Abstract
Acrolein is generated endogenously during lipid peroxidation and inflammation and is an environmental pollutant. Protein adducts of acrolein are detected in atherosclerotic plaques and neurons of patients with Alzheimer’s disease. To understand vascular effects of acrolein exposure, we studied acrolein vasoreactivity in perfused rodent mesenteric bed. Acrolein induced endothelium-dependent vasodilatation that was more robust and more sensitive than dilation induced by 4-hydroxy-trans-2-nonenal, trans-2-hexenal, or propionaldehyde. Acrolein-induced vasodilatation was mediated by K+-sensitive components, e.g., it was abolished in 0 [K+]o buffer or in 3 mM tetrabutylammonium, inhibited 75% in 50 μM ouabain, and inhibited 64% in 20 mM K+ buffer. Moreover, combined treatment with the Ca2+-activated K+ channel inhibitors 1-[(2-chlorophenyl) diphenylmethyl]-H-pyrazole (TRAM-34, 100 nM) and apamin (5 μM) significantly reduced vasodilatation without altering sensitivity to acrolein. However, acrolein-induced % dilation was unaffected by L-NAME or indomethacin pretreatment indicating mechanistic independence of NO and prostaglandins. Moreover, acrolein induced vasodilatation in cirazoline-precontracted mesenteric bed of eNOS-null mice confirming eNOS-independence. Pretreatment with 6-(2-propargyloxyphenyl) hexanoic acid (PPOH 50 μM), an epoxygenase inhibitor, or the superoxide dismutase mimetic Tempol (100 μM) significantly attenuated acrolein-induced vasodilatation. Collectively, these data indicate that acrolein stimulates mesenteric bed vasodilatation due to endothelium-derived signal(s) that is K+-, ouabain-, PPOH-, and tempol-sensitive, and thus, a likely endothelium-derived hyperpolarizing factor (EDHF). These data indicate that low level acrolein exposure associated with vascular oxidative stress or inflammation stimulates vasodilatation via EDHF release in medium-sized arteries – a novel function.
Keywords: aldehyde, endothelium-derived hyperpolarization factor (EDHF), endothelium-derived relaxing factor, eNOS-null mice, potassium channels, ouabain-sensitive dilation
INTRODUCTION
Several disease states associated with lipid peroxidation lead to the generation and accumulation of acrolein and its protein and DNA adducts. Increased levels of acrolein have been measured in plasma of patients with renal failure (Sakata et al., 2003) and in Alzheimer’s patients’ brain tissues (Lovell et al., 2001). Increased acrolein-protein adducts have been detected in vascular tissues of patients with diabetes (Daimon et al., 2003; Suzuki and Miyata, 1999; Uesugi et al., 2004), Alzheimer’s disease (Calingasan et al., 1999; Lovell et al., 2001), and atherosclerosis (Uchida et al., 1998a). Inflammation can also generate acrolein, a highly reactiveα,β-unsaturated aldehyde. Acrolein is generated upon oxidation of L-threonine by myeloperoxidase and its production by neutrophils could contribute to vascular damage at sites of inflammation (Anderson et al., 1997). Moreover, oxidation of unsaturated fatty acids of lipoproteins and lipids results in the formation of alkoxyl and peroxyl radical that decompose into several metastable carbonyl end products including acrolein (Grosch, 1987; Porter et al., 1995). Other sources of tissue acrolein include glucose oxidation of fatty acids (Medina-Navarro et al., 2004) and the oxidation of polyamines by amine oxidase (Gahl and Pitot, 1982). Generation of acrolein by amine oxidase has been linked to ability of polyamines to induce phase 2 enzymes (Kwak et al., 2003) and produce cytotoxicity (Sharmin et al., 2001).
Acrolein is also present in the environment. Pollutants and drugs that contain acrolein or those that generate acrolein during metabolism represent significant sources of acrolein exposure. In polluted urban air, acrolein represents 5% of the total aldehyde formed from photochemical reactions and combustion of organic materials (Beauchamp, Jr. et al., 1985; Ghilarducci and Tjeerdema, 1995). It is present in high amounts in cigarette smoke, automobile exhaust, and heated cooking oil (Beauchamp, Jr. et al., 1985; Ghilarducci and Tjeerdema, 1995). Levels of free acrolein ranging from <0.01 to 4 ppm have been measured in several food substances including cheeses, wines, vegetables, and bread (Feron et al., 1991). Because acrolein is used as a herbicide and a slimicide, human exposures are frequent and extensive (Beauchamp, Jr. et al., 1985), however, the extent of the environmental impact of acrolein generation has not been fully ascertained. Acrolein is also generated during metabolism of drugs and toxins. Metabolism of cyclophosphamide produces acrolein, which has been implicated in the severe toxicity of the drug (Kehrer and Biswal, 2000; Ren et al., 1999). Similarly, acrolein is a metabolic product of allylamine; an aliphatic amine used extensively in the dye industry and a potent cardiovascular toxin in vivo (Boor et al., 1979) and in vitro (Conklin et al., 1998).
Although acrolein is not carcinogenic (Ghilarducci and Tjeerdema, 1995), its non-carcinogenic effects may be significant, particularly within the context of neurological and cardiovascular disease. Generation of acrolein and related aldehydes has been linked to the development of lesions in the brain (Arlt et al., 2002; Butterfield et al., 2002; Montine et al., 2002) and accumulation of acrolein-protein adducts in atherosclerotic plaques could affect vascular lesion progression and rupture (Uchida et al., 1998b; Uchida, 1999). Yet, the consequences of enhanced acrolein in the vasculature are not known. Previous attempts to characterize the systemic effects of acrolein were confounded by overlapping adrenergic and parasympathetic responses and show that acrolein elicits pressor or depressor responses depending upon the dose and the autonomic tone (Egle, Jr. and Hudgins, 1974; Green and Egle, Jr., 1983). We previously reported that acrolein evokes NO- and prostacyclin-dependent relaxation in isolated rat aortic rings (Tsakadze et al., 2003). However, since such effects in aorta cannot be generalized to other vascular beds, we examined the effects of acrolein on medium resistance arteries because they participate in regulation of systemic arterial blood pressure and organ perfusion. Our studies show that acrolein produces vasodilatation of rodent mesenteric bed via release of endothelium-derived hyperpolarizaton factor (EDHF). These effects of acrolein may be of significance in vasoactive responses to oxidative stress and inflammation.
METHODS
Chemicals and Solutions
Acetylcholine chloride (ACh), acrolein (90%), cirazoline, iberiotoxin (IBTX), indomethacin (INDO), Nω-nitro-d-arginine methyl ester hydrochloride (D-NAME), Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME), ouabain, L-phenylephrine hydrochloride (PHE), propionaldehyde (97%), sodium nitroprusside (SNP), Tempol, tetrabutyl ammonium (TBA), and trans-2-hexen-1-al (98%) were purchased from Sigma-Aldrich (St. Louis, MO) and apamin and 6-(2-propargyloxyphenyl) hexanoic acid (PPOH) were from Cayman Chemicals (Ann Arbor, MI). 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) was a gift from Dr. H. Wulff (University of California, Irvine). 4-hydroxy-trans-2-nonenal acetal (4-HNE) was synthesized as reported previously (Chandra and Srivastava, 1997).
Normal Krebs physiological salt solution (PSS) was of the following composition (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 12.5, and glucose 11.1. Twenty mM K+ PSS were prepared by substituting an equimolar amount of K+ for Na+ while 0 K+ PSS was prepared by substituting an equimolar amount of NaH2PO4 for KH2PO4 and KCl was omitted.
Animals
Animal research adhered to Guiding Principles in the Use of Animals in Toxicology and protocols were approved by local IACUC. .Male Sprague-Dawley rats (250–300g) and male mice (C57BL/6 and eNOS−/−; 18–26 weeks old; 25–30g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN) or The Jackson Laboratories (Bar Harbor, ME), respectively.
Isolated Perfused Mesenteric Bed Preparation
In anesthetized rodents, the mesenteric bed was isolated and cannulated at the confluence of the superior mesenteric artery and the dorsal aorta (Awe and Adeagbo, 2002). The mesenteric vascular bed was flushed immediately with heparinized (5%) Krebs PSS and then carefully trimmed off the small intestine border. The isolated mesenteric preparation was transferred within 5 min into a temperature-controlled chamber and perfused with PSS (37 oC, pH 7.4, 95% O2:5% CO2 mix) at a constant flow rate of 5 ml/min (rat) or 0.5 ml/min (mice) using a peristaltic pump (Masterflex, Cole-Parmer Instruments)(Adeagbo et al., 2004; Awe and Adeagbo, 2002). Perfusion pressure was measured with a Statham pressure transducer coupled to a Grass polygraph recorder (model 7H) and basal perfusion pressure was between 5–10 mmHg (rats) or 1–4 mmHg (mice). Tissues were allowed to equilibrate for 1h before the start of the experiments.
