Abstract
Mycoplasma pneumoniae is a leading cause of pneumonia and is associated with asthma. Evidence links M. pneumoniae respiratory disease severity with interleukin-12 (IL-12) concentration in respiratory secretions. We evaluated the microbiologic, inflammatory, and pulmonary function indices of M. pneumoniae pneumonia in IL-12 (p35) knockout (KO) mice and wild-type (WT) mice to determine the role of IL-12 in M. pneumoniae respiratory disease. Eight-week-old wild-type BALB/c mice and 8-week-old IL-12 (p35) KO BALB/c mice were inoculated once intranasally with 107 CFU of M. pneumoniae. Mice were evaluated at days 2, 4, and 7 after inoculation. Outcome variables included quantitative bronchoalveolar lavage (BAL) M. pneumoniae culture, lung histopathologic scores (HPS), BAL cytokine concentrations determined by enzyme-linked immunosorbent assay (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, and granulocyte-macrophage colony-stimulating factor) and plethysmography, before and after methacholine, to assess airway obstruction (AO) and airway hyperreactivity (AHR). IL-12 (p35) KO mice infected with M. pneumoniae were found to have significantly lower BAL M. pneumoniae concentrations compared with M. pneumoniae-infected WT mice. Lung HPS and the parenchymal pneumonia subscores (neutrophilic alveolar infiltrate), as well as AO, were significantly lower in infected KO mice. No difference was found for AHR. Infected KO mice had significantly lower BAL concentrations of IFN-γ than WT mice; a trend toward lower BAL concentrations was observed for IL-10 (P = 0.065) and TNF-α (P = 0.078). No differences were found for IL-1β, IL-2, IL-4, IL-5, or IL-6. The lack of IL-12 in experimental M. pneumoniae pneumonia was associated with less severe pulmonary disease and more rapid microbiologic and histologic resolution.
For decades Mycoplasma pneumoniae has been recognized as a common etiologic agent of acute lower respiratory infection accounting for 20 to 30% of community-acquired pneumonia in the general population (3, 6, 32, 47). More recently M. pneumoniae respiratory infection has been associated with reactive airway disease and asthma (27). While the clinical significance of Mycoplasma respiratory infections is becoming clearer, the involved immunopathogenesis remains to be understood.
A critical process of the innate immune response is the differentiation of naïve CD4+ T cells into Th1 and Th2 effector cells. In general, the stimulation of Th1 cells is promoted as a response to intracellular pathogens, while Th2 cells are protective against extracellular microorganisms and are increased in allergic asthmatic patients. Even though evidence suggests a direct association between allergic asthma and Th2 cytokines, a recent study suggests otherwise for asthma associated with infection (42). In a murine model of Mycoplasma respiratory infection, the absence of interleukin-4 (IL-4), a key Th2 cytokine, resulted in exacerbated airway obstruction (AO) and airway hyperreactivity (AHR), instead of being protective (42). In addition, other in vivo studies have shown that M. pneumoniae infection induces a Th1 cytokine response in the lungs with associated AO, AHR, and lung inflammation (12, 17).
As IL-4 has a key role in Th2 signaling, IL-12 has a pivotal role in the Th1-mediated immune response. IL-12 is a heterodimeric cytokine that is composed of two subunits (p40 and p35) and is produced by antigen-presenting cells (phagocytes and dendritic cells) and targets natural killer and the T cells inducing the production of gamma interferon (IFN-γ; the Th1 effector cytokine) (15, 26, 37). To evaluate the role of IL-12 in the pathogenesis of M. pneumoniae respiratory infection, we studied the effect of IL-12 deletion on airway dysfunction and inflammation using IL-12 (p35) knockout animals in our established murine model of M. pneumoniae pneumonia (12, 13, 17, 18).
