Abstract
The protein McaP was previously shown to be an adhesin expressed by the Moraxella catarrhalis strain O35E, which also displays esterase and phospholipase B activities (J. M. Timpe et al., Infect. Immun. 71:4341-4350, 2003). In the present study, sequence analysis suggests that McaP is a conventional autotransporter protein that contains a 12-stranded β-barrel transporter module (amino acids [aa] 383 to 650) linked to a surface-exposed passenger domain exhibiting lipolytic activity (aa 62 to 330). An in-frame deletion removing most of this predicted N-terminal passenger domain was engineered, and Escherichia coli expressing the truncated McaP protein exhibited greatly reduced adherence to A549 human lung epithelial cells compared to E. coli expressing wild-type McaP. Site-directed mutagenesis of a serine residue at position 62 of McaP, predicted to be important for the lipolytic activity of the protein, resulted in loss of hydrolysis of p-nitrophenyl ester of caproate. E. coli expressing this mutated McaP, however, adhered to A549 monolayers at levels greater than recombinant bacteria expressing the wild-type adhesin. These results indicate that the predicted passenger domain of McaP is involved in both the binding and the lipolytic activity of the molecule and demonstrate that the adhesive properties of McaP do not require its lipolytic activity. Sequence analysis of mcaP from eight Moraxella catarrhalis strains revealed that the gene product is highly conserved at the amino acid level (98 to 100% identity), and Western blot analysis demonstrated that a panel of 16 isolates all express McaP. Flow cytometry experiments using antibodies raised against various portions of McaP indicated that its predicted passenger domain as well as transporter module contain surface-exposed epitopes. In addition to binding to the surface of intact bacteria, these antibodies were found to decrease adherence of M. catarrhalis to A549 human lung cells by up to 47% and to reduce binding of recombinant E. coli expressing McaP by 98%. These results suggest that McaP should be considered as a potential vaccine antigen.
The gram-negative bacterium Moraxella catarrhalis is a significant health problem, causing approximately 20% of all episodes of bacterial otitis media in children (23) and up to 10% of instances of lower respiratory tract infections in elderly patients suffering from chronic obstructive pulmonary disease (COPD) (45). Furthermore, diseases such as sinusitis (8) and conjunctivitis (7) can be added to the growing list of ailments caused by the organism. The development of a vaccine to reduce the risks of M. catarrhalis infections is therefore desirable and would have a substantial impact on the overall health status of the young and elderly.
Several surface antigens expressed by M. catarrhalis have been studied for their vaccinogenic potential. Proteins such as OMPE (6, 46, 47), OMPCD (28, 44, 48-50), and OMPG1a and OMPG1b (1-3) are promising candidates because they are highly conserved among strains, expressed by most isolates tested to date, and contain surface epitopes. Furthermore, immunization with these outer membrane (OM) proteins elicits the production of antibodies that bind to the surface of intact bacteria, and COPD patients recovering from M. catarrhalis infections produce antibodies against OMPCD, OMPE, and OMPG1a/OMPG1b (1-3, 6, 28, 44, 46-50). The adhesins UspA1 (15, 35, 39, 41, 43) and Hag/MID (10, 27, 39, 41-43, 61), the serum resistance factor UspA2 (5, 15, 39, 41, 43, 61), and the iron acquisition proteins CopB (39, 41, 43, 59, 61), TbpA (52), TbpB (14, 43, 52, 67), LbpA (18), and LbpB (18, 67) also exhibit most of the aforementioned vaccinogenic qualities, with the exception that these proteins are more variable at the amino acid level among isolates of various origins. Nevertheless, these types of molecules play key roles in pathogenesis by most bacterial pathogens (e.g., adherence, serum resistance, and iron acquisition) and targeting them in a vaccine may have the added benefit of interfering with the ability of M. catarrhalis to establish itself in the respiratory tract of individuals that are at risk of infection by the bacterium. This hypothesis is supported by the recent demonstration that UspA1, Hag, and UspA2 are the major targets of new immunoglobulin A antibodies in the sputum of COPD patients with M. catarrhalis infections who have successfully cleared the bacterium (43). This protective immune response, however, appears to be strain specific, as COPD patients often get reinfected by different strains of M. catarrhalis (45).
These observations suggest that an effective vaccine for M. catarrhalis will need to include a mixture of antigens expressed by this unencapsulated bacterium. There is clearly a need to identify the regions of vaccine candidates having the best vaccinogenic properties, as well as to identify new and highly conserved antigens expressed by the bacterium which preferably contain surface-exposed epitopes that would be readily available for recognition by the immune system. The present study demonstrates that McaP, an adhesin also exhibiting phospholipase B activity, is a highly conserved OM protein expressed by all M. catarrhalis isolates tested which elicits the production of antibodies that bind to the surface of intact bacteria in addition to reducing adherence to epithelial cells.
MATERIALS AND METHODS
Strains, plasmids, tissue culture cell lines, and growth conditions.
The strains and plasmids described in this study are listed in Table 1. M. catarrhalis was cultured at 37°C using Bacto Todd-Hewitt medium (BD Diagnostic Systems); agar plates were incubated in an atmosphere of 92.5% air-7.5% CO2. The mcaP isogenic mutant strain O35E.M was grown in Todd-Hewitt medium supplemented with 20 μg/ml kanamycin. Recombinant Escherichia coli strains were grown at 37°C using Luria-Bertani (LB) medium (Fisher Bioreagents) supplemented with 15 μg/ml chloramphenicol. For adherence and lipolytic assays, flow cytometry experiments, and purification of Sarkosyl-insoluble outer membrane (OM) proteins, recombinant E. coli bacteria were grown in broth and induced with CopyControl induction solution (Epicentre) as reported by Holm and colleagues (26). A549 cells (type II alveolar lung pneumocytes, ATCC CCL85) were cultured as previously described (26).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| M. catarrhalis | ||
| O35E | Wild-type isolate | 4 |
| O35E.M | Isogenic mcaP mutant, Kanr | 63 |
| O12E | Wild-type isolate | 35 |
| O46E | Wild-type isolate | 35 |
| P44 | Wild-type isolate | 35 |
| TTA24 | Wild-type isolate | 15 |
| TTA37 | Wild-type isolate | 35 |
| V1171 | Wild-type isolate | 15 |
| FIN2404 | Wild-type isolate | Eric Hansen |
| 11P29B1 | Wild-type isolate (Buffalo, NY) | Tim Murphy |
| 32P11B1 | Wild-type isolate (Buffalo, NY) | Tim Murphy |
| 13P18B1 | Wild-type isolate (Buffalo, NY) | Tim Murphy |
| 7P94B1 | Wild-type isolate (Buffalo, NY) | Tim Murphy |
| McGH | Wild-type isolate (Toledo, OH) | George Hageage |
| Mc34F | Wild-type isolate (Toledo, OH) | George Hageage |
| McGHS1 | Wild-type isolate | 10 |
| McGHS2 | Wild-type isolate (Toledo, OH) | George Hageage |
| E. coli | ||
| EPI300 | Cloning strain | Epicentre |
| TUNER | Expression strain | Novagen |
| Plasmids | ||
| pETcoco-1 | Protein expression vector, Cmr | Novagen |
| pSLmcaP51.650 | pETcoco-1 expressing McaP's aa 51 to 650 joined to six N-terminal histidine residues, Cmr | This study |
| pSLmcaP51.333 | pETcoco-1 expressing McaP's aa 51 to 333 joined to six N-terminal histidine residues, Cmr | This study |
| pSLmcaP333.650 | pETcoco-1 expressing McaP's aa 333 to 650 joined to six N-terminal histidine residues, Cmr | This study |
| pCC1 | Cloning vector, Cmr | Epicentre |
| pCC1.3 | pCC1 in which the control insert provided by the manufacturer was cloned; adherence negative control | 26 |
| pIFmcaP17 | pCC1 containing and expressing the O35E-mcaP gene; adherence positive and lipolytically active | 65 |
| pSVmcaPS62N | pIFmcaP17 derivative in which serine 62 of McaP was replaced by an asparagine using site-directed mutagenesis; adherence positive and lipolytically inactive | This study |
| pSVmcaPΔ53-336 | pIFmcaP17 derivative in which aa 53 to 336 were deleted using site-directed mutagenesis; adherence negative and lipolytically inactive | This study |
Recombinant DNA methods, PCR, and cloning.