Acrolein Study Series
Series I: Effects of acrolein and other aldehydes on unconstricted and PHE-preconstricted perfused mesenteric bed
The effects of bolus injections of acrolein (0.01–10 nmol in 0.1 ml PSS) on perfusion pressure were studied in unconstricted and PHE-preconstricted mesenteric bed (1 or 5 μM). Fresh stock acrolein or other aldehyde was diluted in PSS immediately prior to use and then warmed to 37 °C before manual injection of 0.1 ml via 1 ml syringe over 5 secs. Under these conditions acrolein stability was >95% as determined by GC-MS using solid-phase microextraction with on-fiber derivatization methodology as developed for measuring aldehydes in blood (Deng and Zhang, 2004)(data not shown). Additionally, PHE-preconstricted mesenteric beds were exposed to increasing amounts of 4-HNE (1–100 nmol), trans-2-hexenal (1–1000 nmol), or propionaldehyde (1–100 nmol) and changes in perfusion pressure recorded.
Series II: Role of endothelium in acrolein-induced dilator responses in mesenteric bed
The role of the endothelium was assessed by obtaining dilator responses to increasing amounts of acrolein in intact and endothelium-denuded perfused mesenteric bed. The endothelium was denuded by perfusing the mesenteric beds with distilled water for 5 min and confirmed by abolition of ACh-induced dilation of PHE-preconstricted perfused mesenteric bed. SNP (1 nmol) dilator response was obtained before and after endothelium denudation on PHE-preconstricted mesenteric bed to determine the effects of distilled water on NO donor-mediated dilation (Adeagbo and Malik, 1990; Awe and Adeagbo, 2002). Similar experiments were performed with 4-HNE for comparison.
Series III: Role of nitric oxide synthase (NOS) and cyclooxygenase (COX)
To evaluate the role of NOS or COX, PHE-preconstricted mesenteric beds were constantly perfused with L-NAME (0.1 mM), D-NAME (0.1 mM), or INDO (5 μM). The preparation was then exposed to ACh, acrolein, or SNP. In addition, the role of eNOS in acrolein-induced vasodilatation was assessed in cirazoline-preconstricted (1 μM) perfused mesenteric bed of C57BL/6 and eNOS-null mice.
Series IV: Role of EDHF
Effects of acrolein were determined in PHE-preconstricted mesenteric bed perfused with normal PSS, 0 and 20 mM K+ PSS, or normal PSS plus ouabain (50 μM) to minimize activation of K+ channels and Na+/K+ ATPase (Adeagbo and Triggle, 1993). For comparison, parallel experiments were performed with KCl (0.3–200 μmol; in 0 K+ PSS with and without ouabain), ACh, and SNP (in normal PSS and 20 mM K+ PSS). The effects of acrolein on K+-induced dilation were determined in 0 K+ PSS by determining the effects of repeated 100 μmol KCl injections (3 times) followed by injection of 3 nmol acrolein and 5 min later a final injection of 100 μmol KCl.
Series V: Role of specific K+-channels
The role of K+-channels in acrolein-induced dilator responses was determined by comparing effects of ACh, acrolein, and SNP in the perfused mesenteric bed before and after 30 min perfusion of vascular beds with apamin (5 μM; small-conductance Ca2+-activated K+-channel [SKCa] inhibitor), iberiotoxin (IBTX; 100 nM; big-conductance Ca2+-activated K+-channel [BKCa] inhibitor), tetrabutyl ammonium (TBA; 3 mM; a non-selective K+-channel inhibitor), TRAM-34 (100 nM; intermediate-conductance Ca2+-activated K+-channel [IKCa] inhibitor), or the combination of apamin plus TRAM-34 (Crane et al., 2003; Hamilton and Weston, 1989).
Series VI: Role of cytochrome P450 epoxygenase
The role of cytochrome P450 epoxygenase in acrolein-induced vasodilatation of rat mesenteric bed was tested by perfusing the bed with PPOH (50 μM). While PPOH at 3 μM effectively attenuates endothelium-dependent ACh-induced relaxation of isolated rat aorta (Woodman and Boujaoude, 2004), 50 μM PPOH had no effect on PHE-induced vasomotion in isolated rat mesenteric artery (Mauban and Wier, 2004).
Series VII: Role of superoxide anions
The role of superoxide anions was tested by perfusing the bed with superoxide dismutase mimetic Tempol (2,2,6,6-tetramethylpiperidine-N-oxyl; 100 μM) (Romanko and Stepp, 2005). The dilatory effects of ACh and acrolein were determined in the absence and then in the presence of Tempol (100 μM) in PHE-preconstricted mesenteric bed.
Data Analysis
Data are expressed as mean ± SEM. The maximum % reduction in perfusion pressure (Emax), the amount producing 50% reduction in perfusion pressure (ED50), and the duration of vasodilator responses (min) were determined. Group means were compared using Student’s t-test or one-way ANOVA with Student-Newman-Keuls post-test where appropriate (Sigma Stat, SPSS, Inc. Chicago, IL). Statistical significance was assumed where p< 0.05.
RESULTS
Acrolein-induced endothelium-dependent relaxations in perfused mesenteric bed
Bolus addition of pre-warmed acrolein to the mesenteric bed led to concentration-dependent reduction of PHE-induced perfusion pressure (Fig. 1A). Acrolein produced a slow decline in perfusion pressure that was spontaneously reversible. The effects of acrolein were similar to those of ACh (Fig. 1B), although changes produced by ACh were more prompt and recovered more rapidly, especially at low amounts of ACh (Fig. 1C). The mean recovery times after bolus injections of 0.3, 1, 3 and 10 nmol acrolein were: 5.8 ± 0.6, 9.1 ± 0.3, 13.4 ± 1.0, and 16.9 ± 1.0 min (n = 9; Fig. 1C), respectively. The mesenteric bed was remarkably sensitive to acrolein and appreciable responses were observed with 0.3 to 1 nmol and nearly complete (>90%) vasodilation was observed at 10 nmoles. A similar magnitude of dilation was observed with 10 nmoles of ACh (Fig. 1D). Based on these data, we conclude that within the same concentration-range as ACh, acrolein produced robust dilatation of the mesenteric bed. In comparison to acrolein, 4-HNE (10–100 nmol) and trans-2-hexenal (10–1,000 nmol) induced less potent and less efficacious dilations of PHE-preconstricted rat mesenteric bed while no dilation was obtained with propionaldehyde (100 nmol; 4-HNE, 19±1%, n=4; trans-2-hexenal, 41±1%, n=2; propionaldehyde, 0±0%, n=2).
Fig. 1. Acrolein reduces PHE-induced perfusion pressure in a concentration-dependent manner.
