Abstract
Nontypeable (NT) Haemophilus influenzae is an important cause of otitis media in children. We have shown previously that NT H. influenzae mutants defective in their ability to sialylate lipopolysaccharide (LPS), called siaB mutants, show attenuated virulence in a chinchilla model of experimental otitis media (EOM). We show that complement is a key arm of host innate immunity against NT H. influenzae-induced EOM. Depleting complement in chinchillas by use of cobra venom factor (CoVF) rendered two otherwise avirulent siaB mutants fully virulent and able to cause EOM with severity similar to that of wild-type strains. Clearance of infection caused by siaB mutants in CoVF-treated animals coincided with reappearance of C3. Wild-type strains were more resistant to direct complement-mediated killing than their siaB mutants. The serum-resistant strain bound less C3 and C4 than the serum-sensitive strain. Neither NT H. influenzae strain tested bound factor H (alternative complement pathway regulator). Selective activation of the alternative pathway resulted in more C3 binding to siaB mutants. LPS sialylation had a more profound impact on the amount of alternative-pathway-mediated C3 binding (∼5-fold decrease in fluorescence) when LPS was the main C3 target, as occurred on the more serum-resistant strain. In contrast, only an ∼1.5-fold decrease in fluorescence intensity of C3 binding was seen with the serum-sensitive strain, where surface proteins predominantly bound C3. Differences in binding sites for C3 and C4 may account for variations in serum resistance between NT H. influenzae strains, which in turn may impact their virulence. These data demonstrate a central role for complement in innate immune defenses against NT H. influenzae infections and specifically EOM.
The pathogenesis of acute otitis media (OM) most often requires colonization with a bacterial otopathogen and concurrent viral respiratory tract infection resulting in Eustachian tube dysfunction, middle ear negative pressure, and ascension of bacteria into the middle ear. The successful ascension of bacterial otopathogens into the middle ear, bacterial replication, and subsequent inflammatory response require overcoming host defense mechanisms within the nasopharynx and middle ear.
Nontypeable (NT) Haemophilus influenzae is a major respiratory pathogen in otitis media and sinusitis in infants and toddlers (7, 40) and may be increasing in countries where immunization with heptavalent pneumococcal conjugate vaccine is universal. Specific host defenses against respiratory infection with NT H. influenzae have been described. The importance of Toll-like receptor 4 in promoting clearance of NT H. influenzae following nasopharyngeal inoculation (41) has been demonstrated by significant reductions in cytokine responses and bacterial clearance in Toll-like receptor 4-deficient animals (17). Small antimicrobial peptides that demonstrate dose-dependent killing have been identified in nasopharyngeal secretions (18), and their relevance to middle ear invasion has been proven by observing attenuation of disease and reduced survival of a sapA (sensitivity to antimicrobial peptides) mutant in the chinchilla model of experimental otitis media (EOM) (19).
Complement is a major component of innate host defenses, and deficiencies of complement proteins are associated with an increased risk of invasive infections. The role of complement in protection of the middle ear against infections is less well established. Stenfors and Raisanen identified C3-coated bacteria in a minority of middle ear exudates from children with acute otitis media. Most notably, C3 binding to bacteria was not seen in children less than 20 months of age (35). They observed immunoglobulin G (IgG) and C3b on the surface of bacterial otopathogens in only 3/37 culture-positive middle ear specimens (35). Bernstein et al. have demonstrated cleavage products of C3, C4, and factor B in patients with otitis media with effusions, suggesting that both the classical and alternative pathways of complement are active in middle ear inflammation fluid (3).
Previously, we have investigated the role of sialic acid as a virulence factor of NT H. influenzae in a well-described chinchilla model of OM (2, 13). By comparing isogenic wild-type (WT) and sialic acid-deficient strains, we demonstrated that sialylation of lipopolysaccharide (LPS) is a critical virulence feature in pathogenesis of EOM due to NT H. influenzae (5). This was revealed based on the profound attenuation of middle ear infection in animals inoculated with isogenic sialic acid-deficient mutants (disrupted sialyltransferase gene [lic3A] or cytidinemonophospho-N-acetylneuraminic acid [CMP-Neu5Ac] synthetase gene [siaB]). In this new study, we further characterize the role played by sialylated LPS, showing the mechanism by which sialic acid of NT H. influenzae may enable the bacterium to resist host defenses, as well as the critical nature of complement in host defenses against EOM due to NT H. influenzae in the chinchilla model.
MATERIALS AND METHODS
Bacterial isolates.
Two genetically distinct NT H. influenzae isolates, wild-type strains 375 and 486 (375 WT and 486 WT), and their sialic acid-deficient siaB mutants (375siaB and 486siaB, respectively) (see below) were selected from a phylogenetically organized collection of 107 OM isolates (5) obtained by tympanocentesis (10). NT H. influenzae strains were cultured at 37°C in brain heart infusion (BHI) broth supplemented with 10 μg/ml hemin and 2 μg/ml NAD (sBHI) or on chocolate agar plates. sBHI was supplemented with 10 μg/ml kanamycin as appropriate when growing the siaB mutants. Neisseria gonorrhoeae strain 15253 (45), used as a positive control for factor H binding (27), was grown on chocolate agar plates supplemented with IsoVitaleX overnight at 37°C in an atmosphere enriched with 5% CO2.
Construction of mutant strains unable to sialylate LPS.
Isogenic mutants of NT H. influenzae strains 375 and 486 that were unable to activate sialic acid and were therefore deficient in LPS sialylation were constructed by insertional inactivation of the gene encoding CMP-Neu5Ac synthetase (siaB) as described previously (10).
Experimental otitis media model.
An experimental chinchilla model of acute otitis media was used (2). All procedures and manipulations were performed using sedation analgesia with a mixture of ketamine and xylazine given intramuscularly in accordance with approved IACUC protocols at Boston University Medical Center (5). Baseline blood samples were obtained through the cephalic sinus 24 h prior to bacterial inoculation. Animals were randomly assigned to receive either one dose of cobra venom factor (CoVF) (CompTech, Tyler, TX) at a dose of 300 to 600 μg/kg of body weight administered intraperitoneally 24 h prior to middle ear inoculation to deplete complement or sterile normal saline (control animals). Serial plasma samples were obtained to confirm the extent and duration of C3 depletion (described below). Isolates of NT H. influenzae grown to the mid-log phase were diluted in Hank's balanced salt solution (HBSS), and 50 to 100 CFU in 100 μl was inoculated through the left superior bulla with a 25-gauge tuberculin needle (5). Forty-eight hours after inoculation of NT H. influenzae, tympanometry, otomicroscopy, and middle ear cultures were performed to determine the presence of infection. The middle ear cavity was accessed as described previously (2). A direct culture of middle ear was obtained with a calcium alginate swab 48 h after middle ear inoculation and immediately streaked onto a chocolate agar plate. A kanamycin disk was used to confirm the presence of the Kanr marker in the recovered siaB mutants. Middle ear fluid (MEF) was obtained with a 22-gauge angiocatheter connected to an empty tuberculin syringe, 10 μl of MEF was diluted 1:10 in HBSS, and three serial 10-fold dilutions were prepared. One hundred microliters of each dilution was plated onto chocolate agar. The lower limit of detection of viable organisms in MEF using this dilution series was 100 CFU/ml (5). If MEF was absent, the middle ear was flushed with 0.5 ml HBSS and the contents sampled as described above. Direct and indirect ear examination was performed every 3 to 4 days until the middle ear cultures were sterile on two consecutive samples.
