A-induced clinical illness and H10407-induced clinical illness. Clinical presentation, the effect of ciprofloxacin, and the immune responses to CS6, CFA/I, and LT are described below. The mucosal immune response was measured using antibody-secreting cells (ASC), and the systemic response from sera was measured using quantitative methods. This is the first time that the immune response to CS6 has been measured quantitatively after infection with wild-type ETEC.
MATERIALS AND METHODS
Inocula.
H10407 and B7A were provided by Myron M. Levine of the Center for Vaccine Development, University of Maryland School of Medicine, Baltimore. Seed stocks of H10407 and B7A were prepared using current good manufacturing practices at the WRAIR Pilot Bioproduction Facility, Silver Spring, MD. Challenge inocula were prepared as described by Levine et al. (36), bacteria were harvested after overnight incubation on tryptic soy agar and suspended in sterile saline, and the concentrations were adjusted to obtain target concentrations of 1010 CFU/ml B7A and 109 CFU/ml H10407. The number of bacteria administered to volunteers was determined retrospectively pre- and postchallenge. Volunteers were challenged with 1.5 × 109 or 1.4 × 1010 CFU of strain B7A and with 1.2 × 108 or 1.4 × 109 CFU of H10407.
Volunteers.
Volunteers were recruited from the local community, and written informed consent was obtained using a protocol approved by appropriate institutional review boards. White and African American males (n = 20) and nonpregnant females (n = 11) ranging from 18 to 55 years old volunteered for this study. The volunteers were determined to be in good health with regular bowel habits, with at least three stools of normal consistency per week and two or fewer stools per day without frequent use of laxatives or antidiarrheal agents.
Challenge and clinical assessment.
Two 13-day inpatient studies were carried out at the United States Army Medical Research Institute of Infectious Diseases. The study was not blinded for strain; however, neither the investigators nor the volunteers were informed of the dose that a volunteer received. Volunteers drank 120 ml of 1.3% sodium bicarbonate buffer, followed 2 min later by 30 ml of buffer containing bacteria. Volunteers fasted for at least 90 min before and after challenge.
The volunteers were assessed, and vital signs were recorded. Symptoms were graded as follows: mild (no limitation in activity, no intervention needed), moderate (mild to moderate limitation in activity, no or minimal treatment and/or intervention needed), or severe (inability or difficulty performing daily activities, treatment and/or intervention needed). Fever was defined as an oral temperature of ≥100.5°F. Each stool was collected, weighed, assessed to determine the presence of blood, and graded as previously described (35), as follows: grade 1, firm; grade 2, soft but did not take the shape of the container; grade 3, predominantly thick liquid that took the shape of the container; grade 4, opaque watery liquid; grade 5, clear watery liquid. Diarrhea was classified as mild (two or three loose stools and not more than 400 g of diarrheal stools/24 h), moderate (four or five loose stools and 401 to 799 g of diarrheal stools/24 h), or severe (six or more loose stools and 800 g or more of diarrheal stools/24 h or two loose stools/day, regardless of stool weight, that occurred with fever and at least two of the following symptoms graded as severe: headache, nausea, generalized aches, abdominal cramps, gas, emesis, malaise, and fatigue).
To clear the infection, all volunteers were treated with ciprofloxacin administered orally at a dose of 500 mg twice a day for 5 days. Antibiotic treatment began when a volunteer had moderate diarrhea for three consecutive days or had severe diarrhea. If neither of these criteria was met, the volunteer was treated with antibiotics on day 6.5 after challenge. Volunteers were discharged from the ward after completing the 5-day course of antibiotics and when two consecutive stool cultures were found to be negative.
Shedding.
Stool samples were graded, weighed, and streaked for isolation directly onto MacConkey agar. One gram of stool was diluted in sterile phosphate-buffered saline and plated on MacConkey agar for qualitative culture of the stool flora. If no stool was passed, rectal swabs were plated. The identities of presumptive ETEC isolates were confirmed by colony blotting using antisera raised in rabbits against H10407 or B7A. A stool was considered negative for ETEC if no E. coli was isolated or if 10 colonies were negative as determined by immunoblotting. Colonization was defined as isolation of challenge strain B7A or H10407 in two stool specimens collected at least 24 h after challenge.
Test antigens.
CFA/I was provided by P. Askolof, SBL Vaccine, Stockholm, Sweden. LTB was provided by J. D. Clements, Tulane University, New Orleans, LA. CS6 (20 mg/ml) was provided by F. Cassels, Walter Reed Army Institute of Research, Silver Spring, MD.
Antibody-secreting cells.
ASC responses to CS6, CFA/I, and LT were chosen as a surrogate for an intestinal mucosal immune response. Blood was collected on days 0, 5, 7, 10, 14, and 28. Peripheral blood mononuclear cells (PBMC) were isolated from EDTA tubes (Becton Dickinson Vacutainer Systems, Rutherford, NJ) containing venous blood by gradient centrifugation on Ficoll-Hypaque (Sigma Co., St. Louis, Mo). Cryopreservation of PBMC was accomplished by suspending isolated PBMC at a concentration of 2 × 107 cells/ml in ice-cold Cell Freezing Medium dimethyl sulfoxide 1× (Sigma) and freezing them using a controlled-rate freezing device (1). Frozen cells were stored in liquid or vapor-phase nitrogen until they were assayed to determine the ETEC-specific numbers of IgA ASC by the enzyme-linked immunospot assay.
PBMC were assayed to determine the total number of ASC and the number of ETEC-specific IgA ASC by the enzyme-linked immunospot technique as previously described for CS6 and LT (21). For CFA/I responses, the plates were coated with 0.1 ml of purified CFA/I (20 μg/ml).