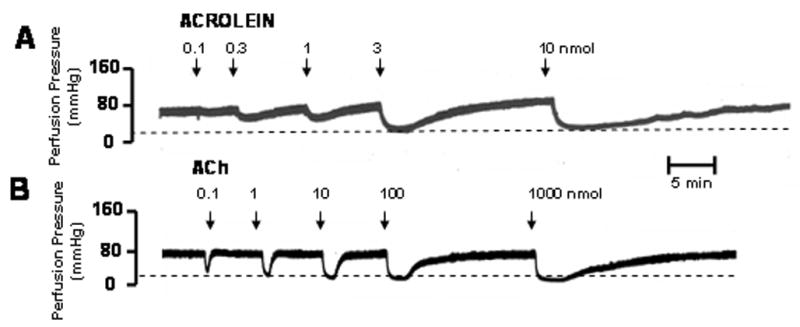
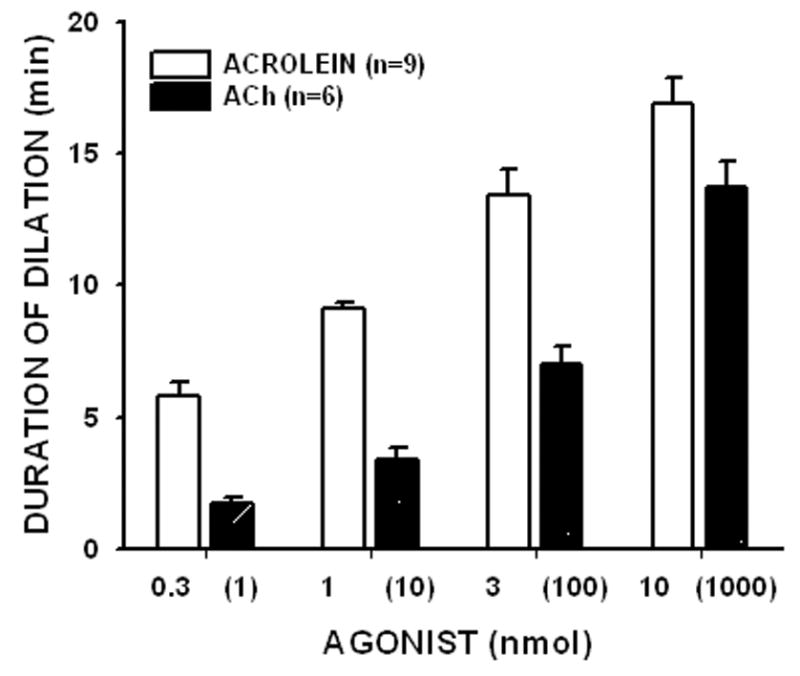
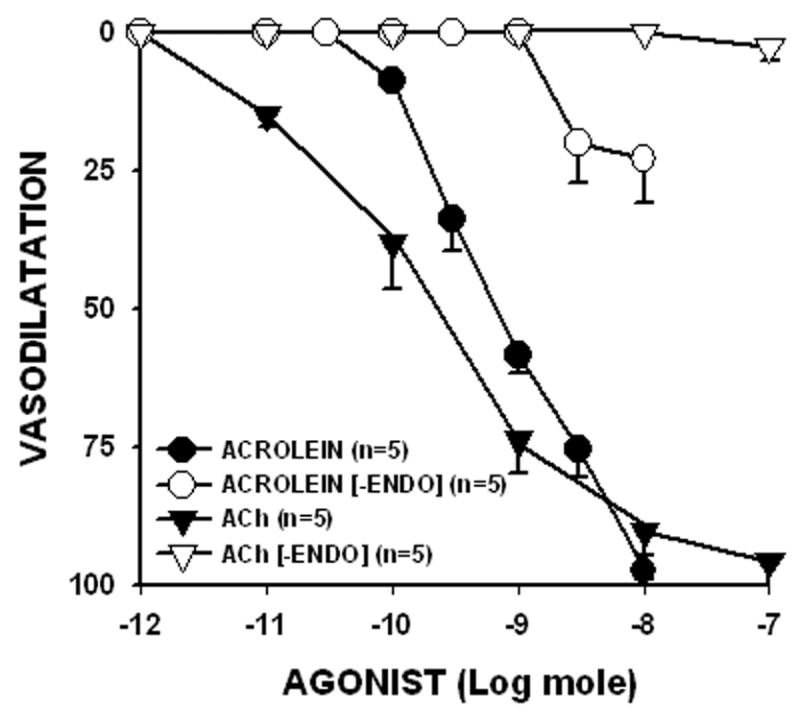
The rat mesenteric bed was preconstricted with PHE (1 μM) by perfusing the preparation at a rate of 5 ml/min. Acrolein (0.3 to 10 nmol) was added as a bolus at the indicated times (A). Similar responses were observed with ACh (B). Panel C shows the duration of dilation induced by acrolein or ACh. The amount of ACh is shown within parenthesis. The % reduction in perfusion pressure induced by different amounts of acrolein and ACh are shown in panel D for the intact mesenteric bed and after endothelium denudation [-ENDO]. Values are shown as mean ± SEM. n = number of rats for each dose. * = P < 0.05 versus the response obtained with the non-denuded mesenteric bed.
Next, we tested whether the endothelium was involved in mediating the effects of acrolein. For this the endothelium was denuded by perfusing the mesenteric bed with distilled water (Awe and Adeagbo, 2002). After 5 min of perfusion, the ACh dilatory response was completely abolished (Emax decreased from 90.4 ± 4.2% to 2.5 ± 2.5%; n = 4; Fig. 1D). The denudation procedure did not affect the underlying smooth muscle cell layer since the magnitude of PHE-preconstriction or dilation to the NO-donor SNP (1 nmol) was unaltered. The Emax of SNP was 86.1 ± 5.1% before and 81.4 ± 7.1% after denudation (n = 4), indicating that the NO-induced vasodilatation of the mesenteric bed was unaffected. In contrast, the response to acrolein also was dramatically blunted by the denudation procedure. The Emax of acrolein decreased from 97.4 ± 1.7% to 22.9 ± 8.0% (n = 4; Fig. 1D). These results suggest that to a large extent acrolein-mediated dilatation requires the presence of intact endothelium. Similarly, endothelial denudation abolished 4-HNE-induced dilation (100 nmol, 0±0%; n=4).
Role of NOS and COX in acrolein-induced dilator responses
Because NO and prostaglandins play an important role in endothelium-mediated relaxation, we tested whether inhibition of these mediators would attenuate or prevent the dilator response elicited by acrolein. To test the role of NO, the preparation was pre-treated with the NO synthase inhibitor L-NAME (0.1 mM). Treatment with l-NAME significantly attenuated ACh-induced Emax from 91.7 ± 4.3% to 42.3 ± 6.5% (n = 4), but it did not affect SNP-induced Emax (93.4 ± 1.9% versus 93.0 ± 4.4%; n = 4), suggesting that it inhibits the release, but not the effects, of NO. Treatment with L-NAME did not affect acrolein-induced dilator responses. Acrolein Emax responses were 86.9 ± 2.4% before and 82.4 ± 3.3% (n = 4) after L-NAME treatment. Addition of D-NAME (0.1 mM) had no effect on acrolein-, ACh-, or SNP-induced Emax (data not shown). Similarly, INDO (5 μM) treatment did not affect Emax induced by acrolein, ACh, or SNP in the PHE-preconstricted mesenteric bed (data not shown), suggesting that the effect of acrolein was independent of COX activity.
To probe the role of NO further, we examined the effects of acrolein on the mesenteric bed of WT and eNOS-null mice. Using treatment protocols as for rat, we found that acrolein produced concentration-dependent and robust vasodilatation in cirazoline-preconstricted mesenteric bed of mice, thus, acrolein effects in the mesenteric bed were species-independent and similarly eficacious in rats and mice. As expected, response to ACh was blunted in the eNOS-null mice. In eNOS-null mice, 10 nmol of ACh produced 59.9 ± 6.5 % relaxation (n = 5) of the WT mice. In contrast, the mesenteric bed of eNOS-null mice relaxed robustly to acrolein (Fig. 2). Overall, the acrolein ED50 was not significantly different between WT and eNOS-null mice (WT, 0.29 ± 0.08 nmol; eNOS-null, 0.22 ± 0.04 nmol) indicating that eNOS plays little role in acrolein-induced dilation of mesenteric vessels.
Fig: 2. Acrolein-induced vasodilatation is independent of eNOS.
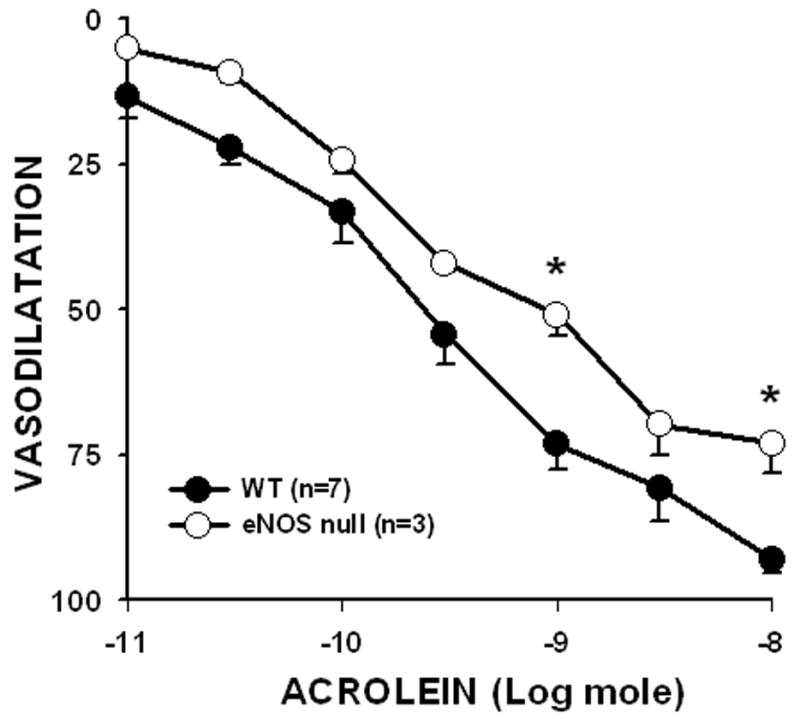
The mesenteric beds of wild-type (WT) and eNOS-null mice were preconstricted with 1 μM cirazoline and the extent of acrolein-induced vasodilatation was determined at the indicated amounts as a % of cirazoline-induced tone. Values are mean ± SEM. n = number of mice in each series. * P< 0.05 versus WT.
