Abstract
Exosomes activate T cells in vivo, but whether exosomes are able to induce humoral immune responses is still unknown. We found that dendritic cells, but not other immune cells, constitutively release an exosome-associated glycoconjugate that is cross-reactive with the capsular polysaccharide of Streptococcus pneumoniae type 14 (Cps14-CRA). Cps14-CRA was localized to the cholesterol-enriched microdomains or rafts of the exosomes and was mapped to the β1→6 branched N-acetyl-lactosamine derivatives of the Cps14-CRA. Injection of CFA-primed naive mice with purified dendritic cell exosomes induced immunoglobulin (Ig) anti-Cps14 responses composed predominantly of IgM, IgG3, and IgG1. These responses were associated with protection against a lethal challenge with live S. pneumoniae type 14, but not with type 3 bacteria, and was correlated with the titer of elicited IgM and IgG3 anti-Cps14. These data show, for the first time, that exosomes can induce a humoral immune response to an associated unprocessed, autologous antigen. Although anti-Cps14 Ig responses are specifically demonstrated, these could reflect a broader mechanism that modulates both natural immunity and autoimmunity to other glycotopes.
Exosomes are small vesicles (60 to 90 nm in diameter) formed by inward budding of the membrane within late multivesicular endosomal compartments, released to the extracellular environment upon fusion of the endosomes with the cell surface (10, 42). The composition of exosomes is roughly similar to that of the cell membrane, but considerable sorting of proteins occurs during exosome biogenesis (9). Thus, dendritic cell (DC)-derived exosomes are enriched in major histocompatibility complex (MHC), T-cell costimulatory and adhesion molecules, chaperonins, and tetraspans and depleted of transferrin and Fcγ receptors and lysosome-associated membrane proteins (9, 10, 35, 40). Cholesterol content in the exosomes is very similar to that in the plasma membrane (23), and therefore it has been suggested that MHC class II (MHC-II) molecules could be organized in both tetraspan-enriched microdomains (22) and cholesterol-rich membrane microdomains or rafts (37).
DC-derived exosomes have been shown to induce in vivo antigen-specific priming of both CD4+ and CD8+ T cells that also appears to require the participation of mature DC in the recipient host (3, 5, 41). In addition to the host DC, the maturation state of the DC releasing the exosomes is also critical for the in vivo function of exosomes (20, 29, 35). Thus, exosomes could be considered vehicles for cell-to-cell spread of the functional status of the DC that produced them. Although this ability of DC-derived exosomes to prime T cells has been studied in detail (3, 5, 41), whether exosomes can be efficient inducers of primary humoral immune responses remains unresolved. We recently demonstrated that bone marrow dendritic cell (BMDC)-derived exosomes containing processed diphtheria toxoid induce primary immunoglobulin M (IgM) and IgG anti-diphtheria toxoid responses in vivo (7). The primary IgG response for this processed protein, presented in association with MHC molecules, was biased toward induction of type 1 IgG isotypes (IgG2b and IgG2a). However, whether exosomes can also induce humoral responses to autologous or neoantigens expressed on their surface, and not associated with MHC-II, is unknown.
Invasive infections with Streptococcus pneumoniae are a leading cause of meningitis and a major cause of otitis media and bacteremia in children and pneumonia in the elderly (17, 43). Vaccine-mediated protection against S. pneumoniae infection is based on humoral immunity specific for S. pneumoniae capsular polysaccharides (Cps) (17). More than 90 Cps serotypes have been described, with no cross-reaction among each other (16, 36). Globally, capsular serotype 14 is one of the most frequent clinical isolates of S. pneumoniae, and Cps14 has been included in all Cps vaccine formulations (11, 28). Cps14 is a ramified heteropolymer with a tetrasaccharide as a repeat unit, formed by a linear backbone of →6)-β-d-GlcpNAc-(1→3)-β-d-Galp-(1→4)-β-d-Glcp-(1→, with monosaccharide side chains of a β-d-Galp-(1→ linked to C-4 of the GlcpNAc residue (24). This structure is similar to the Cps of type III group B Streptococcus, which contains an additional terminal αNAcNeu-(2→ linked to C-3 of the β-d-Galp residue in the side chain (12, 19). Thus, cross-reactivity between these two Cps has been observed (13). However, no cross-reactivity between Cps14 and other S. pneumoniae Cps or host antigens has been described.
In this study, we show that exosomes derived from BMDC express in their cholesterol-enriched microdomains a glycoconjugate that is cross-reactive with the Cps14 (Cps14-CRA). Furthermore, these purified exosomes injected during an inflammatory response can induce S. pneumoniae protective Cps14-specific IgM and IgG3 responses in naive recipients. These results demonstrate that exosomes can induce a humoral immune response to an associated unprocessed, autologous antigen. Although anti-Cps14 Ig responses are specifically demonstrated, these could reflect a more general mechanism that regulates natural immunity and autoimmunity to other glycotopes.
MATERIALS AND METHODS
Mice.
Female BALB/cJ and B6129PF2/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME), and C57BL/6N mice were obtained from the National Cancer Institute (Gaithersburg, MD). Mice were used at 8 to 10 weeks of age and were maintained in a pathogen-free environment at the Uniformed Services University of the Health Sciences (USUHS, Bethesda, MD). The experiments in this study were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (27a).
MAbs specific for bacterial polysaccharides.
A mouse IgG1(κ) monoclonal antibody (MAb) (clone 44.1) and two IgM(κ) MAbs (clones 23.1 and 17.1) specific for Cps14, and one IgM(κ) MAb specific for the Cps of Neisseria meningitidis group A (clone 8F11.1) were kindly provided by Alexander H. Lucas (Children's Hospital Oakland Research Institute, Oakland, CA). The Cps14-specific MAbs had close but different fine specificities (A. H. Lucas, personal communication) and interacted with different avidities with N-acetyl-lactosamine but not with melibiose (Galα1→6Glc). However, lactose or aminophenyl-lactoside interacted significantly only with 44.1, suggesting that the recognized epitope is contained within the major chain, whereas the epitopes recognized by IgM anti-Cps14 MAb are more closely associated with the side chains. A purified mouse anti-dextran IgM(λ) MAb (clone MOPC-104E) and two IgG1 MAbs of unknown specificity (clones S1-68.1 and MOPC-21) were obtained from BD Pharmingen (San Diego, CA).
S. pneumoniae strains and bacterial antigens.
The Pn14 strain was generously provided by S. Wilson (USUHS), and the type 3 strain, WU-2, was kindly provided by J. Yother (University of Alabama, Birmingham, AL). Purified Cps14 and Cps3 were purchased from ATCC (Manassas, VA). Cps14 and Cps3 were biotinylated by activation with cyanogen bromide and coupling to biotin-LC-Hydrazide (Pierce, Rockford, IL) essentially as described by Lucas et al. (25). Cps14 biotinylation did not depolymerize nor significantly affect the antigenic structure of the Cps14.
BM cell culture and granulocyte depletion.