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after 24 to 48 h in a flask containing 20 ml of SP4 medium at 37°C. Approximately 72 h later, the broth turned an orange hue, the supernatant was decanted, and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 108 to 109 CFU/ml (determined by plating dilutions on SP4 agar). Aliquots were stored at −80°C. All SP4 media contained nystatin (50 units/ml) and ampicillin (1.0 mg/ml) to inhibit growth of potential contaminants.
Animals and inoculation.
Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee. Mice were obtained from commercial vendors [BALB/c mice from Charles River Laboratories and IL-12 (p35) knockout (KO) mice with BALB/c background from Jackson Laboratories], who confirmed their mycoplasma- and murine virus-free status. Mice were housed in the animal care facility of our institution in filter-top cages and allowed to acclimate to their new environment for 1 week. Isofluorane, an inhaled anesthetic, was used for sedation during inoculation. Two-month-old female wild-type (WT) and IL-12 KO mice were intranasally inoculated once (day 0) with 107 CFU of M. pneumoniae in 50 μl of SP4 broth. Control mice (WT and IL-12 KO) were inoculated with sterile SP4 broth. All mice were housed in the same animal room and received identical daily care for the duration of the experiment.
Experimental design and sample collection.
Groups of mice were evaluated at 2, 4, and 7 days after inoculation. Samples were obtained from 8 mice per group (the four groups were infected WT, infected IL-12 KO, SP4-inoculated WT, and SP4-inoculated IL-12 KO) at each time point; in addition, whole-body, unrestrained, nonsedated plethysmography was performed at each time point.
Mice were anesthetized with an intraperitoneal injection of 75 mg of ketamine per kg of body weight and 5 mg of acepromazine per kg before cardiac puncture. Bronchoalveolar lavage (BAL) specimens were obtained by infusing 500 μl of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Whole-lung specimens (including the trachea and both lungs) were collected and fixed with a 10% buffered formalin solution for histologic evaluation.
Culture.
Twenty-five microliters of undiluted sample and serial 10-fold dilutions of BAL fluid in SP4 broth (50 μl of undiluted sample was used for the initial dilution) were immediately cultured on SP4 agar plates at 37°C, while the remaining undiluted BAL fluid specimens were stored at −80°C. Quantification was performed by counting colonies on plated specimens, and results are expressed as log10 CFU per milliliter.
Histopathology.
Lung tissue was fixed in buffered formalin, and whole-mount sections of paraffin-embedded lungs were stained with hematoxylin and eosin. The histopathologic scores (HPS) were determined by a single pathologist who was unaware of the infection status and genetic status of the animals from which specimens were taken. The HPS was based on grading of peribronchiolar/bronchial infiltrate (percentage of sites and quality), bronchiolar/bronchial luminal exudate, perivascular infiltrate (percentage of sites), and parenchymal pneumonia (neutrophilic alveolar infiltrate). This HPS system assigned values from 0 to 26: the higher the score, the greater the inflammatory changes in the lung (8).
Plethysmography.
Whole-body, unrestrained, nonsedated plethysmography (Buxco Electronics, Wilmington, NC) was used to monitor the respiratory dynamics of mice in a quantitative manner at baseline (AO) and after methacholine exposure (AHR). Prior to methacholine exposure, mice were allowed to acclimate to the chamber, and then plethysmography readings were recorded to establish baseline values. Next, the mice were exposed to aerosolized methacholine (100 mg/ml); after exposure, plethysmography readings were recorded again. Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh as measured by plethysmography has been previously validated in animal models of AHR (14, 16, 36, 40). AO and AHR were measured in 8 mice per group at 2, 4, and 7 days after inoculation.
Measurement of cytokines in BAL samples.
Concentrations of cytokines in BAL specimens were assessed using Multiplex Bead Immunoassays (BioSource International, Camarillo, CA) in conjunction with a Luminex LabMAP system, following the manufacturer's instructions. The cytokines examined and their levels of sensitivity were as follows: tumor necrosis factor alpha (TNF-α), 5 pg/ml; IFN-γ, 1 pg/ml; IL-1β, 10 pg/ml; IL-2, 15 pg/ml; IL-4, 5 pg/ml; IL-5, 10 pg/ml; IL-6, 10 pg/ml; IL-10, 15 pg/ml, and granulocyte-macrophage colony-stimulating factor, 10 pg/ml.