Role of K+-sensitive components in acrolein-induced dilator responses
Given our observation that the effects of acrolein were mediated by the endothelium, but independent of NO and prostaglandins, we next examined the contribution of EDHF. To abolish the effects of hyperpolarization, the preparation was perfused with 0 [K+]o PSS or treated with ouabain to inhibit the Na+/K+ ATPase pump. As shown in Fig. 3B, acrolein-induced dilatation was abolished in the presence of 0 [K+]o PSS (acrolein in 0 [K+]o, 0 ± 0%, n = 6). This was not due to rapid deterioration of the preparation since Emax of 10 nmol acrolein was restored upon reperfusion with normal PSS (Emax, 77.8 ± 5.2%, n = 6). The effects of acrolein were also inhibited by ouabain. As shown in Fig. 3C, treatment with ouabain (50 μM) significantly reduced acrolein-induced Emax from 80.8 ± 4.0% (n = 10) before ouabain to 9.9 ± 4.6% (n = 6) after ouabain. Addition of KCl (0.3–200 μmol) stimulated concentration-dependent dilations in 0 [K+]o PSS, which were also abolished by ouabain pretreatment (data not shown), indicating that activation of Na+/K+ ATPase is essential for hyperpolarization-mediated dilation. These observations are consistent with the notion that acrolein-induced vasodilatation requires hyperpolarization (of the endothelium) and activation of the Na+/K+ ATPase pump. However, to determine further whether acrolein directly targets Na+/K+ ATPase function, we determined the effect of acrolein pretreatment (3 nmol; 5 min) on KCl-induced dilation in 0 [K+]o PSS. KCl-induced dilation was highly repeatable (3 times over 1h) but was significantly inhibited ( 40%) by acrolein pretreatment (100 μmol KCl, 1st exposure, 49.9 ± 2.7%, 2nd exposure, 46.4 ± 2.0%, 3rd exposure, 47.9 ± 2.1%, 4th exposure 5 min after acrolein, 31.1 ± 2.8; n = 5). These data suggest acrolein does not directly “prime” the Na+/K+ ATPase pump but acts indirectly in a K+-sensitive manner.
Fig: 3. Acrolein-induced dilation of rat perfused mesenteric bed is abolished in the presence of zero [K+]o PSS or ouabain.
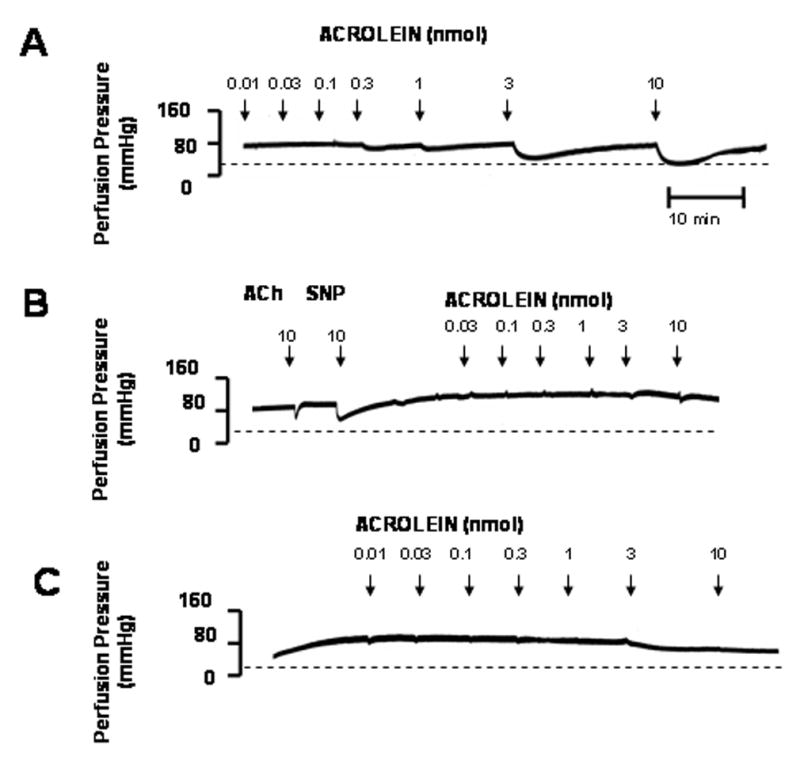
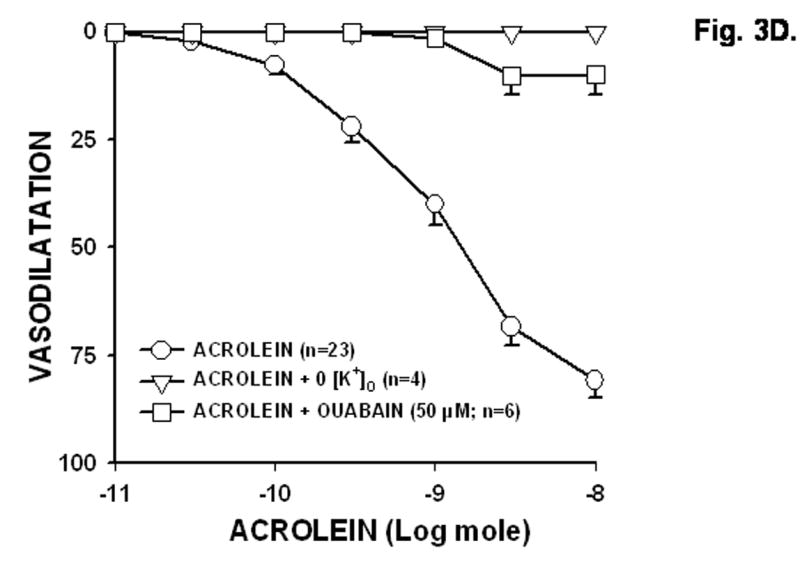
The rat mesenteric bed was precontracted by 1 μM phenylephrine in normal PSS (A), 0 [K+]o PSS (B), or normal PSS containing 50 μM ouabain (C) and changes in perfusion pressure were determined after treatment with acrolein at the indicated times and amounts. Basal perfusion pressure is indicated by a dashed line. (D) Summary concentration response curves of acrolein in PHE-preconstricted mesenteric bed constructed in 0 [K+]o PSS and in normal PSS in the absence and presence of ouabain. Values are mean ± SEM. n = number of rats in each series. * P< 0.05 versus control.
To determine how depolarization affects responses to acrolein, the preparation was perfused with 20 mM [K+]o PSS and the effects of acrolein were determined as % change in PHE-induced tone. As shown in Fig. 4A, perfusion with 20 mM [K+]o PSS significantly reduced both acrolein- (Table 1) and ACh-induced Emax (data not shown) but did not affect SNP-induced Emax (data not shown). Moreover, 20 mM [K+]o PSS significantly reduced the sensitivity of mesenteric bed to ACh (data not shown) but not to acrolein (Table 1).
Fig. 4. K+-sensitive mechanisms mediated the vasodilatory responses of perfused rat mesenteric bed to acrolein.
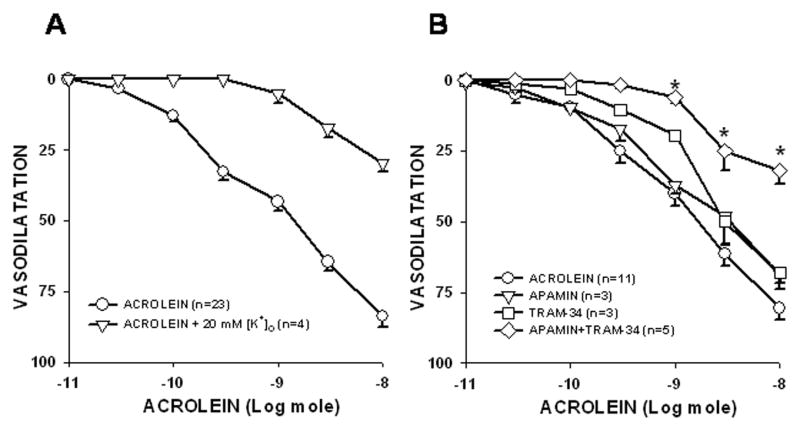
The effect of perfusion with 20 mM K+ PSS (A) or K+-channel blockers (B) on acrolein-induced dilator responses were determined in PHE-preconstricted perfused rat mesenteric bed. The combined treatment of apamin (5 μM) and TRAM-34 (100 nM) significantly inhibited acrolein-induced vasodilatation although each agent alone had no effect (B). Concentration response curves were first performed with acrolein in normal PSS and then repeated in the presence of 20 mM K+ PSS or K+-channel blockers. Values are mean ± SEM. n = number of rats in each series. * P< 0.05 versus control.