BMDC were obtained from bone marrow (BM) cells cultured in media supplemented with 10 ng/ml of murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), essentially as described previously (6). BM-derived macrophages (BMM) were similarly obtained from BM cultured in media supplemented with 10 ng/ml of murine recombinant macrophage colony-stimulating factor. The same pooled BM cells were used to obtain the BMDC and BMM used in the experiments. Cell culture media were supplemented with 6% fetal calf serum that was ultracentrifuged two times at 100,000 × g for 18 h at 10°C and filtered through a 0.20-μm-pore-size filter to remove bovine exosomes and protein aggregates. In some experiments, the contaminant granulocytes (<7% of the cells) were negatively selected by magnetic bead cell sorting using streptavidin-coated beads (Biosource, Camarillo, CA) and biotinylated anti-Gr1 MAb (clone RB6-8C5; BD Pharmingen) to target the granulocytes. The granulocyte-depleted BMDC cultures contained <0.1% Gr-1+ cells.
BMM cell line.
A macrophage cell line obtained by transformation of BM cells with J2 retrovirus (14) was kindly provided by G. Dveksler (USUHS, Bethesda, MD). BMM cell line supernatants were collected after 48 to 72 h of nonconfluent culture seeded at 106 BMM/ml.
B-cell hybridoma and splenocyte culture.
The B-cell hybridoma, clone 28.6.20, secreting an anti-idiotypic MAb specific for the VH1/Vκ 24 of the antiphosphorylcholine-specific MOPC167 myeloma (36), was obtained from James J. Kenny (Beth-Israel Deaconess Medical Center, Boston, MA). Red blood cell-lysed splenocytes from naive C57BL/6 mice were obtained using ACK lysing buffer (Gibco). Splenocytes were cultured at 107cells/ml.
Concentration of cell culture supernatant and purification of exosomes.
BMDC collected at day 7 of the primary BM cell culture in GM-CSF were washed and plated at 106 BMDC/ml. In some experiments, BMDC were stimulated with 20 ng/ml of lipopolysaccharide (LPS) during the culture. Culture supernatants, collected at intervals between 0 h to 72 h, were clarified by centrifugation at 300 × g for 10 min at 4°C and filtered through 0.22-μm cellulose acetate filters (GE Osmonics, Inc., Minnetonka, MN). In some experiments, BM cell supernatants from days 2 and 7 of culture (2 days after the last change of media) were used to compare the supernatant from cultures rich in granulocytes (day 2) and in BMDC (day 7). Clarified supernatants were 50-fold concentrated by ultrafiltration through 100,000-molecular-weight cutoff (100K MWCO; Amicon), and diafiltered in 1 volume of phosphate-buffered saline (PBS) up to a final 10-fold-concentrated volume. Alternatively, to purify exosomes, the clarified BMDC culture supernatants were centrifuged at 10,000 × g for 30 min, and the exosomes were collected from the supernatant by ultracentrifugation at 100,000 × g for 1 h at 4°C. Pelleted exosomes were washed in 1 volume of cold PBS at 100,000 × g for 1 h at 4°C and resuspended in a 1/500 volume of PBS. Protein content was determined by the bicinchoninic acid method (Pierce).
Detergent treatment of the cell culture supernatants.
Concentrated supernatants were treated for 30 min at room temperature with 1% Triton X-100 or 0.0025 to 0.5% saponin from quillaja bark (Sigma, Saint Louis, MO), or at 37°C with 0.05 to 50 mM methyl-β-cyclodextrin (MbCD; Sigma). The detergent-treated supernatants were directly used in the enzyme-linked immunosorbent assays (ELISAs).
Detection and quantitation of tetraspan-containing microvesicles.
The presence of complexed tetraspans was detected by capture-sandwich ELISA. Briefly, Immulon-4 HB-X microtiter plates (Dynex Technologies, Milford, MA) were coated with 5 μg/ml of anti-CD9 MAb (clone KMC8) or an isotype-matched rat IgG2a(κ) control (clone R35-95), both obtained from BD Pharmingen. Wells were blocked with 2% bovine serum albumin (BSA), washed with PBS, and then incubated overnight at 4°C with the crude or concentrated BMDC culture supernatant. Captured tetraspan-containing microvesicles were incubated with 1 μg/ml of biotinylated MAb specific for CD9 (same as used for capture) or specific for CD81 (clone Eat2) overnight at 4°C for detection. Thus, these ELISAs detected only multimeric CD9 or CD9-CD81 complexes, respectively. Both tetraspans (CD9 and CD81) are surface membrane proteins. To quantify the concentration of tetraspan-containing microvesicles in BMDC supernatants, a preparation of purified exosomes of known total protein content was used in the ELISA as a standard.
Detection of Cps14-CRA.
The Cps14-CRA was detected using a capture-sandwich ELISA similar to that indicated above for the detection of tetraspan-containing microvesicles, but in this ELISA, the microvesicles were captured using wells directly coated with 5 μg/ml of the 44.1 IgG1 anti-Cps14 MAb. The captured microvesicles were then detected with 1 μg/ml of biotinylated MAb specific for CD9. Wells coated with mouse IgG1 MAb (clone S1-68.1) served as a negative control. The specificity of the detected Cps14-CRA was tested by competitive inhibition, supplementing the concentrated BMDC culture supernatants, or purified exosomes, with decreasing concentrations of purified Cps14 (10 μg/ml to 0.1 ng/ml).
Alternatively, MAbs specific for Cps14 were captured with purified goat IgG specific for mouse IgG1 (for clone 44.1) or IgM (for clones 17.1 and 23.1) before incubation with concentrated culture supernatants or purified exosomes. To minimize the effects produced by differences in avidity and fine specificity of the anti-Cps14 MAbs on the capture of Cps14-CRA-containing microvesicles, the IgM anti-Cps14 MAbs were used in this ELISA at pretitered concentrations that resulted in the capture of amounts of biotinylated Cps14 similar to 0.1 μg/ml of MAb 44.1 captured on anti-mouse IgG1-precoated wells or 5 μg/ml of MAb 44.1 directly coated to the well. Control MAbs of unrelated specificity were used at concentrations matching those used for IgG1 or IgM anti-Cps14 MAbs.
Mouse immunizations.
BALB/c mice were immunized intraperitoneally (i.p.) with 250 μl of an emulsion of complete Freund's adjuvant (CFA) and PBS at day 0. Twenty-four days later, mice received one intravenous injection of 25 μg of BMDC-derived exosomes in 200 μl PBS or an equal volume of the material collected after the ultracentrifugation of fresh culture media following a protocol identical to that for the purification of exosomes (“media”). This preparation, used as a control for potential trace contaminants of culture media components during the purification of exosomes, contained no detectable protein (<6 μg/ml). All mice were bled at days 0, 14, 38, and 43.
Measurement of serum titers of Ig isotypes specific for Cps14 by ELISA.
Titers of Ig isotypes specific for Cps14 were determined by ELISA using microtiter plates directly coated with 0.5 μg/well of Cps14, essentially as previously described (6). Due to the low responses expected in the experiments of this study and to exclude the detection of Igs interacting with minor contaminants of the purified Cps14 preparations, we tested the sera also in plates indirectly coated with biotinylated Cps14. Briefly, microtiter plates were coated with 10 μg/ml of streptavidin (Sigma) in PBS, pH 7.2, and then blocked with PBS plus 2% BSA for 1 h at 37°C. Plates were then coated with 5 ng/ml of biotinylated Cps14 in PBS plus 2% BSA. The following steps were similar in both ELISAs. Briefly, threefold dilutions of the serum samples containing 20 μg/ml of Cps22F were then added, and the plates were incubated overnight at 4°C. Cps22F was used to block nonspecific binding. Cps22F is structurally unrelated to Cps14 and is highly contaminated with cell wall polysaccharide C. The plates were washed three times with PBS and incubated for 1 h at 37°C with polyclonal goat anti-mouse Ig isotypes conjugated to alkaline phosphatase, and the enzymatic reaction was developed using p-nitrophenyl phosphate as a substrate. Titers were expressed as the dilution of sera giving an absorbance at 405 nm equal to 1.0.