Standard molecular biology techniques were performed as reported elsewhere (10, 26, 57, 63). Platinum Pfx DNA polymerase was used in all experiments per the manufacturer's recommended conditions (Invitrogen). DNA fragments of 2.1 kb containing entire mcaP genes were amplified from M. catarrhalis strains by use of oligonucleotide primers P1 (5′-CGC AAT AAA GAT CAC CAT GCT TG-3′) and P2 (5′-CGG GAT CCC GCT GAC ACA TTG CAT TGA TAA A-3′); these PCR products were used as templates for sequencing. An amplicon of 1.8 kb specifying amino acids (aa) 51 to 650 of O35E-McaP was generated with oligonucleotides P3 (5′-CAG GCG CGC CTA CAA GAA TTT AGC CAA ACC G-3′; AscI site underlined) and P4 (5′-CCT TAA TTA AGG TCA AAA TGC CAT TTG TGC ACC CAC-3′; PacI site underlined). This PCR product was purified, restricted with the endonucleases AscI and PacI (New England Biolabs), and ligated into the AscI and PacI sites of the vector pETcoco-1 (Novagen), yielding the plasmid pSLmcaP51-650. This plasmid was sequenced to verify that no mutations were introduced during PCR and to confirm that the protein expressed from pSLmcaP51.650 corresponds to residues 51 to 650 of O35E-McaP fused to six N-terminal histidine residues (encoded by pETcoco-1). A similar approach was used to obtain the plasmids pSLmcaP51-333 and pSLmcaP333-650, which express residues 51 to 333 and 333 to 650 of O35E-McaP joined to six N-terminal histidine residues, respectively. The PCR product cloned into pSLmcaP51-333 was amplified with primers P3 (see above) and P5 (5′-CCT TAA TTA AGG TCA CCC TGA AGG GTG AAT TTT ATC AGC-3′; PacI site underlined), whereas that inserted in pSLmcaP333-650 was generated with P6 (5′-CAG GCG CGC CTA GGG CGT ACG CAT CGC ATT TTG G-3′; AscI site underlined) and P4 (see above). M. catarrhalis chromosomal DNA was used as the template in all PCR experiments.
Site-directed mutagenesis.
Mutations were introduced in the O35E-mcaP open reading frame (ORF) harbored by the plasmid pIFmcaP17 by use of a QuickChange II SL site-directed mutagenesis kit according to the manufacturer's instructions (Stratagene). The mutagenic primers P7 (5′-ATT TTT TGG TGA TAA CCT AAC TGA CAC AGG-3′) and P8 (5′-CCT GTG TCA GTT AGG TTA TCA CCA AAA AAT-3′) were used in order to replace a serine residue at position 62 with an asparagine, yielding the plasmid pSVmcaPS62N. The oligonucleotides P9 (5′-GCA TCG CCA CCG CCC AAG AAC GCA TTT TGG CAC AAT ATT ATC G-3′) and P10 (5′-CGA TAA TAT TGT GCC AAA ATG CGT TCT TGG GCG GTG GCG ATG C-3′) were used to generate plasmid pSVmcaPΔ53-336, which encodes a truncated McaP protein in which residues 53 through 336 were deleted. Both plasmids were sequenced to verify that only the intended mutations were introduced in the mcaP ORF.
Nucleotide sequence analysis.
Plasmids and PCR fragments were sequenced at the University of Michigan sequencing core (http://seqcore.brcf.med.umich.edu/). Chromatograms were analyzed and assembled with ChromaTool software (BioTools, Inc). Sequence analysis was performed using Vector NTI 10.1.1 (Invitrogen) and the various tools available at the ExPASy proteomics server (http://us.expasy.org).
Protein preparation and analysis of selected antigens.
OM vesicles were obtained from M. catarrhalis strains by using the EDTA procedure of Murphy and Loeb (51). Sarkosyl-insoluble OM proteins were extracted from recombinant E. coli bacteria following a rapid procedure described by Carlone and colleagues (11). The method used to prepare whole-cell lysates is described elsewhere (15, 55). Western blot experiments were performed as described by Bullard et al. (10).
The plasmids pSLmcaP51.650, pSLmcaP51.333, and pSLmcaP333.650 were introduced in the E. coli strain TUNER (Novagen) for the purpose of overexpressing and purifying the recombinant proteins His.McaP51.650, His.McaP51.333, and His.McaP333.650, respectively. All three proteins were extracted from inclusion bodies by use of Bugbuster HT protein extraction reagent (Novagen) and rLysozyme solution (Novagen) according to the manufacturer's suggested guidelines. The recombinant proteins were then purified under denaturing conditions by using a His-Bind resin system per the manufacturer's instructions (Novagen). The composition of refolding buffers was determined using an AthenaES protein refolding kit (Athena Enzyme Systems), and urea was gradually removed by dialyzing the purified recombinant protein preparations at 4°C. Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce).
Antibodies.
The antibodies raised against residues 577 to 596 of the M. catarrhalis O35E McaP protein (i.e., synthetic peptide PEP1) have been described elsewhere (10). For the production of antibodies, female BALB/c mice were immunized with the purified recombinant protein His.McaP51.650, His.McaP51.333, or His.McaP333.650 emulsified in Freund's adjuvants (complete and incomplete; Fisher Scientific) as previously reported (36). Murine antibodies were demonstrated to specifically recognize McaP in Western blot experiments using whole-cell lysates of the wild-type (WT) strain O35E and its mcaP mutant O35E.M (data not shown).
Flow cytometry.
Bacterial cells were suspended to an optical density of 250 Klett units (109 CFU/ml), and 50-μl portions were incubated with diluted murine sera for 30 min with shaking at 37°C. Bacteria were washed three times with 1 ml phosphate-buffered saline-0.15% gelatin (PBSG), and these cells were suspended in 100 μl PBSG to which 1 μl of goat anti-mouse immunoglobulin heavy plus light chains conjugated with fluorescein isothiocyanate (FITC) (Southern Biotech) was added. These mixtures were incubated at 37°C for 30 min with shaking and washed three times with PBSG. Bacteria were suspended in 700 μl of PBSG and subsequently mixed with 700 μl of PBS supplemented with 4% (wt/vol) paraformaldehyde by adding the latter in small increments and vortexing. These fixed cells were stored at 4°C overnight, diluted by adding 700 μl PBSG, and analyzed by the Flow Cytometry Core Laboratory at the University of Toledo Health Sciences Campus using an EPICS Elite ESP (Beckman-Coulter) flow cytometer. The FITC fluorescence from the labeled bacteria was measured through a 525-nm band pass filter in which 25,000 events were measured. These experiments were repeated on at least two separate occasions.
Lipolytic and adherence assays.
Lipolytic assays were performed as described by Timpe and colleagues (63), using p-nitrophenyl ester of caproate (Sigma). In these assays, the lipolytic cleavage of p-nitrophenyl ester of caproate releases nitrophenol, which can be measured spectrophotometrically at a wavelength of 410 nm. Optical measurements were taken 15 min after adding p-nitrophenyl ester of caproate to the recombinant bacteria. These lipolytic assays were performed in triplicate on at least two separate occasions, and the results are expressed as the mean (±standard error) optical density at 410 nm.