Table 1.
Role of [K+]o and K+ channels in acrolein-induced vasodilatation.
| Modulator | Emax | % Inhibition | ED50 | pD2 |
|---|---|---|---|---|
| None (control) | 83.8±3.8 (n=23) | 0 | 1.69±0.29 | 8.90±0.07 |
| 0 [K+]o | 0.0±0.0* (n=4) | 100 | ND | ND |
| TBA | 0.0±0.0* (n=4) | 100 | ND | ND |
| 20 mM [K+]o | 29.6±3.2* (n=4) | 63.6 | 2.90±0.79 | 8.59±0.13 |
| Ouabain | 9.9±4.6* (n=6) | 74.7 | ND | ND |
| IBTX | 66.8±5.7 (n=4) | 17.8 | 2.56±0.94 | 8.75±0.26 |
| TRAM-34 | 68.1±3.7 (n=3) | 16.2 | 1.97±0.14 | 8.71±0.03 |
| APAMIN | 68.6±5.0 (n=3) | 15.6 | 0.90±0.06 | 9.05±0.03 |
| TRAM-34+APAMIN | 31.9±4.8* (n=5) | 60.3 | 2.46±0.59 | 8.65±0.09 |
Initial agonist responses to acrolein were measured in normal PSS or in 0 [K+]o and then repeated in the presence of 20 mM [K+]o PSS, ouabain (50 μM), or specific K+ channel modulators. Agonist sensitivity was determined by interpolation of the effective nmol amount producing 50% dilation (ED50) from individual response curves in the absence and presence of K+ modulators and converted to pD2, i.e., (-log[ED50]). Values = means±SEM. (n) = number of rats in each data series. ND = not determined. % Inhibition was calculated as relative inhibition of the acrolein response in untreated vessels in normal PSS.
= P<0.05 vs control.
Since the [K+]o-sensitive components seem to contribute to both acrolein- and ACh-induced dilations, we examined the role of specific K+ channels in these dilatory responses. The non-specific K+-channel blocker, TBA (3 mM), abolished both acrolein- (Table 1) and ACh-induced (data not shown) dilator responses confirming a vital role of K+-channel opening in dilatory responses. TBA treatment did not affect SNP-induced Emax indicating that the activation of TBA-sensitive channels is essential for the endothelium-dependent nature of the repsonse and not smooth muscle cell relaxation per se (data not shown). To identify the specific K+ channels involved, three different Ca2+-activated K+ channel inhibitors -- apamin, IBTX, and TRAM-34 --were used to inhibit SKCa, BKCa, and IKCa, respectively. Apamin, IBTX, or TRAM-34 alone did not affect Emax of acrolein (Table 1) or SNP (data not shown). However, treatment with apamin combined with TRAM-34 significantly inhibited Emax of acrolein by 60% without altering the sensitivity of the mesenteric bed to acrolein (Fig. 4B; Table 1). These data suggest that the activities of both IKCa and SKCa were required for the acrolein-induced dilation.
Role of cytochrome P450 epoxygenase in acrolein-induced dilator response
Products of P450 epoxygenase activity (e.g., EETs) may be EDHF compounds. Thus to test for the role of specific epoxygenase products, we perfused PHE-preconstricted mesenteric bed with PPOH (50 μM). PPOH pretreatment had no affect on perfusion pressure but it significantly attenuated (by 35.2%) the Emax of acrolein from 84.3 ± 1.8% to 54.6 ± 5.6% (n = 6; Fig. 5). PPOH pretreatment slightly shifted mesenteric bed sensitivity to acrolein by 1.7-times (ED50: acrolein = 1.07 ± 0.27 nmol; acrolein + PPOH = 1.70 ± 0.47 nmol; n = 6). However, PPOH pretreatment significantly decreased sensitivity of mesenteric bed to ACh by >90-times (ED50: ACh, 0.09 ± 0.03 nmol; ACh + PPOH, 8.6 ± 3.9 nmol; n = 6) but not Emax (ACh, 92.5 ± 2.4%; ACh + PPOH, 76.6 ± 8.1%; n = 6; P = 0.089). Moreover, PPOH pretreatment did not alter Emax of SNP (SNP, 92.2 ± 3.6%; SNP + PPOH, 91.6 ± 6.4%; n = 5) indicating that acrolein-mediated dilation of the mesenteric bed is in part mediated by P450 epoxygenase.
Fig. 5. Epoxygenase inhibitor PPOH (50 μM) pretreatment decreases acrolein-induced vasodilatation.
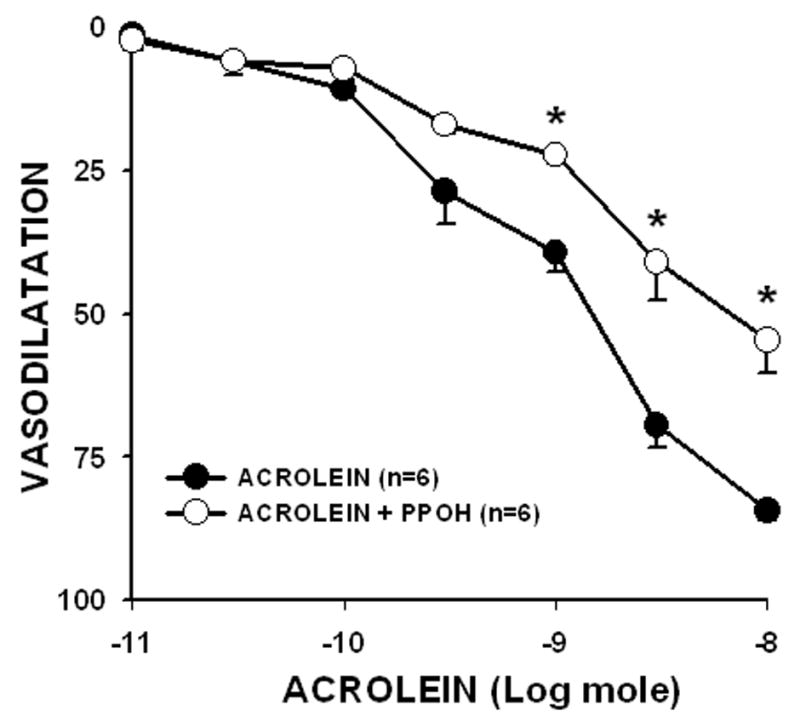
The PHE-preconstricted perfused rat mesenteric beds were pretreated with PPOH for 30 min and acrolein-induced Emax was determined. Values are mean ± SEM. n = number of rats in each series. * P< 0.05 versus control.
Role of superoxide anion in acrolein-induced dilator responses
Since acrolein has been shown to increase ROS generation, which could also stimulate P450 epoxygenase, we also perfused PHE-preconstricted mesenteric bed with the superoxide dismutase mimetic Tempol (100 μM). Tempol pretreatment did not affect perfusion pressure but it significantly affected Emax of acrolein between 0.3 to 10 nmoles (Fig. 6). Tempol decreased acrolein-mediated dilation (10 nmol) from 88.2 ± 4.3% to 71.6 ± 5.5%, but had no effect on the sensitivity of the mesenteric bed to acrolein (ED50: acrolein, 1.03 ± 0.28 nmol; acrolein+Tempol, 1.87 ± 0.28 nmol; n = 6; P=0.06). Tempol had no effect on Emax of ACh (ACh, 100 ± 0%; ACh+Tempol, 95.9 ± 1.9%; n = 6) nor Emax of SNP (%; SNP, 93.3 ± 3.1%; SNP+Tempol, 94.9 ± 4.1%; n = 6), indicating that Tempol lacked non-specific effects. Taken together, these data suggest that acrolein-mediated vasodilatation is in part mediated by an increase in superoxide anion generation.
Fig: 6. Pretreatment with the superoxide dismutase mimetic Tempol (100 μM) attenuates acrolein-induced vasodilatation.
