Abstract
Liver-stage antigen 1 (LSA1) is expressed by Plasmodium falciparum only during the intrahepatic cell stage of the parasite's development. Immunoepidemiological studies in regions where malaria is endemic suggested an association between the level of LSA1-specific humoral and cell-mediated immune responses and susceptibility to clinical malaria. A recombinant LSA1 protein, FMP011, has been manufactured as a preerythrocytic vaccine to induce an immune response that would have the effect of controlling parasitemia and disease in humans. To evaluate the immunogenicity of FMP011, we analyzed the immune response of three inbred strains of mice to antigen immunization using two different adjuvant formulations, AS01B and AS02A. We report here the ability of BALB/c and A/J mice, but not C57BL/6J mice, to mount FMP011-specific humoral (antibody titer) and cellular (gamma interferon [IFN-γ] production) responses following immunization with FMP011 formulated in AS01B or AS02A. Immunization of BALB/c and A/J mice with FMP011/AS01B induced more antigen-specific IFN-γ-producing splenocytes than immunization with FMP011/AS02A. A slightly higher titer of antibody was induced using AS02A than AS01B in both strains. C57BL/6J mice did not respond with any detectable FMP011-specific IFN-γ splenocytes or antibody when immunized with FMP011 in AS01B or AS02A. Intracellular staining of cells isolated from FMP011/AS01B-immunized BALB/c mice indicated that CD4+ cells, but not CD8+ cells, were the main IFN-γ-producing splenocyte. However, inclusion of blocking anti-CD4+ antibody during the in vitro restimulation ELISpot analysis failed to completely abolish IFN-γ production, indicating that while CD4+ T cells were the major source of IFN-γ, other cell types also were involved.
Plasmodium falciparum liver-stage antigen 1 (LSA1) is expressed exclusively in the liver stage of parasite development (17). The native LSA1 protein has a large central repeat region containing over 83 slightly degenerate 17-amino-acid repeat segments, flanked by two highly conserved N- and C-terminal regions. The lsa1 gene encodes a 230-kDa protein whose expression begins shortly after sporozoite invasion of the liver hepatocyte and increases rapidly with liver-stage development (13, 17). The LSA1 protein has been described as forming a flocculent mass surrounding the thousands of developing parasite nuclei and pseudocytomeres and is microscopically observed localized between the plasmalemma and the parasitophorous vacuole membranes inside the infected hepatocyte cytoplasm. Later in development the LSA1 protein has been detected adhering to emerging mature liver merozoites (13).
It has been demonstrated that complete resistance to malaria infection can be induced in rodents and humans by prior injection, by needle or mosquito bite, of gamma-irradiated sporozoites (γ-spz) and that the mechanism of immunity is, in large part, due to major histocompatibility complex class I-restricted CD8+ gamma interferon (IFN-γ)-secreting T cells directed against antigens expressed by malaria-infected hepatocytes (8-10, 12, 16, 17, 22, 23). Furthermore, the effector cells of this immune response target primarily liver-stage developing merozoites and not sporozoites before their entry into hepatocytes. In mice, the developing attenuated liver-stage parasites resulting from the irradiated sporozoites must be present at the time of normal sporozoite challenge for immunity to be manifested (16, 22, 27, 39). In humans, the protection induced by the gamma-irradiated sporozoite model is effective against homologous and heterologous parasite strain challenge, indicating that the antigen(s) responsible is relatively conserved. The role of specific anti-LSA1 immune responses in this protection is evident. It has been reported that human volunteers exposed to a protective dose of P. falciparum irradiated sporozoites develop LSA1 peptide-specific proliferative T cells (17, 20). In addition, epidemiologic studies have reinforced the association of LSA1 to disease resistance or severity among individuals living in areas where malaria is endemic. Studies of antibody (Ab) and CD4+ or CD8+ T-cell responses measured by antigenic specific cellular proliferation, cytokine production (primarily IFN-γ and interleukin-10 [IL-10]), and presence of cytotoxic lymphocytes has been demonstrated in residents of various areas where malaria is endemic (6, 11, 25, 35). Although these data should be interpreted cautiously given the differing ethnicities and ages of the subjects and variety of immunologic assays used, it appears that IFN-γ and IL-10 responses by CD8+ and/or CD4+ T cells stimulated with LSA1 correlate with resistance to reinfection (18, 24, 29-31).
In order to assess the ability of a recombinant LSA1 protein to induce a potentially protective immune response, our laboratory has made a parasite-to-bacteria codon-harmonized gene encoding part of the P. falciparum (3D7 strain) LSA1 protein and expressed it in bacteria (15). This recombinant protein, Falciparum Malaria Protein-011 (FMP011), is a 54-kDa, 456-amino-acid hexahistadine affinity-tagged polypeptide containing the N terminus, two slightly differing 17-amino-acid repeat units, and the C-terminal portion of the LSA1 protein (Fig. 1). We have purified the clinical-grade recombinant product under current Good Manufacturing Practice conditions and are evaluating its safety and immunogenicity in mice before advancing it into primate and, ultimately, human clinical trials. For the preclinical and clinical trials, we have chosen to test FMP011 formulated with GlaxoSmithKline Biologicals' (GSK) proprietary adjuvants AS02A and AS01B. AS02A has been used previously as an adjuvant with three other malaria vaccine candidates, RTS,S (1, 2), MSP1, and AMA1 (3). AS02A is an oil-in-water emulsion mixed with the two immunostimulants monophosphoryl lipid A (MPL; GSK, Seattle, WA) and the saponin derivative QS-21 (Antigenics, Lexington, MA). AS01B, a new experimental adjuvant, is a manufacturing proprietary liposomal formulation with the same proportions of MPL and QS-21 found in AS02A.