Sera and complement reagents.
Normal human serum (NHS) was pooled from 12 healthy volunteers, aliquoted, and stored at −80°C. Factor H, CoVF, and purified iC3b were purchased from CompTech. To study the effects of the alternative pathway exclusively, the classical and lectin pathways of complement were selectively blocked by chelating NHS with 10 mM EGTA and 10 mM MgCl2 (MgEGTA-NHS) (25).
Antibodies.
Goat anti-guinea pig C3 was purchased from Immunology Consultants Laboratory, Newberg, OR. Polyclonal sheep anti-human C4 (Biodesign, Saco, ME) was used at a dilution of 1:1,000 in phosphate-buffered saline (PBS)-0.05% Tween 20, and tissue culture supernatants containing anti-human iC3b monoclonal antibody (MAb) C-3E (gift from Kyoko Iida, Takeda Chemical Industries, Ltd., Tsukuba, Japan) (12) were used in Western blotting experiments. Fluorescein isothiocyanate (FITC)-conjugated sheep anti-human C3 and anti-human C4 (Biodesign) were used in flow cytometry assays as described previously (26, 27). Affinity-purified goat anti-human fH was made by Bethyl Laboratories, Inc. (Montgomery, TX), using purified fH (CompTech, Tyler, TX) as both the immunogen and the immunoadsorbant. Anti-goat IgG, anti-sheep IgG, and anti-mouse IgG conjugated to alkaline phosphatase (Sigma, St. Louis, MO) were used as secondary disclosing antibodies.
Serum bactericidal assays.
Serum bactericidal assays were performed as described previously (14, 20). Briefly, NT H. influenzae isolates grown to the mid-log phase were diluted to 1,000 CFU/ml and incubated for 30 min with various dilutions of NHS. The final volume of reaction mixtures in every tube was 500 μl. Duplicate 100-μl aliquots were plated at the start of the assay (t = 0 min) and after incubation in a 37°C shaking water bath for 30 min (t = 30 min). The plates were incubated overnight at 37°C in a candle extinction jar. Bacteria incubated with heat-inactivated serum served as a negative control. Survival was expressed as the percentage of bacteria surviving at 30 min relative to 0 min (start of the assay). Heat-inactivated NHS failed to demonstrate any killing of strain 375, strain 486, or the respective isogenic mutants.
Western blotting.
Western blotting to examine binding of C4 and C3 activation products was performed as previously described (26). Briefly, 3 × 108 bacteria suspended in HBSS containing 0.15 mM CaCl2 and 1 mM MgCl2 (HBSS++) were incubated with NHS (concentration specified for each experiment) in a final reaction volume of 500 μl for 30 min at 37°C. In some experiments, EGTA and MgCl2 were added to NHS to a final concentration of 10 mM each in order to block classical and lectin pathway activation, leaving only the alternative pathway activation intact. In this instance, the bacteria were suspended in HBSS containing only 1 mM MgCl2. Following incubation with serum, bacteria were washed twice in HBSS++ and divided into two aliquots, incubated with either HBSS++ (leaving all C4 and C3 fragments bound to bacterial targets via both ester or amide linkages intact) or 1 M methylamine, pH 11, for 1 h at 37°C, which releases ester-linked but not amide-linked C4b and C3b/iC3b. Direct determination of the specific amide-bound C4b and C3b/iC3b is not possible, because they cannot be separated intact from acceptor surfaces without altering their primary structures. The final reaction volume of each reaction mixture was 70 μl. Samples were then digested with lithium dodecyl sulfate sample buffer (NuPAGE LDS sample buffer; Invitrogen, Carlsbad, CA) containing 2-mercaptoethanol (10% final concentration) and resolved on NuPAGE Novex 4 to 12% Bis-Tris gradient gels using NuPAGE 3-morpholinopropanesulfonic acid (MOPS) running buffer (Invitrogen) (100 mV for 15 h at 4°C).
Western blotting was performed as described previously. C4b was detected using anti-C4 antibody (Ab) (Biodesign, Saco, ME) at a dilution of 1:1,000 in PBS containing 0.05% Tween 20, and iC3b was detected using MAb G-3E. Disclosure of the bound primary antibodies was performed using the appropriate secondary antibodies conjugated to alkaline phosphatase as described previously (4).
We also used Western blotting to monitor C3 depletion in CoVF-treated chinchillas. Plasma from chinchillas was immediately diluted 1:100 in PBS containing 10 mM EDTA to prevent C3 activation and processing ex vivo. Following the addition of 4× NuPAGE LDS sample buffer (Invitrogen) containing 10% 2-mercaptoethanol, proteins were separated on a 4 to 12% NuPAGE Bis-Tris gel (Invitrogen) by use of NuPAGE MOPS running buffer and transferred to an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). C3 was detected using anti-guinea pig C3 antibody, followed by anti-goat IgG-alkaline phosphatase, both at dilutions of 1:1,000 in PBS-0.05% Tween 20.
Flow cytometry.
Factor H, C3, and C4 bound to bacteria were measured by flow cytometry as described previously (26, 27). Briefly, ∼108 CFU bacteria was incubated with pure fH (2 μg) or NHS (concentration specified for each experiment) in a final reaction volume of 100 μl for 30 min at 37°C. Surface-bound factor H was detected using affinity-isolated goat anti-human factor H antibody (Bethyl Laboratories), followed by anti-goat IgG-FITC (Sigma). C4 and C3 bound to bacteria were detected using FITC-conjugated anti-C4 and anti-C3 antibodies as described above.
Statistical analysis.
Fisher's exact test was used to calculate statistical significance for the differences in the proportions of culture-positive middle ears in animals challenged with 375siaB or 486siaB and treated with CoVF compared with untreated animals.
We used the t test to compare survival of 375 WT with that of 375siaB, survival of 486 WT with that of 486siaB, and survival of the respective wild-type and siaB pairs.