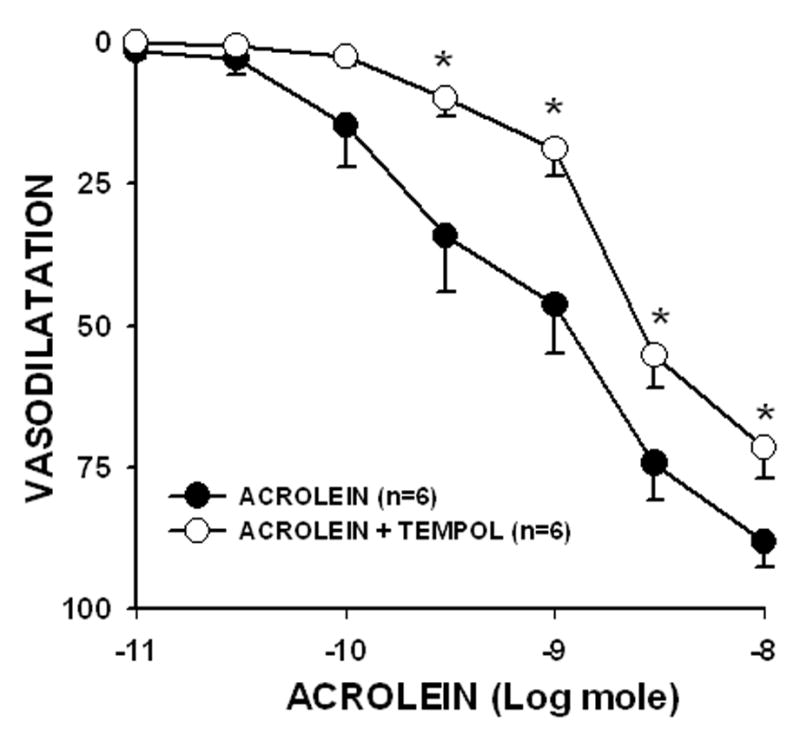
The mesenteric bed was preconstricted with PHE and treated with Tempol for 30 min before addition of the indicated amounts of acrolein. Values are mean ± SEM. n = number of rats in each series. * P< 0.05 versus control.
DISCUSSION
This study demonstrates for the first time that acrolein exerts a direct vasodilatory effect on the mesenteric bed. We show the mesenteric bed is extremely sensitive to acrolein, and unlike its effects on rat aorta (Tsakadze et al., 2003) acrolein produces dilation of the mesenteric bed via mechanisms mediated in part by EDHF and superoxide but independent of NO and prostaglandins.
Previous studies have examined the vasoactive effects of acrolein or related aldehydes and results show that systemic responses to aldehydes are a summation of autonomic (cardiac and vascular) and local effects. In anesthetized rat, ip injections of acrolein or formaldehyde evoke dose dependent depressor and pressor responses due to the stimulation of sympathetic and vagal responses (Egle, Jr. and Hudgins, 1974; Green and Egle, Jr., 1983). In the dog no pressor response to formaldehyde was observed and its direct vasodilatory effect was not affected by atropine or vagotomy (Tani et al., 1978). So even though systemic effects of aldehydes vary with their ability to stimulate autonomic and autocrine responses, aldehydes in general (acrolein [this study and Tsakadze et al., 2003], 4-HNE [this study; Romero et al., 1997], formaldehyde [Tani et al., 1978; Conklin et al., 2004] and acetaldehyde [Ren et al., 2002]) produce vascular relaxation. However, acrolein also induces contraction in isolated rat aorta and coronary artery and hypercontraction in human internal mammary artery at concentrations ≥100 μM possibly due to calcium overload (Conklin et al., 2001; Conklin, unpublished data). Since endogenous aldehydes such as acrolein and 4-HNE are produced by lipid peroxidation among other reactions, their local effects may be significant at the site of injury or inflammation (e.g., vasodilatation and edema); for instance during sepsis, which is associated with increased generation of lipid peroxidation-derived aldehydes (Minamiyama et al., 2004; Portoles et al., 1993; Sewerynek et al., 1995). Moreover, because the endothelium of coronary and mesenteric arteries generates high levels of H2O2 upon stimulation with ACh (Matoba et al., 2000, 2003), concurrent lipid peroxidation and the generation of aldehydes such as acrolein and 4-HNE could modulate agonist-evoked responses under physiological conditions.
The rodent mesenteric bed proved particularly sensitive to acrolein exposure. Acrolein-induced dilation of the rat mesenteric bed has an ED50 value of <2 nmol (Table 1) and is the most sensitive biological response to acrolein or any other aldehyde described to date. The range of acrolein (nmol) used in the present study compares favorably with measurements of acrolein and acrolein-protein adduct levels measured under physiological and pathological settings in vivo. Normal human plasma contains 30 to 50 μM equivalent of protein-acrolein adducts (Satoh et al., 1999) that are increased to 180 μM during renal failure (Sakata et al., 2003) and the concentration of free acrolein in brain tissue of patients with Alzheimer’s disease is between 100 to 200 μM (Lovell et al., 2001). Thus, the effective range of acrolein observed in the present study supports the view that endogenously generated acrolein could modulate vascular tone. Although acrolein is perhaps less abundant than 4-HNE, the perfused rat mesenteric bed is significantly more sensitive to acrolein. Moreover, the EC50 of 4-HNE in the human mesenteric artery is in the micromolar range (Romero et al., 1997), therefore, when generated in equimolar amounts, acrolein is likely to be more effective than 4-HNE in reducing vascular tone.
The relaxant effects of acrolein and 4-HNE are dependent upon the endothelium since they could be abolished by removing the endothelium (this study; Romero et al., 1997;Tsakadze et al., 2003). In our present study, this is not an injury response to acrolein exposure since subsequent ACh-induced endothelial-dependent dilation was unimpaired. In isolated human mesenteric arteries, 4-HNE-induced relaxation is prevented by NOS inhibition (Romero et al., 1997), whereas in the present study the effects of acrolein were independent of NOS activity, e.g., L-NAME, eNOS deficiency. This is also in contrast to acrolein-induced relaxation in rat aorta, which was inhibited by L-NAME (Tsakadze et al., 2003). Thus, the endothelium appears to be a sensitive target of acrolein action and thus, could serve as a sensor in regulating changes in vascular tone in response to endogenous or xeno-oxidants.
Our evidence indicates that acrolein-induced dilation of the mesenteric bed is mediated by an EDHF. The effects of acrolein were prevented by increasing [K+]o (which prevents membrane hyperpolarization) and by inhibiting the Na+/K+ ATPase pump. Previous studies show that part of smooth muscle cell relaxation induced by the endothelium is mediated by EDHF (Busse et al., 2002; Fleming, 2001; Zeldin, 2001), the chemical nature and mode of action of which are currently under investigation. Nevertheless, since EDHF induces smooth muscle cell relaxation by producing hyperpolarization, inhibition of acrolein-mediated relaxation by depolarizing the membrane provides strong support that this is an EDHF-dependent response.
The effects of EDHF are mediated by an increase in potassium conductance (Feletou and Vanhoutte, 1999) and it has been reported that high [K+]o inactivates EDHF-dependent mechanisms in mesenteric arteries by partially depolarizing the smooth muscle cell (Adeagbo and Triggle, 1993). In the present study we found that perfusion with 20 mM [K+]o PSS attenuated acrolein-induced dilation indicating that the response to acrolein is sensitive to changes in [K+]o and could be prevented by inhibiting membrane hyperpolarization. More striking was the observation that 0 [K+]o abolished acrolein-induced dilation suggesting that the response depends critically on [K+]o and that perhaps K+ released to the interstitial space acts as an EDHF (Weston et al., 2002). This scenario is supported by the similarity between dilatory profiles of acrolein and KCl addition.
Both acrolein- and KCl-induced dilations were inhibited 80–90% by ouabain pretreatment further underscoring the dependence of the responses on [K+]o and implicating Na+/K+ ATPase in acrolein-induced dilation. Although the site of the ouabain-sensitive site in our preparation is unknown, EDHF activates Na+/K+ ATPase in cultured canine coronary artery endothelial cells (Feletou and Vanhoutte, 1988) and [K+]o induces hyperpolarization in endothelium-denuded rat mesenteric artery via activation of smooth muscle cell Na+/K+ ATPase (Weston et al., 2002). Thus, Na+/K+ ATPase appears as a critical effector of acrolein action; consistent with a role of EDHF in mediating the dilatory response of acrolein.
