Abstract
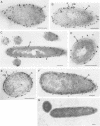
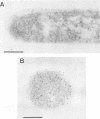
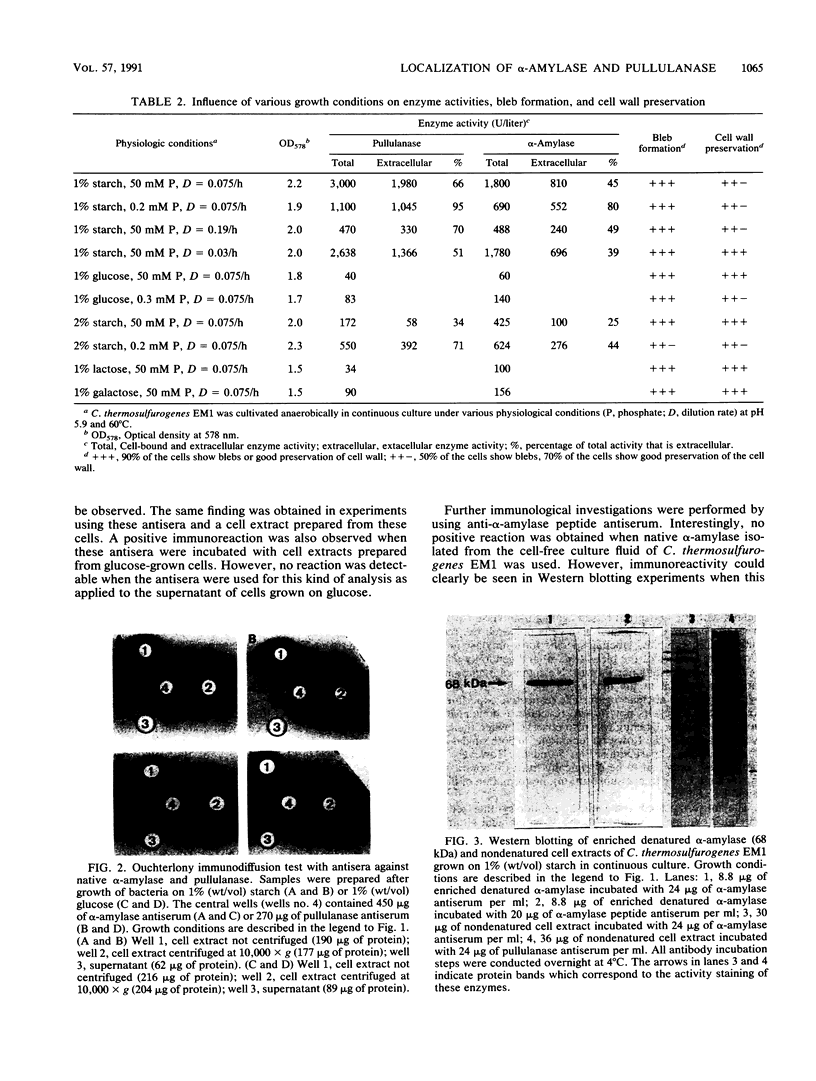
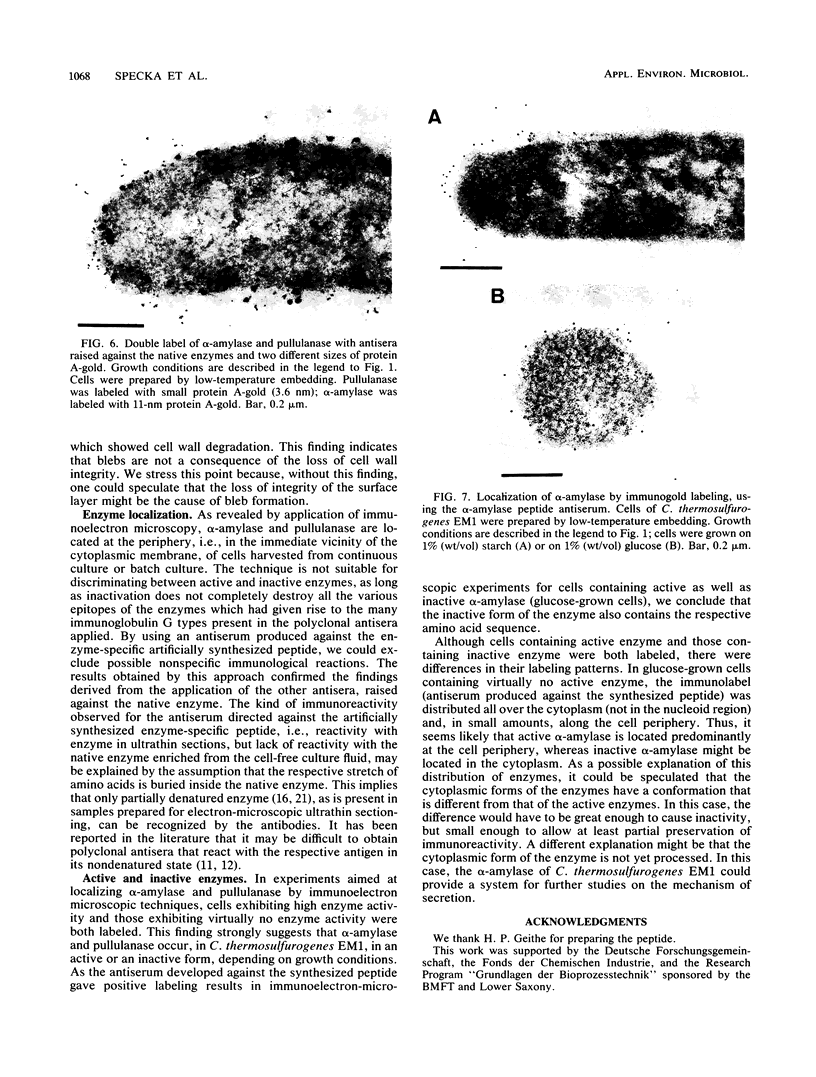
Clostridium thermosulfurogenes EM1 formed blebs, i.e., protrusions still in contact with the cytoplasmic membrane, that originated from the cytoplasmic membrane during growth in batch culture and continuous culture. They could be observed squeezed between the cell wall and cytoplasmic membrane in cells with seemingly intact wall layers (surface layer and peptidoglycan layer) as well as in cells with wall layers in different states of degradation caused by phosphate limitation or high dilution rates. Blebs were found to turn into membrane vesicles by constriction in cases when the cell wall was heavily degraded. Bleb and vesicle formation was also observed in the absence of substrates that induce α-amylase and pullulanase synthesis. No correlations existed between bleb formation and the presence of active enzyme. Similar blebs could also be observed in a number of other gram-positive bacteria not producing these enzymes, but they were not observed in gram-negative bacteria. For immunoelectron-microscopic localization of α-amylase and pullulanase in C. thermosulfurogenes EM1, two different antisera were applied. One was raised against the enzymes isolated from the culture fluid; the other was produced against a peptide synthesized, as a defined epitope, in analogy to the N-terminal amino acid sequence (21 amino acids) of the native extracellular α-amylase. By using these antisera, α-amylase and pullulanase were localized at the cell periphery in samples taken from continuous culture or batch culture. In samples prepared for electron microscopy by freeze substitution followed by ultrathin sectioning, blebs could be seen, and the immunolabel