RESULTS
Complement depletion by CoVF.
CoVF is the complement-activating protein in the venom of the Indian cobra (Naja naja) and related Asian cobras of the genus Naja (36). CoVF is a structural and functional analog of complement component C3. It forms a complex with factor B in the presence of Mg++ (9) and when acted upon by factor D yields CoVF,Bb (34, 38), which has the capacity to function as a C3/C5 convertase (34, 37, 39). Unlike native C3b,Bb, which has a very short half-life at 37°C of about 1.5 min (21, 24), CoVF,Bb has a half-life of about 7 h. In addition, CoVF (unlike native C3b) is resistant to degradation by factor H and factor I, and CoVF,Bb (in contrast to C3b,Bb) is not dissociated by factor H (1, 16, 23). CoVF has been administered safely to laboratory animals (6) to induce a functional complement-depleted state by exhaustively activating C3 and C5 components of complement.
Following injection into the bloodstream, CoVF will form CoVF,Bb as described above, which will result in further C3 activation to form C3b. C3b will proceed to form more C3 convertases, which in turn will further activate more C3. The C3b is then cleaved to iC3b by factors H and I, with further degradation of iC3b to C3d mediated by complement receptor 1.
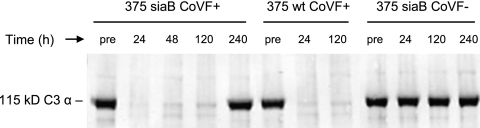
We monitored the extent of complement consumption by Western blotting. C3 in normal serum shows an ∼115-kDa α-chain and a 75-kDa β-chain when electrophoresed under reducing conditions. Activation to C3b results in release of the ∼9-kDa C3a fragment from the N terminus of the C3 α-chain to yield the C3b α′-chain. Further cleavage of the α′-chain results in formation of the ∼68-kDa α1′-chain of iC3b, which subsequently gets degraded into smaller fragments, including the ∼35-kDa C3d molecule. Therefore, complement activation by CoVF results in loss of the intact ∼115-kDa C3 α-chain. As seen in Fig. 1, administration of CoVF results in loss of the C3 115-kDa α-chain within 24 h after injection, and the effects of a single injection resulted in C3 depletion for up to 5 days. Figure 1 shows results with two representative animals: one received a WT strain, and the other received the siaB mutant (similar results were seen with all CoVF-treated chinchillas). Also shown is a control animal that did not receive CoVF.
FIG. 1.
Detection of C3 α-chain in chinchillas following treatment with CoVF (CoVF+) to assess the extent and duration of complement depletion. pre, samples collected 24 h prior to CoVF administration; 375siaB CoVF−, control animal that did not receive CoVF.
Complement depletion renders siaB mutants virulent.
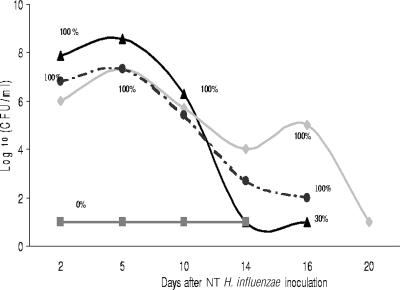
The role of complement in middle ear infection due to NT H. influenzae was evaluated in the EOM chinchilla model by comparing the effect of CoVF on the virulence of WT NT H. influenzae strains to the effect on that of their respective isogenic sialic acid-deficient (siaB) mutants. After middle ear inoculation of approximately 70 CFU in 100 μl of 486siaB into the bulla of 15 chinchillas, the 10 animals that received CoVF developed high-density middle ear infection and the 5 control animals challenged with 486siaB but not administered CoVF showed an absence of middle ear disease (P = 0.0003) (Fig. 2). We also compared the natural history of EOM due to 486siaB in animals receiving CoVF to that due to 486 WT in animals that did not receive CoVF. The course of middle ear disease was shorter in the 486siaB-challenged cohort, with a rapid decrease in MEF bacterial density observed in association with recovery of C3 on day 10 following inoculation. On day 14, 100% of animals challenged with 486 WT were culture positive, compared to 30% of CoVF-treated animals challenged with 486siaB. Although we evaluated the natural history of disease due to 486 WT in only two animals, the course of disease depicted in Fig. 2 is typical of disease caused by this isolate (5).
FIG. 2.
Natural history of EOM due to 486 WT and 486siaB in control animals and chinchillas treated with CoVF. The proportion of animals with culture-positive MEF at the time points indicated in each group is represented by the percentage. Symbols: ▴, 486siaB with CoVF (n = 10); ▪, 486siaB without CoVF (n = 5); ⧫, 486 WT without CoVF (n = 2); •, 486 WT with CoVF (n = 2).
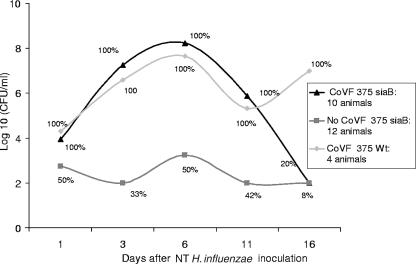
Similar results were observed after middle ear inoculation of 60 CFU in 100 μl of 375siaB compared with 375 WT. In contrast to animals inoculated with 486siaB, 6 of 12 animals challenged with 375siaB and not treated with CoVF developed culture-positive middle ear disease; however, bacterial density was substantially lower than disease due to 375 WT in animals not administered CoVF (Fig. 3). The attenuated virulence of strain 375siaB confirms previous studies (5). No enhancement in burden of infection was observed in the CoVF-treated animals challenged with 486 WT or 375 WT, although only six animals were studied.
FIG. 3.
Natural history of EOM due to 375 WT and 375siaB in control animals and chinchillas treated with CoVF. The proportion of animals with culture-positive MEF at the time points indicated in each group is represented by the percentage.
NT H. influenzae strains differ in baseline serum resistance, and siaB mutants are more serum sensitive than corresponding WT strains.
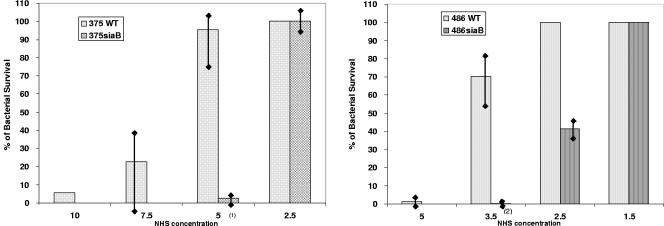
Figure 4 demonstrates results from quadruplicate assays comparing rates of bacterial survival in the presence of pooled NHS for wild-type and siaB dyads. Sialic acid-deficient mutants (siaB) are more serum sensitive than the wild type, as demonstrated by reduced survival in the presence of NHS (P of 0.0002 comparing 375 WT with 375siaB at a 5% NHS concentration and P of 0.0007 comparing 486 WT with 486siaB at 3.5% NHS). The serum bactericidal assay also demonstrated that 375 WT and 375siaB were more serum resistant than 486 WT and 486siaB, respectively (P of 0.0002 comparing 375 WT with 486 WT at a 5% NHS concentration and P of <0.0001 comparing 375siaB with 486siaB at 2.5% NHS), which may at least in part explain why challenge with 375siaB resulted in transient EOM while 486siaB failed to elicit any disease.