Statistics.
For statistical analysis, Sigma Stat 2003 software (SPSS Science, San Rafael, CA) was used. Two-way analysis of variance (ANOVA) was used to compare values of the different groups of animals over 2, 4, and 7 days if the data were normally distributed. For nonparametric data, a two-way factorial ANOVA was conducted on the normal scores for the ranked data (this analysis was done by a biostatistician consultant in the University of Texas Southwestern Medical Center Division of Biostatistics). A comparison was considered statistically significant if the P value was <0.05.
RESULTS
Clinical.
By visual inspection, IL-12 KO mice inoculated with M. pneumoniae had less ruffled fur compared with WT infected mice for the first 2 to 3 days after inoculation. They were also slightly more active. There was no other visible difference among the groups.
BAL culture.
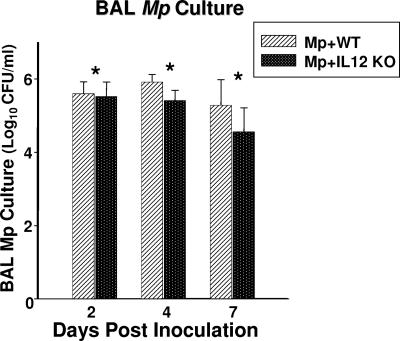
BAL cultures were positive in all mice infected with M. pneumoniae. IL-12 KO mice infected with M. pneumoniae had significantly lower BAL M. pneumoniae concentrations compared with M. pneumoniae-infected WT mice (Fig. 1). All control mice had negative BAL cultures.
FIG. 1.
Quantitative M. pneumoniae (Mp) cultures of BAL fluid samples of WT and IL-12 KO mice inoculated with M. pneumoniae. Values shown are the means ± standard deviations (error bars). Data represent results from two separate experiments with eight mice in each group per time point. *, P = 0.002 between WT and IL-12 KO mice inoculated with M. pneumoniae (two-way ANOVA).
Histopathology.
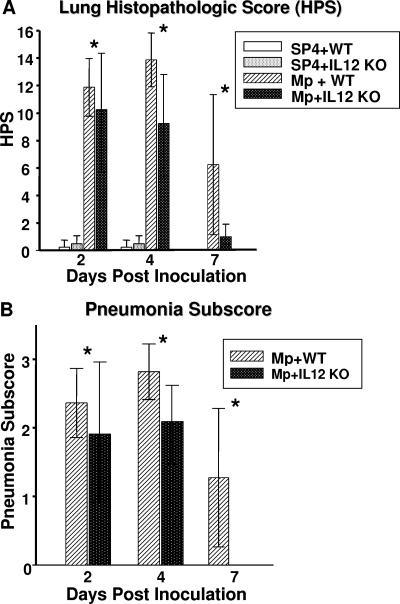
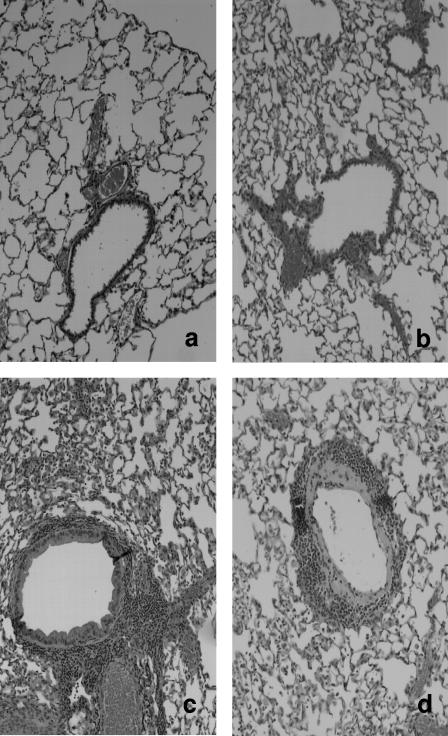
M. pneumoniae-inoculated IL-12 KO mice had significantly lower lung HPS compared with M. pneumoniae-inoculated WT mice (Fig. 2). The parenchymal pneumonia subscore (neutrophilic alveolar infiltrate) of the overall HPS was statistically lower in infected KO mice compared with infected WT mice (Fig. 2). Other subscores of the overall HPS were not significantly different for the infected mice. WT mice and IL-12 KO mice inoculated with SP4 broth did not display signs of histologic lung inflammation (HPS range, 0 to 1). Figure 3 shows the histopathologic appearance of a representative mouse lung inoculated with SP4 broth (WT and IL-12 KO) compared with that of a representative mouse lung inoculated with M. pneumoniae (WT and IL-12 KO) at day 4 of M. pneumoniae infection.
