Abstract
Human monocytic ehrlichiosis is caused by a tick-transmitted rickettsia, Ehrlichia chaffeensis. We recently reported that E. chaffeensis grown in tick cells expresses different proteins than bacteria grown in macrophages. Therefore, we tested the hypothesis that immune responses against E. chaffeensis would be different if the mice are challenged with bacteria grown in macrophages or tick cells. We assessed the E. chaffeensis clearance from the peritoneum, spleen, and liver by C57BL/6J mice using a TaqMan-based real-time reverse transcription-PCR assay. Macrophage-grown E. chaffeensis was cleared in 2 weeks from the peritoneum, whereas the pathogen from tick cells persisted for nine additional days and included three relapses of increasing bacterial load separated by three-day intervals. Tick cell-grown bacteria also persisted in the livers and spleens with higher bacterial loads compared to macrophage-grown bacteria and fluctuated over a period of 35 days. Three-day periodic cycles were detected in T-cell CD62L/CD44 ratios in the spleen and bone marrow in response to infections with both tick cell- and macrophage-grown bacteria and were accompanied by similar periodic cycles of spleen cell cytokine secretions and nitric oxide and interleukin-6 by peritoneal macrophages. The E. chaffeensis-specific immunoglobulin G response was considerably higher and steadily increased in mice infected with the tick cell-derived E. chaffeensis compared to DH82-grown bacteria. In addition, antigens detected by the immunoglobulins were significantly different between mice infected with the E. chaffeensis originating from tick cells or macrophages. The differences in the immune response to tick cell-grown bacteria compared to macrophage-grown bacteria reflected a delay in the shift of gene expression from the tick cell-specific Omp 14 gene to the macrophage-specific Omp 19 gene. These data suggest that the host response to E. chaffeensis depends on the source of the bacteria and that this experimental model requires the most natural inoculum possible to allow for a realistic understanding of host resistance.
Ehrlichia chaffeensis is the causative agent of an emerging infectious disease, human monocytic ehrlichiosis (11). The pathogen is transmitted from the bite of an infected Amblyomma americanum tick (2). E. chaffeensis and other tick-transmitted pathogens have adapted to both tick and vertebrate host cell environments(3, 10, 12, 13, 16, 52, 53, 59). Tick larvae feed on small mammals. They then molt to the nymphal stage off the animal in the environment. Nymphs undergo a similar cycle, feeding on medium-sized mammals such as squirrels. After molting to adults on the ground, they feed on hosts such as white-tailed deer (49). Although E. chaffeensis appears to persist in both hosts for long periods of time, little is known about that process.
Several reports documented that tick feeding results in the modulation of the host immune responses (19, 20, 27, 30, 55). For example, the saliva of the Rhipicephalus sanguineus tick impairs T-cell proliferation and gamma interferon (IFN-γ)-induced macrophage microbicidal activity (18). Similarly, successive tick infestations selectively promote a Th2-type T-helper cell cytokine profile in mice (19). Tick saliva contains immunomodulatory factors that aid in altering the host response (27). Antigens expressed during morphological stages in a host-specific manner by tick-transmitted pathogens may also be an important contributor to the adaptation mechanism that supports their life cycle within tick and vertebrate host environments (4, 7, 26, 46, 47). For example, Borrelia burgdorferi expresses 15 silent sequences of lipoprotein VlsE during infection in mice that do not appear to be expressed in ticks (58). Moreover, B. burgdorferi OspA gene expression may allow adhesion to the midgut, but the expression of OspC genes may allow the invasion of tick salivary glands as a prerequisite to vertebrate host infection (22). Therefore, differential antigen expression may facilitate movement between the arthropods and mammals for tick-transmitted bacteria (14, 21, 41, 45).
In our previous experimental infection studies in mice using E. chaffeensis cultivated in the macrophage cell line, DH82, we concluded that the pathogen is cleared in about 2 weeks, and optimal resolution of the infection requires macrophage activation, major histocompatibility complex class II (MHCII) molecules, and CD4+ helper T-cell responses (24, 25). Antibody-mediated immunity is also important for clearing the organisms from circulation (56). The rapid clearance in the mouse model is contrary to persistent infections in hosts acquiring infection from a tick bite with Ehrlichia species (15, 42, 43). Recently, we presented evidence that E. chaffeensis expresses different p28 isoforms in response to its growth in macrophages and tick cells (46, 47). E. chaffeensis-infected macrophages express primarily the product of the p28-Omp 19 gene, whereas in tick cells the expressed proteins are the product of the p28-Omp 14 gene. In the present study, we tested the hypothesis that the origin of the bacteria, macrophage, or tick cell would impact the course of the immune response and affect the ability of the host to eliminate bacteria. We present evidence that the clearance of E. chaffeensis grown in tick cells is delayed in the murine host compared to that originating from the macrophage culture.
MATERIALS AND METHODS
In vitro cultivation of E. chaffeensis.
The E. chaffeensis Arkansas isolate was cultivated in either the canine macrophage cell line DH82 at 37°C (9) or in the tick cell line ISE6 at 34°C (39). Cultures from T-75 flasks with 80 to 90% infectivity were used for experimental infection studies.
Experimental infections.
E. chaffeensis bacteria from infected macrophages or tick cells were dispersed by vortexing the cells in the presence of glass beads. The suspension was centrifuged at 500 × g for 10 min, and the supernatant was centrifuged at 15,500 × g to collect cell-free Ehrlichia. The cell pellet was resuspended in phosphate-buffered saline (PBS). C56BL/6J (B6) and B6.129-Abbtm1 N5F20 (C2D; MHCII−/−) mice were bred and housed in the vivarium in the Division of Biology at Kansas State University. Five different experiments were done to determine the mouse response to bacteria grown in macrophages or tick cells. Because our experience with the host response to DH82-grown bacteria was more extensive, each individual experiment measured the mouse responses from six mice per time point for ISE6-grown bacteria and four mice per time point for DH82-grown bacteria. Tissues were also collected from two normal mice at all time points to serve as controls for assays of bacterial clearance, antibody concentrations, or cytokine responses. The amount of bacteria injected into mice was estimated by using the TaqMan-based real-time reverse transcription-PCR (RT-PCR) assay (described below). The TaqMan-based real-time RT-PCR assay targeting to E. chaffeensis 16S rRNA was performed as we described recently (48). We opted to estimate the number of bacteria in an inoculum by estimating the amount of rRNA as opposed to the rRNA gene (single-copy gene) because the real-time RT-PCR method using 16S rRNA as the target is about 100 to 180 times more sensitive in detecting E. chaffeensis than an rRNA gene-based real-time PCR method (48). This approach is also supported by Felek et al., who also demonstrated a similar 100 times greater sensitivity using a 16S rRNA-based RT-PCR method compared to a PCR assay (17). To ensure the use of nearly equal numbers of bacteria used in each experimental group, we further evaluated rRNA gene/rRNA ratio and confirmed that the RNA isolated from the organisms cultured in tick cells also contained about 100 times more rRNA copies per each DNA copy of the gene (current study), which is similar to our previous observations for the RNA isolated from E. chaffeensis grown in macrophage cultures (48). Table 1 summarizes the estimated number of bacteria used as an inoculum per mouse in each experimental infection group. Mice used for infection studies were 6 to 8 weeks old at the beginning of each experiment, and they were injected intraperitoneally (i.p.) with an average of 3.5 × 106 bacteria from DH82 cells and 2.3 × 106 bacteria from ISE6 cells. The numbers of bacteria from each treatment group were not statistically different from one another (P > 0.1 by matched Student t test or P > 0.05 for the Wilcoxon signed-rank test). The first experiment used bacteria derived only from tick cells (3.2 × 106), whereas in experiments 2 to 5 mice received E. chaffeensis grown in DH82 and tick cell cultures. The inoculum size varied from as low as 0.7 × 106 to as high as 4.2 × 106 bacteria for the organisms originating from tick cells (the inoculum size varied over a range of sixfold) and the DH82 culture-derived bacteria used as the inoculum ranged from 1.0 × 106 to 7.5 × 106 per mouse (the inoculum size varied in about the sevenfold range).