FIG. 4.
Bactericidal activities of NHS against 375 WT and 375siaB and 486 WT and 486siaB. Percent survival with 95% confidence interval around the mean percentage is shown in the figures. (1), P = 0.0002 (percent survival of 375 WT compared to that of 375siaB at a 5% NHS concentration); (2), P = 0.0007 (percent survival of 486 WT compared to that of 486siaB at 3.5% NHS).
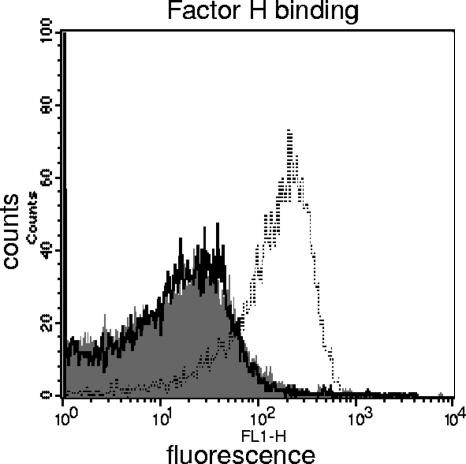
NT H. influenzae LPS sialylation does not enhance factor H binding.
Sialylation of NT H. influenzae LPS results in enhanced resistance to complement-dependent killing compared to siaB mutants that lack the ability to sialylate LPS. We have shown previously that sialylation of N. gonorrhoeae LPS results in enhanced factor H binding (29), which may constitute at least one mechanism by which sialylation promotes serum resistance. We sought to determine if NT H. influenzae LPS sialylation also resulted in increased factor H binding. As seen in Fig. 5, no binding of pure factor H to strain 375 WT was detected, and the histogram was identical to that seen with the siaB mutant. Similar results were obtained with strain 486 and its isogenic siaB mutant (data not shown). As a positive control for factor H binding, we used N. gonorrhoeae strain 15253 (28). Another important soluble-phase complement regulatory molecule is C4b-binding protein, which acts as a cofactor for the factor I-mediated cleavage of C4b and also accelerates the decay of the classical pathway C3 convertase (C4b2a). We did not detect C4b-binding protein binding to either WT NT H. influenzae strain (data not shown).
FIG. 5.
Factor H binding to 375 and 375siaB by flow cytometry. The shaded histogram represents binding to 375 WT, the solid line binding to 375siaB, and the broken line binding to the positive-control strain (N. gonorrhoeae strain 15253). The isotype control for strain 375 (no factor H in the reaction mixture) was identical to the histograms observed for that strain with factor H and has been omitted to simplify the illustration. One experiment representative of two separate experiments is shown. The x axis represents fluorescence on a log10 scale, and the y axis the number of events (counts).
Complement C4 and C3 activation on the WT strains and their siaB mutants.
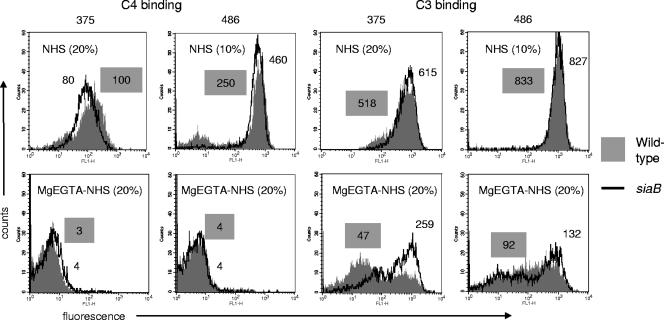
We hypothesize that conditions where only the alternative pathway is functional are likely to be relevant in situations where antibody to NT H. influenzae has yet to develop, such as in infants as well as chinchillas naïve to Haemophilus, as used in our animal model. We measured C3 and C4 binding to the two wild-type strains and their siaB mutants after incubation with either NHS (all complement pathways functional) or MgEGTA-NHS (alternative pathway alone active). Based on results of the serum bactericidal assays (Fig. 4) and preliminary dose-response experiments, we used 20% NHS with strains 375 and 375siaB and 10% NHS with strains 486 and 486siaB to illustrate differences in complement activation among strains. Because alternative pathway activation is concentration dependent and occurs at serum concentrations above 10% (11), we used 20% MgEGTA-NHS for both strains.
(i) Strains 375 and 486 differ in their abilities to activate the classical pathway of complement.
We measured C4 binding to both WT strains and their siaB mutants after incubation with either NHS or MgEGTA-NHS. As seen in Fig. 6, strain 486 bound more C4 than strain 375 (∼4-fold-higher geometric mean fluorescence), despite incubation of the latter with a higher concentration of NHS. We did not observe any difference in C4 binding between WT strains and their corresponding siaB mutants. There were no differences in IgG or IgM binding among the strains (data not shown). As expected, chelation of Ca++ in NHS with MgEGTA abrogated C4 binding (Fig. 6). Collectively, these data suggest that NT H. influenzae strains differ in their abilities to activate C4 and that LPS sialylation does not appear to regulate the amount of C4 binding.
FIG. 6.
C4 and C3 binding to strains 375 WT and 486 WT (shaded histograms) and their siaB mutants (solid lines). Bacteria were incubated with NHS (20%, vol/vol, for strains 375 and 375siaB and 10%, vol/vol, for strains 486 and 486siaB) or with NHS chelated with 10 mM MgEGTA (20%, vol/vol, for all strains). Numbers beside each histogram represent geometric mean fluorescence. Values for the wild-type strains are indicated in shaded boxes. Controls (either no serum or heat-inactivated serum in the reaction mixture) yielded fluorescence values that lay within the first decade (<10) in every instance and have been omitted for simplicity. One experiment representative of two separate and comparable experiments is shown. Axes are described in the legend for Fig. 5.
(ii) LPS sialylation regulates the alternative pathway of complement.