pinpointing α-amylase enzyme particles was seen not only randomly distributed in the cell periphery, but also lining the surface of the cytoplasmic membrane and the blebs. Cells exhibiting high or virtually no enzyme activity were labeled similarly with both antisera. This finding strongly suggests that α-amylase and pullulanase may occur in both active and inactive forms, depending on growth conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antranikian G., Herzberg C., Gottschalk G. Production of Thermostable alpha-Amylase, Pullulanase, and alpha-Glucosidase in Continuous Culture by a New Clostridium Isolate. Appl Environ Microbiol. 1987 Jul;53(7):1668–1673. doi: 10.1128/aem.53.7.1668-1673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F., Judd R. C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989 May;171(5):2499–2505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madi E., Antranikian G., Ohmiya K., Gottschalk G. Thermostable amylolytic enzymes from a new clostridium isolate. Appl Environ Microbiol. 1987 Jul;53(7):1661–1667. doi: 10.1128/aem.53.7.1661-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Chapon C., Schwartz M. Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol. 1986 Jun;166(3):1083–1088. doi: 10.1128/jb.166.3.1083-1088.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M., Mayer F., Meyer O. Immunocytochemical localization of carbon monoxide oxidase in Pseudomonas carboxydovorans. The enzyme is attached to the inner aspect of the cytoplasmic membrane. J Biol Chem. 1984 Dec 10;259(23):14788–14792. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Wöhner G., Wöber G. Pullulanase, an enzyme of starch catabolism, is associated with the outer membrane of Klebsiella. Arch Microbiol. 1978 Mar;116(3):303–310. doi: 10.1007/BF00417856. [DOI] [PubMed] [Google Scholar]