Characteristic of an EDHF response is activation of endothelial IKCa and SKCa channels leading ultimately to smooth muscle cell hyperpolarization (Bolz et al., 1999; Busse et al., 2002). EDHF-mediated relaxation is abolished by a combination of apamin and charybdotoxin but not by apamin and iberiotoxin combination indicating endothelial IKCa and SKCa channels are necessary for EDHF response (Busse et al., 2002). Both IKCa and SKCa participate in mediating hyperpolarization, while SKCa produces hyperpolarization, IKCa facilitates repolarization (Crane et al., 2003). Endothelial IKCa and SKCa channels appear necessary in acrolein response since combination of apamin and TRAM-34 (a more specific IKCa inhibitor than charybdotoxin) is required to attenuate acrolein-induced vasodilatation. Moreover, although activation of BKCa in smooth muscle cells can mediate EDHF responses in some vascular beds (Archer et al., 2003; Huang et al., 2005), in others the EDHF response is insensitive to iberiotoxin (Mauban and Wier, 2004; Zygmunt and Hogestatt, 1996). Our findings show that acrolein-mediated vasodilatation is insensitive to iberiotoxin alone. Since both IKCa and SKCa are exclusive to the endothelium, these data further support the role of the endothelium in acrolein action (vide supra).
The dilatory effects of acrolein are attenuated by inhibiting P450 epoxygenase with PPOH, indicating an epoxygenase product may be the EDHF. The P450 enzymes generate many vasoactive compounds including EETs, DHETs, and HETEs (Fitzpatrick and Murphy, 1988; Fleming, 2001; Zeldin, 2001). PPOH is a specific inhibitor of epoxygenation catalyzed by several P450 enzyme and it inhibits the formation of vasorelaxant metabolites of arachidonic acid (5,6- and 11,12-epoxides; EETs) but not the vasoconstrictor 20-HETE (Adeagbo and Triggle, 1993; Wang et al., 1998). Hence, acrolein-induced dilation may be mediated by an epoxygenase product. EDHF-mediated relaxation in some vascular beds is attenuated by PPOH (Archer et al., 2003) and increased EET synthesis is associated with the EDHF response (Campbell et al., 1996; Fisslthaler et al., 1999).
The complete mechanism of acrolein-induced EDHF response remains unclear, although the ability of acrolein to increase superoxide could be involved. Previous studies have shown that acrolein as well as its glutathione conjugate increase the generation of superoxide in a xanthine oxidase- and aldehyde dehydrogenase-dependent manner (Adams, Jr. and Klaidman, 1993), and hence acrolein could enhance superoxide generation, which in turn could activate the epoxygenase to synthesize EETs. This scenario agrees with our observation that acrolein-mediated vasodilatation is inhibited by the SOD mimetic Tempol. Alternatively, Tempol could affect the synthesis of H2O2, which has been suggested to be an EDHF in some rodent arteries (Matoba et al., 2000). However, treatment with Tempol or Tiron facilitates H2O2 formation (Matoba et al., 2003) and enhances bradykinin-induced H2O2 production and EDHF-mediated relaxation (Matoba et al., 2003; Morikawa et al., 2003). In our study, Tempol decreased acrolein-induced dilatation, suggesting that superoxide, not H2O2, is involved in the dilatory response. Previous studies have shown that exposure of endothelial cells to superoxide stimulates bradykinin-induced calcium influx and membrane hyperpolarization due to calcium release and activates the microsomal epoxygenase activity (Graier et al., 1998), and thus, an increase in endothelial cell superoxide generation by acrolein could be one mechanism by which acrolein affects EET synthesis/release.
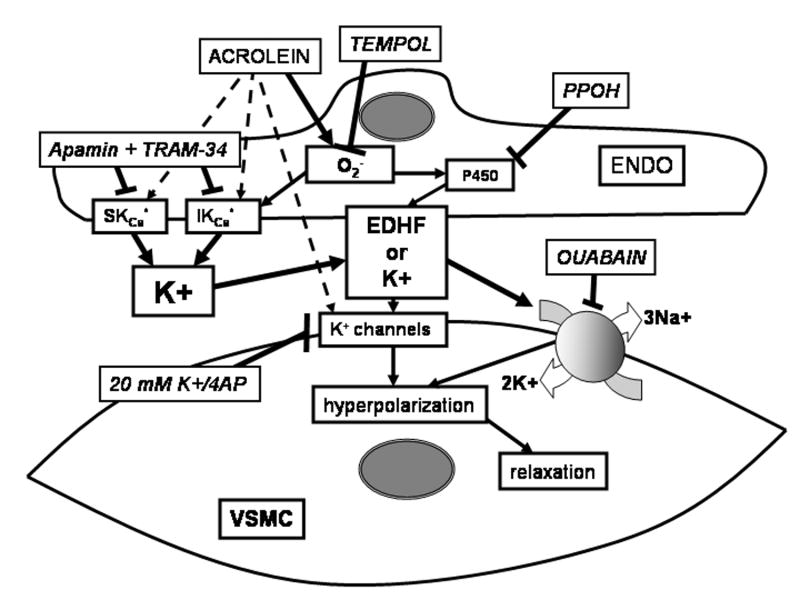
In conclusion, acrolein stimulates mesenteric bed vasodilatation via: 1) endothelium-derived K+-sensitive signals due to EDHF release, and 2) signaling, in part, dependent on superoxide, epoxygenase activity, and Na+/K+ ATPase (summary in Fig. 7). Clearly, additional work is needed to elucidate the mechanism of acrolein-induced dilation and to assess the contribution of acrolein to vasodilatation associated with oxidative stress and inflammation.
Fig. 7.
Schematic showing potential pathways stimulated by acrolein in mediating vasodilation of phenylephrine-preconstricted perfused rodent mesenteric bed. Acrolein-induced vasodilation was endothelium-dependent and sensitive to extracellular [K+], ouabain, epoxygenase inhibitor, Tempol, and calcium-sensitive K+ channel inhibitors indicating a role for EDHF synthesis and/or release. Abbreviations: EDHF, endothelium-derived hyperpolarizing factor, PPOH, P450 epoxygenase inhibitor; VSMC, vascular smooth muscle cell; ENDO, endothelial cell; apamin and TRAM-34, calcium-sensitive K+ channel inhibitors; 4AP, voltage-gated K+ channel inhibitor.
Acknowledgments
This study was supported in part by NIEHS grants ES12062 and ES11860 and a grant from the University of Louisville (#E0566).