Lethality studies.
Isolated colonies of Pn14 in Columbia blood agar were grown in Todd-Hewitt broth and collected in mid-log phase. After washing, the bacterial suspension was adjusted with Dulbecco's PBS to give an absorbance reading at 650 nm of 0.6, which corresponded to 109 CFU/ml, and used immediately for the lethality test. Viable bacterial numbers were determined by colony count on Columbia blood agar. BALB/c mice were injected i.p. with 200 μl of the bacterial suspension containing 25 × 107 CFU (≈10 50% lethal doses [LD50]) or 8 × 107 CFU (≈3 LD50) 20 days after the injection of exosomes. Mice challenged with 9,000 to 12,000 CFU of Pn3 (about 1 LD50) were used as specificity controls. Mice were inspected every 12 h for 6 days.
Statistics.
Data were expressed as arithmetic means ± standard errors of the means (SEM) of the individual titer. Levels of significance of the differences between the groups were determined by the Student's t test. P values of <0.05 were considered statistically significant.
RESULTS
BMDC constitutively release CD9-containing microvesicles.
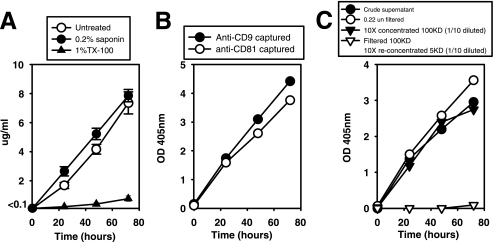
CD9 is a tetraspan of about 24 to 27 kDa highly expressed in the plasma membrane and in endosome-derived exosomes (10). BMDC continually release CD9 into the culture media at a constant rate of ∼0.07 ± 0.01 μg/h/106 BMDC and close to linearity during the first 72 h of culture (Fig. 1), as determined by a sandwich ELISA in which the same anti-CD9 MAb was used as both the capture and detection antibody. Thus, the detected CD9 likely represents multimeric complexes, not monomeric CD9. Furthermore, when a MAb specific for another tetraspan (CD81) was used as a capture antibody, followed by detection with biotinylated anti-CD9 MAb, almost identical signals and kinetics of release were obtained (Fig. 1B). Thus, both tetraspans (CD9 and CD81) are in the same multimeric complex, likely membrane associated.
FIG. 1.
BMDC constitutively release CD9-containing microvesicles. (A) BMDC supernatants collected at different times during the culture period were filtered through 0.22-μm filters and treated with 0.2% saponin or 1% Triton X-100 for 30 min at room temperature. Microvesical CD9 content was estimated by sandwich-capture ELISA using the same anti-CD9 MAb for capture and detection. Results are expressed as μg/ml of protein exosomes using a preparation of purified BMDC-derived exosomes as a standard. Data represent the arithmetic means ± SEM of three wells tested separately. (B) CD9 in BMDC supernatants was captured using anti-CD9 (“anti-CD9 captured”) or anti-CD81 MAbs (“anti-CD81 captured”). In both cases, captured microvesicles were detected with biotinylated anti-CD9 MAb. (C) Culture supernatants filtered through 0.22-μm filters were concentrated 10-fold by ultrafiltration through 100K MWCO units, and the eluted solution was 10-fold concentrated in 5K MWCO units. Both concentrated samples were diluted to the initial volume to obtain a direct comparison with the nonconcentrated culture supernatant. As a reference, crude supernatant (unfiltered through 0.22-μm filters) was included in the assay. Data displayed in each graph are from three separate experiments. OD 405 nm, optical density at 405 nm.
To further investigate this hypothesis, we treated the supernatants with Triton X-100, which extracts proteins from cell membranes without affecting ionic interactions, or with saponin, which complexes to and sequesters cholesterol but with little removal of proteins from the cell membrane (34). Triton X-100 treatment completely abrogated the detection of CD9 in culture supernatants (Fig. 1A), supporting the notion that CD9 was bound to membranes and that free, solubilized, monomeric CD9 was not detected by this ELISA. In contrast, saponin treatment did not reduce (Fig. 1A) but instead slightly enhanced CD9 detection, probably due to the disruption of cholesterol-rich membrane microdomains that are well known to be enriched in tetraspans (9, 10). This disruption likely makes CD9 more accessible for anti-CD9 MAb binding. These results demonstrate that tetraspan complexes detected in BMDC supernatants are associated with membrane-derived vesicles.
Detection of CD9 was not affected by the filtration of supernatants from 48-h BMDC cultures through 0.22-μm filters, suggesting that CD9 is expressed in vesicles <200 nm in size. Filtration through 0.22-μm filters of supernatants from 72-h cultures, however, resulted in a slightly reduced detection of CD9, likely due to the onset of spontaneous apoptosis (8) and the generation of large apoptotic bodies (>200 nm). Ultrafiltration of 0.22-μm prefiltered supernatants through 100K MWCO units retained all the CD9-associated material (105% ± 15%). Moreover, the filtrate passing through 100K MWCO filters, once reconcentrated through 5K MWCO filters (that retain monomeric and polymeric CD9) contained negligible amounts of CD9 (Fig. 1C). Thus, CD9 is contained in microvesicles of <200 nm and >100K MWCO (≈5 nm), compatible with the size of exosomes. In summary, these data demonstrate that BMDC actively and constitutively release tetraspan-containing microvesicles, similar in size to exosomes.
BMDC-derived microvesicles contain a component cross-reactive with Cps14.
Upon analysis of the phenotypic composition of BMDC-derived microvesicles we observed that an IgG1 MAb (44.1) specific for the Cps of Pn14, when used as a capture antibody in a sandwich ELISA, bound material that was detected with biotinylated anti-CD9 MAb (Fig. 2). This reactivity, low in crude supernatants, was readily detectable after 10-fold concentration of the supernatants by ultrafiltration through 100K MWCO units (Fig. 2A). Therefore, both CD9 and Cps14-CRA are most likely carried on the same complex. This was further supported by the observation that the concentration of Cps14-CRA-containing material progressively increased with the time of culture (Fig. 2A to C), showing similar kinetics of increase as tetraspan-expressing microvesicles (Fig. 1) and was roughly similar in size (Fig. 2). Culture media alone did not exhibit reactivity with IgG1 anti-Cps14 MAb. Additionally, one IgG1 MAb of unrelated specificity (clone S1-68.1) did not capture detectable CD9 (Fig. 2B). Supernatants from BALB/cJ, C57BL/6N, or B6129PF2/J BMDC cultures all contained Cps14-CRA (data not shown), thus excluding it as an allogeneic antigen.
FIG. 2.
An IgG1 anti-Cps14 MAb captures CD9-containing microvesicles. ELISA plates coated with 5 μg/ml of 44.1 IgG1 MAb specific for the Cps14 of Pn14 were incubated with BMDC supernatants collected at different times during the culture period, and the captured CD9 was detected with 1 μg/ml of biotinylated anti-CD9 MAb. (A) BMDC supernatants were tested directly (crude supernatant), after filtration through 0.22-μm filters, or once concentrated 10-fold in 100K MWCO units by ultrafiltration. (B) In a separate experiment, both the 10-fold 100K MWCO concentrated supernatant and the filtrate from this ultrafiltration were 10-fold reconcentrated in 5K MWCO ultrafiltration units. Wells coated with an IgG1 MAb of unrelated specificity were used as controls. In the same experiment, the concentrated supernatants were treated with 1% Triton X-100 (C) or 0.2% saponin (D) for 30 min at room temperature. OD 405 nm, optical density at 405 nm.