Total C3 binding to the strains was measured by flow cytometry. Again, strain 375 bound less C3 than strain 486 (geometric mean fluorescence was 518 with 375 WT versus 833 with 486 WT). We did not observe any differences between WT strains and their siaB isogenic counterparts by use of NHS. When bacteria were incubated with MgEGTA-NHS, we noted a marked (0.5- to 1-log10 geometric mean fluorescence) decrease in C3 binding compared to levels for the same strain incubated with NHS, suggesting that an intact classical pathway was required for optimal complement activation and C3 deposition. Sialylation of LPS was critical in retarding C3 deposition when only the alternative pathway was active. Sialylation of 375 had a profound impact (∼5-fold decrease in geometric mean fluorescence with the WT compared to the level with its siaB mutant) on alternative pathway-mediated C3 binding, compared to a more modest effect of 486 LPS sialylation (∼1.5-fold decrease in geometric mean fluorescence with 486 WT compared to the level with its siaB mutant).
Examination of C4 and C3 targets on bacteria.
In order to better understand the reason for the differences in complement C4 and C3 activation between the two strains, we sought to determine if the ligands for these two complement components on the two strains were different.
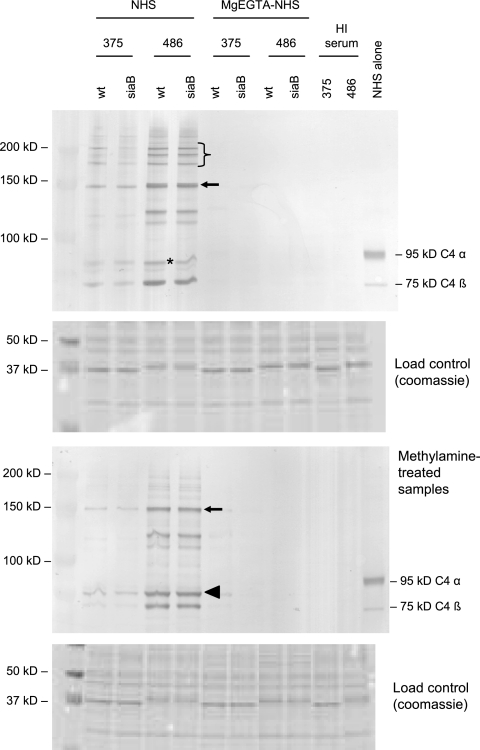
We first examined C4b binding to the strains. Figure 7 shows fewer and less intense anti-C4-reactive bands with 375 and 375siaB than with 486 and its siaB mutant. We did not notice any differences between either WT strain and its siaB mutant, confirming results seen by flow cytometry. The majority of C4b bound to both strains was detected as part of an ∼145-kDa complex (Fig. 7). Therefore, the molecular mass of the target can be estimated by subtracting the mass of the C4b α′-chain (87 kDa) from that of the complex, which amounts to ∼58 kDa. In addition, we noted two other C4b-target complexes, most readily seen with 486 and its siaB mutant, which migrated between the 100-kDa and 150-kDa markers. Finally, a C4b-target adduct was seen just below the location of the 95-kDa C4 α-chain (Fig. 7), which represents the 87-kDa C4b α′-chain linked to an ∼5-kDa molecule, most likely LPS. An anti-LPS monoclonal antibody that reacts with 375 LPS in Western blots, called MAb D2 (National Research Council of Canada), as well as an anti-KDO antibody, did not bind to the ∼92-kDa complex. As a result, we could not definitively identify the low-molecular-mass bacterial molecule in that complex as LPS. Complexes migrating above the 150-kDa marker likely represented C4b-containing heterodimers (i.e., C5 convertases) linked to bacterial targets. Methylamine treatment, which dissociates ester-linked C4b by nucleophilic attack and results in the appearance of the free 87-kDa α′-chain, did not affect C4b-containing complexes between 100 kDa and 150 kDa but dissociated the low-molecular-mass C4b complex and the higher-molecular-mass C5 convertases. These data suggest that C4b binds mainly to proteins on these NT H. influenzae strains via amide linkages and that a relatively small amount of C4b binds to the ∼5-kDa target (likely LPS), but via ester bonds. As expected, no C4b was detected on samples treated with MgEGTA-NHS or controls using heat-inactivated serum.
FIG. 7.
C4b binding to strains 375 and 486 and their siaB mutants. Bacteria were incubated either with NHS (20%, vol/vol, for strains 375 and 375siaB and 10%, vol/vol, for strains 486 and 486siaB) or with NHS chelated with 10 mM MgEGTA (20%, vol/vol, for all strains). The upper blot shows samples not treated with methylamine (both amide and ester linkages intact), while the lower blot shows methylamine-treated samples, where ester linkages are disrupted by nucleophilic attack while amide linkages are unaffected. Coomassie-stained gels, where 10 μl of each sample was loaded per lane, were used as loading controls and are shown below each blot. The asterisks indicate binding of C4b α′-chain to an ∼5-kDa target. The arrow indicates the C4b α′-chain bound to an ∼55-kDa target. The bracket indicates the location of C4b-containing heterodimers (C5 convertases). The arrowhead in the lower blot indicates the 87-kDa C4b α′-chain that was ester linked to its targets and has been released by nucleophilic attack by methylamine. The C4 α-chain (95 kDa) and C4 β-chain (75 kDa) are indicated in the lane marked NHS alone (serum with no bacteria). The lanes marked HI serum represent bacteria incubated with heat-inactivated serum. The far left lane indicates the molecular mass marker.