FIG. 2.
Lung HPS and pneumonia subscores of WT and IL-12 KO mice inoculated with M. pneumoniae (Mp) or sterile SP4 broth (controls). Values shown are the means ± standard deviations (error bars). Data represent results from two separate experiments with eight mice in each group per time point. *, P < 0.001 for HPS and P = 0.002 for the pneumonia subscore between WT and IL-12 KO mice inoculated with M. pneumoniae (two-way ANOVA).
FIG. 3.
Comparative histopathological appearance of the lungs of uninfected wild type mice (a; HPS = 0), IL-12 KO mice (b; HPS = 0), M. pneumoniae-infected WT mice (c; HPS = 16) and M. pneumoniae-infected IL-12 KO mice (d; HPS = 9) on day 4 postinoculation. Magnification, ×20.
Plethysmography.
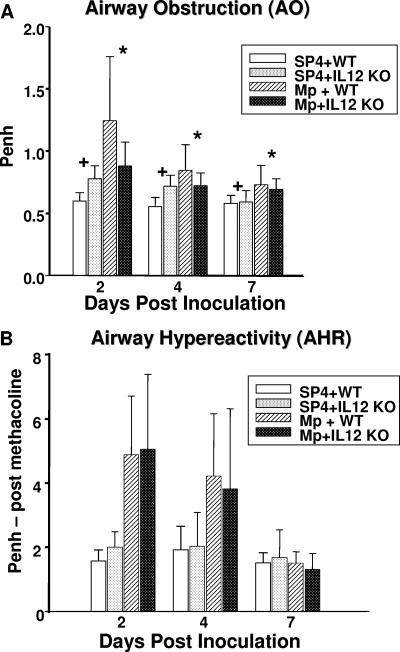
AO as measured by baseline Penh was significantly lower in infected IL-12 KO mice compared with infected WT mice (Fig. 4 A). In contrast, uninfected WT mice displayed significantly less AO than uninfected IL-12 KO mice (Fig. 4). Penh after standardized methacholine challenge (AHR) was not statistically different between KO and WT mice.
FIG. 4.
AO and AHR were assessed by whole-body plethysmography by measuring Penh in WT and IL-12 KO mice inoculated with M. pneumoniae (Mp) or sterile SP4 broth (controls). Values shown are the means ± standard deviations (error bars). Data represent results from two separate experiments with eight mice in each group per time point. *, P = 0.022 between WT and IL-12 KO mice inoculated with M. pneumoniae (two-way ANOVA). +, P < 0.001 between WT and IL-12 KO mice inoculated with SP4 broth (controls) (two-way ANOVA).
BAL fluid cytokines.