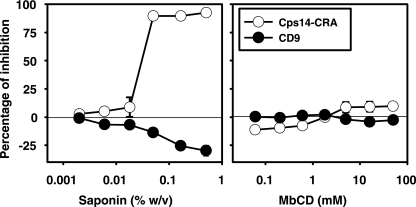
Treatment with 1% Trition X-100 abolished the detection of Cps14-CRA (Fig. 2C), in addition to CD9, in microvesicles (Fig. 1A). However, in contrast to CD9, which remains insoluble by treatment with 0.2% saponin (Fig. 1A), the Cps14-CRA was completely solubilized at this saponin concentration (Fig. 2D), suggesting that the Cps14-CRA is located within the cholesterol-rich lipid microdomains. To explore further the nature of the interaction between Cps14-CRA and cholesterol, we determined the solubility of Cps14-CRA at various concentrations of saponin or MbCD. Saponin is an amphiphilic molecule that solubilizes cholesterol domains by creating micelles. Proteins in proximity to cholesterol may thus become soluble by being incorporated into these micelles (39). In contrast, MbCD removes cholesterol without physically binding to the membranes. Therefore, proteins that require cholesterol for association with membrane microdomains would be expected to become at least partially soluble following MbCD treatment. Saponin treatment completely solubilized the Cps14-CRA at concentrations above 0.02%, with a concomitant increased detection of CD9 (Fig. 3). This is the concentration normally used to permeabilize cell membranes with minimal structural disruption. Interestingly, an increased detection of CD9 was observed at the same concentrations of saponin that solubilized Cps14-CRA (Fig. 3), suggesting that the disruption of the lipid rafts by saponin favors a reorganization of the tetraspan microdomains. In contrast, neither CD9 nor Cps14-CRA was extracted from microvesicle membranes after MbCD treatment (Fig. 3), even at concentrations (50 mM) that are more than 5 to 10 times the concentration used for cholesterol depletion from cell membranes. In summary, these results strongly support the notion that the Cps14-CRA is located in close proximity to, but not directly associated with, the cholesterol of the microvesicle membrane.
FIG. 3.
Detergent solubility of the Cps14-CRA. Supernatants collected at 48 h of BMDC culture were 10-fold concentrated in 100K MWCO filters units and treated with increasing concentrations of saponin or MbCD. Microvesicles were captured in ELISA plates coated with 5 μg/ml of anti-Cps14 IgG1 MAb 44.1 (“Cps14-CRA”) or with anti-CD9 MAb (“CD9”). Microvesicles captured with either MAb were detected with 1 μg/ml of anti-CD9 MAb. Data shown represent the arithmetic means ± SEM of three independent experiments run in duplicate.
Cps14-CRA expression is restricted to myeloid BMDC-derived microvesicles.
The expression of Cps14-CRA in CD9-containing microvesicles from different cell types was determined next. As shown in Fig. 4, only concentrated culture supernatants from BMDC contained significant levels of Cps14-CRA. Thus, depletion of granulocytes (to <0.1%) from BMDC cultures actually increased, to a modest degree, Cps14-CRA detection (Fig. 4). Furthermore, the supernatants of granulocyte-rich cultures such as BM cells cultured for 2 days in GM-CSF, did not contain detectable levels of Cps14-CRA (Fig. 4). This result also suggests that Cps14-CRA is expressed late in BMDC ontogeny. BMM culture supernatants obtained from the same pooled BM cells used to obtain the BMDC, while releasing similar amounts of CD9 as BMDC, contained barely detectable levels of Cps14-CRA (Fig. 4). Similar results were obtained for the supernatants of a BMM cell line (Fig. 4). Thus, although CD9 and Cps14-CRA can be coexpressed on the same vesicle, they are independently expressed. Finally, culture supernatants of a B-cell hybridoma (28.6.20) that releases CD9 microvesicles did not contain Cps14-CRA. In this regard, splenocytes released only modest amounts of Cps14-CRA (Fig. 4), likely due to the presence of a small number of DC. Thus, within the immune cell population, our data suggest that Cps14-CRA may be specifically expressed in DC microvesicles.
FIG. 4.
Detection of Cps14-CRA in supernatants of different cultured cell types. Cell culture supernatants filtered through 0.22-μm filters and concentrated 120-fold in 100K MWCO ultrafiltration units were diluted to pretitered normalized dilutions containing amounts of CD9 microvesicles similar to those of the undiluted supernatants of BMDC cultures at day 7. These dilutions were 12-fold concentrated for the supernatant of the B-cell hybridoma expressing CD9, 6-fold concentrated for the supernatants of BM cells at day 2 of culture in GM-CSF, 2-fold concentrated for the BMM cell line supernatant (BMM cell line), 1.2-fold concentrated for the BM-derived macrophages supernatants at day 7 of the primary culture (BMM, d7), and 1-fold concentrated for the respective BMDC at day 7 of culture. Control culture media and the supernatant from splenocyte cultures were used at the maximal concentration (12-fold concentrated) due to their absent or very low content of CD9-containing microvesicles. Supernatant samples at those dilutions were incubated in ELISA plates coated with anti-CD9 MAb (“anti-CD9 captured”) and at a 10-fold-higher concentration in plates coated with the anti-Cps14 MAb 44.1 (“44.1 captured”). The captured microvesicles were detected in both ELISAs by incubation with biotinylated anti-CD9 MAb. All supernatants were collected after 48 h of cell culture, except for the supernatant of the B-cell hybridoma (28.6.20) that was collected after 4 days of culture. Bars represent the arithmetic means ± SEM of the optical density at 405 nm (OD405) obtained in the triplicate samples in one of three independent experiments.
Cps14-CRA is likely present in glycoconjugates containing β1→6 branched N-acetyl-lactosamine in their glycosidic moiety.
To better define the biochemical nature of Cps14-CRA, we compared the ability of MAb 44.1 to capture BMDC CD9-containing microvesicles with two other IgM(κ) anti-Cps14 MAbs (23.1 and 17.1) as well as IgM(κ) MAb 8F11.1 specific for the Cps of Neisseria meningitidis group A and an IgM(λ) MAb specific for dextran (clone MOPC-104E). The fine specificity of the anti-Cps14 MAbs has been described by A. H. Lucas (personal communication). Both IgM anti-Cps14 MAbs and the IgG1 MAb 44.1 interact, with different avidities, with N-acetyl-lactosamine (much lower for 23.1) but not with melibiose. MAb 44.1, but not MAb 23.1 or 17.1, also bound lactose and aminophenyl-lactoside. To minimize the effects, in the ELISA, of avidity and fine specificity of the various MAbs on the capture of Cps14-CRA, all Cps14-specific MAbs were used at concentrations that captured similar amounts of soluble biotinylated Cps14 (Fig. 5A). As shown in Fig. 5B, the IgM MAb 17.1 and IgG1 MAb 44.1 were equally effective at capturing CD9 microvesicles, whereas MAb 23.1 was clearly less efficient. The two control IgM MAbs, 8F11.1 and MOPC-104E, failed to capture any CD9-containing material (Fig. 5B). This profile of reactivity, which coincides with their reactivity with N-acetyl-lactosamine (A. H. Lucas, personal communication), strongly suggests that Cps14-CRA is contained within the N-acetyl-lactosamine branches of the glycoconjugate present in BMDC-derived microvesicles.