TABLE 1.
Inoculum doses used in mouse infections with E. chaffeensis grown in DH82 or ISE6 cells
| Expt | Source of bacteria (cell line) | Inoculum size (106) |
|---|---|---|
| 1 | DH82 | NTa |
| ISE6 | 3.2 | |
| 2 | DH82 | 3.5 |
| ISE6 | 2.3 | |
| 3 | DH82 | 2.0 |
| ISE6 | 1.1 | |
| 4 | DH82 | 7.5 |
| ISE6 | 4.2 | |
| 5 | DH82 | 1.0 |
| ISE6 | 0.7 |
NT, not tested.
Tissue collection and processing.
Blood, peritoneal exudate cells, spleens, livers, and bone marrow were harvested from normal or infected mice (both treatment groups) at different times postinfection. The peritoneal exudate cells and spleen and liver samples were used to assess the presence of E. chaffeensis. Plasma recovered from blood was used for antibody analyses. Spleen and bone marrow cells were used for mapping cell phenotypes. Spleen and peritoneal macrophages were also used to measure cytokine production. Blood was collected from the retro-orbital sinus into a heparanized micropipette and transferred to a microcentrifuge tube containing 25 μl of heparin (10,000 IU/ml). The blood was centrifuged at 3,000 × g, and the plasma was stored at −70°C. Spleens were divided in half. One-half was used to prepare RNA to quantitate bacteria (see below). The second half was homogenized, erythrocytes were lysed, and the cells were counted. A total of 5 × 106 spleen cells per 60-mm plate were cultivated in Dulbecco modified Eagle medium containing 2% fetal bovine serum (DME2) and gentamicin (50 μg/ml). Supernatants were collected after a 20-h incubation at 37°C and frozen at −20°C until cytokine assays were performed (described below). A fraction of the spleen cells was analyzed with flow cytometry (see below). Bone marrow cells were washed from the femora and humeri and were used for culture or flow cytometry. About 100 mg of liver tissue was homogenized and used to isolate RNA according to the Tri-reagent method (see below). Peritoneal exudate cells containing predominantly macrophages were collected from E. chaffeensis-infected and uninfected control mice by peritoneal lavage with 20 ml of ice-cold, sterile PBS. Approximately 2 × 106 cells per sample were seeded into wells of 24-well culture plates in volume of 2 ml. Cells were incubated for 20 h, culture supernatants were collected, and cell-free supernatant was stored at −20°C until the cytokine assays were performed. A fraction of the peritoneal wash cells was also used for RNA isolation to determine the presence of bacteria.
RNA isolation.
Total RNA from a 0.5-ml portion of in vitro cultures or cell pellets collected from 5 ml of peritoneal wash cells or from about 50 mg each of spleen or 100 mg of liver tissue was isolated by using the Tri-Reagent RNA isolation kit according to the manufacturer's protocol (Sigma Aldrich, St. Louis, MO). Purified RNA pellets from in vitro cultures or peritoneal wash cells were resuspended in 50 μl of TE (10 mM Tris, 1 mM EDTA [pH 8.0]), and the pellets from spleen or liver tissue were resuspended in 50 or 100 μl of TE. The purity and concentration of the RNA was assessed using a spectrophotometer (Nanodrop Technologies, Wilmington, DE) by calculating the ratio between the optical densities at 260 and 280 nm. The absorbance ratio for all samples ranged between 1.8 and 2.0. The quality of RNA for a subset of samples was also confirmed by resolving them on a 1.5% formaldehyde agarose gel (38).
Real-time quantitative RT-PCR.
The master mixture for RT-PCR assay is 25 μl in volume containing 2 μl of RNA, 5 pmol each of the TaqMan forward and reverse primers, 3.75 pmol of E. chaffeensis-specific TaqMan probe, 5 nmol of deoxynucleoside triphosphates, 125 nmol of MgCl2, and 1 μl of SS-III and Taq mix (SuperScript-III, One-Step RT-PCR system with platinum Taq DNA polymerase; Invitrogen Technologies, Carlsbad, CA). The temperature cycles used for the assay were one cycle each at 48°C for 30 min and 95°C for 3 min, followed by 45 cycles of 95°C for 15 s, 50°C for 30 s, and 60°C for 60 s. The product formation was monitored in real-time by measuring the emitted fluorescence in the extension phase of the PCR cycles using a SmartCycler system (Cepheid, Sunnyvale, CA). The reaction is regarded as positive for the presence of a template when amplified product formation results in the detection of 10 fluorescent units. The temperature cycle at which this occurs is regarded as the CT value that is template concentration dependent (48).
To determine the inoculum to be used for experimental infections, RNA was isolated from an aliquot of the culture suspension used for injection, and the amount of bacterial 16S rRNA was determined by real-time RT-PCR assay (48). The CT values were compared to a standard curve that was prepared by diluting known concentrations of bacterial 16S rRNA and determining the individual CT values for each dilution. The minimum detection sensitivity was estimated at one organism based on the assumption that there are approximately 100 molecules of 16S rRNA present per one organism for DH82 derived E. chaffeensis (17, 48). We also estimated the ratio of 16S rRNA to rRNA gene in tick cell-derived E. chaffeensis, and it is similar to that found in DH82 culture-derived E. chaffeensis, i.e., ∼1:100. Thus, we estimated bacterial numbers by translating 100 copies of 16S rRNA molecules as equal to one bacteria.
Quantitative ELISA to map IgG subclasses.
Quantitative enzyme-linked immunosorbent assay (ELISA) using whole-cell antigen (20 ng/well) was performed to determine the concentrations of E. chaffeensis-specific immunoglobulin G (IgG) subclass antibodies by following a protocol described previously (24, 25). Antigens used for ELISAs for macrophage-derived E. chaffeensis-infected mouse plasma were made from E. chaffeensis purified from DH82 cultures. Similarly, homologous antigens were used for ELISAs to map IgG subclass antibodies in mice infected with tick cell-derived E. chaffeensis.
Western blot analysis.
The whole-cell protein extract (3 μg/well) of purified E. chaffeensis Arkansas isolate cultured in either macrophage- or tick cell-derived organisms was resolved on a sodium dodecyl sulfate-12% polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and were probed with the plasma from E. chaffeensis-infected mice diluted at 1:128 for the presence of E. chaffeensis-specific antibody (24, 25).
Nitric oxide and cytokine assessment.
As a measure of nitric oxide (NO) production, we estimated peritoneal macrophage secretion of NO2, a stable end product of the NO synthesis pathway (50) using the Griess reaction (51). The sensitivity of the assay was approximately 200 nM. Macrophage cytokines were measured after peritoneal cells were isolated from mice injected with bacteria from either type of cell. Each mouse sample was measured independently, and multiple time points were tested in each experiment. The adherent macrophages were washed free of nonadherent cells, and they were cultured ex vivo for 20 h. Supernatants were collected and stored for assay. For spleen cells, interleukin-2 (IL-2), IL-6, and IL-10 and gamma interferon (IFN-γ) were assayed by using capture ELISAs in 96-well polyvinyl chloride plates as detailed previously (8). The sensitivities of these assays were 50 pg/ml, 20 pg/ml, 30 pg/ml, and 2 ng/ml for IL-2, IL-6, IL-10, and IFN-γ, respectively. The spleen cell supernatants were combined according to harvest date and type of infection. Supernatants produced by cells were also assessed by using Cytomix beads and assayed for IL-1α, IL-2, IL-5, IL-6, IL-10, IFN-γ, tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor, IL-4, and IL-17 by using a Flow Cytomix Multiplex mouse Th1/Th2 10plex kit (Bender MedSystems, Vienna, Austria).