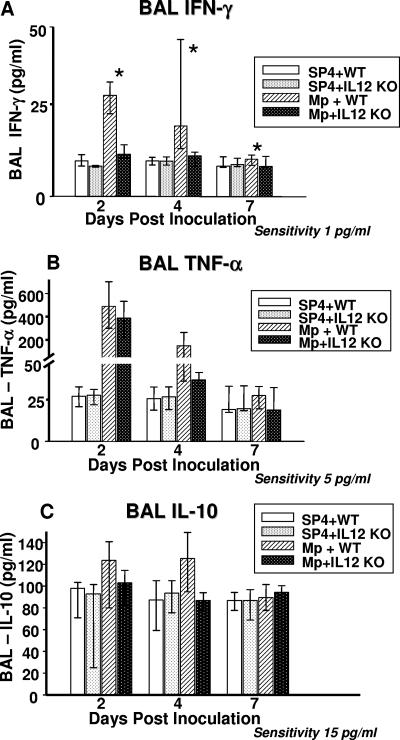
Infected IL-12 KO mice had significantly lower concentrations of IFN-γ in BAL fluid compared with infected WT mice. TNF-α and IL-10 concentrations in BAL samples tended to be lower in the infected IL-12 KO mice compared with the infected WT mice; however, no statistically significant difference was found between the two groups (P = 0.08 and 0.065, respectively) (Fig. 5). No significant differences in the BAL concentrations of IL-1β, IL-2, IL-4, IL-5, IL-6, and granulocyte-macrophage colony-stimulating factor were found in the infected IL-12 KO mice compared with the infected WT mice.
FIG. 5.
Cytokine concentrations in BAL fluid specimens from WT and IL-12 KO mice inoculated with M. pneumoniae (Mp) or sterile SP4 broth (controls). Values shown are the medians and the 25th to 75th percentile (error bars). Data represent results from two separate experiments with eight mice in each group per time point. *, P < 0.001 between WT and IL-12 KO mice inoculated with M. pneumoniae (two-way ANOVA).
DISCUSSION
The Th1 host immune response and, in particular, IL-12 production are considered an essential prerequisite for the protection against intracellular organisms (34, 39). After infection, IL-12 stimulates NK and T cells to produce IFN-γ, which activates macrophage activity and promotes the production of cytotoxic CD8+ lymphocytes (38). Its protective effect is illustrated by the fact that, in mice and humans, deficiency of IL-12 or its receptor increases susceptibility to intracellular pathogens (1, 10, 29, 45, 46).
Much has been learned regarding the role of the innate and adaptive immunity in Mycoplasma respiratory infection (5, 12, 21); however, the immunopathogenic mechanisms of action involved in M. pneumoniae respiratory infection remain to be completely defined (5, 19, 21). Recent studies have shown a significant role of the Th1 cytokines in the pathogenic mechanism of the infection (12, 17, 42, 44). Our purpose in this study was to evaluate the effect of IL-12 deficiency on respiratory disease to better understand immunopathogenesis.
We observed a significantly lower concentration of M. pneumoniae in quantitative BAL cultures from the IL-12 KO mice compared with WT mice. This may indicate that the lack of IL-12 allows more efficient clearing of M. pneumoniae infection than in the infected WT mice. Of note, Woolard et al. demonstrated that in Mycoplasma pulmonis respiratory infection in IFN-γ KO mice, the concentration of Mycoplasma in BAL samples was higher than in the infected WT. However, when NK cells were depleted by anti-asialo GM1 antibody in IFN-γ KO mice, there was a significant reduction in the concentration of Mycoplasma in the BAL culture that was comparable to that of the infected WT mice (44). Based on our results, we speculate that the NK cells in the IL-12 KO mice were only partially stimulated, as IL-12 is a primary trigger for the NK cell activation; this could explain the similar findings of significantly lower amounts of Mycoplasma in the lungs of the IL-12 KO mice and mice depleted of NK cells. To date, the exact mechanism by which M. pneumoniae is cleared more efficiently is unknown.