FIG. 5.
Specificity of the interaction between MAbs specific for the Cps14 of Pn14 and microvesicles derived from BMDC in culture. Purified IgG1 (44.1) or IgM (17.1 and 23.1) MAbs specific for Cps14 and two control IgM MAbs, one specific for the Cps of Neisseria meningitidis group A (“anti-CpsA,” 8F11.1) and the other specific for dextran (“antidextran,” clone MOPC-104E), were captured in wells precoated with anti-IgG1 or anti-IgM antibodies, and then incubated with 40 ng/ml of biotinylated Cps14 (A), 10-fold concentrated BMDC supernatant (B), or 10-fold concentrated BMDC supernatants supplemented with purified Cps14 (C). Captured biotinylated Cps14 was directly detected (A), and the CD9-containing microvesicles with anti-CD9 were biotinylated (B and C). Data in panels A and B show the arithmetic means of results for triplicate samples ± SEM of the optical density at 450 nm (OD450) obtained in one of three independent representative experiments. Data in panel C show the percentages of inhibition obtained in those three experiments.
The interaction between MAbs specific for the Cps14 and BMDC-derived microvesicles is specific and inhibited by purified Cps14.
We next tested the specificity of the interaction between the anti-Cps14 MAbs and Cps14-CRA by competitive inhibition with soluble purified Cps14. Cps14 inhibited the binding of all three anti-Cps14 MAbs to CD9-containing microvesicles (Fig. 5C). In agreement with their fine specificity (Fig. 5B), the interaction of 17.1 and 44.1 was inhibited by similar amounts of purified Cps14 (47 ng/ml and 57 ng/ml, respectively) (Fig. 5C). In contrast, the interaction of MAb 23.1 was not completely inhibited (Fig. 5B) even at very high concentrations of Cps14 (10 μg/ml), consistent with the lower avidity of MAb 23.1 for Cps14. These results demonstrate that similar motifs are involved in the interaction of the combinatory sites of the anti-Cps14 MAbs with both Cps14 and Cps14-CRA.
BMDC microvesicles containing Cps14-CRA exhibit the reported phenotype of exosomes.
The phenotype of the microvesicles captured by the anti-Cps14 MAbs was next determined. As shown in Fig. 6, microvesicles expressing Cps14-CRA expressed high levels of tetraspans (CD9, CD81, and CD53), integrins and adhesion molecules (CD11c, CD11b, CD11a, and CD54), molecules involved in antigen presentation (MHC-II, MHC-I, and CD1d), receptors for antigen adsorptive uptake (DEC-205), and costimulatory molecules (CD40, CD80, and CD86). Furthermore, microvesicles derived from BMDC stimulated with LPS contained higher levels of some DC markers, particularly those involved in antigen presentation (Fig. 6B). This microvesicle phenotype is similar to that of the BMDC surface. However, FcγRII/III and CD44, which are highly expressed on the BMDC surface, were barely detectable on the microvesicles (Fig. 6A), whereas CD71, a marker of vesicles of endosomal origin, was expressed (Fig. 6A). A potential significant contribution of cell types other than BMDC was ruled out by the failure to detect Gr-1 (Fig. 6A), CD8α, B220, CD14, or TER-119 (Ly-76) (data not shown). These results strongly suggest that microvesicles containing Cps14-CRA are derived from the endosomal compartment of the BMDC, not directly from the plasma membrane, and thus are exosomes.
FIG. 6.
Phenotype of BMDC-derived microvesicles. BMDC culture supernatants collected at day 7 of the primary culture (A) or once washed and cultured for 48 h in the absence (media) or in presence of 20 ng/ml of LPS (LPS) (B) were concentrated 10-fold by ultrafiltration in 100K MWCO filter units. Microvesicles were captured in 44.1-coated ELISA plates and detected with MAbs specific for the cell markers indicated on the figure. The plates were developed when CD9 expression reached an optical density at 405 nm (OD405) of 2.0 to normalize the content of microvesicles in the different samples. The data shown are the arithmetic means ± SEM of three separate experiments (A) or the duplicates of one experiment (B).
BMDC-derived Cps14-CRA is expressed on exosomes.
To confirm that Cps14-CRA is indeed expressed within exosomes, we purified exosomes from BMDC culture supernatants by differential ultracentrifugation. As shown in Table 1, Cps14-CRA was not detected in the pellet obtained after ultracentrifugation at 10,000 × g, which contained microvesicles or plasma membrane fragments of greater size than exosomes. Furthermore, Cps14-CRA was even more enriched by the purification of exosomes than total CD9. Thus, 68% ± 8% of the Cps14-CRA versus 45% ± 2% of the CD9 contained in the initial BMDC culture supernatant was collected in the exosomal fraction, whereas 18% ± 4% of Cps14-CRA versus 40% ± 3% CD9 remained soluble following the last (100,000 × g) ultracentrifugation, suggesting that part of the total CD9 detected is contained on microvesicles of higher flotation or lower size than exosomes. As a result of this reduced content of CD9+ Cps14-CRA− microvesicles in purified exosomes, the content of the detected Cps14-CRA increased from 1.8% ± 0.4% in the initial culture supernatant up to 12% ± 3% of the CD9-associated total protein in the purified exosomes. Purified exosomes from a BMM cell line were also enriched in Cps14-CRA with respect to the initial BMM culture supernatant, but the amount remained very low in comparison with BMDC exosomes (data not shown). These results demonstrate that Cps14-CRA is expressed mostly in exosomes derived from BMDC.
TABLE 1.
Cps14-CRA copurification with exosomes
| Sample source | % Recovery froma:
|
|
|---|---|---|
| CD9 | Cps14-CRA | |
| Retentate (0.22-μm filter) | 0.6 ± 0.2 | 0.2 ± 0.1 |
| Pellet (10,000 × g) | 1.4 ± 0.4 | 2.1 ± 0.5 |
| Supernatant (100,000 × g) | 40.6 ± 3 | 18.1 ± 4 |
| Pellet (100,000 × g, exosomes) | 45.1 ± 2 | 68.6 ± 8 |
The CD9 or Cps14-CRA content in the 10-fold-concentrated sample of the starting clarified supernatant (mean ± SEM of three independent purifications) was considered 100% recovery.
BMDC-derived exosomes induced Cps14-specific Ig responses in vivo and specifically protected against a lethal challenge with Pn14.