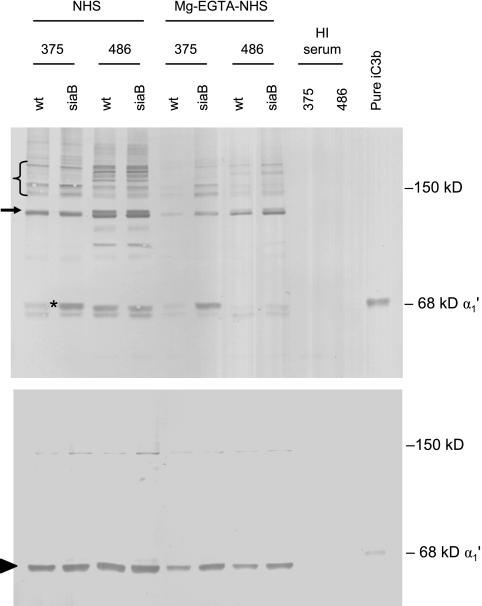
Activation of C3 results in covalent binding of C3b to bacterial targets. C3b is then cleaved to iC3b by the cofactor and enzymatic activities of factor H and I, respectively (24, 33, 43). A Western blot using polyclonal anti-C3 (which recognizes both C3b and iC3b) showed qualitatively similar bands on each WT strain and its siaB mutant, but the bands are of greater intensity on the mutant strain (data not shown). In addition, iC3b was the predominant C3 fragment on the bacterial surface, based on the observation that the released iC3b α1′-chain (68 kDa) was readily visualized whereas the released C3b α′-chain (110 kDa) was barely seen (data not shown). Because the polyclonal anti-C3 Ab cannot distinguish between C3b and iC3b in each complex, which would make estimating the molecular masses of C3b/iC3b-bound NT H. influenzae targets difficult, we selectively detected iC3b bound to the strains using anti-iC3b MAb G-3E (Fig. 8). The most prominent iC3b-target complex with strain 375 WT was seen at ∼125 kDa (Fig. 8), yielding a bacterial target with an estimated molecular mass (derived by deducting the mass of the ∼68-kDa iC3b α1′-chain from that of the complex) of ∼57 kDa. A faint adduct was also seen at ∼68 kDa (Fig. 8). The loss of LPS sialic acid on strain 375 resulted in the enhanced prominence of an ∼68-kDa iC3b-containing complex on 375siaB, while the ∼125-kDa complex remained unchanged. The position of higher-molecular-mass complexes that likely represent iC3b-containing heterodimers (C5 convertases where the C3b has been converted to iC3b) is also indicated (Fig. 8). Strain 486 also bound iC3b via the ∼57-kDa target. Three additional bands reactive with anti-iC3b MAb were also seen. The iC3b binding profiles of 486 and 486siaB were similar. Incubation of bacteria with MgEGTA-NHS revealed minimal iC3b bound to 375 WT; loss of sialic acid resulted in appearance of the ∼68-kDa complex. In contrast, incubation of 486 WT with MgEGTA-NHS resulted in binding of iC3b mainly to the ∼57-kDa target, with an adduct barely visible at ∼68 kDa. A small amount of iC3b α1′-chain was spontaneously released from its target and was visible as a band just beneath the putative iC3b α1′-chain-LPS complex (Fig. 8). Methylamine treatment (Fig. 8, lower blot) resulted in release of almost all iC3b from its targets, with concomitant appearance of the free iC3b α1′-chain. It was noted that the α1′-chain in iC3b made from fluid-phase C3b migrates more slowly than the iC3b α1′-chain released from bacterial targets (Fig. 8, far right lane), raising the possibility that bacterium-bound iC3b may be subjected to additional or altered processing compared to iC3b generated in the fluid phase.
FIG. 8.
iC3b binding to strains 375 WT and 486 WT and their siaB mutants. Bacteria were incubated either with NHS or with MgEGTA-NHS as described in the legend for Fig. 7. The upper blot shows samples not treated with methylamine (both amide and ester linkages intact), while the lower blot shows methylamine-treated samples, where ester linkages are disrupted by nucleophilic attack while amide linkages are unaffected. The asterisk indicates the iC3b α1′-chain bound to an ∼5-kDa target. The arrow at ∼125 kDa indicates the iC3b α1′-chain bound to an ∼57-kDa target. The arrowhead in the lower blot indicates the ∼68-kDa iC3b α1′-chain that was ester linked to its targets and has been released by nucleophilic attack by methylamine. The lanes marked HI serum are controls using bacteria incubated with heat-inactivated serum. The same samples used for Fig. 7 were used in these experiments, and loading controls for these samples are illustrated in Fig. 7. Pure iC3b, iC3b made from fluid-phase C3b.
Collectively, the data described above suggest that the targets for C4b and C3b/iC3b differ among NT H. influenzae strains and that this may, at least in part, account for differences in their susceptibilities to complement-dependent killing.
DISCUSSION
These studies demonstrate a definitive role for complement as an integral part of the host defense against middle ear infection. Animals with an intact complement system that were inoculated with 486siaB failed to develop culture-positive middle ear disease or any evidence of inflammatory exudate, confirming that immune mechanisms are able to prevent the development of acute otitis media. The reduction in culture-positive episodes and overall episodes observed in the Finnish Otitis Media Study of heptavalent pneumococcal conjugate vaccine is consistent with the occurrence of rapid sterilization in the absence of an inflammatory exudate (8). Complement depletion enables the otherwise avirulent sialic acid-deficient isolates to establish disease of intensity similar to that seen with the WT strain. Disease appears to clear in association with the return of complement activity in the animal. Thus, resistance to complement-dependent host defenses is necessary for both development of experimental otitis media and persistent culture-positive disease and supports our previous observation of the presence of sialylated LPS glycoforms on ex vivo isolates on days 5 and 8 following inoculation (5).
Our studies also help to detail the particular role of sialic acid in permitting evasion of host defenses by NT H. influenzae. Although sialic acid on NT H. influenzae LPS has previously been shown to enhance serum resistance (10), the molecular mechanism by which it regulates complement has not been demonstrated previously. Factor H is a key regulatory protein of the alternative complement pathway, and several bacterial species bind factor H to their surface, which results in serum resistance or impedance to phagocytosis (15). Sialylation of N. gonorrhoeae lacto-N-neotetraose-containing LPS results in enhanced binding of factor H directly to the bacterial surface, which results in complement regulation (28). However, we were not able to demonstrate enhanced binding of factor H directly to the surface of NT H. influenzae in either the presence or the absence of sialylated glycoforms (Fig. 5). Thus, the role of sialylated glycoforms in NT H. influenzae in restricting complement binding was shown to differ from that in N. gonorrhoeae and required further clarification.
We observed C4b binding to a low-molecular-mass target (∼5 kDa) in both strain backgrounds (Fig. 7). While it is likely that this target is LPS, which is the most abundant outer membrane component on a molar basis, we were not able to colocalize LPS in these complexes by using available anti-LPS monoclonal antibodies. Circumstantial evidence for LPS being this low-molecular-mass target is provided by the observation that mutations in LPS biosynthesis genes of strain 375 that alter the LPS structure induce concomitant changes in migration of these complexes. As examples, hmg locus and lpsA gene mutant strains that express LPS molecules with masses higher and lower than that of 375 WT LPS result in decreased and increased migration velocities of the complexes, respectively (data not shown). All low-molecular-mass C4b complexes were dissociated with methylamine, suggesting that C4b formed ester linkages and not amide linkages with the LPS of these strains. In contrast to N. meningitidis, which binds C4b via amide linkages when phosphoethanolamine is present at the 6-position of heptose (Hep) II (or at the 3-position when concomitant HepI extensions are restricted to ≤2 hexoses) (26), we did not see any evidence for amide linkages between C4b and LPS of these NT H. influenzae strains. It is likely that the extensions of the other Hep residues in NT H. influenzae strains prevent access of C4b to inner core phosphoethanolamine at the 6-position of HepII.