We were also able to demonstrate that M. pneumoniae respiratory infection in IL-12 KO mice resulted in decreased pulmonary histologic inflammation, particularly, the neutrophilic alveolar infiltrates. Moreover, the lack of IL-12 had a broad and significant effect on the production of pulmonary cytokines. As expected, the production of pulmonary IFN-γ in M. pneumoniae-infected mice was significantly depressed by the absence of IL-12, establishing the validity of our model. A trend for a decrease in the proinflammatory cytokine TNF-α was also noted. In previous studies TNF-α has been directly associated with M. pneumoniae disease severity (12, 13, 17), and its lower concentrations in the BAL fluid may have contributed to the decreased histologic lung inflammation in the IL-12 KO mice. We also found a trend for a decrease in the anti-inflammatory cytokine IL-10 in the IL-12 KO mice; this could be explained by the fact that IL-10 is a known negative feedback regulator of IL-12 production from phagocytic and dendritic cells (50). For this reason, in our study the lack of IL-12 in the KO mice likely had the result that the autoregulatory inhibitory mechanism of IL-10 was not engaged.
Consistent with the reduced inflammation, significantly decreased AO was also noticed in M. pneumoniae-infected IL-12 KO mice. In contrast, AO was worse in the control IL-12 KO mice than in the control WT mice. The effect of IL-12 on the airway may differ in the presence or absence of infection. The lack of IL-12 did not affect AHR.
The noted changes in the pulmonary cytokines associated with the salutary effect on microbiologic, histologic, and airway parameters provide further insight into the immunopathogenesis of M. pneumoniae respiratory infection and suggest that IL-12 plays an important role in orchestrating the pulmonary disease of M. pneumoniae. This result is consistent with our hypothesis of a protective effect of the absence of IL-12 in M. pneumoniae respiratory infection and further supports the idea that, after infection with M. pneumoniae, the innate host response directly contributes to the disease of M. pneumoniae (5, 17, 20).
Our demonstration of improvement of M. pneumoniae disease in the absence of IL-12 is in contrast with what has been shown for diseases with intracellular organisms, such as Mycobacterium tuberculosis (9, 41), Toxoplasma gondii (28), Listeria monocytogenes (4), Brucella spp. (33, 48), Leishmania spp. (30, 31, 35), Francisella tularensis (11), Helicobacter pylori (22), and Cryptococcus (24, 25, 49), in which the absence of IL-12 worsened disease and decreased survival rates. The different mechanisms by which M. pneumoniae interacts with the host in this regard are not clear; perhaps the fact that M. pneumoniae acts as an extracellular pathogen in the acute stages of infection differentiates its immunopathogenesis from these intracellular pathogens.
While in this investigation the absence of IL-12 (a Th1 mediator) in M. pneumoniae respiratory infection was protective, the absence of IL-4 (a Th2 mediator) in a murine model of M. pulmonis respiratory infection resulted in exacerbated AO and AHR (42). Additionally, other Th1- and/or Th2-related investigations have been performed in IL-4 KO, IFN-γ KO, and T-bet KO mice, as well as in mice depleted of CD8 T cells or CD4 T cells (2, 23, 43). Chu and coworkers found that when M. pneumoniae infection occurred after ovalbumin sensitization and challenge, increased IL-4 concentrations in BAL fluid and increased histologic lung inflammation were observed compared with sham-infected mice (7). The sum of these studies indicates that mycoplasma-related airway disease may have various predominant immunopathogenic mechanisms depending on the background milieu of the host. Overlying mechanistic pathways are likely responsible for this phenomenon, although further investigation is required.
Acknowledgments
This work was funded by NIH/NIAID grant 5KO8 AI052262 (R.D.H.).