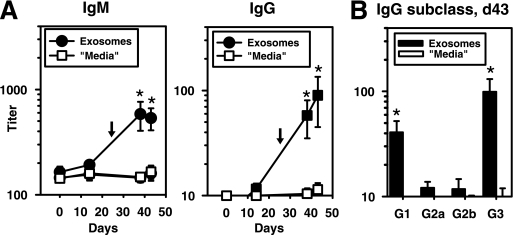
In a final set of experiments, we wished to determine whether the injection of exosomes could induce Cps14-specific Ig responses in naive recipients and protect mice from an otherwise lethal challenge with Pn14. As illustrated in Fig. 7A, BALB/c mice immunized with CFA on day 0 followed by injection 24 days later with 25 μg of purified exosomes in PBS elicited an IgM and IgG response specific for Cps14. IgM anti-Cps14 responses induced by exosomes peaked earlier relative to IgG. This IgG response was restricted to IgG3 (P = 0.03) and IgG1 (P = 0.002) (Fig. 7B). No detectable cross-reaction with Cps3 was observed (data not shown). Using the IgG1 anti-Cps14 MAb as a standard, the serum concentration of anti-Cps14 IgG1 induced by the injection of exosomes was ≈60 ng/ml. Since the injected exosomes were derived from BMDC never exposed to S. pneumoniae, these results demonstrate that exosomes can induce Ig isotype responses to exosome-associated antigens.
FIG. 7.
Cps14-specific Ig isotype responses induced by exosomes. Kinetics of the IgM and IgG anti-Cps14 response (A) or Cps14-specific IgG subclasses (B) at day 43 induced in mice immunized i.p. with an emulsion of CFA and PBS at day 0 and 24 days later (indicated by arrows) injected intravenously with 25 μg of BMDC exosomes (“exosomes”) or an equal volume of the material collected after the ultracentrifugation of fresh culture media following an identical protocol as for the purification of exosomes (“media”). The data shown are the geometric means ± SEM.
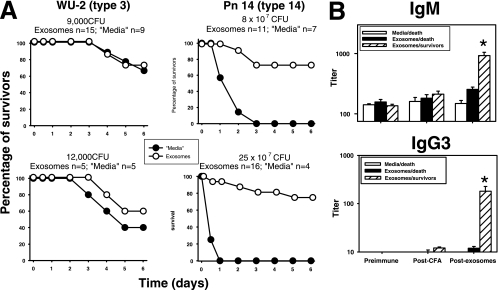
Twenty days after the injection of exosomes, BALB/c mice were challenged i.p. with a relatively high (25 × 107 CFU; ≈10 LD50) or low (8 × 107 CFU; ≈3 LD50) lethal dose of Pn14. All naive mice died within the first 12 to 24 h after challenge with the high bacterial dose, and mice receiving the low bacterial dose had slightly delayed (24 to 48 h) kinetics (Fig. 8A). In contrast, 75% of the mice injected with exosomes survived regardless of the bacterial dose used, and in the remaining mice, the death and symptoms of bacteremia were markedly delayed relative to naive mice (Fig. 8A). No further deaths were recorded over the next 15 days. In contrast, no protection was conferred by exosomes following infection with 9,000 to 12,000 CFU (≈1 LD50) of Pn3 (Fig. 8A). Cps3 is structurally unrelated to Cps14 (18). Exosome-elicited titers of anti-Cps14 IgM and IgG3, but not IgG1, correlated with protection against Pn14. Thus, serum titers of anti-Cps14 IgM in mice that survived were five times higher than in those that died (P = 0.002), whereas serum IgG3 titers in the surviving mice were 15-times higher (P = 0.01) than in those that died. Sera from survivors contained significantly higher anti-Cps14 IgM and IgG3 titers after injection with exosomes than mice receiving CFA alone (IgM, P = 0.0007; IgG3, P = 0.015) or preimmune sera (IgM, P = 0.0006; IgG3, P = 0.003). In contrast, sera from mice that succumbed to Pn14 infection did not significantly increase their IgM or IgG3 anti-Cps14 levels following the injection of exosomes (P > 0.07). Thus, these results strongly suggest that exosomes can induce an Ig-mediated, protective response specific for Pn14.
FIG. 8.
Protection conferred by the injection of BMDC-derived exosomes. (A) BALB/c mice immunized as indicated in the legend to Fig. 7 were challenged i.p. with 9,000 CFU or 12,000 CFU of strain WU-2 of Pn3 or 8 × 107 CFU or 2.5 × 108 CFU of Pn14 20 days after being injected with exosomes or control media. The number of mice per group injected with exosomes or “media” is indicated in each graph. Data shown are from three independent experiments. (B) Geometric means ± SEM of IgM and IgG3 anti-Cps14 titers in the sera of mice at day 0 (preimmune), at day 14 (post-CFA), and at day 43 (postexosomes), 1 day before the mice received the challenge with 25 × 107 CFU of Pn14. These mice are the same shown in the lethality studies for this bacterial dose (A). IgM and IgG3 titers of the survivors and animals that died after the bacterial challenge were considered separately to determine the potential association between protection and anti-Cps14 Ig responses induced by BMDC-derived exosomes.
DISCUSSION
DC exosomes are formed within late multivesicular endosomal compartments where exogenous antigens are processed for their further presentation on the DC surface (10, 42). Thus, exosomes can be loaded with processed antigens during their biogenesis. This led to the hypothesis that exosomes released by DC could act in vivo as efficient stimulatory vehicles for antigen delivery to distant cells. This immunostimulatory role of exosomes has been thought to be restricted to the priming of antigen-specific T-cell responses (3, 5, 41), as suggested by their expression of high levels of MHC and T-cell costimulatory molecules (35, 40). Nevertheless, we recently demonstrated that exosomes containing a protein (diphtheria toxoid) processed to serologically undetectable levels can induce primary IgM and IgG anti-diphtheria toxoid responses in a naive recipient in vivo (7), biased towards type 1 IgG isotypes. Thus, exosomes can also activate antigen-specific B cells when the protein is mostly presented as processed peptides complexed with MHC molecules. However, whether autologous or unprocessed antigens not associated with MHC on the surface of the exosome can induce Ig responses has remained unknown. In this report, we describe a novel antigen endogenously expressed on the surface of BMDC-derived exosomes that cross-reacts with the Cps14 of S. pneumoniae (Cps14-CRA) and demonstrate that, indeed, exosomes can elicit Ig responses specific for cross-reactive autologous antigen during an inflammatory response in vivo.
The newly identified Cps14-CRA is expressed mainly, if not exclusively, in exosomes derived from antigen-presenting cells of myeloid origin. Cps14-CRA was only detected at relatively high levels in exosomes derived from BMDC, with much lower levels in those derived from BM macrophages. Cps14-CRA was absent from granulocyte- and B-cell-derived microvesicles. Of note, modest amounts of Cps14-CRA were detected in microvesicles from freshly explanted splenocytes, mostly composed of B and T cells, suggesting that DC-derived exosomes in vivo express Cps14-CRA. However, since DC are a minor cell population, the Cps14-CRA content in naive mice in vivo is anticipated to be at trace levels.
Cps14-CRA expression is largely restricted to exosomes. Thus, microvesicles containing the Cps14-CRA expressed high levels of tetraspans, MHC, integrins, and costimulatory and adhesion molecules characteristic of exosomes (9, 10, 35, 40) but did not contain CD44 or FcγRII/III expressed at high levels at the BMDC surface. Furthermore, we observed an enrichment of Cps14-CRA during the purification of exosomes, a process that excludes larger cell membrane-derived vesicles. In fact, we were unable to detect Cps14-CRA at the surface of the intact BMDC by fluorescence-activated cell sorter analysis (6). Therefore, Cps14-CRA is likely produced within exosomes during their biogenesis in late endosomal compartments.