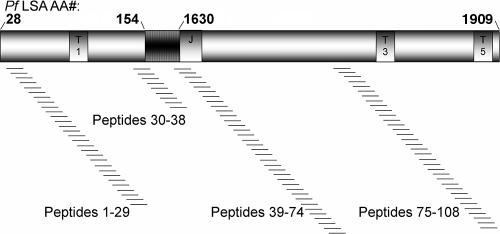
FIG. 1.
Schematic representation of FMP011, a recombinant P. falciparum LSA1 protein. The N-terminal region (amino acids 28 to 154 of the native protein) contains the T1 epitope (20), while the C-terminal region (amino acids 1630 to 1909 of the native protein) contains the T3 and T5 epitopes (20). Between these regions are two copies of the 17-amino-acid degenerate repeat (15). Peptides, 15mers overlapping by 11 and spanning the entire recombinant protein FMP011, were synthesized and used to map cellular immune responses. Not shown is the hex-His tag on the C terminus.
MATERIALS AND METHODS
Animals and protocols.
BALB/c (H-2d), A/J (H-2k), and C57BL/6J (H-2b) female mice, 6 to 8 weeks old, were purchased from Jackson Laboratory (Bar Harbor, ME). All animal-involved protocols were reviewed and approved by the Walter Reed Army Institute of Research IACUC. All work was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Recombinant protein and peptides.
FMP011 expression, purification, and lyophilization has been detailed elsewhere (15). Lot 1204 was used in these experiments. Stability, osmolarity, pH, and potency studies have shown no change in the protein over 18 months. Fifteenmer peptides overlapping by 11 amino acids spanning the entire FMP011 protein were synthesized (GenScript, Piscataway, NJ) to be used in ELISpot analysis (Fig. 1). The peptides, dissolved in dimethyl sulfoxide, were used singly or pooled as described in Table 1.
TABLE 1.
Peptide pool matrixa
| Pool | Pool and peptide
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | |
| R1 | 1 | 5 | 9 | 13 | 17 | 21 | 25 | 29 | 33 | 37 | 41 |
| R2 | 45 | 49 | 53 | 57 | 61 | 65 | 69 | 73 | 77 | 81 | 85 |
| R3 | 89 | 93 | 97 | 101 | 105 | 108 | 2 | 6 | 10 | 14 | 18 |
| R4 | 22 | 26 | 30 | 34 | 38 | 42 | 46 | 50 | 54 | 58 | 62 |
| R5 | 66 | 70 | 74 | 78 | 82 | 86 | 90 | 94 | 98 | 102 | 106 |
| R6 | 7 | 11 | 15 | 19 | 23 | 27 | 31 | 35 | 39 | 43 | 47 |
| R7 | 51 | 55 | 59 | 63 | 67 | 71 | 75 | 79 | 83 | 87 | 91 |
| R8 | 95 | 99 | 103 | 107 | 4 | 8 | 12 | 16 | 20 | 24 | 28 |
| R9 | 32 | 36 | 40 | 44 | 48 | 52 | 56 | 60 | 64 | 68 | 72 |
| R10 | 76 | 80 | 84 | 88 | 92 | 96 | 100 | 104 | 3 | W | W |
| R11 | W | W | W | W | W | W | W | W | W | W | W |
Pool of 15mer peptides. Each row (R) and column (C) pool contained an equal volume of each of the listed peptides (1 mg/ml), as indicated in that row or column. W, distilled water. Boldface entries show peptide pools that gave positive IFN-γ ELISpot reactivity.
Adjuvants and formulation.
Clinical-grade AS01B and AS02A was provided by GSK under a Cooperative Research and Development Agreement between GSK and Walter Reed Army Institute of Research. Liquid adjuvant (0.6 ml) was added to vialed lyophilized protein (60 μg) and mixed by gentle swirling for 1 min for a final antigen concentration of 100 μg/ml. The formulated protein was administered within 1 h after addition of the adjuvant. However, analysis of protein stability and integrity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on formulated samples held at 4°C for up to 24 h has shown no degradation of the FMP011 protein (data not shown).
Immunizations.
FMP011 (10 μg/100 μl) was prepared in AS01B or AS02A. Mice were injected intramuscularly with 50 μl of the prepared formulation in each of the left and right posterior thighs on a 0-, 14-, and 28-day schedule unless otherwise noted. The dosage was chosen after dosage-ranging studies to find the minimum amount needed to induce detectable FMP011-specific antibody by enzyme-linked immunosorbent assay (ELISA) in 100% of the mice. Control animals were similarly injected intramuscularly with 100 μl of AS01B or AS02A adjuvant alone on a schedule similar to that of the test antigen formulation.