The kinetics of the secretion of NO and IL-6 by peritoneal macrophages were very similar among the different experiments conducted. However, the peak secreted amounts of NO and IL-6 varied among the four experiments. Therefore, to illustrate the kinetics of the response, we normalized the data as follows. After we calculated how much cytokine was made by cells from each mouse at each time point, the peak amount of cytokine made in an experiment by a particular sample was valued at 100% of the maximum cytokine made. All other samples were valued at ≤100% of the maximum sample. Samples from the same time point were averaged, and the values at each time for four experiments used for the data analysis.
Flow cytometry.
Mouse spleen and bone marrow cells were resuspended in DME2, and 106 cells were incubated for 30 min in the wells of 96-well plates in 50 μl of goat serum diluted 1:2 in Hanks buffered salt solution. Cells were stained with 10 μl of mouse-specific, anti-CD62L-APC antibodies (BD Pharmingen, Franklin Lakes, NJ) and CD44-PE (eBiosciences, San Diego, CA) and separately stained with 5 μl of mouse-specific, anti-CD3-APC (BD Pharmingen) antibodies. Cells were incubated for 1 h, washed two times in Hanks balanced salt solution, and fixed with 250 μl of PBS containing 1% formalin. The fluorescence intensity was measured on a FACSCalibur analytical flow cytometer (Becton Dickinson, San Jose, CA). Splenic lymphocytes were identified in forward-scatter versus side-scatter plots and assessed for CD44 and CD62L. In the bone marrow, the percentage of CD3+ cells was determined in each population visible on forward-scatter versus side-scatter plots. The population with the highest number of CD3+ cells was used in the analyses of CD44 and CD62L.
In vitro and in vivo monitoring of changes in gene expression of the p28-Omp locus genes 14 and 19.
To evaluate changes in the gene expression, ISE6 tick cell-derived E. chaffeensis was purified (46) and used to infect several T-25 flasks containing DH82 (macrophage) or ISE6 (tick cell) cultures. Similarly, DH82 culture-derived E. chaffeensis was purified and used to infect flasks containing DH82 cultures. Total RNA was isolated from cell cultures at several times postinoculation until the culture reached 80 to 100% infectivity (0.5 to 5 days). Total RNA was recovered by using the Tri-Reagent RNA method (described above). We recently reported that the p28-Omp 14 transcript is the predominant transcript seen in tick cell-derived E. chaffeensis with low-level expression from the p28-Omp gene 19, whereas the macrophage-derived cultures had a predominant expression of gene 19 with low-level expression of gene 14 (determined by a TaqMan-based diplex real-time RT-PCR assay) (47). The TaqMan-based diplex real-time RT-PCR analysis was performed on RNAs to map differences in the gene expression for the p28-Omp 14 and 19 genes by following our recently described protocols (47). We also examined RNA samples that tested positive for E. chaffeensis from peritoneal macrophages isolated from B6 mice infected with macrophage- or tick cell-derived E. chaffeensis to determine the p28-Omp gene expression. C2D mice were also challenged with ISE6 and DH82 culture-derived E. chaffeensis to determine the changes in the gene expression from the p28-Omp genes 14 and 19 in the absence of a curing host response (a persistent infection model for E. chaffeensis [25]). RNA was isolated from peritoneal macrophages at various times after infection and assessed as described above.
Statistical analysis.
In these experiments, mouse age and sex were closely matched between the two treatment groups, and individuals were randomly assigned to each treatment group. Because the mice were drawn from the same population, we assumed that there was a normal distribution in the population variance for the cytokine and clearance assays. Student t tests were performed as indicated to determine differences between treatments. As a check, data were also analyzed using the Wilcoxon signed-rank test for paired data or the Mann-Whitney rank-sum test. Parametric and nonparametric tests agreed for our analyses. Statistical tests were done with the StatMost statistical software package (Dataxiom, Los Angeles, CA). Statistical significance was set at P ≤ 0.05. Trends were noted at significance for P ≤ 0.1.
RESULTS
Evaluation of E. chaffeensis clearance in mice after challenge with bacteria grown in tick cells or canine macrophages.
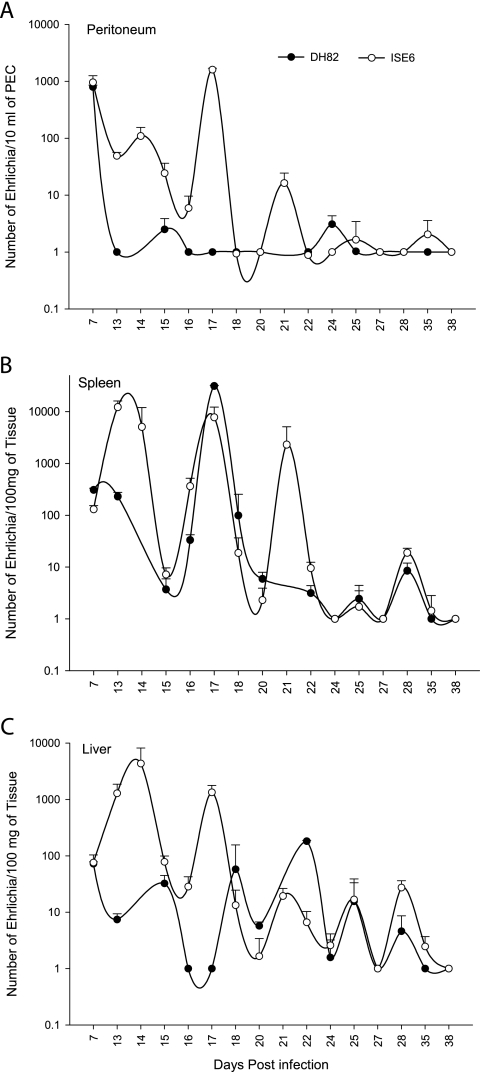
To test the hypothesis that the origin of E. chaffeensis would impact the mouse immune response to the infection, we used a very sensitive, real-time, RT-PCR assay and standard curves to estimate the number of E. chaffeensis bacteria in the peritoneum, liver, and spleen. The B6 mice cleared the macrophage-derived E. chaffeensis from the peritoneum in about 2 weeks, whereas the bacteria from tick cells persisted for approximately nine additional days (Fig. 1A). The bacterial load in mice infected with the tick cell-derived E. chaffeensis fluctuated in the peritoneum with three cyclical peaks that were separated by 3-day intervals. E. chaffeensis persisted in the spleen and liver for longer periods of time for up to 35 days for both the tick cell- and macrophage-derived E. chaffeensis (Fig. 1B and C). The bacteria also fluctuated in the spleen and liver for both infection groups and had peaks separated by about 3 days, a finding similar to what we observed in the peritoneal exudate cells for the tick cell-derived E. chaffeensis-infected mice. There were significantly (P < 0.05) more bacteria in the mouse spleens 13 to 14 days after infection with tick cell-derived bacteria compared to macrophage-grown bacteria. Similarly, significantly higher bacteria load (P < 0.05) was detected in the peritoneum for days 13 to 17 and 21 and for the liver samples for days 13 to 14, 17, 22, and 28 (Fig. 1).
FIG. 1.
Kinetics of B6 mouse infection after i.p. inoculation of E. chaffeensis cultures grown in DH82 (•) or ISE6 (○) cells. E. chaffeensis clearance was assessed by a 16S rRNA-based real-time RT-PCR assay. The number of bacteria were estimated by extrapolation of the RT-PCR cycles at which the samples tested positive (CT values) compared to CT values of known numbers of bacteria in a standard curve. Number of bactaria were plotted against days postinfection on a semilog graph. For the purpose of plotting the graph, a value of one bacteria is given to the samples that tested negative for E. chaffeensis by real-time RT-PCR assay. The data represent the median values ± SD from five independent experiments. Each experimental group contained four to six mice per time point. Bacteria were estimated in peritoneal exudate cells (A), spleen (B), and liver (C).