Adherence was measured using a viable cell count assay previously described by our laboratory (10, 26, 27, 63). Duplicate adherence experiments were performed on at least three independent occasions, and the results are expressed as the mean (±standard error) percentage of inoculated bacteria that bound to A549 pneumocytes. For inhibition assays using antibodies against McaP, bacteria were incubated with the indicated murine sera at 37°C for 30 min prior to inoculating monolayers of A549 cells. Preincubation of bacteria with antibodies did not appreciably increase clumping.
Statistical analysis.
The data were analyzed with the Mann-Whitney test using GraphPad Prism 4.0 software, and P values of <0.05 were reported as statistically significant.
Nucleotide sequence accession numbers.
The nucleotide sequences of the mcaP genes from strains O12E (EF075933), V1171 (EF075934), 11P29B1 (EF075935), Mc34F (EF075936), O46E (EF075937), TTA37 (EF075938), McGHS1 (EF075939), and P44 (EF075940) have been deposited in GenBank under the accession numbers indicated in parentheses.
RESULTS
McaP is a highly conserved OM protein.
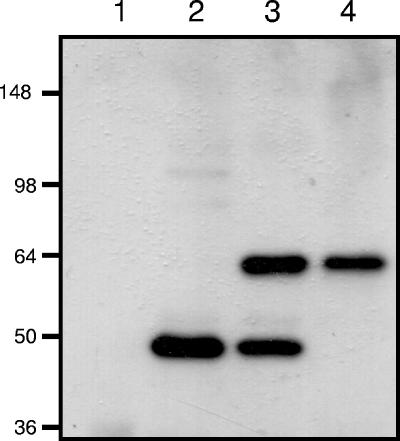
It was demonstrated previously that McaP is a 62-kDa adhesin associated with the OM of the M. catarrhalis strain O35E (63). To study McaP expression, murine antibodies binding to residues 577 to 596 were generated by immunizing mice with a synthetic peptide designated PEP1 (10). Western blot analysis of OM vesicles prepared from strain O35E and heated at 37°C revealed that PEP1 antibodies recognized a 48-kDa antigen (Fig. 1, lane 2). After these OM vesicles were incubated at 60°C for 15 min prior to electrophoresis, the antibodies reacted with two bands of 48 and 62 kDa (Fig. 1, lane 3), whereas heating at 100°C resulted in the appearance of only the 62-kDa form of the antigen (Fig. 1, lane 4). As expected, the PEP1 antibodies did not bind to any OM proteins in the mcaP isogenic mutant O35E.M (Fig. 1, lane 1). These results indicate that McaP is a heat-modifiable protein located in the OM of M. catarrhalis.
FIG. 1.
Western blot analysis of M. catarrhalis OM vesicles. Proteins present in OM vesicles were resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and analyzed by Western blotting with PEP1-specific antibodies at a dilution of 1:10,000. Lane 1 corresponds to OM vesicles extracted from the isogenic mcaP mutant O35E.M and heated at 100°C prior to electrophoresis. Lanes 2, 3, and 4 correspond to OM vesicles extracted from the WT isolate O35E and heated at 37°C, 60°C, or 100°C prior to electrophoresis, respectively. The numbers on the left indicate molecular masses in kilodaltons.
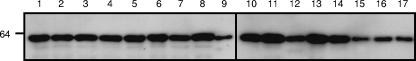
The nucleotide sequence of mcaP was determined from eight M. catarrhalis clinical isolates of diverse geographical origins, namely, O12E, 11P29B1, Mc34F, V1171, O46E, TTA37, McGHS1, and P44. In addition, BLAST searches of patent databases through the NCBI service identified the mcaP ORF of the M. catarrhalis strain ATCC 43617 (nucleotides 18472 to 16523 of AX067443). Sequence analysis indicated that all of these mcaP ORFs specified proteins of 650 residues exhibiting 98 to 100% identity over their entire lengths (not shown). Upon closer examination, we observed that the first 21 aa were identical in 9 of the 10 proteins analyzed; O35E-McaP contained 7 aa substitutions, which resulted in a lower level of identity in this particular portion of the protein (not shown). These 7 residues of McaP, however, are likely part of a leader peptide due to the presence of a potential signal sequence cleavage site between aa 50 and 51, as previously reported by Timpe and colleagues (63). When compared without their putative leader peptides, the McaP proteins were found to be 99.1% to 100% identical. The region encompassing residues 22 to 350 was found to be perfectly conserved whereas the last 300 aa displayed 97.9 to 100% identity; the differences were observed at residues 351 (T↔ N), 363 (H↔ S), 367 (S↔ N), 368 (Q↔ H), 377 (S↔ G), 431 (H↔ Y), 492 (K↔ Q), 510 (T↔ S), and 586 (A↔ T). These observations demonstrate that the mcaP gene product is highly conserved among M. catarrhalis isolates. To test whether McaP is expressed by strains other than O35E, whole-cell lysates were prepared from 15 M. catarrhalis isolates of various clinical and geographical sources and analyzed by Western blotting with PEP1 antibodies. As shown in Fig. 2, all strains were found to express the protein.
FIG. 2.
Western blot analysis of M. catarrhalis isolates. Proteins present in whole-cell preparations were resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and analyzed by Western blotting with PEP1-specific antibodies at a dilution of 1:10,000. Cell lysates were heated at 100°C prior to electrophoresis. The number on the left indicates the molecular mass in kilodaltons. Lanes 1 and 10, O35E; lane 2, O12E; lane 3, TTA24; lane 4, TTA37; lane 5, P44; lane 6, O46E; lane 7, V1171; lane 8, McGHS1; lane 9, FIN2404; lane 11, McGH; lane 12, McGHS2; lane 13, 32P11B1; lane 14, 7P94B1; lane 15, 11P29B1; lane 16, 13P18B1; lane 17, Mc34F.
McaP contains epitopes exposed on the surface of M. catarrhalis.
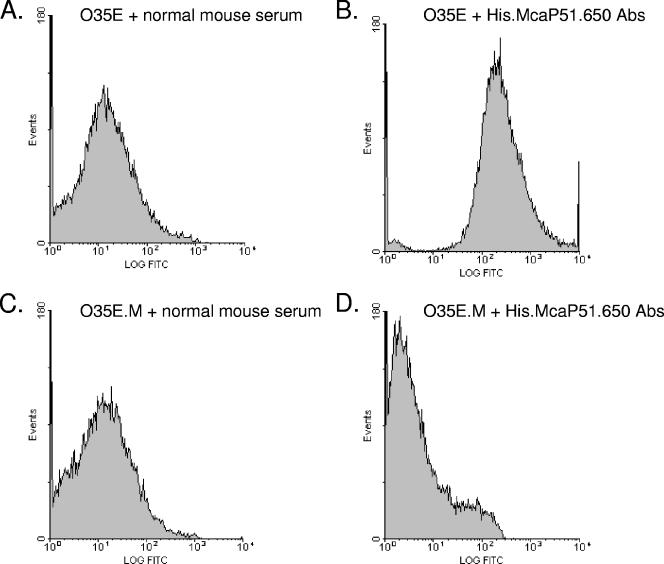
The PEP1 antibodies, used to demonstrate conservation of McaP among M. catarrhalis isolates, did not bind to the surface of intact bacteria in flow cytometry experiments (data not shown). However, the PEP1 antibodies were generated against aa 577 to 596 of McaP, which may not be exposed on the bacterial surface. We therefore generated antibodies against residues 51 to 650 of the protein, which were predicted to contain surface-exposed epitopes (not shown). To achieve this, the O35E-McaP ORF was cloned without its predicted leader peptide (i.e., aa 1 to 50) into the expression vector pETcoco-1, and the purified recombinant protein, His.McaP51.650, was used to immunize mice. In parallel experiments, mice were injected with PBS alone. Figure 3 shows the results of a representative flow cytometry experiment using sera recovered from the aforementioned mice. Figure 3B, which corresponds to strain O35E incubated with His.McaP51.650-specific antibodies, clearly shows reactivity with surface-exposed epitopes. This reactivity was abolished when the antibodies were incubated with M. catarrhalis cells that lack expression of McaP (i.e., O35E.M [Fig. 3D]). As an additional control, bacteria were incubated with the sera recovered from mice immunized with PBS alone and only background levels of fluorescence were observed (Fig. 3A and C).