Blood collection.
Blood (300 μl) was collected into a heparinized tube after incision of the tail vein. Samples were usually collected before each injection and then at 1, 2, 5, and 7 weeks after the final injection, unless otherwise noted. Cells were pelleted by centrifugation, and serum was collected for determination of Ab titer against FMP011 by ELISA.
ELISA.
Reactions were done in 96-well plates (Dynex, Chantilly, VA) as described previously (40). After initial protein coating of the wells with FMP011 (0.25 μg/ml), the plates were blocked with 0.5% casein, 1% Tween (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline, pH 7.1 (PBS). Primary mouse Ab was detected by goat anti-mouse immunoglobulin G-biotinylated horseradish peroxidase-conjugated Ab (Southern Biotechnology Associates, Birmingham, AL) and developed using 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]-peroxidase (ABTS) substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and the optical density (OD) determination was made at 414 nm.
ELISpot assay for enumeration of IFN-γ-producing cells.
Animals were euthanized by exposure to CO2, spleens were removed under sterile conditions, and a single-cell suspension of unlysed splenocytes was prepared by gently pressing the spleens through a 0.2 μM mesh cell strainer. Splenocytes were cultured in RPMI 1640 containing penicillin-streptomycin, sodium pyruvate, and nonessential amino acids (Invitrogen, Carlsbad, CA) supplemented with a final 1% mouse serum (Sigma). The number of antigen-specific IFN-γ-producing cells was determined using an ELISpot IFN-γ kit (Mabtech, Inc., Mariemont, OH) following the manufacturer's recommended procedure and conditions. Briefly, 96-well ELISpot plates (Millipore Corp., Bedford, MA) were coated with 1.5 μg/ml of capture monoclonal antibody (MAb) against IFN-γ (AN18). One-hundred microliters of a cell suspension (2 × 106 cells/ml) was added to the wells and incubated with 100 μl media only or 100 μl media containing either recombinant protein FMP011 (1 or 10 μg/ml), a pool of 9 to 11 different peptides (final concentration of each peptide was 0.9 μg/ml) as indicated in Table 1, peptide 102 (2 μg/ml), or concanavalin A (5 μg/ml) for 35 to 48 h. In some experiments, either 1 μg/ml of anti-CD4+ (NA/LE GK1.5) or anti-CD8+ (NA/LE 53-6.7) MAb or 1 μg/ml of both MAbs was added. After incubation, plates were washed with PBS, and 100 μl of secondary biotinylated MAb R4-6A2 against IFN-γ (1 μg/ml) was added. After washing, plates were incubated with 100 μl alkaline phosphatase-conjugated streptavidin for 1.5 h, washed, and developed by adding 100 μl of ABTS substrate. Spots, corresponding to cells secreting IFN-γ, were enumerated by an automated AID-EliSpot Reader ELHR03 (Cell Technology, Inc., Columbia, MD) and then verified by reexamination of the digital image of each well by eye. Experiments were done in triplicate, and the average number of spots in the stimulant wells was determined. Results were expressed as spot-forming units (SFU) per million splenocytes.
Intracellular staining and flow cytometry.
Splenocytes were resuspended at 2 × 106 cells/ml and incubated overnight with media containing 10 μg/ml recombinant protein FMP011. All samples received 4 μg/ml of anti-CD28 MAb 37.51 (BD Bioscience Pharmingen, San Diego, CA) prior to starting the incubation. After 2 h at 37°C, Golgi Stop (BD Bioscience) was added to each well (1:500, vol/vol), and incubation continued overnight. Cells were washed in PBS, resuspended in 100 μl PBS, and stained with 2 μg/ml peridin-chlorophyll protein complex (PerCP)-labeled anti-CD4+ MAb RM4-5 or allophyocyanin-conjugated antigen-presenting cell-labeled anti-CD8+ MAb 53-6.7 (BD Bioscience). After 20 min at 4°C, the excess MAb was washed away and the cells were permeabilized by incubating for 20 min at room temperature with cyrofix/cytoperm, washed with perm/wash, and incubated for 20 min at room temperature with phycoerythrin-conjugated anti-IFN-γ or a phycoerythrin-conjugated matching isotype control. After washing away the excess MAb, cells were resuspended in 500 μl PBS and analyzed by flow cytometry with a four-color Becton Dickinson FACS Caliber (BD Bioscience, San Jose, CA). Results are expressed as the percentage of CD4+ or CD8+ T cells specific for FMP011 that also expressed IFN-γ.
Statistics.
The data presented in Fig. 3A and B were evaluated by analysis of variance (ANOVA); those in Fig. 5A were evaluated by a one-way ANOVA of rank-transformed differences of the FMP011-treated sample minus the corresponding media sample for each mouse; those of Fig. 5B were evaluated by application of the Welch 2 Sample t test; and those of Fig. 6 were evaluated by an ANOVA and a Dunnett's (two-sided) multiple-comparison test.