IgG response against E. chaffeensis.
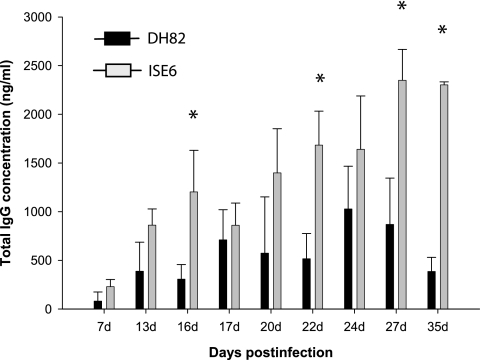
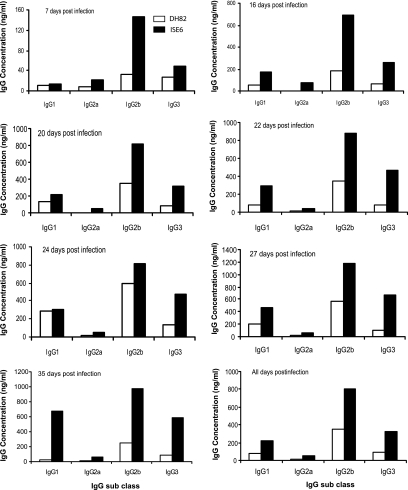
E. chaffeensis-specific IgG responses and IgG subclass differences were evaluated by quantitative ELISA to determine whether mice responded to tick cell-grown bacteria differently than macrophage-grown bacteria. Total IgG levels were higher and persisted longer after tick cell bacteria challenge (Fig. 2). The IgG levels were considerably higher and steadily increased in mice infected with tick cell-derived E. chaffeensis, peaking at a mean of 2,120 (standard deviation [SD] = 814) ng/ml and 2,108 (SD = 476) ng/ml on days 27 and 35 (P < 0.05 compared to DH82). In contrast, the total IgG response declined after reaching a peak at a mean value of 654 (SD = 370) ng/ml after 24 days postinfection in mice infected with the macrophage-derived E. chaffeensis (Fig. 2). Both infection groups developed E. chaffeensis-specific IgG responses with predominant expression (>90%) of the complement-fixing IgG molecules IgG2a, IgG2b, and IgG3 for all postinfection day samples (Fig. 3) . The IgG2 and IgG3 concentrations of mice injected with bacteria grown in ISE6 tick cells were usually at least twice as high as those in mice injected with bacteria grown in macrophages.
FIG. 2.
Total IgG made by B6 mice infected with E. chaffeensis cultivated DH82 or ISE6 cells. Plasma samples were analyzed by quantitative ELISA as described in Materials and Methods. The data are presented by days postinfection and are pooled from 8 to 12 mice for all postinfection dates. Each bar represents the mean ± the SD IgG concentration determined from the samples analyzed. The data in solid bars represent the total IgG concentration assessed for macrophage-derived plasma using antigens derived from E. chaffeensis cultured in DH82 cells, while shaded bars represent data for plasma from mice infected with ISE6 culture derived from E. chaffeensis using purified homologous antigen.
FIG. 3.
Quantitative analysis of IgG subclass antibodies made in mice infected with E. chaffeensis derived from DH82 and ISE6 cultures. Plasma samples from all postinfection days were assessed by quantitative ELISA. Each bar represents the mean IgG concentration at different postinfection dates. The IgG concentrations were calculated from standard curves plotted using IgG subclass standards and are presented as ng/ml of plasma.
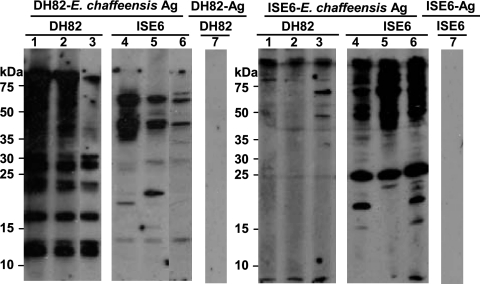
Western blot analysis using whole-cell E. chaffeensis antigens showed considerable variation in the antigens recognized by the immunoglobulins made in response to tick cell-derived E. chaffeensis compared to those made against macrophage-derived E. chaffeensis (Fig. 4). Antibodies from macrophage- and tick cell-derived E. chaffeensis-infected mice recognized considerably more antigens from the bacteria they were immunized with (homologous antigens) compared to antigens detected in bacteria grown in the other cell line (heterologous antigens). The antibodies recognized few shared antigens and also recognized several unique antigens. Mouse-to-mouse variation within the same experimental group was also visible in their ability to bind antigens and the concentration of the antibodies made (Fig. 4). The intensity of detection signals resulting from antigen and antibody complex to homologous antigens was also higher than those with heterologous antigens.
FIG. 4.
Western blot profile showing the response to E. chaffeensis whole-cell antigens. Representative data for three mice each for 28 days postinfection were presented. Lanes 1 to 3 had plasma for three mice infected with DH82 culture-derived E. chaffeensis; lanes 4 to 6 had plasma for three mice infected with ISE6 culture-derived E. chaffeensis. Lane 7 had plasma from one of the mice infected with DH82 culture-derived E. chaffeensis. Similarly, lane 8 had plasma from one of the mice infected with ISE6 culture-derived E. chaffeensis. The first set of six samples contained antigens derived from DH82-grown E. chaffeensis. The second set of six lanes included purified E. chaffeensis antigens from ISE6 cultures. Lanes 7 and 8 had antigens derived from uninfected DH82 and ISE6 cultures that were subjected to a purification protocol similar to that used for E. chaffeensis from infected cultures.
Peritoneal macrophage activation in E. chaffeensis-infected mice.
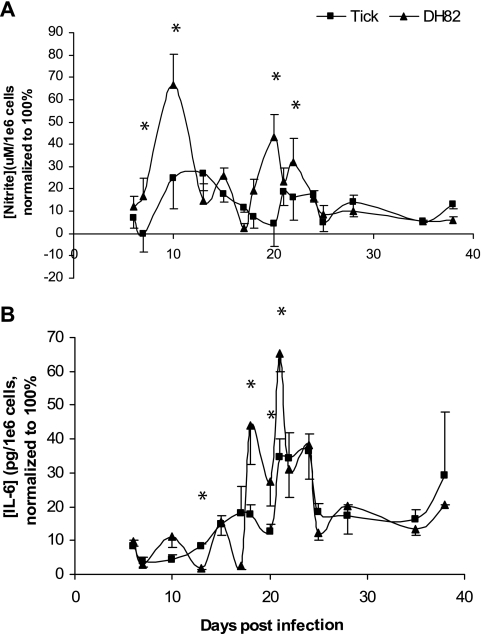
The kinetics of the secretion of NO and IL-6 in peritoneal macrophages were very similar for the normalized data for four independent experiments (Fig. 5). Peritoneal macrophages from mice infected with tick cell-grown E. chaffeensis secreted peak amounts of nitric oxide at about the same time as that observed for macrophage-grown pathogen, except that the magnitude of the peak response was lower at days 7, 10, 20, and 22 (P < 0.05; Fig. 5A). Macrophages from both treatment groups showed two peaks of secretion, one each at 10 to 12 days and at 20 to 22 days after infection.
FIG. 5.