Although sialic acid in LPS is a key NT H. influenzae virulence factor (5), we observed that the siaB mutant of strain 375 was capable of causing disease, although the rate and burden of disease were attenuated compared to those for the WT strains. One possible explanation for this phenomenon could be the higher resistance of strain 375 to complement-mediated killing (Fig. 4). Assembly of C5 convertases on the bacterial surface is necessary for C5b-9 formation, and it is the latter that mediates bacterial killing. It has been shown that the classical pathway C5 convertase (C4b2aC3b) is about nine times more efficient in cleaving C5 than the alternative pathway C5 convertase (C3bBbC3b) (30, 31). In addition, the classical pathway C5 convertase is more stable than its alternative pathway counterpart because C3b that is bound to C4b (as occurs in the C4b2aC3b complex) is resistant to degradation by factors H and I (22). Therefore, binding of C4b to the bacterial surface, effected either by Ab or in some instances by C-reactive protein binding to phosphorylcholine on LPS (42), is essential for bacterial killing. Greater C4b binding was detected with strain 486, which is consistent with its greater sensitivity to killing by serum (Fig. 4). The reason for the higher C4b binding to strain 486 was not because of differences in levels of Ab binding (data not shown). The primary targets for C4b on strain 486 are proteins, and definitive identification of these molecules, while beyond the scope of this study, would shed light on the reason for differences among strains to resist complement.
While the alternative pathway alone does not mediate bactericidal activity (44), it plays a key role in amplification of C3 that is initially deposited via the classical pathway. The requirement of Ab in mediating bactericidal activity is illustrated by the observation that absorbing bacterium-specific Ab from serum by use of fixed bacteria or depleting serum of IgG by use of protein G abrogates killing (data not shown). In the absence of anti-NT H. influenzae Ab, the alternative pathway and possibly the mannan-binding lectin pathway are responsible for depositing C3b on bacteria, which is important in aiding opsonophagocytic dispatch of bacteria by polymorphonuclear leukocytes. A situation where there is little or no Ab-dependent classical pathway activation in response to infection may occur in infants and young children and also in naïve chinchillas as we have used in our animal model, where natural Ab (the result of colonization) has not yet been elicited. We therefore studied C3 binding in the absence of the Ab-dependent classical and lectin pathways by using NHS chelated with MgEGTA. As expected, the total amount of C3 that bound to both strains and their mutant derivatives was smaller in chelated serum than in intact serum (Fig. 6). The siaB mutants bound more C3 than WT strains in the presence of MgEGTA-treated serum. It is noteworthy that the presence of LPS sialic acid in strain 375 had a more profound effect on C3 binding than did the LPS sialylation of strain 486 (Fig. 6). This may be explained by the observation that most of the iC3b on 375siaB was bound to the low-molecular-mass molecule, most probably LPS (Fig. 7), therefore enabling sialylation to have a more direct impact on C3b binding by probably obscuring a target on LPS for C3b (C3b is converted to iC3b, and we detected the latter fragment). In contrast, the protein target (Fig. 8) on strain 486 bound iC3b, and as a result LPS sialylation may have had a lesser impact on C3b deposition. The restoration of virulence to both otherwise avirulent isogenic siaB mutants of NT H. influenzae provides definitive evidence for an active role for complement in local host defenses against middle ear infection. This observation is consistent with the further delineated role sialylated LPS glycoforms play in restricting complement deposition on the surface, especially when the classical pathway for complement activation has been incapacitated. This appears relevant to naïve hosts, such as infants, who have yet to develop strain-specific antibody to NT H. influenzae. Whether sialylation is necessary to establish colonization requires further evaluation. Shakhnovich and colleagues have reported that neuraminidase produced by Streptococcus pneumoniae has the capacity to desialylate NT H. influenzae in vitro and suggested that the mechanism may permit S. pneumoniae to compete with NT H. influenzae in the nasopharynx (32). Our observations also provide evidence of the diversity of NT H. influenzae both in susceptibility to bactericidal activity of NHS and in the preferred ligand for complement C3 and C4 binding on its surface. Further studies are planned to identify if variability in outer membrane proteins contributes to differences in susceptibility to complement activity.
Acknowledgments
These studies were supported by NIH NIDCD research grant award DC005855 to R.G. and in part by a research grant to S.I.P. from the Shereta R. Seelig Charitable Foundation Trust. S.R. was supported in part by NIH awards AI054544 and AI032725. D.W.H. and E.R.M. were supported by a program grant from the United Kingdom Medical Research Council.
We thank Beiyang Ma and Loc Truong for expert technical assistance. We thank the Otitis Media Study Group (National Public Health Institute, Finland) for nontypeable Haemophilus influenzae OM isolates 375 and 486.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Alper, C. A., and D. Balavitch. 1976. Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science 191:1275-1276. [DOI] [PubMed] [Google Scholar]
- 2.Babl, F. E., S. I. Pelton, and Z. Li. 2002. Experimental acute otitis media due to nontypeable Haemophilus influenzae: comparison of high and low azithromycin doses with placebo. Antimicrob. Agents Chemother. 46:2194-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein, J. M., H. A. Schenkein, R. J. Genco, and W. R. Bartholomew. 1978. Complement activity in middle ear effusions. Clin. Exp. Immunol. 33:340-346. [PMC free article] [PubMed] [Google Scholar]
- 4.Blake, M. S., and E. C. Gotschlich. 1982. Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect. Immun. 36:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochrane, C. G., H. J. Muller-Eberhard, and B. S. Aikin. 1970. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J. Immunol. 105:55-69. [PubMed] [Google Scholar]
- 7.Dagan, R., A. Hoberman, C. Johnson, E. L. Leibovitz, A. Arguedas, F. V. Rose, B. R. Wynne, and M. R. Jacobs. 2001. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr. Infect. Dis. J. 20:829-837. [DOI] [PubMed] [Google Scholar]
- 8.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 9.Hensley, P., M. C. O'Keefe, C. J. Spangler, J. C. Osborne, Jr., and C. W. Vogel. 1986. The effects of metal ions and temperature on the interaction of cobra venom factor and human complement factor B. J. Biol. Chem. 261:11038-11044. [PubMed] [Google Scholar]