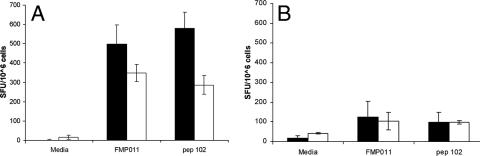
FIG. 3.
ELISpot detection of IFN-γ-producing splenocytes from BALB/c mice in response to FMP011. BALB/c mice were immunized with 10 μg/mouse of FMP011 formulated in 100 μl AS01B (A) or AS02A (B). The IFN-γ-releasing cells, upon in vitro restimulation with recombinant protein FMP011 (10 μg/ml) or peptide 102 (2 μg/ml), were determined. Results shown are the averages ± standard deviations of eight determinations for the time point 2 weeks after the third immunization (solid bars) and four determinations for the time point 7 weeks after the third immunization (open bars). ANOVA analysis of data obtained show that the responses for splenocytes stimulated with FMP011 or peptide 102 at either 2 or 7 weeks after the third immunization was significantly different from media-alone stimulation (P < 0.05).
FIG. 5.
(A) IFN-γ production in splenocytes isolated from mice immunized with FMP011 in AS01B. BALB/c, A/J, or C57BL/6J mice were injected with 10 μg/mouse of FMP011 formulated in 100 μl AS01B or 100 μl adjuvant alone at day 0 and at 2 and 4 weeks. Splenocytes were harvested 3 weeks after the third immunization, and IFN-γ secretion was determined by ELISpot upon incubation with media control or FMP011. Control animal (adjuvant only) splenocytes responded to media incubation or FMP011 stimulation with less than 10 SFU/106 cells (results not shown). (B) Antibody response (total IgG) of mice immunized with FMP011 in AS02A or AS01B. BALB/c, A/J, or C57BL/6J mice were injected with 10 μg/mouse of FMP011 formulated in 100 μl AS01B or AS02A at weeks 0 and 4. Two weeks after the second immunization, the antibody titer of sera diluted 1:100 was determined by ELISA. Results shown are the averages ± standard deviations for four mice; each ELISpot assay was done in triplicate. The values obtained show that the antibody responses of the BALB/c and A/J mice were each significantly different from that of the C57BL/6 mice (P < 0.05) in response to FMP011 delivered in either AS01B or AS02A, but the responses of BALB/c and A/J mice were not significantly different from each other.
FIG. 6.
IFN-γ production in the presence of anti-CD4+ or anti-CD8+ antibodies. BALB/c mice were immunized with 10 μg FMP011 formulated in 100 μl AS01B. Three weeks after the third immunization, splenocytes were harvested and IFN-γ-producing cells were determined by ELISpot analysis. Before in vitro stimulation with FMP011 blocking antibody against CD4+ and/or CD8+, markers were added to the culture. Results shown are the averages of four determinations ± standard deviations done in triplicate. There is no significant difference in the response seen in cells treated with anti-CD8 antibodies and control FMP011 cells or between cells treated with anti-CD4 antibodies only and those treated with anti-CD4 and anti-CD8 antibodies. However, the level of IFN-γ production was significantly reduced when cells were treated with any anti-CD4 antibody (P < 0.05).
RESULTS
FMP011 induces humoral and cellular responses in BALB/c mice.
BALB/c mice (n = 20/group) were injected with FMP011-adjuvanted AS01B or AS02A. Two additional control groups (n = 10/group) received AS01B or AS02A adjuvant only. Animals from each experimental group and adjuvant control group were sacrificed at specified time points after immunizations. Serum antibodies against FMP011 were measured prior to immunization for all animals and then at indicated times for each set of experiments. The results are reported as the optical density (OD) for a serum dilution of 1:100. Often serum and spleen cells were recovered from the same mouse. As shown in Fig. 2, all mice receiving FMP011 had detectable antibody titers after a single dose. No individual mouse in the group of mice receiving adjuvant only developed detectable anti-FMP011 antibodies during the duration of the experiment (data not shown). After the second dose of FMP011/AS01B or FMP011/AS02A, the titer of antibody in each group increased. At 1 week after the second dose there was no difference in the mean titer of antibody between the two groups (two-sided t test; P = 0.94). The mean titer levels of the groups did not increase after the third immunization and remained high at least 7 weeks after the third dose.
FIG. 2.
Antibody response in BALB/c mice to FMP011 in AS01B (diamond) or AS02A (square) adjuvant. Mice were immunized at 0, 2, and 4 weeks with 10 μg/mouse of FMP011 formulated in 100 μl of adjuvant as shown by arrows. All mice receiving adjuvant only had titers below 0.5 at a 1:50 serum dilution throughout the study (data not shown). Results shown are the averages ± standard deviations of 3 to 10 determinations.
The number of IFN-γ-producing cells (Fig. 3) upon in vitro stimulation with FMP011 or immunodominant peptide 102 2 weeks after the third immunization was 550 per 1 × 106 cells and 600 per 1 × 106 cells, respectively, when AS01B was used as the adjuvant for FMP011 immunizations. In a similar assay, splenocytes from animals immunized using AS02A responded to the in vitro stimulation with FMP011 or peptide 102 with <200 SFU per 1 × 106 IFN-γ-producing cells. Seven weeks after the third immunization, the number of IFN-γ-producing cells upon restimulation with FMP011 or peptide 102 was 350 per 1 × 106 cells and 300 per 1 × 106 cells, respectively, from animals immunized with AS01B. Mice receiving FMP011 or peptide 102 in AS02A developed 100 splenocytes per 1 × 106 IFN-γ producing cells. Naïve control animals responded to in vitro stimulation with <10 splenocytes per 1 × 106 IFN-γ-producing cells (data not shown).