Kinetics of nitrite (A) and IL-6 (B) secretion by peritoneal exudate macrophages in response to experimental challenge with E. chaffeensis. Numbers represent the mean percentage ± the SD (four independent experiments) of the maximum concentration of cytokine made during each experiment at each time interval. The IL-6 and nitrite concentrations detected ranged from 23 to 45 ng/106 cells and from 24 to 140 μM/106 cells, respectively. Cytokines were secreted by cells taken from four to six mice (measured independently) per time point per experiment. An asterisk indicates a significant difference (P < 0.05).
Macrophages from mice challenged with both types of bacteria also secreted IL-6 with both treatment groups having peak secretions at approximately 19 to 21 days after infection. Macrophages from mice challenged with DH82-grown bacteria produced significantly more IL-6 than macrophages from mice challenged with ISE6 tick cell-grown bacteria at 12, 19, 20, and 21 days after infection (P < 0.05; Fig. 5B).
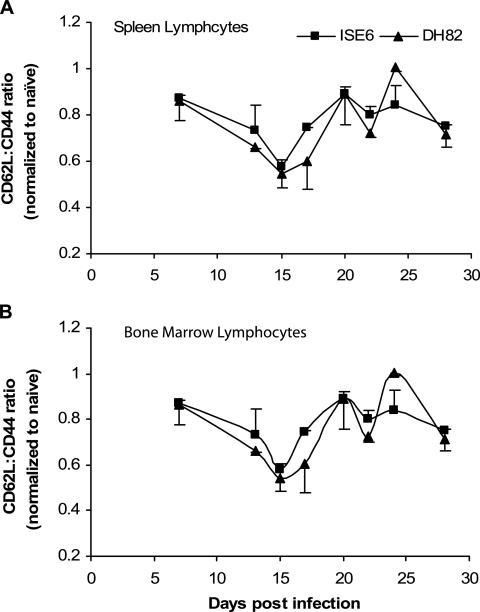
Lymphocyte assessment for CD62L and CD44.
To determine the progression of the immune response to E. chaffeensis, the expression of cell surface molecules CD62L and CD44 was investigated. We analyzed both molecules simultaneously, and the data are presented in Fig. 6 as a ratio of the percent CD62L to the percent CD44 on lymphocytes in the spleen and bone marrow to give a measure of “effector memory” (29). The CD62L/CD44 ratio was very similar for both infection groups in the spleen and decreased until about 15 days after experimental challenge for mice injected with either tick cell-grown or macrophage-grown E. chaffeensis (Fig. 6A). This low point immediately preceded the initial depression in the number of bacteria estimated to be in the spleen by real-time RT-PCR (Fig. 1B). By day 21 after infection, the CD62L/CD44 ratio returned to about 0.9. The CD62L/CD44 ratio then oscillated above and below the 0.8 value for the rest of the infection. The CD62L/CD44 ratio was also very similar for both infection groups for the cells isolated from the bone marrow (Fig. 6B). There was a general trend for an initial depression at around day 15 with an increase that peaked for tick cell-derived bacteria at day 21. There also was some indication for both treatment groups that the bone marrow CD62L/CD44 ratio oscillated with time.
FIG. 6.
CD62L/CD44 ratio versus time after experimental infection with E. chaffeensis for lymphocytes in the spleen (A) and bone marrow (B). The ratios of infected mice were normalized by dividing by the same ratio found in an uninfected mouse. A value of 1 is considered the value found in normal, uninfected mice. Cells from four mice per time point were evaluated.
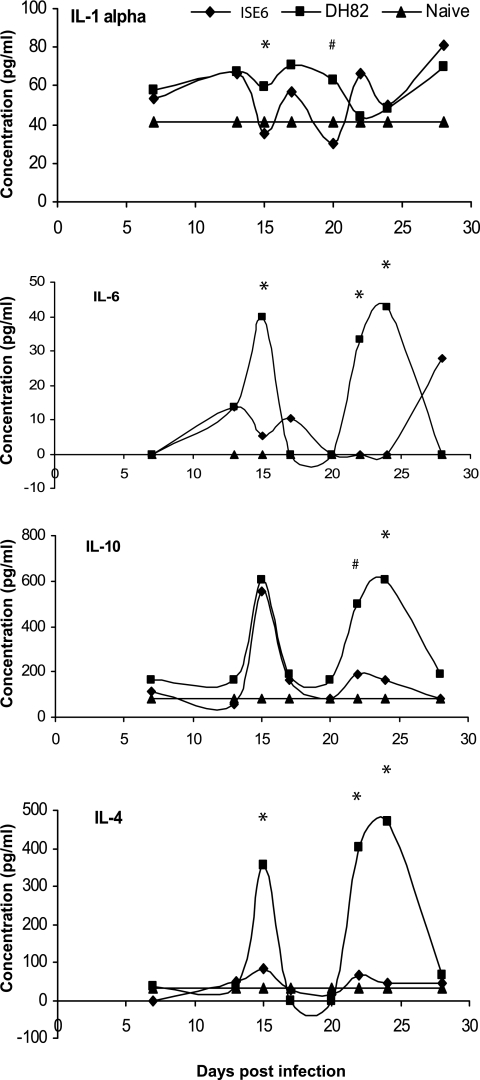
Spleen cell cytokine profiles.
When we measured spleen cell cytokine responses after infection, we detected measurable levels of IL-1α, IL-4, IL-6, and IL-10 (Fig. 7), but IFN-γ and IL-2 were undetectable by capture ELISA or by flow cytometry. Secretion of the four detectable cytokines also followed a cyclical pattern similar to that observed for the bacterial load, the CD62L/CD44 ratio in the spleen, and other measurements. The cyclical cytokine expression trends remained very similar for both of the infection groups, except that the secretion levels were significantly lower (P < 0.05 unless indicated otherwise) for the tick cell-derived E. chaffeensis.
FIG. 7.
Concentrations of IL-1-α, IL-6, IL-10, and IL-4 cytokines produced by spleen cells ex vivo after isolation from mice challenged with E. chaffeensis grown in ISE6 or DH82 cells (*, P ≤ 0.05; #, P ≤ 0.1). Spleen cell supernatants were also assayed for IFN-γ, IL-2, granulocyte-macrophage colony-stimulating factor, TNF-α, and IL-5, but there were no appreciable amounts of these cytokines at any time point.
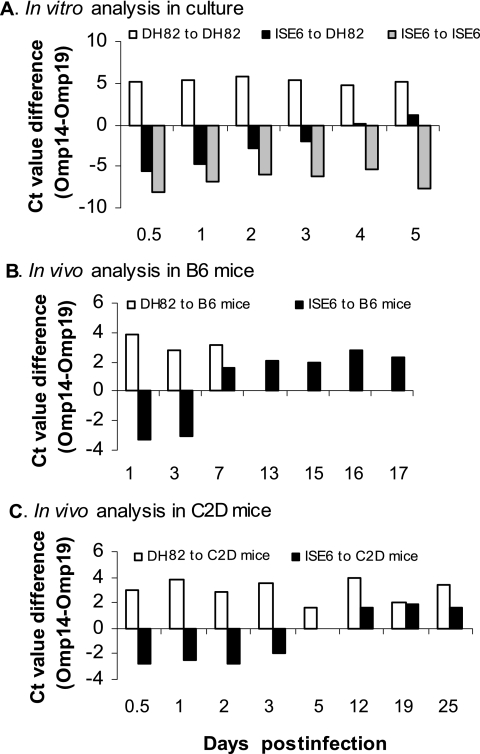
Expression of p28-Omp locus genes 14 and 19 in vitro and in vivo.