FIG. 3.
Flow cytometry analysis of M. catarrhalis strains O35E and O35E.M. M. catarrhalis cells were incubated with normal mouse serum (A and C) or murine serum containing His.McaP51.650 antibodies (Abs) (B and D) at a dilution of 1:25. Bacteria were washed, incubated with FITC-conjugated secondary antibody, and processed as described in Materials and Methods. The x axes represent the level of fluorescence, and the y axes correspond to the particles counted in arbitrary units.
Selected structural features of the mcaP gene product.
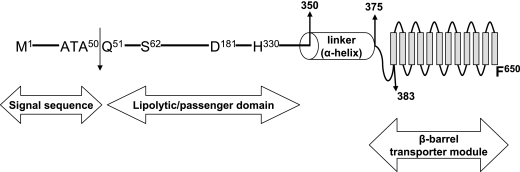
Previous sequence analysis (63) revealed that the McaP C terminus resembles the transporter domain of autotransporter proteins. These molecules exhibit conserved structural features, such as an N-terminal signal peptide, a surface-exposed passenger domain specifying the function of the protein, a C-terminal transporter module that translocates the passenger domain on the surface of bacterial cells and anchors the molecule in the OM, and an α-helical linker region of 20 to 40 residues connecting the passenger domain to the transporter module (24). Recent studies have also revealed that autotransporters can be divided in two subfamilies based on the structure of their porin-like transporter module: conventional or trimeric (16). The C-terminal transporter domain of conventional autotransporters generally consists of ∼300 aa forming 12 antiparallel β-strands, while the transporter domain of the trimeric subfamily is substantially shorter (∼70 residues) and is predicted to contain only four β-strands. Further analysis of McaP with the PSIPRED secondary structure prediction algorithm (31) suggests that McaP is a conventional autotransporter. The last 267 aa of McaP are predicted to contain 12 β-strands, and this potential OM anchor is immediately preceded by a helical region of 34 residues (not shown). These structural features of McaP are highly reminiscent of the β-barrel transporter domain of the Neisseria meningitidis conventional autotransporter protein NalP, for which the crystal structure has been determined previously (54).
Previous database searches (63) also indicated that McaP, which exhibits esterase and phospholipase B activities, belongs to a family of lipolytic enzymes designated GDSL (64) due to the presence of these four highly conserved residues in its N terminus (aa 60 to 63 in McaP). While the substrates of GDSL enzymes are diverse, the amino acids directly involved in their activity appear to be conserved in the molecules that have been characterized thus far, such as the Aeromonas hydrophila lipase/acyltransferase GCAT (25), an aryl transferase of Vibrio mimicus (13), and a thioesterase/protease expressed by E. coli (37). These active-site residues form a catalytic triad and include the highly conserved serine nucleophile found in the GDSL motif as well as downstream histidine and aspartate residues. The predicted amino acid sequence of McaP was therefore compared to that of other GDSL lipolytic enzymes, and residues S62, D181, and H330 were identified as being potentially important for McaP lipolytic activities (not shown). McaP thus appears to be a conventional autotransporter protein containing a β-barrel transporter module, composed of 12 β-strands (aa 383 to 650), that is linked to a surface-exposed passenger domain exhibiting lipolytic activity (aa 62 to 330) by an α-helical region of 34 residues (aa 350 to 383). These predicted structural features of McaP are illustrated in Fig. 4.
FIG. 4.
Structural features of McaP. Different regions of the McaP protein are depicted, with the position of amino acid residues defining key structural as well as functional features.
The N-terminal half of McaP contains surface-exposed epitopes and is involved in adherence.
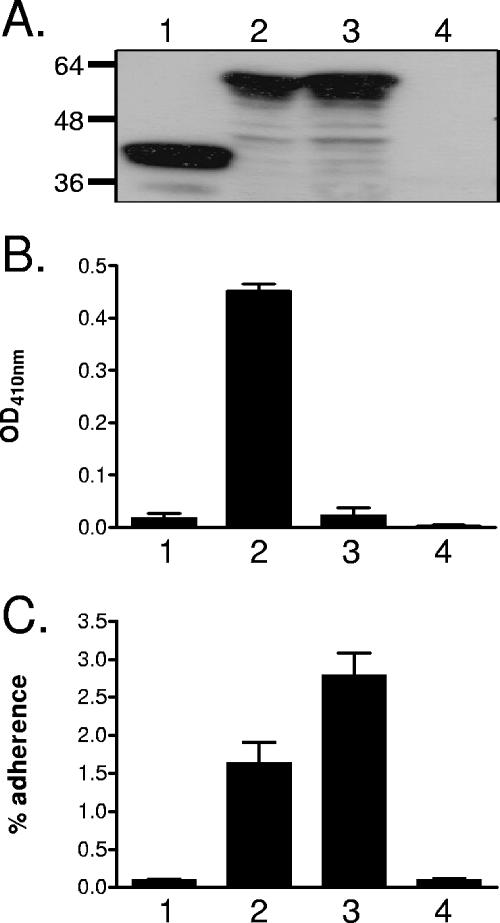
Based on the predicted structural similarities to autotransporter proteins, we hypothesized that McaP has a passenger domain located on the surface of M. catarrhalis cells that is important for adhesive as well as lipolytic functions. To test this hypothesis, an in-frame deletion of aa 53 to 336 was introduced into the O35E-mcaP ORF harbored by the plasmid pIFmcaP17 (65), yielding the plasmid pSVmcaPΔ53.336, and E. coli cells expressing the mutated protein were tested for their ability to hydrolyze p-nitrophenyl caproate and bind to A549 human lung epithelial cells. As shown in Fig. 5B and C, deletion of the McaP putative passenger domain abolished the ability of recombinant bacteria to hydrolyze the lipolytic substrate and adhere to A549 cells. Western blot analysis of OM proteins extracted from these recombinant bacteria by use of McaP-specific antibodies confirmed that the truncated protein was still transported to the OM (Fig. 5A, lane 1). E. coli cells expressing the WT O35E-mcaP gene product (i.e., harboring the plasmid pIFmcaP17) and recombinant bacteria lacking McaP (i.e., containing the plasmid pCC1.3) were used as controls in these experiments. These data demonstrate that the McaP N-terminal passenger domain is involved in both the adhesive and lipolytic properties of the molecule.
FIG. 5.
Western blot analysis (A), lipolytic activity (B), and adherence (C) of recombinant E. coli bacteria. (A) Sarkosyl-insoluble OM proteins were purified from E. coli cells harboring the plasmid pSVmcaPΔ53-336 (lane 1), pIFmcaP17 (lane 2), pSVmcaPS62N (lane 3), or pCC1.3 (lane 4), resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and analyzed by Western blotting with PEP1-specific antibodies at a dilution of 1:10,000. Molecular mass markers are shown to the left in kilodaltons. (B) E. coli cells carrying the plasmid pSVmcaPΔ53-336 (lane 1), pIFmcaP17 (lane 2), pSVmcaPS62N (lane 3), or pCC1.3 (lane 4) were incubated with the lipolytic substrate p-nitrophenyl caproate. The absorbance of triplicate samples at a wavelength of 410 nm (OD, optical density), which is indicative of lipolytic activity, was measured 15 min after the addition of the substrate. (C) E. coli cells carrying the plasmid pSVmcaPΔ53-336 (lane 1), pIFmcaP17 (lane 2), pSVmcaPS62N (lane 3), or pCC1.3 (lane 4) were incubated with monolayers of A549 cells for 3 h prior to washing off unbound bacteria. The results are expressed as the means (±standard errors) of the percentages of inoculated bacteria that bound to A549 cells.