Mapping the cellular response.
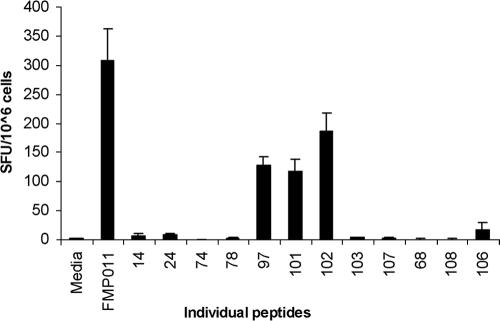
BALB/c mice were immunized with FMP011 in AS01B, and 3 weeks after the third immunization spleens were harvested for determination of IFN-γ production by splenocyte restimulation for 35 to 48 h with 22 different pools of FMP011 peptides. The layout matrix for setting up the peptide pools quickly identified the individual peptides that specifically stimulated the splenocytes (41). After the initial round of stimulation with peptide pools, individual peptides found in overlapping pools on the matrix were tested singly for their ability to stimulate splenocytes to produce IFN-γ. Peptides 97 (DKSLYDEHIKKYKND), 101 (QVNKEKEKFIKSLFH), 102 (EKEKFIKSLFHIFDG), and, to a lesser extent, 106 (NEILQIVDELSEDIT), each at 0.2 μg/ml, had the ability to significantly stimulate splenocytes to produce IFN-γ when tested alone, thus mapping T-cell epitopes of FMP011 (Fig. 4). The sequence which is shared between peptide 101 and peptide 102, the most stimulatory peptides, is EKFIKSLFH.
FIG. 4.
ELISpot detection of IFN-γ-producing splenocytes from BALB/c mice having received 10 μg FMP011 formulated in 100 μl AS01B. Cells were stimulated in vitro by individual 15mer peptides. Initial peptide mapping was done with pools of peptides as indicated in Table 1. Results represent the average of four separate determinations, each done in triplicate. ELISpot analysis showed that peptides 97, 101, 102, and 106 were able to induce IFN-γ production in a significant number of splenocytes.
Different strains of mice respond differently to FMP011 immunization.
We had previously reported the nonresponsiveness of C57BL/6J mice to FMP011 in Montanide ISA720 (15). We wanted to determine if this nonresponsiveness was observed when FMP011 was administered with AS01B, an adjuvant shown to induce higher T-cell responses. BALB/c, A/J, or C57BL/6J strains of mice were each divided into two groups (n = 5) and injected with either FMP011 formulated in AS01B adjuvant or adjuvant alone on days 0, 14, and 28. Three weeks after the third immunization animals were sacrificed, splenocytes were harvested, and IFN-γ-producing T cells were enumerated after in vitro restimulation with FMP011 (Fig. 5). BALB/c mice responded better than A/J mice to FMP011 in vitro restimulation; however, C57BL/6J mice did not respond at all (Fig. 5A). C57BL/6J mice also failed to produce antibodies against FMP011 (Fig. 5B), while A/J and BALB/c mice produced nearly equivalent antibodies to the antigen in AS01B or AS02A.
CD4+ lymphocytes are the major producers of IFN-γ in BALB/c mice.
To determine if the lymphocytes producing IFN-γ were CD4+ or CD8+ T cells, BALB/c mice were immunized with FMP011 in AS01B (n = 4) or with adjuvant alone (n = 4) as described above and were sacrificed 3 weeks after the third immunization. IFN-γ producing cells were enumerated by ELISpot after in vitro restimulation with recombinant FMP011 in the presence or absence of 1 μg/ml of anti-CD4+ or anti-CD8+ MAb or a combination of the two (Fig. 6). Inclusion of anti-CD4+ MAb resulted in an approximately 70% reduction, but not complete elimination, of the number of IFN-γ-producing cells. However, all counts were significantly higher than the number of cells stimulated with media only (<10 per 1 × 106 IFN-γ producing cells [data not shown]). On the contrary, inclusion of anti-CD8+ MAb did not have any affect on the number of cells producing IFN-γ.
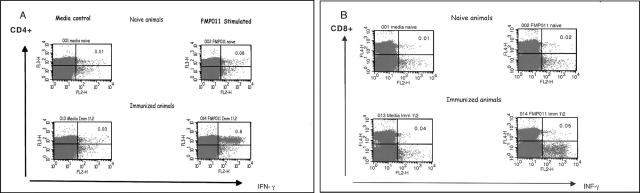
Splenocytes of similarly immunized mice were analyzed by intracellular staining and fluorescent-activated cell sorter analysis (Fig. 7). Splenocytes from naïve or immunized mice were cultured in vitro for 24 h in media containing anti-CD28 Ab and Golgi Stop in the presence or absence of 10 μg/ml FMP011. Control cells were in media with all except test protein. After incubation, cells were the membrane stained for CD4+ or CD8+ markers and intracellularly for IFN-γ. Stimulation of splenocytes from immunized animals resulted in 0.8% CD4+ IFN-γ-containing cells, 10-fold more than the number identified from animals receiving adjuvant only. Only background levels of CD8+ IFN-γ-containing cells were detected from mice receiving identical immunizations.