Footnotes
Conflict of Interest Statements for Authors
There are no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free RadicBiolMed. 1993;15:187–193. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- Adeagbo AS, Awe SO, Wulff H, Joshua IG. Mesenteric arterial dilation induced by 11,12-epoxyeicosatrienoic acid involves BKCa and IKCa channels. FASEB J. 2004;18:A1281. [Google Scholar]
- Adeagbo AS, Malik KU. Mechanism of vascular actions of prostacyclin in the rat isolated perfused mesenteric arteries. JPharmacolExpTher. 1990;252:26–34. [PubMed] [Google Scholar]
- Adeagbo AS, Triggle CR. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. JCardiovascPharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- Arlt S, Beisiegel U, Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer’s disease. CurrOpinLipidol. 2002;13:289–294. doi: 10.1097/00041433-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Awe SO, Adeagbo AS. Analysis of tert-butyl hydroperoxide induced constrictions of perfused vascular beds in vitro. Life Sci. 2002;71:1255–1266. doi: 10.1016/s0024-3205(02)01845-3. [DOI] [PubMed] [Google Scholar]
- Beauchamp RO, Jr, Andjelkovich DA, Kligerman AD, Morgan KT, Heck HD. A critical review of the literature on acrolein toxicity. Crit RevToxicol. 1985;14:309–380. doi: 10.3109/10408448509037461. [DOI] [PubMed] [Google Scholar]
- Bolz SS, de Wit C, Pohl U. Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. BrJPharmacol. 1999;128:124–134. doi: 10.1038/sj.bjp.0702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor PJ, Moslen MT, Reynolds ES. Allylamine cardiotoxicity: I. Sequence of pathologic events. ToxicolApplPharmacol. 1979;50:581–592. doi: 10.1016/0041-008x(79)90413-7. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends PharmacolSci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. NeurobiolAging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. JNeurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. CircRes. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Chandra A, Srivastava SK. A synthesis of 4-hydroxy-2-trans-nonenal and 4-(3H) 4-hydroxy-2-trans-nonenal. Lipids. 1997;32:779–782. doi: 10.1007/s11745-997-0100-6. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Boyce CL, Trent MB, Boor PJ. Amine metabolism: a novel path to coronary artery vasospasm. ToxicolApplPharmacol. 2001;175:149–159. doi: 10.1006/taap.2001.9238. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Cowley HR, Wiechmann RJ, Johnson GH, Trent MB, Boor PJ. Vasoactive effects of methylamine in isolated human blood vessels: role of semicarbazide-sensitive amine oxidase, formaldehyde, and hydrogen peroxide. AmJPhysiol Heart CircPhysiol. 2004;286:H667–H676. doi: 10.1152/ajpheart.00690.2003. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Langford SD, Boor PJ. Contribution of serum and cellular semicarbazide-sensitive amine oxidase to amine metabolism and cardiovascular toxicity. ToxicolSci. 1998;46:386–392. doi: 10.1006/toxs.1998.2528. [DOI] [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. JPhysiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimon M, Sugiyama K, Kameda W, Saitoh T, Oizumi T, Hirata A, Yamaguchi H, Ohnuma H, Igarashi M, Kato T. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. EndocrJ. 2003;50:61–67. doi: 10.1507/endocrj.50.61. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang X. A simple, rapid and sensitive method for determination of aldehydes in human blood by gas chromatography/mass spectrometry and solid-phase microextraction with on-fiber derivatization. Rapid CommunMass Spectrom. 2004;18:1715–1720. doi: 10.1002/rcm.1544. [DOI] [PubMed] [Google Scholar]
- Egle JL, Jr, Hudgins PM. Dose-dependent sympathomimetic and cardioinhibitory effects of acrolein and formaldehyde in the anesthetized rat. ToxicolApplPharmacol. 1974;28:358–366. doi: 10.1016/0041-008x(74)90221-x. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. BrJPharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. The third pathway: endothelium-dependent hyperpolarization. JPhysiol Pharmacol. 1999;50:525–534. [PubMed] [Google Scholar]
- Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. MutatRes. 1991;259:363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick FA, Murphy RC. Cytochrome P-450 metabolism of arachidonic acid: formation and biological actions of “epoxygenase”-derived eicosanoids. PharmacolRev. 1988;40:229–241. [PubMed] [Google Scholar]
- Fleming I. Cytochrome p450 and vascular homeostasis. CircRes. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- Gahl WA, Pitot HC. Polyamine degradation in foetal and adult bovine serum. BiochemJ. 1982;202:603–611. doi: 10.1042/bj2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. RevEnvironContam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Graier WF, Hoebel BG, Paltauf-Doburzynska J, Kostner GM. Effects of superoxide anions on endothelial Ca2+ signaling pathways. ArteriosclerThrombVascBiol. 1998;18:1470–1479. doi: 10.1161/01.atv.18.9.1470. [DOI] [PubMed] [Google Scholar]
- Green MA, Egle JL., Jr The effects of acetaldehyde and acrolein on blood pressure in guanethidine-pretreated hypertensive rats. ToxicolApplPharmacol. 1983;69:29–36. doi: 10.1016/0041-008x(83)90115-1. [DOI] [PubMed] [Google Scholar]
- Grosch W. Reactions of hydroperoxides-products of low molecular weights. In: Chan HWS, editor. In Autoxidation of Unsaturated Lipids. Academic Press; London: 1987. pp. 95–139. [Google Scholar]
- Hamilton TC, Weston AH. Cromakalim, nicorandil and pinacidil: novel drugs which open potassium channels in smooth muscle. GenPharmacol. 1989;20:1–9. doi: 10.1016/0306-3623(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. CircRes. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. ToxicolSci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Kensler TW, Casero RA., Jr Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: effect of the polyamine metabolite acrolein. BiochemBiophysResCommun. 2003;305:662–670. doi: 10.1016/s0006-291x(03)00834-9. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. NeurobiolAging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. ArteriosclerThrombVascBiol. 2003;23:1224–1230. doi: 10.1161/01.ATV.0000078601.79536.6C. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. JClinInvest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauban JR, Wier WG. Essential role of EDHF in the initiation and maintenance of adrenergic vasomotion in rat mesenteric arteries. AmJPhysiol Heart CircPhysiol. 2004;287:H608–H616. doi: 10.1152/ajpheart.01084.2003. [DOI] [PubMed] [Google Scholar]
- Medina-Navarro R, Duran-Reyes G, Diaz-Flores M, Hicks JJ, Kumate J. Glucose-stimulated acrolein production from unsaturated fatty acids. HumExpToxicol. 2004;23:101–105. doi: 10.1191/0960327104ht416oa. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Takemura S, Toyokuni S, Imaoka S, Funae Y, Hirohashi K, Yoshikawa T, Okada S. CYP3A induction aggravates endotoxemic liver injury via reactive oxygen species in male rats. Free RadicBiolMed. 2004;37:703–712. doi: 10.1016/j.freeradbiomed.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer’s disease. Free RadicBiolMed. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. JClinInvest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Portoles MT, Ainaga MJ, Pagani R. The induction of lipid peroxidation by E. coli lipopolysaccharide on rat hepatocytes as an important factor in the etiology of endotoxic liver damage. BiochimBiophysActa. 1993;1158:287–292. doi: 10.1016/0304-4165(93)90027-6. [DOI] [PubMed] [Google Scholar]
- Ren J, Wang GJ, Petrovski P, Ren BH, Brown RA. Influence of hypertension on acetaldehyde-induced vasorelaxation in rat thoracic aorta. PharmacolRes. 2002;45:195–199. doi: 10.1006/phrs.2002.0947. [DOI] [PubMed] [Google Scholar]
- Ren S, Kalhorn TF, Slattery JT. Inhibition of human aldehyde dehydrogenase 1 by the 4-hydroxycyclophosphamide degradation product acrolein. Drug Metab Dispos. 1999;27:133–137. [PubMed] [Google Scholar]
- Romanko OP, Stepp DW. Reduced constrictor reactivity balances impaired vasodilation in the mesenteric circulation of the Obese Zucker rat. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.00213.2005. [DOI] [PubMed] [Google Scholar]
- Romero FJ, Romero MJ, Bosch-Morell F, Martinez MC, Medina P, Lluch S. 4-hydroxynonenal-induced relaxation of human mesenteric arteries. Free RadicBiolMed. 1997;23:521–523. doi: 10.1016/s0891-5849(97)00119-6. [DOI] [PubMed] [Google Scholar]
- Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. BiochemBiophysResCommun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- Satoh K, Yamada S, Koike Y, Igarashi Y, Toyokuni S, Kumano T, Takahata T, Hayakari M, Tsuchida S, Uchida K. A 1-hour enzyme-linked immunosorbent assay for quantitation of acrolein- and hydroxynonenal-modified proteins by epitope-bound casein matrix method. AnalBiochem. 1999;270:323–328. doi: 10.1006/abio.1999.4073. [DOI] [PubMed] [Google Scholar]
- Sewerynek E, Melchiorri D, Chen L, Reiter RJ. Melatonin reduces both basal and bacterial lipopolysaccharide-induced lipid peroxidation in vitro. Free RadicBiolMed. 1995;19:903–909. doi: 10.1016/0891-5849(95)00101-3. [DOI] [PubMed] [Google Scholar]
- Sharmin S, Sakata K, Kashiwagi K, Ueda S, Iwasaki S, Shirahata A, Igarashi K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. BiochemBiophysResCommun. 2001;282:228–235. doi: 10.1006/bbrc.2001.4569. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. InternMed. 1999;38:309–314. doi: 10.2169/internalmedicine.38.309. [DOI] [PubMed] [Google Scholar]
- Tani T, Satoh S, Horiguchi Y. The vasodilator action of formaldehyde in dogs. ToxicolApplPharmacol. 1978;43:493–499. doi: 10.1016/s0041-008x(78)80008-8. [DOI] [PubMed] [Google Scholar]
- Tsakadze NL, Srivastava S, Awe SO, Adeagbo AS, Bhatnagar A, D’Souza SE. Acrolein-induced vasomotor responses of rat aorta. AmJPhysiol Heart CircPhysiol. 2003;285:H727–H734. doi: 10.1152/ajpheart.00269.2003. [DOI] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends CardiovascMed. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. JBiolChem. 1998a;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. ProcNatlAcadSciUSA. 1998b;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi N, Sakata N, Nangaku M, Abe M, Horiuchi S, Hisano S, Iwasaki H. Possible mechanism for medial smooth muscle cell injury in diabetic nephropathy: glycoxidation-mediated local complement activation. AmJKidney Dis. 2004;44:224–238. doi: 10.1053/j.ajkd.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. JPharmacolExpTher. 1998;284:966–973. [PubMed] [Google Scholar]
- Weston AH, Richards GR, Burnham MP, Feletou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. BrJPharmacol. 2002;136:918–926. doi: 10.1038/sj.bjp.0704787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman OL, Boujaoude M. Chronic treatment of male rats with daidzein and 17 beta-oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. BrJPharmacol. 2004;141:322–328. doi: 10.1038/sj.bjp.0705603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. JBiolChem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Hogestatt ED. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. BrJPharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]