To determine whether deletion of the predicted passenger domain or the resulting lack of lipolytic activity is responsible for the loss of McaP adhesive properties, site-directed mutagenesis was used to replace the conserved serine at position 62 of McaP with an asparagine, yielding the plasmid pSVmcaPS62N. Sequence comparison to other GDSL enzymes suggests that this serine is the catalytic nucleophile for the lipolytic activity of McaP. As demonstrated by results shown in Fig. 5B, the Ser-Asn substitution abolished the lipolytic activity of recombinant bacteria. Surprisingly, E. coli expressing the lipolytically inactive McaP in its OM (Fig. 5A, lane 3) showed no loss of adherence and in fact attached to A549 cells at levels greater than those for recombinant bacteria expressing wild-type McaP (Fig. 5C). These results demonstrate that McaP lipolytic activity is separate from its adhesive properties. In an attempt to further delineate the region(s) of McaP relevant to adherence, a series of consecutive in-frame deletions encompassing aa 53 to 336 were constructed and recombinant bacteria expressing the mutated proteins were tested in quantitative attachment assays. These deletions, each removing 20 residues at a time, were all found to abolish McaP lipolytic as well as adhesive properties (data not shown), which is consistent with both functions being specified by the N-terminal half of the protein.
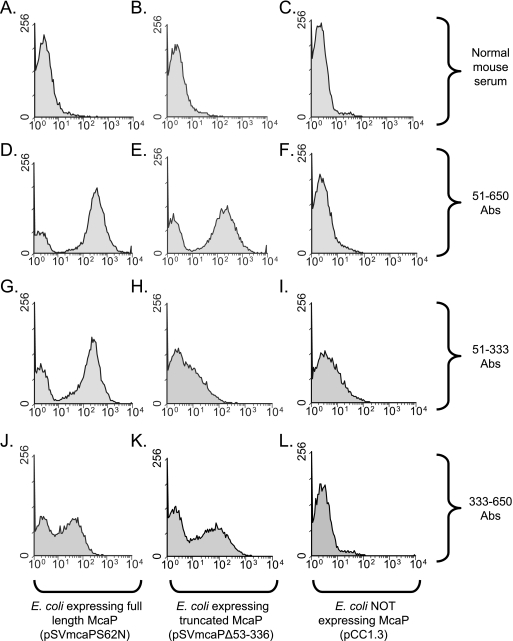
To support the hypothesis that the predicted passenger domain of McaP is located on the surface of bacterial cells, antibodies raised against residues 51 to 333 of the protein were tested for their ability to bind to intact bacteria. To achieve this, mice were immunized with the purified recombinant protein designated His.McaP51.333 and the sera recovered from these animals were tested by flow cytometry. In parallel experiments, mice were immunized with a purified recombinant protein that corresponds to aa 333 to 650 of McaP fused to a histidine tag (His.McaP333.650) to generate antibodies binding to the C-terminal half of the molecule. As shown in Fig. 6, antibodies against aa 51 to 333 (Fig. 6G) as well as against aa 333 to 650 (Fig. 6J) caused a shift in fluorescence, indicating that they bind to the surface of intact E. coli expressing full-length McaP. Antibodies against residues 51 to 650 (Fig. 6E) and antibodies against aa 333 to 650 (Fig. 6K) were also discovered to bind to the surface of E. coli expressing the truncated McaP protein that lacks most of its passenger domain (i.e., harboring the plasmid pSVmcaPΔ53.336); as expected, the antibodies raised against aa 51 to 333 did not bind to these recombinant cells (Fig. 6H). No shifts in fluorescence were observed when recombinant bacteria were incubated with normal mouse serum (Fig. 6A, B, and C) or when E. coli that lacks expression of McaP was incubated with the various McaP antibodies (Fig. 6F, I, and L). These results are consistent with the McaP predicted passenger domain (i.e., aa 51 to 330) being exposed on the surface of bacterial cells. Our data also demonstrate that the McaP C-terminal transporter module contains surface-exposed epitopes.
FIG. 6.
Flow cytometry analysis of recombinant E. coli bacteria. E. coli cells were incubated with normal mouse serum or with murine serum containing antibodies against His.McaP51.650 (51-650 Abs), His.McaP51.333 (51-333 Abs), or His.McaP333.650 (333-650 Abs) at a dilution of 1:25. Bacteria were washed, incubated with FITC-conjugated secondary antibody, and processed as described in Materials and Methods. The x axes represent the level of fluorescence, and the y axes correspond to the particles counted. These recombinant E. coli cells expressed full-length McaP, truncated McaP that lacks aa 53 to 336, or no McaP at all, as indicated at the bottom of the figure.
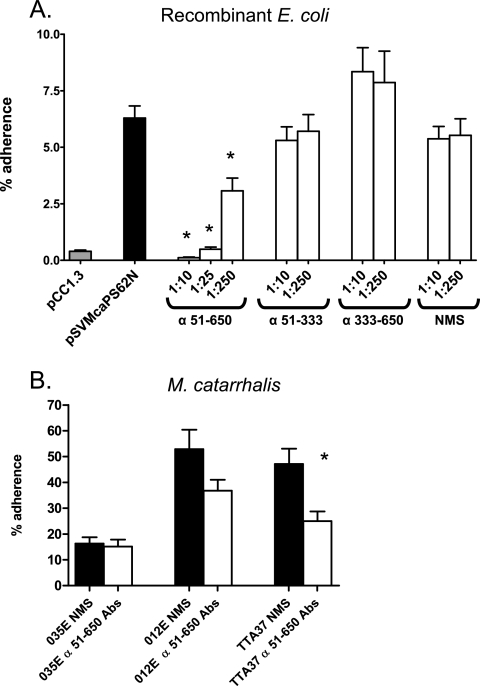
Anti-McaP antibodies were tested for their ability to block adherence to lung epithelial cells since they were found to bind epitopes located on the surface of intact bacteria. As shown in Fig. 7A, preincubating E. coli expressing McaP with antibodies against aa 51 to 333 or aa 333 to 650 did not significantly reduce adherence to A549 monolayers. Antibodies against aa 51 to 650, however, reduced binding to pneumocytes by 98%, and this inhibition of adherence was found to be dose dependent (compare preincubation with a 1:10 dilution to that with a 1:250 dilution). Preincubation of recombinant bacteria with normal mouse serum did not adversely affect attachment to A549 cells. Interestingly, the M. catarrhalis WT strains O12E and TTA37 were found to attach to these lung cells substantially better than O35E, and antibodies against aa 51 to 650 reduced the adherence of these two isolates by 30% and 47%, respectively (Fig. 7B). Thus, these results suggest that McaP may be responsible for this increased adherence. The antibodies against residues 51 to 650 did not interfere with the ability of strain O35E to attach to monolayers, which is consistent with our previous report showing that lack of McaP expression by strain O35E does not decrease binding to epithelial cells because of other adhesins compensating for the loss of McaP (63).
FIG. 7.