FIG. 7.
BALB/c mice were immunized with 10 μg FMP011 formulated in 100 μl AS01B. Three weeks after the third immunization, splenocytes from naïve or immunized mice were cultured in vitro with media only or media containing recombinant protein (10 μg/ml FMP011). Cells were membrane stained for CD4 and CD8 markers and intracellularly for IFN-γ. Gated lymphocytes were analyzed for (A) CD4+ and IFN-γ double positivity or (B) CD8+ and IFN-γ double positivity. Results are representative of four independent determinations, each from a pool of cells from two spleens.
DISCUSSION
Of equal importance in the selection of a protein antigen in the development of a malaria vaccine is the selection of an adjuvant. Two clinically tested adjuvants, AS01B and AS02A, contain equivalently proportioned amounts of MPL and QS-21. The two clinically tested adjuvants, AS01B and AS02A, both consist of the two immunostimulants MPL and QS-21 and contain either liposomes or an oil-in-water emulsion, respectively. AS02A has been tested in thousands of human volunteers and has been used in several clinical (14, 32, 34, 43) and field studies of malaria protein antigens (1, 40) as well as clinical trials of vaccines for tuberculosis (26), hepatitis B virus (42), and human immunodeficiency virus (28). AS01B is a new adjuvant formulation being tested as an improvement to AS02A. This study, an investigation of the immunogenicity of FMP011, an P. falciparum LSA1-based protein, in three inbred strains of mice, compares the effectiveness of the two adjuvants to induce cellular and humoral responses.
Administration of FMP011 in either adjuvant, AS01B or AS02A, induced strong humoral and cellular responses in BALB/c mice and, to a lesser extent, A/J mice. C57BL/6J mice, however, produced no antibodies against FMP011 nor developed IFN-γ-producing splenocytes upon immunization with FMP011 in either AS02A or AS01B (Fig. 5). The genetically restricted nonresponsiveness observed in C57BL/6J mice could not be attributed to the adjuvant alone, as we had previously observed that FMP011 responded similarly in Montanide ISA720 (data not shown). Different haplotypes have different abilities to process and/or present proteins, as could be demonstrated by the excellent generation of LSA1-specific T cells in BALB/c mice (haplotype H-2d) and moderate levels in A/J mice (haplotype H-2k) but complete abrogation in C57BL/6J mice (haplotype H-2b). In addition, using ELISpot assays we were unable to detect IFN-γ, IL-4, IL-5, or IL-10 cytokines in response to FMP011 in C57BL/6J mice (data not shown). Lack of specific T cells also implied a lack of help for T-cell-mediated antibody production in C57BL/6J mice. Interestingly, Joshi et al. (19) were able to get C57BL/6 mice to respond to synthetic LSA1 peptides, sequences which are in FMP011. However, the peptides were administered in Freund's complete adjuvant, an extremely strong adjuvant that is not approved for human use. Also, we did not try to immunize C57BL/6J mice with peptides in AS01B or AS02A, therefore it is possible that the nonresponsiveness we observed could have been due to the inability of the mice to process the recombinant protein. It is possible that the failure of C57BL/6J to mount an immune response to FMP011 was that antigen-specific clones, once formed, were not able to survive in the C57BL/6J background either for lack of growth factors or because of the generation of mostly regulatory T cells, which would have caused a suppression of the immune response (38). However, in order to further investigate the nonresponsiveness of C57BL/6J mice to FMP011 antigen, we immunized Black6/d (H-2d) mice (BALB/c [H-2d haplotype] on a C57BL/6J [H-2b] background). These mice responded with good cellular and humoral immune responses to FMP011/AS01B. On the contrary, BALB/b (H-2b) mice (C57BL/6J [H-2b haplotype] on a BALB/c [H-2d] background) failed to develop either cellular or humoral responses after three immunizations with FMP011/AS01B (data not shown). These results indicate that the lack of an immune response in C57BL/6J mice lies, at least in part, in the inability of the H-2b major histocompatibility complex to present peptides and/or in the inability of antigen-presenting cells to generate proper peptides to be presented.
After harvesting splenocytes from BALB/c or A/J mice immunized with FMP011/AS01B or FMP011/AS02A, we were able to use pools of peptides in a matrix analysis (Table 1) to identify a very limited number of 15mer peptides and were able to restimulate cells in an ELISpot assay. Further analysis (Fig. 4) using single peptides revealed that only peptides 97, 101, and 102 were able to stimulate cells in an ELISpot assay. Peptides 101 and 102 share the common sequence EKFIKSLFH. In vitro IFN-γ production by T cells upon restimulation with recombinant FMP011 or peptide 102 was significantly but not completely abrogated by inclusion of an anti-CD4+-specific MAb (Fig. 6), while inclusion of an anti-CD8+-specific MAb did not affect the response. Thus, the major producer of the cytokine IFN-γ in response to immunization with FMP011 in AS01A or AS02B adjuvant appears to be CD4+ T cells. This conclusion is consistent with the observation that intracellular staining of FMP011 in vitro-stimulated splenocytes from immunized mice show that only CD4+, and not CD8+ T cells, produce IFN-γ (Fig. 7).