The antibody responses to tick cell-derived bacteria were different from the antibody responses raised against macrophage-grown bacteria. We hypothesized that these differences were caused by the differential antigen expression, such as Omp gene 14 in ISE6 tick cells compared to Omp gene 19 expression in DH82 macrophages and the level of expression of these genes when bacteria were introduced into a new host environment. To test this hypothesis, bacteria grown in ISE6 cells or DH82 cells were assayed for the transcripts for p28-Omp 14 and 19 genes after the bacteria were used to infect new cells (Fig. 8A). When DH82 cells were infected with E. chaffeensis from ISE6 cells, the expression of gene 14 was high after 12 h and was expressed in a declining pattern for an additional 3 days. On day 4, the expression of genes 14 and 19 equalized, and by day 5, the p28-Omp gene 14 became the dominantly expressed form (Fig. 8A). Bacteria maintained in consecutive DH82 passages maintained high-level expression of gene 19, whereas bacteria maintained in ISE6 cells retained high-level expression of gene 14 (Fig. 8A).
FIG. 8.
In vitro and in vivo gene expression of p28-Omp locus genes 14 and 19. A TaqMan-based, diplex real-time RT-PCR analysis was performed to assess the expression for p28-Omp multigene locus genes 14 and 19. (A) RNA was isolated from in vitro cultures infected with tick cell- or macrophage culture-derived E. chaffeensis. (B and C)The RNA analysis was also performed on the RNAs isolated from the B6 (B) and C2D (C) mice infected with macrophage- or tick cell-derived E. chaffeensis. CT value differences (i.e., the gene 14 CT value − the gene 19 CT value) in the amplification cycles were plotted for analyzed RNA samples collected at different times after infection. The negative values refer to high-level transcription of gene 14 relative to gene 19, whereas the positive values indicate that the transcription of gene 19 was higher. In panel A, DH82 to DH82 refers to E. chaffeensis organisms grown in DH82 cells that were used to infect DH82 cells; ISE6 to DH82 indicates that ISE6 cell-grown bacteria were used to infect DH82 cells, whereas ISE6 to ISE6 represents ISE6 culture-derived E. chaffeensis that was used to infect ISE6 cells. In panel B, DH82 to B6 mice indicates that DH82-grown bacteria were used as the inoculum to infect B6 mice; ISE6 to B6 represents B6 mice infected with ISE6 culture-derived E. chaffeensis. In panel C, DH82 to C2D mice and ISE6 to C2D mice are similar to the descriptions for the captions in panel B except that the C2D mice were used for infection.
To determine whether similar delays in gene shifts occurred in mouse macrophages in vivo, we examined RNA samples from B6 mouse peritoneal macrophages that tested positive for E. chaffeensis after mice were infected with DH82 or ISE6 cell-derived bacteria (Fig. 8B). In B6 mice infected with ISE6 cell-derived E. chaffeensis, the transcripts for gene 14 were present at high levels 1 and 3 days after experimental challenge similar to what we observed in vitro. The expression of gene 19 increased and the expression of gene 14 decreased between days 7 and 17 postinfection. A similar analysis was not possible using B6 mice infected with DH82-derived E. chaffeensis after day 7 because the bacteria were not detectable in peritoneal exudate cells beyond this day postinfection. Because B6 mice cleared infections of DH82 derived-bacteria so quickly, we did similar experiments in C2D mice. These mice do not cure the infection (25) and allowed us to isolate bacteria for long periods of time (Fig. 8C). In these experiments, gene 14 transcripts remained high in C2D mice infected with ISE6-derived E. chaffeensis for 3 days after i.p. experimental challenge. By 5 days, transcription of genes 14 and 19 were equal. However, on subsequent days postinfection, we detected more gene 19 transcripts than those for gene 14.
DISCUSSION
Human monocytic ehrlichiosis is an emerging zoonotic infection that has doubled in incidence since 1983 (40). In the present study, we presented data showing that E. chaffeensis grown in tick cells induces an immune response in mice that is distinct from that observed for the pathogen grown in macrophages. Although E. chaffeensis grown in both cell types is cleared by the murine host and induced a similar range of macrophage and T-cell cytokines, the host responses to tick cell-grown bacteria were slower, and the concentrations of cytokines measured in the present study were lower. In addition, there were distinct humoral immune responses detected.
Mice infected with tick cell-derived E. chaffeensis exhibited higher rickettsemia and slower clearance. This was best exemplified by the data showing that mice injected with tick cell-grown bacteria resolved peritoneal bacteremia about 9 days later than mice injected with bacteria grown in macrophage cultures. The slow clearance in mice injected with tick cell-derived bacteria was not because these mice were injected with more bacteria. In fact, although it is not statistically different, the mice consistently received more DH82-grown bacteria than tick cell-derived E. chaffeensis (Table 1), yet the clearance of DH82-derived bacteria was faster. In addition, we assessed the clearance kinetics in five independent experiments with inocula as low as 0.7 × 106 to as high as 4.2 × 106 for tick cell-derived Ehrlichia and inocula ranging from 1.0 × 106 to 7.5 × 106 for mice injected with DH82 culture-derived E. chaffeensis. Independent of the dose used for either group of bacteria, the general kinetics of clearance were the same. For example, the clearance of bacteria from the peritonea of mice challenged with DH82-culture-derived bacteria was consistently in the range of 14 to 17 days. Therefore, the range of challenge doses did not change the kinetics of the mouse response. More importantly, the clearance kinetics for DH82-derived bacteria was consistent with our previous reports in which we used inocula of 5 × 106 infected DH-82 cells (24, 25), where we did not quantitate the actual numbers of bacteria. Finally, our estimations of inocula given to the mice did not appear to be skewed because of differences in the ratio of the rRNA gene to rRNA for E. chaffeensis grown in the two different host cell backgrounds. Although it is possible that an organism can make different levels of rRNA in different environments (i.e., rates of rRNA transcriptions can vary in different host environments to support varying needs of protein synthesis), we did not find evidence for this based on the rRNA gene-to-rRNA ratios for tick cell- and DH82-derived E. chaffeensis.
The delay in clearance was more pronounced in the peritoneum than in the liver and spleen, which may reflect the fact that the peritoneum is the site of injection and that the impact of the origin of the bacteria is on the initial host response compared to responses that occur later. This hypothesis is supported by the observations that ISE6-derived bacteria do not activate macrophages to the same extent as bacteria grown in DH82 cells, as judged from the NO and IL-6 secretions by the peritoneal macrophages (Fig. 5). Macrophages are also not stimulated as well by an Ehrlichia injection in C3H/HeJ mice compared to wild-type mice, and this poorer stimulation delays clearance 2 to 3 weeks (24).
In the present study, we used a real-time, RT-PCR assay for the first time to assess the presence of bacteria after experimental infections. This assay is highly specific and sensitive for detecting E. chaffeensis (48). It allowed us to make a more sensitive quantitation of rickettsemia after the experimental challenges with E. chaffeensis compared to previous studies. The delayed clearance in liver and spleen compared to the peritoneum is consistent with our previous observations (24, 25). Our hypothesis for bacteria persisting longer in the liver and spleen, regardless of the source of the E. chaffeensis inoculum used to infect the mice, is that bacteria initially injected in the peritoneum are drained to the spleen and liver, and it takes the activation of acquired immunity to clear those organs.
An interesting observation made during the present study was that mice infected with bacteria from both treatment groups relapsed with a periodicity of about 3 days. Although considerably longer, cyclical rickettsemia has been reported for two related tick-transmitted rickettsiales of the genus Anaplasma. Anaplasma marginale and A. platys rickettsemia fluctuates in vertebrate hosts acquiring infections by both natural and experimental methods (23, 28, 33). French et al. found differences in expressed outer membrane proteins during each wave of rickettsemia (23). To our knowledge, this is the first study to demonstrate cyclical rickettsemia for an Ehrlichia species. However, it is still not clear whether or how antigen expression contributes to the oscillations. Although we have not reported the cyclical pattern of clearance in the past, a review of our older data set reveals hints of the same type of response (24).