Inhibition of adherence with anti-McaP antibodies. (A) E. coli cells carrying the plasmid pCC1.3 (gray bar; adherence negative control) or pSVmcaPS62N (all other bars) were preincubated with no antibodies (black bar; adherence positive control) or with normal mouse serum (NMS) as well as murine sera against aa 51 to 333 (α 51-333), aa 333 to 650, and aa 51 to 650 at dilutions of 1:10, 1:25, or 1:250 (open bars). These bacteria were then incubated with monolayers of A549 cells for 3 h prior to washing off unbound bacteria. (B) The WT M. catarrhalis strains O35E, O12E, and TTA37 were incubated with NMS (black bars) or murine sera against aa 51 to 650 (α 51-650 Abs) (open bars) at a dilution of 1:10. These bacteria were then incubated with A549 cells for 5 min. The results are expressed as the means (±standard errors) of the percentages of inoculated bacteria that bound to A549 cells. The asterisks indicate statistically significant decreases in adherence compared to levels for bacteria incubated with NMS.
DISCUSSION
Autotransporter proteins are the largest known family of virulence factors expressed by gram-negative bacteria and play prominent roles in processes such as serum resistance, cell-to-cell aggregation, biofilm formation, and adherence to host cells (24). Most members of this family exhibit conserved structural features that include an N-terminal surface-exposed passenger domain responsible for the autotransporter's biological function followed by a short helical region of up to 40 residues that connects the passenger domain to a C-terminal transporter module containing several β-strands and serves to anchor the molecule in the OM of bacterial cells (24). Because of their structure and role in virulence, autotransporters are potential targets for the development of therapeutic agents as well as vaccines. Large portions of these autotransporter proteins are located on the surface of bacteria (especially the passenger domain) and thus are readily accessible to the immune system. Furthermore, most autotransporters studied to date play important roles in pathogenesis, so targeting them may interfere with the ability to cause disease. For instance, immunization with the passenger domain of the Haemophilus influenzae autotransporter adhesin Hap confers protection in a mouse nasopharyngeal colonization model and elicits the production of antibodies that reduce adherence to human epithelial cells in vitro (17, 38). Immunization with the passenger domain of the Bordetella pertussis autotransporter Brk, a serum resistance factor, also yields biologically relevant antibodies that enhance the bactericidal activity of human complement against B. pertussis (53). Of note, another autotransporter expressed by B. pertussis, designated Pertactin (32), is a component of four out of the five pertussis vaccines currently licensed for use in the United States (12).
Based on the predicted structure of its C terminus, McaP can be classified as a conventional autotransporter. The prototypical member of this subfamily is N. meningitidis NalP, for which the crystal structure has been determined previously (54). The NalP transporter module consists of 12 antiparallel β-strands embedded in the OM of Neisseria, each connected by loops of various lengths that are exposed on the periplasmic side of the OM or on the surface of bacteria (54). This transporter domain of NalP folds into a β-barrel conformation that creates a hydrophilic pore in the OM through which the N-terminal passenger domain is secreted. McaP is also predicted to contain 12 β-strands connected by loops ranging in lengths from 3 to 28 residues. Six of these loops are predicted to be surface exposed, and this hypothesis is supported by flow cytometry data demonstrating that antibodies against aa 333 to 650, corresponding to the McaP predicted transporter domain, bind to the surface of intact E. coli cells expressing the protein (Fig. 6J). Furthermore, it was discovered that E. coli expressing only the McaP transporter module in its OM (i.e., harboring the plasmid pSLVmcaPΔ53-336 [Fig. 5A]) still bound McaP antibodies to their surface (Fig. 6E and K). These results indicate that the β-barrel portion of McaP contains surface-exposed epitopes. The heat-modifiable characteristics of McaP (Fig. 1) are also consistent with its classification as a conventional autotransporter. The secondary and tertiary structures of proteins rich in β-strands are stable enough to withstand incubation at room temperature in the presence of sodium dodecyl sulfate, but denaturation may be induced by heating at 100°C (40, 54, 62). Because intact and denatured β-barrels exhibit different electrophoretic mobilities when resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the presence of a β-barrel can be detected by using heat modification followed by SDS-PAGE analysis. Intact β-barrels linked to a denatured passenger domain tend to migrate more rapidly in SDS-PAGE than their completely denatured equivalents (40, 54, 62), which is what was observed for McaP (Fig. 1).
Though the McaP transporter domain contains surface-exposed epitopes which could interact with epithelial cells, this portion of the protein is unlikely to be responsible for its adhesive properties. This belief is based on the results of two sets of adherence assays. First, antibodies against McaP residues 333 to 650 do not reduce adherence. Second, E. coli cells expressing the McaP transporter module without a linked passenger domain (e.g., harboring pSLVmcaPΔ53-336) do not attach to A549 cells (Fig. 5C), even though the truncated McaP is present in the OM (Fig. 5A), and flow cytometry experiments suggest that this mutated McaP is displayed on the surface of intact cells (Fig. 6E and K). These observations support the hypothesis that the McaP passenger domain contains the major epithelial cell binding determinant(s). Flow cytometry experiments with antibodies against residues 51 to 333 clearly demonstrate that the McaP passenger domain contains surface-exposed epitopes (Fig. 6G), which could have the ability to mediate binding to epithelial cells. In addition, small consecutive deletions that span the entire passenger domain of McaP were all found to abrogate the adhesin function of the protein (data not shown). Furthermore, the passenger domain contains the adherence epitopes of most autotransporter adhesins that have been characterized, including Yersinia enterocolitica YadA (56), H. influenzae Hia (34, 66), E. coli Ag43 (33), and H. influenzae Hap (19, 38). Although both immune sera clearly bind on the surface of intact bacteria (Fig. 6), it is not clear why antibodies against residues 51 to 333 did not block adherence to epithelial cells, while antibodies against aa 51 to 650 effectively reduced binding (Fig. 7A). One possible explanation is that the His.McaP51.333 protein was purified under denaturing conditions and may not have refolded in a manner that retained the immunogenicity of McaP's adherence epitope(s). Alternatively, antibodies against aa 51 to 333 bind to immunodominant epitopes that are not relevant to adherence.
In addition to its adhesive function, McaP exhibits esterase/phospholipase B activities and resembles members of a family of lipolytic enzymes termed GDSL (63). Previous studies of the mechanism of these proteins suggest the presence of an active-site charge relay system that is common to non-GDSL lipases (9, 13, 25, 30, 37). Similarly to catalysis by other lipases and serine proteases, GDSL lipolytic activity involves a catalytic triad of serine, histidine, and aspartate residues (30), all of which are predicted to be present in McaP (Fig. 4). The active-site serine residue is central to this mechanism in that it performs a nucleophilic attack that results in the formation of a transient covalent intermediate with the substrate (30). Once the substrate is bound in the active site, hydrolysis may proceed. By accepting a proton from the nucleophilic serine, the histidine and aspartate residues increase its nucleophilicity and reactivity. Thus, each member of the Ser-His-Asp catalytic triad plays an essential role in this process such that a mutation of one of these residues renders the enzyme inactive (9, 25, 30). Our data strongly suggest that McaP serine 62 corresponds to this active-site serine, as mutagenesis of this residue abolished the lipolytic activity of the molecule (Fig. 5B). Interestingly, the lipolytically inactive McaPS62N was found to possess increased adhesive properties (Fig. 5C), demonstrating that the two functions of this protein are separable. Similar observations were made by Fink et al. for the autotransporter adhesin Hap (20, 21). These investigators discovered that Hap is a serine protease that mediates its own proteolytic cleavage through a catalytic triad consisting of histidine, aspartate, and serine residues at positions 98, 140, and 243 of the protein, respectively (20). In addition, a Hap mutant protein containing an alanine residue in lieu of serine at position 243 and consequently unable to undergo autoproteolytic cleavage to release its passenger domain from the bacterial surface conferred increased adherence to human epithelial cells (21). Recently, Ganendren and colleagues (22) demonstrated that the Cryptococcus neoformans-secreted phospholipase B PLB1 was involved in adherence to A549 cells. The role of PLB1 in adherence, however, was found to be dependent upon its ability to enzymatically degrade phospholipids. By contrast, the two functions are clearly separable in McaP and the molecular basis by which the lipolytically inactive McaP confers increased adherence to epithelial cells remains to be elucidated. The identification of the ligand for McaP on the surface of human cells may shed some light on this unexpected result.