The important and complex roles of CD8+ and CD4+ T cells in liver-stage immunity to malaria has been investigated in both murine and human systems (4, 21, 22, 33). Harvesting cells from animals or humans immunized with γ-spz or collecting peripheral blood mononuclear cells from individuals living in areas where malaria is endemic provides some insight into the contributions of these cells to parasite or disease control (23). The presence of CD8+ cytotoxic cells has been described in human volunteers after immunization with γ-spz (46), and CD8+ T cells specific for LSA1 peptides have been found in humans exposed to P. falciparum in areas where malaria is endemic (11). In the murine γ-spz model, both CD4+ and CD8+ cells are involved in protection from challenge after immunization. The importance of CD4+ cells is enforced by results from studies of P. yoelii γ-spz in mice (45). In vivo depletion of CD4+ cells by infusion of anti-CD4+ antibodies results in suppression of antibody production and loss of protection to challenge. Passive transfer of hyperimmune sera back to these animals did not restore protection, nor did infusion of IL-12. The generation of antigen-specific CD4+ T cells following exposure to P. falciparum has been established (36, 37). In addition, it has been shown that antigen-specific CD4+ T cells can eliminate P. yoelii-infected hepatocytes from in vitro culture (36) as well as protect against P. yoelii sporozoite challenge by adoptive transfer (36) or induction by active immunization (7, 44).
At the same time, a large number of murine malaria models have revealed the critical role of IFN-γ-producing CD8+ T cells in the protection induced by immunization with γ-spz, whereas depletion of CD4+ T cells had no effect (5, 12). Furthermore, a study in seven inbred strains and outbred mice strongly showed that in the γ-spz model protection was absolutely dependent on CD8+ T cells, because in vivo depletion of CD8+ T cells completely eliminated protective immunity. In some of the mouse strains the CD4+ T cells were neither sufficient nor required for the effector mechanism (12). In our model of immunization, however, we used a large protein as the immunogen, thus, we were more likely to generate CD4+ cells. Our data indicate that CD4+ T cells produced the majority of the detectable IFN-γ (Fig. 7). Blocking of the CD4 coreceptor in the ELISpot mixture resulted in a significant abrogation of IFN-γ production, which is consistent with CD4+ cells being the major IFN-γ producers in this experimental vaccine model.
However, we cannot exclude a role for CD8+ cells, as they may produce IFN-γ at a concentration not detectable by intracellular staining but still be biologically relevant in vivo. The failure to detect a function of the anti-CD8+ blocking antibody may be due to an incomplete block of the coreceptor. More important, we have not investigated in this study other effector functions, such as cytotoxicity. It is possible that specific CD8+ cells generated by immunization with FMP011 are cytotoxic and/or produce cytokines other than IFN-γ. Nevertheless, the appearance of an immune response to the vaccination with FMP011 is encouraging. Moreover, CD4+ macrophages may contribute to IFN-γ production. Although preliminary experiments indicate that the IFN-γ-secreting CD4+ cells are CD3+ (data not shown), a more complete investigation on the phenotype of IFN-γ-producing cells is in progress.
Taken as a whole, experimental and epidemiological evidence has demonstrated that distinct T-cell-dependent immune responses against liver-stage antigens can be part of a sterile and protective immune response. Furthermore, the data suggest that a vaccine based on liver-stage antigens that are effective in stopping the development of parasites following a sporozoite challenge could be feasible. Tantamount to success, however, is the requirement that the vaccine should induce IFN-γ-mediated immune responses. The FMP011 protein, delivered in AS02A, and even more so if delivered in AS01B, fulfills that requirement in two of the mouse strains tested here.
Acknowledgments
We thank Katarina Radosevic (Crucell) for devising the peptide matrix table and D'arcy Sloe and Cristina Fernandez for excellent technical assistance.
This work was supported by funds from the Military Infectious Disease Research Program and the Malaria Vaccine Initiative, PATH, through a tripartite Cooperative Research and Development Agreement with WRAIR and GSK.