Intact exosomes captured with Cps14-specific MAbs can be detected with antitetraspan Igs, suggesting that Cps14-CRA is expressed at the exosome surface. Further, solubilization of the exosomes membrane with Triton X-100 abrogated Cps14-CRA detection, suggesting that it is directly inserted into the membrane. However, in contrast to the tetraspan CD9, Cps14-CRA is completely removed by saponin, indicating that it is located within the cholesterol-rich microdomains or rafts (9, 37), segregated from the tetraspan-enriched microdomains (22). The anchoring of Cps14-CRA in the raft does not directly depend on cholesterol, as suggested by Cps14-CRA insolubility in MbCD. Thus, Cps14-CRA is unlikely to be a proteolipid, since these require cholesterol for insertion into the membrane and, therefore, are very efficiently solubilized by MbCD (30). However, it has also been suggested that, at least within the cell membrane, there is a subtle degree of order within rafts, such that MbCD cannot extract cholesterol from the raft core but can do so from lower-ordered domains of the glycerophospholipid bilayer (16). Thus, Cps14-CRA could be located in the raft core of the exosomes in close proximity to MHC.
The precise identity and nature of Cps14-CRA cannot be directly determined from the presented data, but it is clearly a glycoconjugate, as suggested by its cross-reactivity with Cps14. The mapping, with MAbs, of this specificity strongly suggests that the cross-reactive epitope is formed by β1→6 branched N-acetyl-lactosamine. Thus, the avidity of the interaction of the MAbs specific for Cps14 with exosomes paralleled that shown for their interaction with N-acetyl-lactosamine. The MAbs 44.1 and 17.1 were equally effective at capturing exosomes, despite the fact that only 44.1 interacts with lactose and that neither interacts with melibiose (Galα1→6Glc), suggesting that both type of linkage and the N-acetyl group are critical parts of the cross-reactive epitope. Moreover, a MAb specific for dextran, poly(α1→6Glc), did not interact with the Cps14-CRA.
This Cps14 cross-reactive epitope likely represents a posttranslational modification. Thus, posttranslational substitution with β1→6 branched N-acetyl-lactosamine and poly-N-lactosaminoglycans is a relatively common phenomenon that is thought to modulate the structural and functional properties of the carrier molecule (21). We were unable to detect Cps14-CRA by sandwich ELISA using the same Cps14-specific antibody for capture and detection, suggesting that the epitope in Cps14-CRA is not multimeric, and that Cps14 is expressed at low levels in exosomes.
Interestingly, the Cps14-CRA epitope closely resembles the motif recognized by some galectins, a highly conserved family of β-galactoside-binding animal lectins, particularly galectin-3 (15, 38). Specific interaction of a galectin from chicken liver (CG-16) and Cps14 has been reported (44). Of interest, galectin-3 has been identified by proteomic analysis to be located within DC exosomes (40). This suggests the possibility that galectins could interact with Cps14-CRA within the exosomes and therefore significantly mask the epitope recognized by the anti-Cps14 MAbs. This could explain, at least in part, the low level of Cps14-CRA detected in the exosomes with anti-Cps14 MAbs. Although the role played by exosome-associated galectins is unknown, galectins acting at the cell surface have been reported to exert multiple specialized functions that may exert both positive and negative effects on immunity, including a role as a critical mediator of B-cell differentiation and survival (1, 31-33). The binding of Cps14-CRA to galectins within exosomes could potentially modulate these galectin-mediated effects.
Cps14-CRA expression is low and confined to exosomes of DC. Therefore, its content in the body is likely minute, perhaps precluding the induction of tolerance to this epitope. The injection of relatively high amounts of Cps14-CRA in the form of purified exosomes following an inflammatory stimulus mediated by CFA likely accounts for the induction of immunity to this epitope. Inflammatory responses are associated with both autoimmune disorders and immune responses to pathogens, and this promotes the activation of host DC. During a similarly induced inflammatory response, we demonstrated that the injection of exosomes containing diphtheria toxoid processed to serologically undetectable levels induces a primary IgM and IgG anti-diphtheria toxoid response in vivo, biased towards type 1 IgG isotypes (IgG2b and IgG2a), in contrast to the preferential type 2 isotype IgG1, induced by intact diphtheria toxoid (7). In contrast, the anti-Cps14-CRA responses induced by exosomes were mostly IgM and IgG3, with a minor contribution of IgG1 and barely, if any, IgG2a/IgG2b response. This latter Ig isotype profile is the same as that elicited in response to many purified bacterial polysaccharides and other thymus-independent type 2 antigens in which cognate T-cell help is not involved (27). Thus, it is likely that exosomes, in the absence of exogenous antigen, were directly activating Cps14-specific B cells in a T-cell-independent manner, whereas the presence of processed diphtheria toxoid in association with MHC additionally recruited T-cell help.
The cross-reactive Ig responses induced by exosomes were Cps14 specific and conferred protection against a further challenge with live Pn14. Exosomes neither induce Cps3-specific Ig responses nor confer protection against challenge with a low dose of live Pn3. Furthermore, protection was associated with the levels of serum Cps14 cross-reactive IgM and IgG3, but not IgG1, induced by the exosomes. Protection against invasive infections with S. pneumoniae is largely correlated with the opsonic activity of antibacterial polysaccharide-specific Ig (2, 4). The critical value of antipolysaccharide IgG3 in protection against fatal S. pneumoniae sepsis has been demonstrated in mice genetically deficient in IgG3 (26). IgG3 was shown to be particularly important in protection when low IgG1 responses were induced by bacterial polysaccharide immunization (26), similar to the case in which the Cps14-specific responses were induced by exosomes.
In summary, these results demonstrate, for the first time, that exosomes can induce a humoral immune response to an associated unprocessed, autologous antigen. Although anti-Cps14 Ig responses are specifically demonstrated, these could reflect a broader mechanism that modulates both natural immunity and autoimmunity to other glycotopes.
Acknowledgments
This work was supported by NIH grant 1R01 AI49192.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Acosta-Rodriguez, E. V., C. L. Montes, C. C. Motran, E. I. Zuniga, F. T. Liu, G. A. Rabinovich, and A. Gruppi. 2004. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 172:493-502. [DOI] [PubMed] [Google Scholar]
- 2.Alonso De Velasco, E., B. A. Dekker, A. F. Verheul, R. G. Feldman, J. Verhoef, and H. Snippe. 1995. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J. Infect. Dis. 172:562-565. [DOI] [PubMed] [Google Scholar]
- 3.Andre, F., N. Chaput, N. E. Schartz, C. Flament, N. Aubert, J. Bernard, F. Lemonnier, G. Raposo, B. Escudier, D. H. Hsu, T. Tursz, S. Amigorena, E. Angevin, and L. Zitvogel. 2004. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172:2126-2136. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., C. Forman, S. Hudak, and J. L. Claflin. 1984. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J. Mol. Cell. Immunol. 1:305-309. [PubMed] [Google Scholar]
- 5.Chaput, N., N. E. Schartz, F. Andre, J. Taieb, S. Novault, P. Bonnaventure, N. Aubert, J. Bernard, F. Lemonnier, M. Merad, G. Adema, M. Adams, M. Ferrantini, A. F. Carpentier, B. Escudier, T. Tursz, E. Angevin, and L. Zitvogel. 2004. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J. Immunol. 172:2137-2146. [DOI] [PubMed] [Google Scholar]
- 6.Colino, J., Y. Shen, and C. M. Snapper. 2002. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 195:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colino, J., and C. M. Snapper. 2006. Exosomes from bone-marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J. Immunol. 177:3757-3762. [DOI] [PubMed] [Google Scholar]
- 8.Colino, J., and C. M. Snapper. 2003. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 171:2354-2365. [DOI] [PubMed] [Google Scholar]
- 9.de Gassart, A., C. Geminard, B. Fevrier, G. Raposo, and M. Vidal. 2003. Lipid raft-associated protein sorting in exosomes. Blood 102:4336-4344. [DOI] [PubMed] [Google Scholar]
- 10.Escola, J. M., M. J. Kleijmeer, W. Stoorvogel, J. M. Griffith, O. Yoshie, and H. J. Geuze. 1998. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273:20121-20127. [DOI] [PubMed] [Google Scholar]
- 11.Fedson, D. S., D. M. Musher, and J. Eskola. 1999. Pneumococcal vaccine, p. 553-608. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines. WB Saunders Company, Philadelphia, Pa.