In summary, our data demonstrate that McaP is a highly conserved OM protein expressed by M. catarrhalis. Immunization with a polypeptide corresponding to aa 51 to 650 of McaP elicited the production of antibodies that bind to the surface of M. catarrhalis and that substantially reduce adherence to human lung epithelial cells. These results warrant further studies aimed at evaluating the vaccinogenic potential of McaP, since antibodies against this protein may enhance clearance of M. catarrhalis by opsonizing the bacterium and by interfering with adherence to mucosal surfaces of the respiratory tract. Our results indicate that the N-terminal half of McaP specifies both the lipolytic and adherence properties of the molecule and that these two biological functions are independent of one another. Since adherence and lipolytic activity are frequently associated with virulence in other organisms (58, 60), further structure-function analyses of McaP should yield important information pertaining to pathogenesis by M. catarrhalis. This knowledge is important to the development of novel therapeutic approaches to combat the high level of antibiotic resistance observed for M. catarrhalis isolates (29).
Acknowledgments
This study was supported by a grant from NIH/NIAID (AI051477) to E.R.L.
We thank Eric Hansen at the University of Texas Southwestern Medical Center in Dallas and Tim Murphy at the State University New York at Buffalo for providing M. catarrhalis strains and antibodies. We thank Brian Bullard, Rachel Balder, Robert Blumenthal, and Randall Worth for their helpful comments on the manuscript. We also thank Tom Sawyer and Karen Domenico at the University of Toledo Health Sciences Campus for their assistance with the flow cytometry experiments.
Editor: D. L. Burns
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Adlowitz, D. G., T. Hiltke, A. J. Lesse, and T. F. Murphy. 2004. Identification and characterization of outer membrane proteins G1a and G1b of Moraxella catarrhalis. Vaccine 22:2533-2540. [DOI] [PubMed] [Google Scholar]
- 2.Adlowitz, D. G., C. Kirkham, S. Sethi, and T. F. Murphy. 2006. Human serum and mucosal antibody responses to outer membrane protein G1b of Moraxella catarrhalis in chronic obstructive pulmonary disease. FEMS Immunol. Med. Microbiol. 46:139-146. [DOI] [PubMed] [Google Scholar]
- 3.Adlowitz, D. G., S. Sethi, P. Cullen, B. Adler, and T. F. Murphy. 2005. Human antibody response to outer membrane protein G1a, a lipoprotein of Moraxella catarrhalis. Infect. Immun. 73:6601-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 65:4367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attia, A. S., S. Ram, P. A. Rice, and E. J. Hansen. 2006. Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect. Immun. 74:1597-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhushan, R., C. Kirkham, S. Sethi, and T. F. Murphy. 1997. Antigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalis. Infect. Immun. 65:2668-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingen, E., R. Cohen, N. Jourenkova, and P. Gehanno. 2005. Epidemiologic study of conjunctivitis-otitis syndrome. Pediatr. Infect. Dis. J. 24:731-732. [DOI] [PubMed] [Google Scholar]
- 8.Brook, I., P. A. Foote, and E. H. Frazier. 2005. Microbiology of acute exacerbation of chronic sinusitis. Ann. Otol. Rhinol. Laryngol. 114:573-576. [DOI] [PubMed] [Google Scholar]
- 9.Brumlik, M. J., and J. T. Buckley. 1996. Identification of the catalytic triad of the lipase/acyltransferase from Aeromonas hydrophila. J. Bacteriol. 178:2060-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullard, B., S. L. Lipski, and E. R. Lafontaine. 2005. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect. Immun. 73:5127-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlone, G. M., M. L. Thomas, H. S. Rumschlag, and F. O. Sottnek. 1986. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J. Clin. Microbiol. 24:330-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2000. Epidemiology and prevention of vaccine-preventable diseases. Chapter 7. Pertussis. http://www.cdc.gov/nip/publications/pink/pert.pdf.
- 13.Chang, R. C., J. C. Chen, and J. F. Shaw. 1996. Site-directed mutagenesis of a novel serine arylesterase from Vibrio mimicus identifies residues essential for catalysis. Biochem. Biophys. Res. Commun. 221:477-483. [DOI] [PubMed] [Google Scholar]
- 14.Chen, D., J. C. McMichael, K. R. VanDerMeid, A. W. Masi, E. Bortell, J. D. Caplan, D. N. Chakravarti, and V. L. Barniak. 1999. Evaluation of a 74-kDa transferrin-binding protein from Moraxella (Branhamella) catarrhalis as a vaccine candidate. Vaccine 18:109-118. [DOI] [PubMed] [Google Scholar]
- 15.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, G. H. McCracken, Jr., and E. J. Hansen. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter, S. E., N. K. Surana, and J. W. St. Geme III. 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 13:199-205. [DOI] [PubMed] [Google Scholar]
- 17.Cutter, D., K. W. Mason, A. P. Howell, D. L. Fink, B. A. Green, and J. W. St. Geme III. 2002. Immunization with Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J. Infect. Dis. 186:1115-1121. [DOI] [PubMed] [Google Scholar]
- 18.Du, R. P., Q. Wang, Y. P. Yang, A. B. Schryvers, P. Chong, M. H. Klein, and S. M. Loosmore. 1998. Cloning and expression of the Moraxella catarrhalis lactoferrin receptor genes. Infect. Immun. 66:3656-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink, D. L., A. Z. Buscher, B. Green, P. Fernsten, and J. W. St. Geme III. 2003. The Haemophilus influenzae Hap autotransporter mediates microcolony formation and adherence to epithelial cells and extracellular matrix via binding regions in the C-terminal end of the passenger domain. Cell. Microbiol. 5:175-186. [DOI] [PubMed] [Google Scholar]
- 20.Fink, D. L., L. D. Cope, E. J. Hansen, and J. W. St. Geme III. 2001. The Haemophilus influenzae Hap autotransporter is a chymotrypsin clan serine protease and undergoes autoproteolysis via an intermolecular mechanism. J. Biol. Chem. 276:39492-39500. [DOI] [PubMed] [Google Scholar]
- 21.Fink, D. L., and J. W. St. Geme III. 2003. Chromosomal expression of the Haemophilus influenzae Hap autotransporter allows fine-tuned regulation of adhesive potential via inhibition of intermolecular autoproteolysis. J. Bacteriol. 185:1608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganendren, R., E. Carter, T. Sorrell, F. Widmer, and L. Wright. 2006. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 8:1006-1015. [DOI] [PubMed] [Google Scholar]
- 23.Giebink, G. S., Y. Kurono, L. O. Bakaletz, J. M. Kyd, S. J. Barenkamp, T. F. Murphy, B. Green, P. L. Ogra, X. X. Gu, J. A. Patel, T. Heikkinen, S. I. Pelton, M. Hotomi, and P. Karma. 2005. Recent advances in otitis media. 6. Vaccine. Ann. Otol. Rhinol. Laryngol. Suppl. 194:86-103. [PubMed] [Google Scholar]
- 24.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilton, S., and J. T. Buckley. 1991. Studies on the reaction mechanism of a microbial lipase/acyltransferase using chemical modification and site-directed mutagenesis. J. Biol. Chem. 266:997-1000. [PubMed] [Google Scholar]