Editor: J. L. Flynn
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi, C. S., M. Malik, P. M. Carrico, and T. J. Sellati. 2006. T-bet deficiency facilitates airway colonization by Mycoplasma pulmonis in a murine model of asthma. J. Immunol. 177:1786-1795. [DOI] [PubMed] [Google Scholar]
- 3.Block, S., J. Hedrick, M. R. Hammerschlag, G. H. Cassell, and J. C. Craft. 1995. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr. Infect. Dis. J. 14:471-477. [DOI] [PubMed] [Google Scholar]
- 4.Brombacher, F., A. Dorfmuller, J. Magram, W. J. Dai, G. Kohler, A. Wunderlin, K. Palmer-Lehmann, M. K. Gately, and G. Alber. 1999. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int. Immunol. 11:325-332. [DOI] [PubMed] [Google Scholar]
- 5.Cartner, S. C., J. R. Lindsey, J. Gibbs-Erwin, G. H. Cassell, and J. W. Simecka. 1998. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect. Immun. 66:3485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassell, G. H., and B. C. Cole. 1981. Mycoplasmas as agents of human disease. N. Engl. J. Med. 304:80-89. [DOI] [PubMed] [Google Scholar]
- 7.Chu, H. W., J. M. Honour, C. A. Rawlinson, R. J. Harbeck, and R. J. Martin. 2003. Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice. Infect. Immun. 71:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. van Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 11.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca-Aten, M., A. M. Rios, A. Mejias, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am. J. Respir. Cell Mol. Biol. 32:201-210. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca-Aten, M., C. M. Salvatore, A. Mejias, A. M. Rios, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 49:4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubler, U., A. O. Chua, D. S. Schoenhaut, C. M. Dwyer, W. McComas, R. Motyka, N. Nabavi, A. G. Wolitzky, P. M. Quinn, P. C. Familletti, and et al. 1991. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. USA 88:4143-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766-775. [DOI] [PubMed] [Google Scholar]
- 17.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy, R. D., A. M. Rios, S. Chavez-Bueno, H. S. Jafri, J. Hatfield, B. B. Rogers, G. H. McCracken, and O. Ramilo. 2003. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob. Agents Chemother. 47:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, R. D., T. Prescot Atkinson, and G. H. Cassell. 2005. Mucosal immunology, 3rd ed., vol. 2. Elsevier Academic Press, New York, NY.
- 20.Hayakawa, M., H. Taguchi, S. Kamiya, Y. Fujioka, H. Watanabe, S. Kawai, and H. Kobayashi. 2002. Animal model of Mycoplasma pneumoniae infection using germfree mice. Clin. Diagn. Lab. Immunol. 9:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickman-Davis, J. M. 2002. Role of innate immunity in respiratory mycoplasma infection. Front. Biosci. 7:d1347-1355. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman, P. S., N. Vats, D. Hutchison, J. Butler, K. Chisholm, G. Sisson, A. Raudonikiene, J. S. Marshall, and S. J. Veldhuyzen van Zanten. 2003. Development of an interleukin-12-deficient mouse model that is permissive for colonization by a motile KE26695 strain of Helicobacter pylori. Infect. Immun. 71:2534-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, H. P., L. Tabor, X. Sun, M. D. Woolard, and J. W. Simecka. 2002. Depletion of CD8+ T cells exacerbates CD4+ Th cell-associated inflammatory lesions during murine mycoplasma respiratory disease. J. Immunol. 168:3493-3501. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami, K., Y. Koguchi, M. H. Qureshi, A. Miyazato, S. Yara, Y. Kinjo, Y. Iwakura, K. Takeda, S. Akira, M. Kurimoto, and A. Saito. 2000. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-gamma production by NK cells. J. Immunol. 165:941-947. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami, K., M. Tohyama, Q. Xie, and A. Saito. 1996. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin. Exp. Immunol. 104:208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170:827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraft, M. 2000. The role of bacterial infections in asthma. Clin. Chest Med. 21:301-313. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman, L. A., F. Cardillo, A. M. Owyang, D. M. Rennick, D. J. Cua, R. A. Kastelein, and C. A. Hunter. 2004. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 173:1887-1893. [DOI] [PubMed] [Google Scholar]