The host cyclical immune response to E. chaffeensis corresponded with the peaks of rickettsemia. The oscillatory nature of bacterial clearance, cellular activation, and cytokine secretion after infection most likely reflects the natural resonance of intracellular responses (1, 34, 35) and/or the manifestation of amplification loops of the expanding immune response (6, 36). Wodarz et al. (57) called this process “dynamic elimination.” In that model, the length of the oscillatory pulse was dependent on the magnitude of the virus infection and the strength of the cytotoxic T-cell response. In an E. chaffeensis infection, it is easy to envision an initial nonspecific response to the bacteria, followed by the activation of more specific Th1 effectors (24). The generation of helper T cells and their feedback on macrophages, which form the core response against the intracellular pathogen, represent one of the quintessential demonstrations of cytokine amplification of the immune response. Th1 cells secrete IFN-γ, which upregulates MHCII expression on macrophages, which leads to enhanced antigen presentation. Indeed, when Wigginton and Kirschner modeled the clearance of Mycobacterium tuberculosis, another macrophage-tropic intracellular pathogen, they predicted oscillations in the clearance, cytokines secreted, macrophages isolated from alveoli and macrophage activation (54). Our data are consistent with their predictions. The recurring rickettsemia in our experiments may represent transitions in the host immune response that allow opportunities for the bacteria to reemerge.
We measured CD62L and CD44 to measure the development of effector memory (37, 44). The development of effector memory cells (CD44+ CD62L−) peaked just after bacteria reemerged during infection regardless of the source of bacteria. This point in time might be when the initial innate immune response is waning and the adaptive response is developing, but not fully functional. The kinetics of the peritoneal macrophage secretion of NO would support this hypothesis. The detectable NO response peaked at day 10 and then waned until day 17, when there was a second wave of activity. Interestingly, IL-6 also fluctuated once it was produced at peak amounts around day 20, and its production corresponded with the final waves of infection.
Our inability to detect IL-2 or IFN-γ after 7 days of infection with E. chaffeensis from either cell line appears to be inconsistent with the growing evidence that IFN-γ and a Th1-type immune response plays a role in host resistance to monocytic ehrlichiosis (5, 18, 24, 25, 31). We did detect Th1 cytokines in plasma within 10 h after infection, and we have observed transcriptional activation of a number of Th1-associated genes immediately after infection with both types of bacteria (data not shown). Therefore, we feel that the difference between our system and other investigations has to do with when we are looking for the cytokine. We are measuring the host response at a time when it is transitioning from early Th1 responses to the production of Th2-type cytokines later in the response (32). It is also important to point out that Bitsaktsis et al. (5) demonstrated a Th1 response to E. chaffeensis by showing IFN-γ production at the single-cell level. Their technique allows for the detection of small amounts of cytokine that might be missed when screening plasma or culture supernatants the way we did.
There was very low ex vivo secretion of TNF-α by spleen cells or peritoneal macrophages from mice injected with either type of bacteria and culture of cells in vitro. This was similar to our recent study on experimental infections in several mouse strains (25). Other researchers have suggested that TNF-α is necessary for the resolution of Ixodes ovatus Ehrlichia infection (5, 18). However, different methods were used to make the inference. For example, these methods either used antibody to deplete cytokine or knockout animals to deduce an impact. Alternatively, Bitsaktsis et al. measured TNF-α after a 4-day in vitro sensitization assay which makes it possible to detect small amounts of cytokine (5). It is also possible that the difference is due to the fact that Ixodes ovatus Ehrlichia is more pathogenic than E. chaffeensis (31).
The lower concentrations of cytokine production by mice challenged with tick cell-grown bacteria and the distinct differences in the development of the humoral response in those mice have significant implications about the model system currently being used by investigators studying E. chaffeensis. The poorer responses in the absence of tick saliva suggest that host cell environment (macrophage versus tick cells) plays a role in activating the host response. The distinct kinetics of antibody production and the specificity of the antibodies made against E. chaffeensis antigens by mice from the two treatment groups are also intriguing. It is not clear why tick cell-grown bacteria induce a more prolonged IgG response. Our in vitro and in vivo analyses of the changes in gene expression suggest that the transition from tick-specific antigen expression to macrophage-specific antigen expression is a very slow process for E. chaffeensis. The delayed clearance of tick cell-derived E. chaffeensis with distinct immune responses may reflect the host's inability to alter its immune response to the changing antigenic makeup of the bacteria. This differential protein expression might be one of the mechanisms used by E. chaffeensis to persist longer in vertebrate hosts. A number of groups have used DH82 cells to produce E. chaffeensis for in vivo experimental challenges, including our own (24, 25). Perhaps tick cell-derived bacteria would be a better source of inoculum to assess the true host immune responses against E. chaffeensis. In addition, although it appears that there is no difference in the immune response to various isolates of E. chaffeensis (25), it is not clear whether isolates other than the Arkansas strain of E. chaffeensis will behave in a similar fashion. Additional studies will be necessary to resolve this issue. Nevertheless, it is clear that much more remains to be determined about this intriguing intracellular pathogen.
Acknowledgments
This study was supported by the National Institutes of Health grants AI50785, AI052206, AI55052, RR16475, and RR17686; NASA grants NAG2-1274 and NAGW-1197; the Howard Hughes Foundation; and the Kansas Agricultural Experiment Station.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 23 October 2006.
Kansas Agricultural Experiment Station contribution 06-269-J.
REFERENCES
- 1.Adachi, Y., A. L. Kindzelskii, N. Ohno, T. Yadomae, and H. R. Petty. 1999. Amplitude and frequency modulation of metabolic signals in leukocytes: synergistic role of IFN-gamma in IL-6- and IL-2-mediated cell activation. J. Immunol. 163:4367-4374. [PubMed] [Google Scholar]
- 2.Anderson, B. E., K. G. Sims, J. G. Olson, J. E. Childs, J. F. Piesman, C. M. Happ, G. O. Maupin, and B. J. Johnson. 1993. Amblyomma americanum: a potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 49:239-244. [DOI] [PubMed] [Google Scholar]
- 3.Andrew, H. R., and R. A. Norval. 1989. The carrier status of sheep, cattle, and African buffalo recovered from heartwater. Vet. Parasitol. 34:261-266. [DOI] [PubMed] [Google Scholar]
- 4.Barbet, A. F., A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer. 2000. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 68:6133-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitsaktsis, C., J. Huntington, and G. Winslow. 2004. Production of IFN-gamma by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 172:6894-6901. [DOI] [PubMed] [Google Scholar]
- 6.Bonecchi, R., S. Sozzani, J. T. Stine, W. Luini, G. D'Amico, P. Allavena, D. Chantry, and A. Mantovani. 1998. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood 92:2668-2671. [PubMed] [Google Scholar]
- 7.Brayton, K. A., P. F. Meeus, A. F. Barbet, and G. H. Palmer. 2003. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect. Immun. 71:6627-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapes, S. K., and A. A. Beharka. 1995. Lipopolysaccharide is required for the lethal effects of enterotoxin B after d-galactosamine sensitization. J. Endotoxin Res. 2:263-271. [Google Scholar]