- 26.Holm, M. M., S. L. Vanlerberg, I. M. Foley, D. D. Sledjeski, and E. R. Lafontaine. 2004. The Moraxella catarrhalis porin-like outer membrane protein CD is an adhesin for human lung cells. Infect. Immun. 72:1906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 71:4977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsiao, C. B., S. Sethi, and T. F. Murphy. 1995. Outer membrane protein CD of Branhamella catarrhalis: sequence conservation in strains recovered from the human respiratory tract. Microb. Pathog. 19:215-225. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs, M. R., S. Bajaksouzian, A. Windau, C. E. Good, G. Lin, G. A. Pankuch, and P. C. Appelbaum. 2004. Susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis to 17 oral antimicrobial agents based on pharmacodynamic parameters: 1998-2001 U S surveillance study. Clin. Lab. Med. 24:503-530. [DOI] [PubMed] [Google Scholar]
- 30.Jaeger, K. E., S. Ransac, B. W. Dijkstra, C. Colson, M. van Heuvel, and O. Misset. 1994. Bacterial lipases. FEMS Microbiol. Rev. 15:29-63. [DOI] [PubMed] [Google Scholar]
- 31.Jones, D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195-202. [DOI] [PubMed] [Google Scholar]
- 32.Junker, M., C. C. Schuster, A. V. McDonnell, K. A. Sorg, M. C. Finn, B. Berger, and P. L. Clark. 2006. Pertactin beta-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc. Natl. Acad. Sci. USA 103:4918-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klemm, P., L. Hjerrild, M. Gjermansen, and M. A. Schembri. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51:283-296. [DOI] [PubMed] [Google Scholar]
- 34.Laarmann, S., D. Cutter, T. Juehne, S. J. Barenkamp, and J. W. St. Geme. 2002. The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Mol. Microbiol. 46:731-743. [DOI] [PubMed] [Google Scholar]
- 35.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lafontaine, E. R., N. J. Wagner, and E. J. Hansen. 2001. Expression of the Moraxella catarrhalis UspA1 protein undergoes phase variation and is regulated at the transcriptional level. J. Bacteriol. 183:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, T. H., C. Chen, R. F. Huang, Y. L. Lee, J. F. Shaw, and T. H. Huang. 1998. Multinuclear NMR resonance assignments and the secondary structure of Escherichia coli thioesterase/protease I: a member of a new subclass of lipolytic enzymes. J. Biomol. NMR 11:363-380. [DOI] [PubMed] [Google Scholar]
- 38.Liu, D. F., K. W. Mason, M. Mastri, M. Pazirandeh, D. Cutter, D. L. Fink, J. W. St. Geme III, D. Zhu, and B. A. Green. 2004. The C-terminal fragment of the internal 110-kilodalton passenger domain of the Hap protein of nontypeable Haemophilus influenzae is a potential vaccine candidate. Infect. Immun. 72:6961-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathers, K., M. Leinonen, and D. Goldblatt. 1999. Antibody response to outer membrane proteins of Moraxella catarrhalis in children with otitis media. Pediatr. Infect. Dis. J. 18:982-988. [DOI] [PubMed] [Google Scholar]
- 40.Maurer, J., J. Jose, and T. F. Meyer. 1999. Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions. J. Bacteriol. 181:7014-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier, P. S., R. Troller, I. N. Grivea, G. A. Syrogiannopoulos, and C. Aebi. 2002. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine 20:1754-1760. [DOI] [PubMed] [Google Scholar]
- 42.Mollenkvist, A., T. Nordstrom, C. Hallden, J. J. Christensen, A. Forsgren, and K. Riesbeck. 2003. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J. Bacteriol. 185:2285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect. Immun. 73:8161-8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect. Immun. 73:3471-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy, T. F., A. L. Brauer, B. J. Grant, and S. Sethi. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy, T. F., A. L. Brauer, N. Yuskiw, and T. J. Hiltke. 2000. Antigenic structure of outer membrane protein E of Moraxella catarrhalis and construction and characterization of mutants. Infect. Immun. 68:6250-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy, T. F., A. L. Brauer, N. Yuskiw, E. R. McNamara, and C. Kirkham. 2001. Conservation of outer membrane protein E among strains of Moraxella catarrhalis. Infect. Immun. 69:3576-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy, T. F., C. Kirkham, E. DeNardin, and S. Sethi. 1999. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect. Immun. 67:4578-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy, T. F., C. Kirkham, and A. J. Lesse. 1993. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol. Microbiol. 10:87-97. [DOI] [PubMed] [Google Scholar]
- 50.Murphy, T. F., C. Kirkham, D. F. Liu, and S. Sethi. 2003. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect. Immun. 71:1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy, T. F., and M. R. Loeb. 1989. Isolation of the outer membrane of Branhamella catarrhalis. Microb. Pathog. 6:159-174. [DOI] [PubMed] [Google Scholar]
- 52.Myers, L. E., Y. P. Yang, R. P. Du, Q. Wang, R. E. Harkness, A. B. Schryvers, M. H. Klein, and S. M. Loosmore. 1998. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect. Immun. 66:4183-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver, D. C., and R. C. Fernandez. 2001. Antibodies to BrkA augment killing of Bordetella pertussis. Vaccine 20:235-241. [DOI] [PubMed] [Google Scholar]
- 54.Oomen, C. J., P. Van Ulsen, P. Van Gelder, M. Feijen, J. Tommassen, and P. Gros. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 23:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patrick, C. C., A. Kimura, M. A. Jackson, L. Hermanstorfer, A. Hood, G. H. McCracken, Jr., and E. J. Hansen. 1987. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect. Immun. 55:2902-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roggenkamp, A., N. Ackermann, C. A. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 185:3735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 58.Schmiel, D. H., and V. L. Miller. 1999. Bacterial phospholipases and pathogenesis. Microbes Infect. 1:1103-1112. [DOI] [PubMed] [Google Scholar]
- 59.Sethi, S., J. M. Surface, and T. F. Murphy. 1997. Antigenic heterogeneity and molecular analysis of CopB of Moraxella (Branhamella) catarrhalis. Infect. Immun. 65:3666-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St. Geme, J. W., III. 1997. Bacterial adhesins: determinants of microbial colonization and pathogenicity. Adv. Pediatr. 44:43-72. [PubMed] [Google Scholar]
- 61.Stutzmann Meier, P., N. Heiniger, R. Troller, and C. Aebi. 2003. Salivary antibodies directed against outer membrane proteins of Moraxella catarrhalis in healthy adults. Infect. Immun. 71:6793-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surana, N. K., S. Grass, G. G. Hardy, H. Li, D. G. Thanassi, and J. W. St. Geme III. 2004. Evidence for conservation of architecture and physical properties of Omp85-like proteins throughout evolution. Proc. Natl. Acad. Sci. USA 101:14497-14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Timpe, J. M., M. M. Holm, S. L. Vanlerberg, V. Basrur, and E. R. Lafontaine. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71:4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upton, C., and J. T. Buckley. 1995. A new family of lipolytic enzymes? Trends Biochem. Sci. 20:178-179. [DOI] [PubMed] [Google Scholar]
- 65.Vanlerberg, S. L., C. Akimana, and E. R. Lafontaine. 2004. Cloning and expression of Moraxella catarrhalis adhesins using the CopyControlTM PCR cloning system. Epicentre Forum 11:21-22. [Google Scholar]
- 66.Yeo, H. J., S. E. Cotter, S. Laarmann, T. Juehne, J. W. St. Geme III, and G. Waksman. 2004. Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J. 23:1245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu, R. H., R. A. Bonnah, S. Ainsworth, and A. B. Schryvers. 1999. Analysis of the immunological responses to transferrin and lactoferrin receptor proteins from Moraxella catarrhalis. Infect. Immun. 67:3793-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]