A positive ASC response was defined as a ≥2-fold increase over the baseline value for the number of ASC per 106 PBMC, when the number of ASC was 0.5 cell per 106 PBMC in the baseline sample. If the number of preimmune ASC was less than 0.5 cell per 106 PBMC, a value of more than 1.0 cell per 106 PBMC after dosing was considered a positive response.
Serum antibody.
Sera were collected on days 0, 5, 7, 10, 14, and 28. The IgA and IgG antibody titers against LT were determined by the GM1 enzyme-linked immunosorbent assay (ELISA) method (27, 53), and the titers against CFA/I and CS6 were determined by an ELISA as previously described (22, 52). The endpoint titers were defined as the interpolated dilutions of the samples giving an absorbance at 450 nm that was 0.4 absorbance unit above the background value. The titers were adjusted in relation to the results obtained with a reference specimen included in each test to compensate for variations between assays performed on different days. For all antigens, pre- and postchallenge serum samples from the same individual were always tested side by side. The antibody titer ascribed to each sample represented the geometric mean for duplicate determinations performed on different days. Serum samples with undetectable titers (i.e., a reciprocal endpoint titer of <5.0) were assigned a value of 2.5 for computational purposes. Based on calculations of the methodological error of each ELISA, a significant response was defined as a ≥2-fold increase in the endpoint titer between pre- and postchallenge specimens, with the added criterion that the postchallenge reciprocal titer had to be >10 (28).
Statistical analyses.
Exploratory data analyses were performed using the STA statistical software (version 6; SAS Institute Inc.). Data were analyzed by using the Fisher exact two-tailed test at a 95% confidence level or by using Student's t test. Comparisons were made within and between strains. The SPSS Base 10 software (SPSS Inc., Chicago, IL) was used to analyze the immunological data.
RESULTS
Clinical symptoms.
The most common adverse events were diarrhea, nausea, loss of appetite, intestinal cramping, intestinal gurgling, headaches, malaise, and fatigue (Table 1). These symptoms are similar to those reported for previous challenges with B7A (9, 35, 36) and H10407 (2, 11, 12, 14, 18, 20, 35, 37, 54). Symptoms were less frequently reported and less severe after B7A challenge than after H10407 challenge (Table 1), and they were milder in volunteers challenged with the lower dose of B7A than in volunteers challenged with the higher dose. For the volunteers who had diarrhea, the mean time from challenge to the first diarrheal stool was significantly shorter for the B7A groups than for the H10407 groups (P = 0.0001) (Table 2) and was shorter than the times in previous studies (14, 55, 58). For these volunteers, the mean total stool weights were significantly higher for the H10407 groups than for the B7A groups for the same dose (109 cells) or any dose (P = 0.0123). The stool volume and duration of symptoms were likely reduced by antibiotic treatment. Oral ciprofloxacin administration generally diminished or stopped symptoms by the second day of antibiotic treatment.
TABLE 1.
Comparison of clinical symptoms for the B7A and H10407 groups
| Symptom | No. of volunteersa
|
|||||
|---|---|---|---|---|---|---|
| B7A
|
H10407
|
|||||
| 109 CFU (n = 8) | 1010 CFU (n = 8) | Total (n = 16) | 108 CFU (n = 7) | 109 CFU (n = 8) | Total (n = 15) | |
| Diarrhea | 3 | 8 | 11 | 6 | 7 | 13 |
| Cramps | 2 | 3 | 5 | 5 | 8 | 13 |
| Fatigue | 0 | 4 | 4 | 5 | 5 | 10 |
| Emesis | 0 | 3 | 3 | 3 | 4 | 7 |
| Malaise | 0 | 2 | 2 | 4 | 4 | 8 |
| Lightheadedness | 0 | 0 | 0 | 3 | 5 | 8 |
| Headache | 2 | 3 | 5 | 4 | 5 | 9 |
| Gurgling | 1 | 4 | 5 | 5 | 7 | 12 |
| Nausea | 1 | 3 | 4 | 3 | 5 | 8 |
| Appetite loss | 1 | 0 | 1 | 5 | 6 | 11 |
| Body ache | 0 | 3 | 3 | 4 | 4 | 8 |
| Subjective feverb | 0 | 2 | 2 | 4 | 4 | 8 |
| Objective fever | 1 | 2 | 3 | 3 | 3 | 6 |
The values are the numbers of volunteers who were ill (moderate or severe illness).
Fever was defined as a body temperature of ≥100.5°F.
TABLE 2.
Comparison of diarrhea after challenge with B7A or H10407
Gender differences in clinical symptoms.
Although there was no gender difference for the occurrence of diarrhea in the B7A and H10407 groups, the ratios of sick individuals to total infected volunteers for fever, nausea, cramps, and appetite loss were significantly different for the females and males (Table 3). In the H10407 group, the incidence of fever for males (5/9) was significantly higher than the incidence of fever for females (0/6) (P = 0.04). In contrast, in the B7A group, the complaints of gastrointestinal disturbances, including nausea, abdominal cramps, and appetite loss, were significantly higher for females than for males (P = 0.026, P = 0.034, and P = 0.001, respectively).
TABLE 3.