- 29.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 30.Mattner, F., K. Di Padova, and G. Alber. 1997. Interleukin-12 is indispensable for protective immunity against Leishmania major. Infect. Immun. 65:4378-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 32.Michelow, I. C., K. Olsen, J. Lozano, N. K. Rollins, L. B. Duffy, T. Ziegler, J. Kauppila, M. Leinonen, and G. H. McCracken, Jr. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113:701-707. [DOI] [PubMed] [Google Scholar]
- 33.Murphy, E. A., J. Sathiyaseelan, M. A. Parent, B. Zou, and C. L. Baldwin. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raupach, B., and S. H. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417-428. [DOI] [PubMed] [Google Scholar]
- 35.Satoskar, A. R., S. Rodig, S. R. Telford III, A. A. Satoskar, S. K. Ghosh, F. von Lichtenberg, and J. R. David. 2000. IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic immunopathology. Eur. J. Immunol. 30:834-839. [DOI] [PubMed] [Google Scholar]
- 36.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern, A. S., F. J. Podlaski, J. D. Hulmes, Y. C. Pan, P. M. Quinn, A. G. Wolitzky, P. C. Familletti, D. L. Stremlo, T. Truitt, R. Chizzonite, et al. 1990. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 87:6808-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008-4027. [PubMed] [Google Scholar]
- 39.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 40.van Schaik, S. M., G. Enhorning, I. Vargas, and R. C. Welliver. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 177:269-276. [DOI] [PubMed] [Google Scholar]
- 41.Wakeham, J., J. Wang, J. Magram, K. Croitoru, R. Harkness, P. Dunn, A. Zganiacz, and Z. Xing. 1998. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette-Guerin in IL-12-deficient mice. J. Immunol. 160:6101-6111. [PubMed] [Google Scholar]
- 42.Woolard, M. D., R. D. Hardy, and J. W. Simecka. 2004. IL-4-independent pathways exacerbate methacholine-induced airway hyperreactivity during mycoplasma respiratory disease. J. Allergy Clin. Immunol. 114:645-649. [DOI] [PubMed] [Google Scholar]
- 43.Woolard, M. D., L. M. Hodge, H. P. Jones, T. R. Schoeb, and J. W. Simecka. 2004. The upper and lower respiratory tracts differ in their requirement of IFN-gamma and IL-4 in controlling respiratory mycoplasma infection and disease. J. Immunol. 172:6875-6883. [DOI] [PubMed] [Google Scholar]
- 44.Woolard, M. D., D. Hudig, L. Tabor, J. A. Ivey, and J. W. Simecka. 2005. NK cells in gamma-interferon-deficient mice suppress lung innate immunity against Mycoplasma spp. Infect. Immun. 73:6742-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, C., J. Ferrante, M. K. Gately, and J. Magram. 1997. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J. Immunol. 159:1658-1665. [PubMed] [Google Scholar]
- 46.Wu, C., X. Wang, M. Gadina, J. J. O'Shea, D. H. Presky, and J. Magram. 2000. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 165:6221-6228. [DOI] [PubMed] [Google Scholar]
- 47.Wubbel, L., L. Muniz, A. Ahmed, M. Trujillo, C. Carubelli, C. McCoig, T. Abramo, M. Leinonen, and G. H. McCracken, Jr. 1999. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr. Infect. Dis. J. 18:98-104. [DOI] [PubMed] [Google Scholar]
- 48.Zhan, Y., Z. Liu, and C. Cheers. 1996. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 64:2782-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, T., K. Kawakami, M. H. Qureshi, H. Okamura, M. Kurimoto, and A. Saito. 1997. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect. Immun. 65:3594-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, L., A. A. Nazarian, and S. T. Smale. 2004. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol. Cell. Biol. 24:2385-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]