- 9.Chen, S. M., V. L. Popov, H. M. Feng, J. Wen, and D. H. Walker. 1995. Cultivation of Ehrlichia chaffeensis in mouse embryo, Vero, BGM, and L929 cells and study of Ehrlichia-induced cytopathic effect and plaque formation. Infect. Immun. 63:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson, W. R., J. M. Lockhart, D. E. Stallknecht, E. W. Howerth, J. E. Dawson, and Y. Rechav. 2001. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J. Wildl. Dis. 37:538-546. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, J. E., B. E. Anderson, D. B. Fishbein, C. Y. Sanchez, C. Y. Goldsmith, K. H. Wilson, and C. W. Duntley. 1991. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J. Clin. Microbiol. 29:2741-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson, J. E., K. L. Biggie, C. K. Warner, K. Cookson, S. Jenkins, J. F. Levine, and J. G. Olson. 1996. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human erlichiosis, in dogs from southeast Virginia. Am. J. Vet. Res. 57:1175-1179. [PubMed] [Google Scholar]
- 13.Dawson, J. E., J. E. Childs, K. L. Biggie, C. Moore, D. Stallknecht, J. Shaddock, J. Bouseman, E. Hofmeister, and J. G. Olson. 1994. White-tailed deer as a potential reservoir of Ehrlichia spp. J. Wildl. Dis. 30:162-168. [DOI] [PubMed] [Google Scholar]
- 14.De, S. A., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 16.Dumler, J. S., W. L. Sutker, and D. H. Walker. 1993. Persistent Infection with Ehrlichia chaffeensis. Clin. Infect. Dis. 17:903-905. [DOI] [PubMed] [Google Scholar]
- 17.Felek, S., A. Unver, R. W. Stich, and Y. Rikihisa. 2003. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J. Clin. Microbiol. 39:460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, H. M., and D. H. Walker. 2004. Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect. Immun. 72:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira, B. R., and J. S. Silva. 1998. Saliva of Rhipicephalus sanguineus tick impairs T-cell proliferation and IFN-gamma-induced macrophage microbicidal activity. Vet. Immunol. Immunopathol. 64:279-293. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira, B. R., and J. S. Silva. 1999. Successive tick infestations selectively promote a T-helper 2 cytokine profile in mice. Immunology 96:434-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fikrig, E., U. Pal, M. Chen, J. F. Anderson, and R. A. Flavell. 2004. OspB antibody prevents Borrelia burgdorferi colonization of Ixodes scapularis. Infect. Immun. 72:1755-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fingerle, V., S. Rauser, B. Hammer, O. Kahl, C. Heimerl, U. Schulte-Spechtel, L. Gern, and B. Wilske. 2002. Dynamics of dissemination and outer surface protein expression of different European Borrelia burgdorferi sensu lato strains in artificially infected Ixodes ricinus nymphs. J. Clin. Microbiol. 40:1456-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganta, R. R., C. Cheng, M. J. Wilkerson, and S. K. Chapes. 2004. Delayed clearance of Ehrlichia chaffeensis infection in CD4+ T-cell knockout mice. Infect. Immun. 72:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganta, R. R., M. J. Wilkerson, C. Cheng, A. M. Rokey, and S. K. Chapes. 2002. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect. Immun. 70:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Garcia, J. C., J. de la Fuente, E. F. Blouin, T. J. Johnson, T. Halbur, V. C. Onet, J. T. Saliki, and K. M. Kocan. 2004. Differential expression of the msp1alpha gene of Anaplasma marginale occurs in bovine erythrocytes and tick cells. Vet. Microbiol. 98:261-272. [DOI] [PubMed] [Google Scholar]
- 27.Gillespie, R. D., M. L. Mbow, and R. G. Titus. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite. Immunol. 22:319-331. [DOI] [PubMed] [Google Scholar]
- 28.Harvey, J. W., C. F. Simpson, and J. M. Gaskin. 1978. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J. Infect. Dis. 137:182-188. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa, K., F. Martin, G. Huang, D. Tumas, L. Diehl, and A. C. Chan. 2004. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 303:685-689. [DOI] [PubMed] [Google Scholar]
- 30.Inokuma, H., R. L. Kerlin, D. H. Kemp, and P. Willadsen. 1993. Effects of cattle tick (Boophilus microplus) infestation on the bovine immune system. Vet. Parasitol. 47:107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail, N., L. Soong, J. W. McBride, G. Valbuena, J. P. Olano, H. M. Feng, and D. H. Walker. 2004. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 172:1786-1800. [DOI] [PubMed] [Google Scholar]
- 32.Jiao, X., R. Lo-Man, N. Winter, E. Deriaud, B. Gicquel, and C. Leclerc. 2003. The shift of Th1 to Th2 immunodominance associated with the chronicity of Mycobacterium bovis bacille Calmette-Guerin infection does not affect the memory response. J. Immunol. 170:1392-1398. [DOI] [PubMed] [Google Scholar]
- 33.Kieser, S. T., I. S. Eriks, and G. H. Palmer. 1990. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 58:1117-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kindzelskii, A. L., and H. R. Petty. 1999. Early membrane rupture events during neutrophil-mediated antibody-dependent tumor cell cytolysis. J. Immunol. 162:3188-3192. [PubMed] [Google Scholar]
- 35.Kindzelskii, A. L., and H. R. Petty. 2002. Apparent role of traveling metabolic waves in oxidant release by living neutrophils. Proc. Natl. Acad. Sci. USA 99:9207-9212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Klouche, M., S. Bhakdi, M. Hemmes, and S. Rose-John. 1999. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J. Immunol. 163:4583-4589. [PubMed] [Google Scholar]
- 37.Lau, L. L., B. D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature 369:648-652. [DOI] [PubMed] [Google Scholar]
- 38.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Munderloh, U. G., Y. Liu, M. Wang, C. Chen, and T. J. Kurtti. 1994. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80:533-543. [PubMed] [Google Scholar]
- 40.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ristic, M., and D. L. Huxsoll. 1984. Tribe II. Ehrlichieae Philip 1957, 948AL, p. 704-711. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 44.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 45.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singu, V., H. Liu, C. Cheng, and R. R. Ganta. 2005. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect. Immun. 73:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singu, V., L. Peddireddi, K. R. Sirigireddy, C. Cheng, U. G. Munderloh, and R. R. Ganta. 2006. Unique macrophage and tick cell-specific protein expression from the p28/p30 Omp multigene locus in Ehrlichia species. Cell Microbiol. 8:1475-1487. [DOI] [PubMed] [Google Scholar]
- 48.Sirigireddy, K. R., and R. R. Ganta. 2005. Multiplex detection of Ehrlichia and Anaplasma species pathogens in peripheral blood by real-time reverse transcriptase-polymerase chain reaction. J. Mol. Diagn. 7:308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonenshine, D. E. 1991. Biology of ticks, vol. 1, p. 51-64. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 50.Stamler, J. S., D. J. Singel, and J. Loscalzo. 1992. Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898-1902. [DOI] [PubMed] [Google Scholar]
- 51.Stuehr, D. J., and C. F. Nathan. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169:1543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuen, S., E. O. Engvall, and K. Artursson. 1998. Persistence of Ehrlichia phagocytophila infection in lambs in relation to clinical parameters and antibody responses. Vet. Rec. 143:553-555. [DOI] [PubMed] [Google Scholar]
- 53.Unver, A., Y. Rikihisa, R. W. Stich, N. Ohashi, and S. Felek. 2002. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 70:4701-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wigginton, J. E., and D. Kirschner. 2001. A model to predict cell-mediated immune regulatory mechanisms during human infection with Mycobacterium tuberculosis. J. Immunol. 166:1951-1967. [DOI] [PubMed] [Google Scholar]
- 55.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]
- 56.Winslow, G. M., E. Yager, K. Shilo, E. Volk, A. Reilly, and F. K. Chu. 2000. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wodarz, D., K. M. Page, R. A. Arnaout, A. R. Thomsen, J. D. Lifson, and M. A. Nowak. 2000. A new theory of cytotoxic T-lymphocyte memory: implications for HIV treatment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:329-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, J., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, X. F., J. Z. Zhang, S. W. Long, R. P. Ruble, and X. J. Yu. 2003. Experimental Ehrlichia chaffeensis infection in beagles. J. Med. Microbiol. 52:1021-1026. [DOI] [PubMed] [Google Scholar]