Adverse events after challenge with B7A and H10407, by gender
| Adverse event | B7A
|
H10407
|
||||
|---|---|---|---|---|---|---|
| No. of volunteers
|
P valuea | No. of volunteers
|
P valuea | |||
| Female (n = 5) | Male (n = 11) | Female (n = 6) | Male (n = 9) | |||
| Diarrhea | 5 | 8 | 0.51 | 5 | 8 | 1.00 |
| Tenesmus | 1 | 2 | 1.00 | 3 | 4 | 1.00 |
| Cramps | 5 | 4 | 0.034b | 5 | 9 | 0.40 |
| Fatigue | 3 | 2 | 0.25 | 4 | 8 | 0.53 |
| Emesis | 2 | 0 | 0.08 | 2 | 5 | 1.00 |
| Malaise | 1 | 1 | 1.00 | 2 | 7 | 0.14 |
| Lightheadedness | 1 | 2 | 1.00 | 2 | 8 | 0.09 |
| Headache | 4 | 5 | 0.31 | 2 | 5 | 0.61 |
| Gurgling | 4 | 6 | 0.59 | 5 | 9 | 0.4 |
| Nausea | 5 | 3 | 0.026b | 2 | 8 | 0.09 |
| Appetite loss | 5 | 1 | 0.0014b | 3 | 8 | 0.14 |
| Body ache | 2 | 3 | 1.00 | 2 | 7 | 0.14 |
| Subjective feverc | 1 | 2 | 1.00 | 2 | 6 | 0.32 |
| Objective fever | 2 | 1 | 0.21 | 0 | 5 | 0.044b |
P values for comparisons of females and males within the same group.
Statistically significant.
Fever was defined as a body temperature of ≥100.5°F.
Shedding of ETEC and ciprofloxacin effect.
All volunteers shed ETEC after challenge. ETEC was usually shed in the second stool after challenge, but the second stool occurred earlier in volunteers given B7A than in volunteers given H10407 (Table 2). The shortest time to excretion was 3.4 h, and the longest time to excretion was 59 h. There was no apparent relationship between inoculum size and time to excretion. The higher the grade of stool, the higher the relative proportion of ETEC, but ETEC was detected in stools of all grades.
There was a qualitative decrease in the numbers of bacteria on MacConkey agar between 2 and 93 h (median, 22 h) after ciprofloxacin treatment began. Although the decline in the number of bacteria was swift and dramatic, it took considerably more time for eradication of all facultative aerobes from the stools. The time until the first stool without ETEC ranged from 6 h to 144 h (median, 45 h). Volunteers who received H10407 were asked to submit stools after they were discharged from the ward to assess the recovery of the bacterial flora after ciprofloxacin treatment. By the end of the first week after ciprofloxacin treatment, 5 of 12 volunteers were colonized by lactose fermenters. By 3 weeks after the course of ciprofloxacin, the same proportion of volunteers (9 of 12) was colonized with lactose fermenters that was colonized before the challenge. The nine volunteers who were colonized prechallenge were not the same nine volunteers who were colonized after treatment with ciprofloxacin.
Immune response to CS6 and LT after B7A challenge.
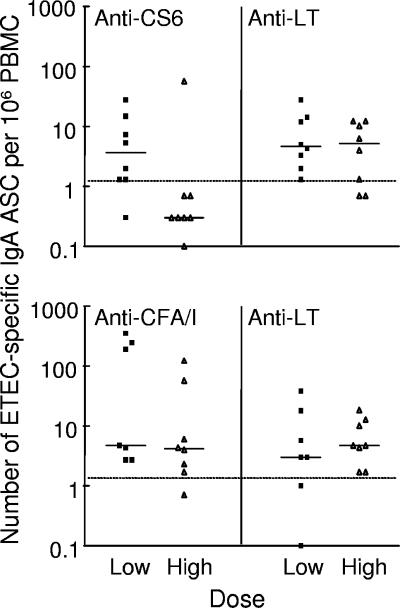
B7A challenge induced significant IgA ASC responses to CS6 in one-half of the volunteers and significant IgA ASC responses to LT in 88% of the volunteers (Table 4). The peak numbers of CS6- and LT-specific IgA ASC are shown in Fig. 1. The frequency of anti-CS6 IgA ASC responders for the volunteers who received 109 B7A cells was significantly higher (P < 0.01) than the frequency for the volunteers who received 1010 B7A cells, but there were no significant differences in the anti-LT ASC response rates between these groups. Among responders, the highest numbers of CS6- and LT-specific IgA ASC were observed on days 7 and 10 after challenge, and the geometric mean increases in the numbers of IgA ASC were >12-fold for CS6 and >9-fold for LT.
TABLE 4.
Immunologic response rates in volunteers after oral challenge with ETEC strain B7A
| Dose (CFU) | n | No. (%) with significant IgA-ASC responses
|
No. (%) with significant serum antibody responses
|
||||
|---|---|---|---|---|---|---|---|
| Anti-CS6 | Anti-LT | IgA anti-CS6 | IgG anti-CS6 | IgA anti-LT | IgG anti-LT | ||
| 109 | 8 | 7 (88)a | 6 (75) | 4 (50) | 3 (38) | 7 (88) | 6 (75) |
| 1010 | 8 | 1 (13) | 8 (100) | 1 (13) | 1 (13) | 5 (63) | 5 (63) |
| All | 16 | 8 (50) | 14 (88) | 5 (31) | 4 (25) | 12 (75) | 11 (69) |
P < 0.01, as determined by Fisher's exact test, for a comparison of the ASC response rates for volunteers who received 109 CFU with the ASC response rates for volunteers who received 1010 CFU.
FIG. 1.
Peak number of ETEC-specific IgA ASC after challenge with E. coli B7A (top panel) or H10407 (bottom panel). Each symbol represents one volunteer. The solid lines indicate the medians, and the dotted lines indicate the threshold for a positive response.