- 12.Gonzalez-Outeirino, J., R. Kadirvelraj, and R. J. Woods. 2005. Structural elucidation of type III group B Streptococcus capsular polysaccharide using molecular dynamics simulations: the role of sialic acid. Carbohydr. Res. 340:1007-1018. [DOI] [PubMed] [Google Scholar]
- 13.Guttormsen, H. K., C. J. Baker, M. H. Nahm, L. C. Paoletti, S. M. Zughaier, M. S. Edwards, and D. L. Kasper. 2002. Type III group B streptococcal polysaccharide induces antibodies that cross-react with Streptococcus pneumoniae type 14. Infect. Immun. 70:1724-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha, C. T., R. Waterhouse, J. Wessells, J. A. Wu, and G. S. Dveksler. 2005. Binding of pregnancy-specific glycoprotein 17 to CD9 on macrophages induces secretion of IL-10, IL-6, PGE2, and TGF-beta1. J. Leukoc. Biol. 77:948-957. [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi, J., T. Hashidate, Y. Arata, N. Nishi, T. Nakamura, M. Hirashima, T. Urashima, T. Oka, M. Futai, W. E. Muller, F. Yagi, and K. Kasai. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572:232-254. [DOI] [PubMed] [Google Scholar]
- 16.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335(Pt 2):433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, C. 1985. Identification of the tetrasaccharide repeating-unit of the Streptococcus pneumoniae type 23 polysaccharide by high-field proton n.m.r. spectroscopy. Carbohydr. Res. 139:75-83. [DOI] [PubMed] [Google Scholar]
- 19.Kasper, D. L., C. J. Baker, R. S. Baltimore, J. H. Crabb, G. Schiffman, and H. J. Jennings. 1979. Immunodeterminant specificity of human immunity to type III group B streptococcus. J. Exp. Med. 149:327-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, S. H., E. R. Lechman, N. Bianco, R. Menon, A. Keravala, J. Nash, Z. Mi, S. C. Watkins, A. Gambotto, and P. D. Robbins. 2005. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 174:6440-6448. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan, V., S. M. Bane, P. D. Kawle, K. N. Naresh, and R. D. Kalraiya. 2005. Altered melanoma cell surface glycosylation mediates organ specific adhesion and metastasis via lectin receptors on the lung vascular endothelium. Clin. Exp. Metastasis 22:11-24. [DOI] [PubMed] [Google Scholar]
- 22.Kropshofer, H., S. Spindeldreher, T. A. Rohn, N. Platania, C. Grygar, N. Daniel, A. Wolpl, H. Langen, V. Horejsi, and A. B. Vogt. 2002. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat. Immunol. 3:61-68. [DOI] [PubMed] [Google Scholar]
- 23.Laulagnier, K., C. Motta, S. Hamdi, S. Roy, F. Fauvelle, J. F. Pageaux, T. Kobayashi, J. P. Salles, B. Perret, C. Bonnerot, and M. Record. 2004. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindberg, B., J. Lonngren, and D. A. Powell. 1977. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr. Res. 58:177-186. [DOI] [PubMed] [Google Scholar]
- 25.Lucas, A. H., K. D. Moulton, V. R. Tang, and D. C. Reason. 2001. Combinatorial library cloning of human antibodies to Streptococcus pneumoniae capsular polysaccharides: variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect. Immun. 69:853-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLay, J., E. Leonard, S. Petersen, D. Shapiro, N. S. Greenspan, and J. R. Schreiber. 2002. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J. Immunol. 168:3437-3443. [DOI] [PubMed] [Google Scholar]
- 27.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 27a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 28.Obaro, S. K. 2002. The new pneumococcal vaccine. Clin. Microbiol. Infect. 8:623-633. [DOI] [PubMed] [Google Scholar]
- 29.Peche, H., M. Heslan, C. Usal, S. Amigorena, and M. C. Cuturi. 2003. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 76:1503-1510. [DOI] [PubMed] [Google Scholar]
- 30.Pitha, J., T. Irie, P. B. Sklar, and J. S. Nye. 1988. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 43:493-502. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovich, G. A. 1999. Galectins: an evolutionarily conserved family of animal lectins with multifunctional properties; a trip from the gene to clinical therapy. Cell Death Differ. 6:711-721. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovich, G. A., and A. Gruppi. 2005. Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of the disease. Parasite Immunol. 27:103-114. [DOI] [PubMed] [Google Scholar]
- 33.Rabinovich, G. A., N. Rubinstein, and M. A. Toscano. 2002. Role of galectins in inflammatory and immunomodulatory processes. Biochim. Biophys. Acta 1572:274-284. [DOI] [PubMed] [Google Scholar]
- 34.Schuck, S., M. Honsho, K. Ekroos, A. Shevchenko, and K. Simons. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA 100:5795-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segura, E., C. Nicco, B. Lombard, P. Veron, G. Raposo, F. Batteux, S. Amigorena, and C. Thery. 2005. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106:216-223. [DOI] [PubMed] [Google Scholar]
- 36.Sieckmann, D. G., E. Martin, G. Guelde, D. L. Longo, and J. J. Kenny. 1997. Anti-idiotype monoclonal antibodies specific for the MOPC167 anti-phosphocholine transgene-encoded antibody. Hybridoma 16:503-511. [DOI] [PubMed] [Google Scholar]
- 37.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 38.Sparrow, C. P., H. Leffler, and S. H. Barondes. 1987. Multiple soluble beta-galactoside-binding lectins from human lung. J. Biol. Chem. 262:7383-7390. [PubMed] [Google Scholar]
- 39.Taylor, C. M., T. Coetzee, and S. E. Pfeiffer. 2002. Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. J. Neurochem. 81:993-1004. [DOI] [PubMed] [Google Scholar]
- 40.Thery, C., M. Boussac, P. Veron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena. 2001. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166:7309-7318. [DOI] [PubMed] [Google Scholar]
- 41.Thery, C., L. Duban, E. Segura, P. Veron, O. Lantz, and S. Amigorena. 2002. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3:1156-1162. [DOI] [PubMed] [Google Scholar]
- 42.Thery, C., L. Zitvogel, and S. Amigorena. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2:569-579. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 1999. Pneumococcal vaccines WHO position paper. Wkly. Epidemiol. Rec. 74:177-184. [PubMed] [Google Scholar]
- 44.Wu, A. M., J. H. Wu, M. S. Tsai, H. Kaltner, and H. J. Gabius. 2001. Carbohydrate specificity of a galectin from chicken liver (CG-16). Biochem. J. 358:529-538. [DOI] [PMC free article] [PubMed] [Google Scholar]