- 10.Hood, D. W., K. Makepeace, M. E. Deadman, R. F. Rest, P. Thibault, A. Martin, J. C. Richards, and E. R. Moxon. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol. Microbiol. 33:679-692. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz, H. I., R. M. Des Prez, and E. W. Hook. 1962. Effects of bacterial endotoxin on rabbit platelets. II. Enhancement of platelet factor 3 activity in vitro and in vivo. J. Exp. Med. 116:619-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida, K., K. Mitomo, T. Fujita, and N. Tamura. 1987. Characterization of three monoclonal antibodies against C3 with selective specificities. Immunology 62:413-417. [PMC free article] [PubMed] [Google Scholar]
- 13.Karasic, R. B., C. E. Trumpp, H. E. Gnehm, P. A. Rice, and S. I. Pelton. 1985. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J. Infect. Dis. 151:273-279. [DOI] [PubMed] [Google Scholar]
- 14.Kasper, D. L., P. A. Rice, and W. M. McCormick. 1977. Bactericidal antibody in genital infection due to Neisseria gonorrhoeae. J. Infect. Dis. 135:243-251. [DOI] [PubMed] [Google Scholar]
- 15.Kraiczy, P., and R. Wurzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 16.Lachmann, P. J., and L. Halbwachs. 1975. The influence of C3b inactivator (KAF) concentration on the ability of serum to support complement activation. Clin. Exp. Immunol. 21:109-114. [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz, E., D. D. Patel, T. Hartung, and D. A. Schwartz. 2002. Toll-like receptor 4 (TLR4)-deficient murine macrophage cell line as an in vitro assay system to show TLR4-independent signaling of Bacteroides fragilis lipopolysaccharide. Infect. Immun. 70:4892-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lysenko, E. S., J. Gould, R. Bals, J. M. Wilson, and J. N. Weiser. 2000. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 68:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuillen, D. P., S. Gulati, and P. A. Rice. 1994. Complement-mediated bacterial killing assays. Methods Enzymol. 236:137-147. [DOI] [PubMed] [Google Scholar]
- 21.Medicus, R. G., O. Gotze, and H. J. Muller-Eberhard. 1976. The serine protease nature of the C3 and C5 convertases of the classical and alternative complement pathways. Scand. J. Immunol. 5:1049-1055. [DOI] [PubMed] [Google Scholar]
- 22.Meri, S., and M. K. Pangburn. 1990. A mechanism of activation of the alternative complement pathway by the classical pathway: protection of C3b from inactivation by covalent attachment to C4b. Eur. J. Immunol. 20:2555-2561. [DOI] [PubMed] [Google Scholar]
- 23.Nagaki, K., K. Iida, M. Okubo, and S. Inai. 1978. Reaction mechanisms of beta1H globulin. Int. Arch. Allergy Appl. Immunol. 57:221-232. [DOI] [PubMed] [Google Scholar]
- 24.Pangburn, M. K., and H. J. Muller-Eberhard. 1986. The C3 convertase of the alternative pathway of human complement. Enzymic properties of the bimolecular proteinase. Biochem. J. 235:723-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platts-Mills, T. A., and K. Ishizaka. 1974. Activation of the alternate pathway of human complements by rabbit cells. J. Immunol. 113:348-358. [PubMed] [Google Scholar]
- 26.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278:50853-50862. [DOI] [PubMed] [Google Scholar]
- 27.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, B. G. Monks, C. O'Connell, R. Boden, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193:281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ram, S., A. K. Sharma, S. D. Simpson, S. Gulati, D. P. McQuillen, M. K. Pangburn, and P. A. Rice. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawal, N., and M. K. Pangburn. 1998. C5 convertase of the alternative pathway of complement. Kinetic analysis of the free and surface-bound forms of the enzyme. J. Biol. Chem. 273:16828-16835. [DOI] [PubMed] [Google Scholar]
- 31.Rawal, N., and M. K. Pangburn. 2003. Formation of high affinity C5 convertase of the classical pathway of complement. J. Biol. Chem. 278:38476-38483. [DOI] [PubMed] [Google Scholar]
- 32.Shakhnovich, E. A., S. J. King, and J. N. Weiser. 2002. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect. Immun. 70:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim, E., A. B. Wood, L. M. Hsiung, and R. B. Sim. 1981. Pattern of degradation of human complement fragment, C3b. FEBS Lett. 132:55-60. [DOI] [PubMed] [Google Scholar]
- 34.Smith, C. A., C. W. Vogel, and H. J. Muller-Eberhard. 1982. Ultrastructure of cobra venom factor-dependent C3/C5 convertase and its zymogen, factor B of human complement. J. Biol. Chem. 257:9879-9882. [PubMed] [Google Scholar]
- 35.Stenfors, L. E., and S. Raisanen. 1992. Opsonization of middle ear bacteria during chronic suppurative and secretory otitis media. Acta Otolaryngol. 112:96-101. [DOI] [PubMed] [Google Scholar]
- 36.Vogel, C. W., R. Bredehorst, D. C. Fritzinger, T. Grunwald, P. Ziegelmuller, and M. A. Kock. 1996. Structure and function of cobra venom factor, the complement-activating protein in cobra venom. Adv. Exp. Med. Biol. 391:97-114. [DOI] [PubMed] [Google Scholar]
- 37.Vogel, C. W., and H. J. Muller-Eberhard. 1982. The cobra venom factor-dependent C3 convertase of human complement. A kinetic and thermodynamic analysis of a protease acting on its natural high molecular weight substrate. J. Biol. Chem. 257:8292-8299. [PubMed] [Google Scholar]
- 38.Vogt, W., L. Dieminger, R. Lynen, and G. Schmidt. 1974. Alternative pathway for the activation of complement in human serum. Formation and composition of the complex with cobra venom factor that cleaves the third component of complement. Hoppe-Seyler's Z. Physiol. Chem. 355:171-183. [DOI] [PubMed] [Google Scholar]
- 39.von Zabern, I., B. Hinsch, H. Przyklenk, G. Schmidt, and W. Vogt. 1980. Comparison of Naja n. naja and Naja h. haje cobra-venom factors: correlation between binding affinity for the fifth component of complement and mediation of its cleavage. Immunobiology 157:499-514. [DOI] [PubMed] [Google Scholar]
- 40.Wald, E. R. 2003. Otitis media. N. Engl. J. Med. 348:363. [DOI] [PubMed] [Google Scholar]
- 41.Wang, X., C. Moser, J. P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 168:810-815. [DOI] [PubMed] [Google Scholar]
- 42.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whaley, K., and S. Ruddy. 1976. Modulation of the alternative complement pathways by beta 1 H globulin. J. Exp. Med. 144:1147-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams, B. J., G. Morlin, N. Valentine, and A. L. Smith. 2001. Serum resistance in an invasive, nontypeable Haemophilus influenzae strain. Infect. Immun. 69:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamasaki, R., D. E. Kerwood, H. Schneider, K. P. Quinn, J. M. Griffiss, and R. E. Mandrell. 1994. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection. Evidence for a new glycosylation pathway of the gonococcal lipooligosaccharide. J. Biol. Chem. 269:30345-30351. [PubMed] [Google Scholar]