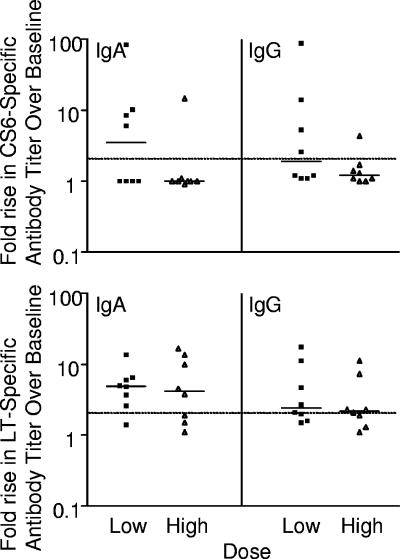
There were no significant differences between the dose groups with respect to the frequency and magnitude of serum anti-CS6 and anti-LT responses. B7A challenge induced significant CS6-specific IgA and IgG responses in 5 of 16 (31%) and 4 of 16 (25%) volunteers, respectively (Table 4). Among the responders, the peak geometric mean increases in CS6-specific IgA and IgG titers compared with the baseline titers were 15-fold (range, 6.0- to 85-fold) and 13-fold (range, 4.3- to 89-fold), respectively (Fig. 2). In the majority of volunteers, the peak anti-CS6 antibody titers were detected on day 10 for IgA and on day 14 for IgG.
FIG. 2.
Peak increases in serum antibody titers to CS6 and LT after challenge with ETEC strain B7A. Each symbol represents one volunteer. The solid lines indicate the medians, and the dotted lines indicate the threshold for a positive response.
B7A challenge induced significant serum IgA and IgG anti-LT responses in 75% and 69% of the volunteers, respectively (Table 4). Among the responders, the peak geometric mean increases in LT-specific IgA and IgG titers compared with the baseline titers were 6.1-fold (range, 2.6- to 17-fold) and 4.0-fold (range, 2.0- to 18-fold), respectively (Fig. 2). In the majority of these volunteers, the peak anti-LT antibody titers were detected on day 10 for IgA and on days 14 and 28 for IgG.
Immune response to CFA/I and LT after H10407 challenge.
ETEC H10407 challenge induced significant IgA ASC responses to CFA/I in 93% of the volunteers and to LT in 87% of the volunteers (Table 5). The peak numbers of CFA/I- and LT-specific IgA ASC after H10407 challenge are shown in Fig. 1. The geometric mean increases in IgA ASC were >26-fold for CFA/I and >10-fold for LT. In contrast to the responses to other antigens, the ASC response to CFA/I remained high from day 7 to day 28 after challenge, and eight volunteers had peak responses on day 14 or 28. The volunteers with “late” ASC responses to CFA/I had no or normal ASC responses to LT (ASC responses to LT peaked on day 7 or 10 and were low on days 14 and 28). One volunteer had 123 ASC per 106 PBMC on day 14, and another had 57 ASC per 106 PBMC on day 28. The volunteers with late ASC responses to CFA/I were not remarkable in terms of the severity of illness, volume of diarrheal stools, onset of diarrhea, or number of days of shedding H10407. There were no significant differences between doses with respect to the frequency and magnitude of anti-CFA/I or anti-LT ASC responses.
TABLE 5.
Immunologic responses rates in volunteers after oral challenge with ETEC strain H10407
| Dose (CFU) | n | No. (%) with significant IgA-ASC responses
|
No. (%) with significant serum antibody responses
|
||||
|---|---|---|---|---|---|---|---|
| Anti-CFA/I | Anti-LT | IgA anti-CFA/I | IgG anti-CFA/I | IgA anti-LT | IgG anti-LT | ||
| 108 | 7 | 7 (100) | 5 (71) | 7 (100) | 7 (100) | 5 (71) | 4 (57) |
| 109 | 8 | 7 (88) | 8 (100) | 8 (100) | 8 (100) | 7 (88) | 8 (100) |
| All | 15 | 14 (93) | 13 (87) | 15 (100) | 15 (100) | 12 (75) | 12 (75) |
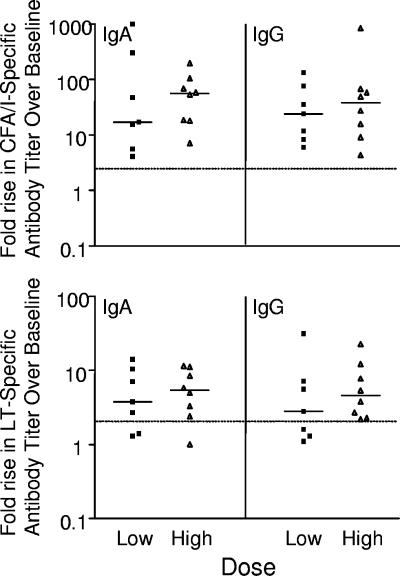
There were no significant differences between the dose groups with regard to the frequency and magnitude of serum anti-CFA/I and anti-LT responses. H10407 challenge induced significant serum IgA and IgG anti-CFA/I responses in all 15 volunteers (Table 5). In these volunteers, the peak geometric mean increases in CFA/I-specific IgA and IgG titers compared with the baseline titers were 40-fold (range, 4.1- to 1,070-fold) and 30-fold (range, 4.3- to 830-fold), respectively (Fig. 3), with the highest anti-CFA/I titers 28 days after challenge.
FIG. 3.
Peak increases in serum antibody titers to CFA/I and LT after challenge with ETEC strain H10407. Each symbol represents one volunteer. The solid lines indicate the medians, and the dotted lines indicate the threshold for a positive response.
H10407 challenge induced significant serum IgA and IgG anti-LT responses in 75% of the volunteers (Table 5). Among the responders, the peak geometric mean increases in LT-specific IgA and IgG titers compared with the baseline titers were 6.3-fold (range, 2.3- to15-fold) and 6.8-fold (range, 2.3- to 32-fold), respectively (Fig. 3). In the majority of the volunteers, the peak anti-LT antibody titers were detected on day 14 for IgA and on days 14 and 28 for IgG.
DISCUSSION
This study was the first direct comparison of the clinical courses following oral inoculation with the same dose (109 CFU) of ETEC strains B7A and H10407 and the first time that the immune response to CS6 was quantified after infection with wild-type ETEC. The onset of shedding and clinical symptoms appeared earlier in volunteers challenged with B7A than in volunteers challenged with H10407, but the number and volume of stools were smaller and the symptoms were milder in volunteers challenged with B7A. Patel et al. (46) suggested that the difference in virulence could result from an autotransporter, EatA, that is present in H10407 but not in B7A. Other possible explanations for differences between the ETEC strains could be differences in the CS6 and CFA/I colonization factors, the amount of LT expressed (10, 56) or released (15, 16, 31), or genes present only in H10407 (15) or B7A (19, 33, 38). The significance of other genes is consistent with recent publications that have suggested that other antigens, yet to be defined, should be included in vaccine development (3, 51).
This paper provides the first quantitative data for the immune response to CS6 after experimental infection with a wild-type ETEC strain. Helander et al. (23) found that four of five Bangladeshis with natural ETEC infection responded to CS6 in fecal IgA and serum IgA. A similar proportion of responders was also found after vaccination with formalin-inactivated ETEC. Our data for responses to CS6 revealed 51% for ACS, 31% for serum IgA, and 25% for serum IgG. In a given volunteer, a response to CS6 did not necessarily correspond to a response to LT. However, the ASC response to CS6 was related to the challenge dose. An immune response to CS6 has been reported after oral delivery of prototype vaccines in microspheres or transcutaneously (21, 30). The frequencies and magnitudes of responses were similar in all three studies (30). This finding, in combination with recent publications that question the protective role of CF (other than CFA/I), cast doubt on the role of CS6 as a component of future vaccine candidates (3, 48, 51).
The serum IgG response to CFA/I after H10407 challenge (100%) was remarkable compared to the responses described in previous reports (40 to 87%) (13, 35, 37, 54). In the present study, ASC also showed substantial responses. Immune responses to CFA/I are likely to be protective, because passive administration of antibody to CFA/I (18) and high titers of preexisting antibody to CFA/I protected volunteers against challenge with H10407 (20). Furthermore, the serum titers to CFA/I were inversely related to the risk of diarrhea caused by ETEC strains bearing CFA/I in Egyptian children (48). CFA/I should be included in candidate vaccines, and these results help set a benchmark for the immune response to CFA/I. A validated assay to measure endpoints in future clinical trials and efficacy studies is needed.
The frequency of ASC responses to CFA/I differed from the frequency for other antigens in that the ASC responses continued to increase throughout the 28 days of follow-up. It was surprising to see an increase in ASC responders on days 14 and 28, since ASC responses generally occur around days 7 to 10, as they did for the LT and CS6 antigens. It may be important in future studies to include data obtained at 14, 28, and 35 days to detect ASC responses.
Using low cutoff points, such as 1 to 2 ASC per 106 PBMC, the ASC response is more sensitive than the serum antibody response. However, serum responses were usually associated with ASC responses in the range from 50 to 100 ASC per 106 PBMC. The clinical significance of ASC responses that are less than 5 ASC per 106 PBMC is questionable.
Although there were differences in immune responses to the two colonization factors, the immune responses to LT were similar in all groups. Formation of antibody to LT after ETEC infection in populations or areas with frequent ETEC infections is well described. In the present study, the serum IgG responses to LT after H10407 and B7A challenges were consistent with previous reports (13, 18, 35, 37, 54).
Early in 1983, Graham et al. (20) reported that the immune response was not directly related to the severity of diarrhea. In the present study, we confirmed that there is no significant correlation between the severity of illness or the presence of colonization and immune responses. This conclusion was based on the following findings: (i) volunteers with no diarrhea mounted an immune response to LT and CS6, (ii) volunteers challenged with H10407 who developed fever (both >1 day and >100.5°F) were no more likely than other volunteers to mount immune responses to LT, and (iii) two volunteers who shed B7A for only 2 days had immune responses to LT and volunteers who shed H10407 for only 2 days responded to LT and CFA/I. These findings suggest that even a short exposure to ETEC is sufficient to cause an immune response to CFA/I and LT in humans.
There was no gender difference in the occurrence of diarrhea for the B7A and H10407 groups, but the severity of other symptoms was related to gender. Males had a significantly higher incidence of fever than females, while females suffered more gastrointestinal disturbances, including nausea, abdominal cramps, and appetite loss. Recent findings suggest that the primary abnormality in visceral sensitivity in irritable bowel syndrome, which is more common in females (32), may be at the level of the parietal mechanoreceptors, which in some patients becomes sensitized by a postinfection inflammatory process (39). Whether this mechanism is responsible for the abdominal complaints during an infection has not been demonstrated. There are other reported factors that warrant further investigation with respect to the etiology of the observed differences in fever and other adverse events. Glutathione and the cytosolic glutathione S-transferases serve as the antitoxic barrier of the gastrointestinal mucosa and may have immunomodulatory functions (17). Hoensch et al. (24) showed that there was a significant difference in the activity of these enzymes between males and females and demonstrated that the activity of the enzymes was stimulated by a diet high in fruits and vegetables. This enzyme system, although not directly involved in the pathophysiology of ETEC infections, serves as a model for gut-associated reactogenicity and immunomodulatory factors influenced by gender and diet. In recent studies workers have investigated the role of stress and hormones on disease outcome. It has been reported that estrogen may influence episodic diseases such as irritable bowel syndrome or premenstrual syndrome, with its associated episodic abdominal pain and fatigue, due to its modulating activity on neurotransmitters such as serotonin (57). The enteric nervous system, which plays a role in several mucosal functions, such as mucosal blood flow, regulation of epithelial permeability, and cell proliferation, is modulated by stress (42); however, it has not been demonstrated that these changes are correlated with systemic symptoms. In our study we did not obtain baseline data regarding a subject's response to gastrointestinal stimuli, hormonal status (premenopausal versus menopausal or premenstrual versus postmenstrual), or perceived level of stress and/or anxiety, nor did we standardize the diet of the volunteers. Future vaccine studies should consider these factors.
The first approach to controlling traveler's diarrhea has been the short-duration use of prophylactic antibiotics. However, with the introduction of each new antibiotic used to protect against traveler's diarrhea, the bacteria, including ETEC, have become resistant. This factor has led investigators to work to develop a vaccine to protect against ETEC. However, the development of a vaccine will not eliminate the need for effective antibiotics to treat diarrhea caused by ETEC. Our results provide standards for immune response after vaccination, antibiotic clearance of shedding, and resolution of symptoms that can be used during vaccine development and development of new antibiotic drugs. Our data also highlight the importance of using both genders and obtaining diet and hormonal history and perceived stress level during investigation of the reactogenicity, tolerance, and efficacy of enteric vaccines.
Acknowledgments
We are indebted to the staff of the Clinical Studies Department and the Biometrics and Information Management Division at USAMRIID for conduct of the study, to the Department of Biometrics at WRAIR for assistance with data analysis, and to T. Larry Hale and Jennifer Thompson for reviewing the manuscript.
This work was supported by the Military Infectious Diseases Research Program of the U.S. Army Medical Research and Materiel Command, Fort Detrick, MD.
The views expressed are those of the authors and do not necessarily represent the views of the Department of the Army or Department of Defense.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Baqar, S., A. A. Nour El Din, D. A. Scott, A. L. Bourgeois, A. S. Mourad, M. T. Kleinosky, M. J. Oplinger, and J. R. Murphy. 1997. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin. Diagn. Lab. Immunol. 4:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, R. E., M. M. Levine, M. L. Clements, L. Cisneros, and V. Daya. 1982. Treatment of experimentally induced enterotoxigenic Escherichia coli diarrhea with trimethoprim, trimethoprim-sulfamethoxazole, or placebo. Rev. Infect. Dis. 4:540-545. [DOI] [PubMed] [Google Scholar]
- 3.Boedeker, E. C. 2005. Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol. 21:15-19. [PubMed] [Google Scholar]
- 4.Bourgeois, A. L., C. H. Gardiner, S. A. Thornton, R. A. Batchelor, D. H. Burr, J. Escamilla, P. Echeverria, N. R. Blacklow, J. E. Herrmann, and K. C. Hyams. 1993. Etiology of acute diarrhea among United States military personnel deployed to South America and west Africa. Am. J. Trop. Med. Hyg. 48:243-248. [DOI] [PubMed] [Google Scholar]
- 5.Cassels, F. J., and M. K. Wolf. 1995. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J. Ind. Microbiol. 15:214-226. [DOI] [PubMed] [Google Scholar]
- 6.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, P. K. Neogy, B. Stanton, N. Huda, M. U. Khan, B. A. Kay, and M. R. Khan. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372-377. [DOI] [PubMed] [Google Scholar]
- 7.Dalton, C. B., E. D. Mintz, J. G. Wells, C. A. Bopp, and R. V. Tauxe. 1999. Outbreaks of enterotoxigenic Escherichia coli infection in American adults: a clinical and epidemiologic profile. Epidemiol. Infect. 123:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels, N. A., J. Neimann, A. Karpati, U. D. Parashar, K. D. Greene, J. G. Wells, A. Srivastava, R. V. Tauxe, E. D. Mintz, and R. Quick. 2000. Traveler's diarrhea at sea: three outbreaks of waterborne enterotoxigenic Escherichia coli on cruise ships. J. Infect. Dis. 181:1491-1495. [DOI] [PubMed] [Google Scholar]
- 9.DuPont, H. L., S. B. Formal, R. B. Hornick, M. J. Snyder, J. P. Libonati, D. G. Sheahan, E. H. LaBrec, and J. P. Kalas. 1971. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 285:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Echeverria, P., O. Serichantalerg, S. Changchawalit, B. Baudry, M. M. Levine, F. Orskov, and I. Orskov. 1992. Tissue culture-adherent Escherichia coli in infantile diarrhea. J. Infect. Dis. 165:141-143. [DOI] [PubMed] [Google Scholar]
- 11.Evans, D. G., D. J. Evans, Jr., A. R. Opekun, and D. Y. Graham. 1988. Non-replicating oral whole cell vaccine protective against enterotoxigenic Escherichia coli (ETEC) diarrhea: stimulation of anti-CFA (CFA/I) and anti-enterotoxin (anti-LT) intestinal IgA and protection against challenge with ETEC belonging to heterologous serotypes. FEMS Microbiol. Immunol. 1:117-125. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. G., D. Y. Graham, and D. J. Evans, Jr. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87:934-940. [PubMed] [Google Scholar]
- 13.Evans, D. G., T. K. Satterwhite, D. J. Evans, Jr., and H. L. DuPont. 1978. Differences in serological responses and excretion patterns of volunteers challenged with enterotoxigenic Escherichia coli with and without the colonization factor antigen. Infect. Immun. 19:883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. J., Jr., D. G. Evans, A. R. Opekun, and D. Y. Graham. 1988. Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiol. Immunol. 1:9-18. [DOI] [PubMed] [Google Scholar]
- 15.Fleckenstein, J. M., D. J. Kopecko, R. L. Warren, and E. A. Elsinghorst. 1996. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 64:2256-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein, J. M., L. E. Lindler, E. A. Elsinghorst, and J. B. Dale. 2000. Identification of a gene within a pathogenicity island of enterotoxigenic Escherichia coli H10407 required for maximal secretion of the heat-labile enterotoxin. Infect. Immun. 68:2766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraternale, A., M. R. Paoletti, A. Casabianca, J. Oiry, P. Clayette, J. U. Vogel, J. Cinatl, Jr., A. T. Palamara, R. Sgarbanti, E. Garaci, E. Millo, U. Benatti, and M. Magnani. 2006. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr. Med. Chem. 13:1749-1755. [DOI] [PubMed] [Google Scholar]
- 18.Freedman, D. J., C. O. Tacket, A. Delehanty, D. R. Maneval, J. Nataro, and J. H. Crabb. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 177:662-667. [DOI] [PubMed] [Google Scholar]
- 19.Girón, J. A., G. I. Viboud, V. Sperandio, O. G. Gómez-Duarte, D. R. Maneval, M. J. Albert, M. M. Levine, and J. B. Kaper. 1995. Prevalence and association of the longus pilus structural gene (lngA) with colonization factor antigens, enterotoxin types, and serotypes of enterotoxigenic Escherichia coli. Infect. Immun. 63:4195-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham, D. Y., M. K. Estes, and L. O. Gentry. 1983. Double-blind comparison of bismuth subsalicylate and placebo in the prevention and treatment of enterotoxigenic Escherichia coli-induced diarrhea in volunteers. Gastroenterology 85:1017-1022. [PubMed] [Google Scholar]
- 21.Güereña-Burgueño, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall, E. R., T. F. Wierzba, C. Åhrén, M. R. Rao, S. Bassily, W. Francis, F. Y. Girgis, M. Safwat, Y. J. Lee, A.-M. Svennerholm, J. D. Clemens, and S. J. Savarino. 2001. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine. Infect. Immun. 69:2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helander, A., C. Wennerås, F. Qadri, and A.-M. Svennerholm. 1998. Antibody responses in humans against coli surface antigen 6 of enterotoxigenic Escherichia coli. Infect. Immun. 66:4507-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoensch, H., I. Morgenstern, G. Petereit, M. Siepmann, W. H. Peters, H. M. Roelofs, and W. Kirch. 2002. Influence of clinical factors, diet, and drugs on the human upper gastrointestinal glutathione system. Gut 50:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoge, C. W., J. M. Gambel, A. Srijan, C. Pitarangsi, and P. Echeverria. 1998. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin. Infect. Dis. 26:341-345. [DOI] [PubMed] [Google Scholar]
- 26.Hyams, K. C., A. L. Bourgeois, B. R. Merrell, P. Rozmajzl, J. Escamilla, S. A. Thornton, G. M. Wasserman, A. Burke, P. Echeverria, K. Y. Green, et al. 1991. Diarrheal disease during Operation Desert Shield. N. Engl. J. Med. 325:1423-1428. [DOI] [PubMed] [Google Scholar]
- 27.Jertborn, M., C. Ahren, J. Holmgren, and A. M. Svennerholm. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255-260. [DOI] [PubMed] [Google Scholar]
- 28.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measure of intestinal immunity after cholera vaccination and natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang, Z.-D., B. Lowe, M. P. Verenkar, D. Ashley, R. Steffen, N. Tornieporth, F. von Sonnenburg, P. Waiyaki, and H. L. DuPont. 2002. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 185:497-502. [DOI] [PubMed] [Google Scholar]
- 30.Katz, D. E., A. J. DeLorimier, M. K. Wolf, E. R. Hall, F. J. Cassels, J. E. van Hamont, R. L. Newcomer, M. A. Davachi, D. N. Taylor, and C. E. McQueen. 2003. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 21:341-346. [DOI] [PubMed] [Google Scholar]
- 31.Kesty, N. C., K. M. Mason, M. Reedy, S. E. Miller, and M. J. Kuehn. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, H. S., P. L. Rhee, J. Park, J. H. Lee, Y.-H. Kim, J. J. Kim, and J. C. Rhee. 2006. Gender-related differences in visceral perception in health and irritable bowel syndrome. J. Gastroenterol. Hepatol. 21:468-473. [DOI] [PubMed] [Google Scholar]
- 33.Knutton, S., D. R. Lloyd, and A. S. McNeish. 1987. Identification of a new fimbrial structure in enterotoxigenic Escherichia coli (ETEC) serotype O148:H28 which adheres to human intestinal mucosa: a potentially new human ETEC colonization factor. Infect. Immun. 55:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine, M. M. 1990. Vaccines against enterotoxigenic Escherichia coli infections. I. Vaccines based predominantly or entirely on antibacterial immunity, p. 649-660. In G. C. Woodrow and M. M. Levine (ed.), New generation vaccines. Marcel Dekker, New York, NY.
- 35.Levine, M. M., R. E. Black, C. C. Brinton, Jr., M. L. Clements, P. Fusco, T. P. Hughes, S. O'Donnell, R. Robins-Browne, S. Wood, and C. R. Young. 1982. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand. J. Infect. Dis. Suppl. 33:83-95. [PubMed] [Google Scholar]
- 36.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Berquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine, M. M., M. B. Rennels, L. Cisneros, T. P. Hughes, D. R. Nalin, and C. R. Young. 1980. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am. J. Epidemiol. 111:347-355. [DOI] [PubMed] [Google Scholar]
- 38.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mach, T. 2004. The brain-gut axis in irritable bowel syndrome—clinical aspects. Med. Sci. Monit. 10:RA125-RA131. [PubMed] [Google Scholar]
- 40.McConnell, M. M., L. V. Thomas, G. A. Willshaw, H. R. Smith, and B. Rowe. 1988. Genetic control and properties of coli surface antigens of colonization factor antigen IV (PCF8775) of enterotoxigenic Escherichia coli. Infect. Immun. 56:1974-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moseley, S. L., M. Samadpour-Motalebi, and S. Falkow. 1983. Plasmid association and nucleotide sequence relationships of two genes encoding heat-stable enterotoxin production in Escherichia coli H-10407. J. Bacteriol. 156:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumlist, M., F. Toumi, T. Oreschkova, M. Denis, J. Leborgne, C. L. Laboisse, J. P. Galmiche, and A. Jarry. 24 July 2003. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein Zo-1 via VIPergic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1028-G1036. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 43.Oldfield, E. C., III, and M. R. Wallace. 2001. The role of antibiotics in the treatment of infectious diarrhea. Gastroenterol. Clin. N. Am. 30:817-836. [DOI] [PubMed] [Google Scholar]
- 44.Orndorff, G. R., and C. Lebron. 1996. Epidemiology of enterotoxigenic Escherichia coli-associated diarrheal disease occurring on board U.S. Navy ships visiting Asian ports. Mil. Med. 161:475-478. [PubMed] [Google Scholar]
- 45.Oyofo, B. A., L. F. Peruski, T. F. Ismail, S. H. El Etr, A. M. Churilla, M. O. Wasfy, B. P. Petruccelli, and M. E. Gabriel. 1997. Enteropathogens associated with diarrhea among military personnel during Operation Bright Star 96, in Alexandria, Egypt. Mil. Med. 162:396-400. [PubMed] [Google Scholar]
- 46.Patel, S. K., J. Dotson, K. P. Allen, and J. M. Fleckenstein. 2004. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 72:1786-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao, M. R., T. F. Wierzba, S. J. Savarino, R. Abu-Elyazeed, N. El-Ghoreb, E. R. Hall, A. Naficy, I. Abdel-Messih, R. W. Frenck, Jr., A. M. Svennerholm, and J. D. Clemens. 2005. Serologic correlates of protection against enterotoxigenic Escherichia coli Diarrhea. J. Infect. Dis. 191:562-570. [DOI] [PubMed] [Google Scholar]
- 49.Satterwhite, T. K., D. G. Evans, H. L. DuPont, and D. J. Evans, Jr. 1978. Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet ii:181-184. [DOI] [PubMed] [Google Scholar]
- 50.Skerman, F. J., S. B. Formal, and S. Falkow. 1972. Plasmid-associated enterotoxin production in a strain of Escherichia coli isolated from humans. Infect. Immun. 5:622-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinsland, H., P. Valentiner-Branth, H. K. Gjessing, P. Aaby, K. Molbak, and H. Sommerfelt. 2003. Protection from natural infections with enterotoxigenic Escherichia coli: longitudinal study. Lancet 362:286-291. [DOI] [PubMed] [Google Scholar]
- 52.Stoll, B. J., A. M. Svennerholm, L. Gothefors, D. Barua, S. Huda, and J. Holmgren. 1986. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhea in an endemic area. J. Infect. Dis. 153:527-534. [DOI] [PubMed] [Google Scholar]
- 53.Svennerholm, A. M., J. Holmgren, R. Black, M. M. Levine, and M. M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 14:7514-7522. [DOI] [PubMed] [Google Scholar]
- 54.Tacket, C. O., G. Losonsky, H. Link, Y. Hoang, P. Guesry, H. Hilpert, and M. M. Levine. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N. Engl. J. Med. 318:1240-1243. [DOI] [PubMed] [Google Scholar]
- 55.Tacket, C. O., R. H. Reid, E. C. Boedeker, G. Losonsky, J. P. Nataro, H. Bhagat, and R. Edelman. 1994. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12:1270-1274. [DOI] [PubMed] [Google Scholar]
- 56.Trachman, J. D., and M. Yasmin. 2004. Thermo-osmoregulation of heat-labile enterotoxin expression by Escherichia coli. Curr. Microbiol. 49:353-360. [DOI] [PubMed] [Google Scholar]
- 57.Warnock, J. K., and A. H. Clayton. 2003. Chronic episodic disorders in women. Psychiatr. Clin. N. Am. 26:725-740. [DOI] [PubMed] [Google Scholar]
- 58.Wennerås, C., A. M. Svennerholm, C. Åhrén, and C. Czerkinsky. 1992. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect. Immun. 60:2605-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto, T., and P. Echeverria. 1996. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect. Immun. 64:1441-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]