The views expressed herein are personal and are not to be construed as official positions of the Department of Defense or the Department of the Army.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411-1420. [DOI] [PubMed] [Google Scholar]
- 3.Ballou, R. W. 2005. Malaria vaccines in development. Expert Opin. Emerging Drugs 10:489-503. [DOI] [PubMed] [Google Scholar]
- 4.Berenzon, D., R. J. Schwenk, L. Letellier, M. Guebre-Xabier, J. Williams, and U. Krzych. 2003. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J. Immunol. 171:2024-2034. [DOI] [PubMed] [Google Scholar]
- 5.Brice, G. T., N. L. Graber, D. J. Carucci, and D. L. Doolan. 2002. Optimal induction of antigen-specific CD8+ T cell responses requires bystander cell participation. J. Leukoc. Biol. 72:1164-1171. [PubMed] [Google Scholar]
- 6.Bucci, K., W. Kastens, M. R. Hollingdale, A. Shankar, M. P. Alpers, C. L. King, and J. W. Kazura. 2000. Influence of age and HLA type on interferon-gamma (IFN-gamma) responses to a naturally occurring polymorphic epitope of Plasmodium falciparum liver stage antigen-1 (LSA-1). Clin. Exp. Immunol. 122:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charoenvit, Y., V. F. Majam, G. Corradin, J. B. Sacci, Jr., R. Wang, D. L. Doolan, T. R. Jones, E. Abot, M. E. Patarroyo, F. Guzman, and S. L. Hoffman. 1999. CD4(+) T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect. Immun. 67:5604-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clyde, D. F. 1990. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971-75. Bull. W.H.O. 68(Suppl.):9-12. [PMC free article] [PubMed] [Google Scholar]
- 9.Clyde, D. F. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24:397-401. [DOI] [PubMed] [Google Scholar]
- 10.Clyde, D. F., H. Most, V. C. McCarthy, and J. P. Vanderberg. 1973. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266:169-177. [DOI] [PubMed] [Google Scholar]
- 11.Connelly, M., C. L. King, K. Bucci, S. Walters, B. Genton, M. P. Alpers, M. Hollingdale, and J. W. Kazura. 1997. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect. Immun. 65:5082-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453-1462. [DOI] [PubMed] [Google Scholar]
- 13.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. Benmohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, J. F. Meis, et al. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 153:190-204. [PubMed] [Google Scholar]
- 14.Heppner, D. G., Jr., K. E. Kester, C. F. Ockenhouse, N. Tornieporth, O. Ofori, J. A. Lyon, V. A. Stewart, P. Dubois, D. E. Lanar, U. Krzych, P. Moris, E. Angov, J. F. Cummings, A. Leach, B. T. Hall, S. Dutta, R. Schwenk, C. Hillier, A. Barbosa, L. A. Ware, L. Nair, C. A. Darko, M. R. Withers, B. Ogutu, M. E. Polhemus, M. Fukuda, S. Pichyangkul, M. Gettyacamin, C. Diggs, L. Soisson, J. Milman, M. C. Dubois, N. Garcon, K. Tucker, J. Wittes, C. V. Plowe, M. A. Thera, O. K. Duombo, M. G. Pau, J. Goudsmit, W. R. Ballou, and J. Cohen. 2005. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine 23:2243-2250. [DOI] [PubMed] [Google Scholar]
- 15.Hillier, C. J., L. A. Ware, A. Barbosa, E. Angov, J. A. Lyon, D. G. Heppner, and D. E. Lanar. 2005. Process development and analysis of liver-stage antigen 1, a preerythrocyte-stage protein-based vaccine for Plasmodium falciparum. Infect. Immun. 73:2109-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingdale, M. R., and U. Krzych. 2002. Immune responses to liver-stage parasites: implications for vaccine development. Chem. Immunol. 80:97-124. [DOI] [PubMed] [Google Scholar]
- 17.Hollingdale, M. R., C. J. McCormick, K. G. Heal, A. W. Taylor-Robinson, P. Reeve, R. Boykins, and J. W. Kazura. 1998. Biology of malarial liver stages: implications for vaccine design. Ann. Trop. Med. Parasitol. 92:411-417. [DOI] [PubMed] [Google Scholar]
- 18.John, C. C., P. O. Sumba, J. H. Ouma, B. L. Nahlen, C. L. King, and J. W. Kazura. 2000. Cytokine responses to Plasmodium falciparum liver-stage antigen 1 vary in rainy and dry seasons in highland Kenya. Infect. Immun. 68:5198-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi, S. K., A. Bharadwaj, S. Chatterjee, and V. S. Chauhan. 2000. Analysis of immune responses against T- and B-cell epitopes from Plasmodium falciparum liver-stage antigen 1 in rodent malaria models and malaria-exposed human subjects in India. Infect. Immun. 68:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krzych, U., J. A. Lyon, T. Jareed, I. Schneider, M. R. Hollingdale, D. M. Gordon, and W. R. Ballou. 1995. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J. Immunol. 155:4072-4077. [PubMed] [Google Scholar]
- 21.Krzych, U., and J. Schwenk. 2005. The dissection of CD8 T cells during liver-stage infection. Curr. Top. Microbiol. Immunol. 297:1-24. [DOI] [PubMed] [Google Scholar]
- 22.Krzych, U., R. Schwenk, M. Guebre-Xabier, P. Sun, D. Palmer, K. White, and I. Chalom. 2000. The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites. Immunol. Rev. 174:123-134. [DOI] [PubMed] [Google Scholar]
- 23.Kurtis, J. D., M. R. Hollingdale, A. J. Luty, D. E. Lanar, U. Krzych, and P. E. Duffy. 2001. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 17:219-223. [DOI] [PubMed] [Google Scholar]
- 24.Kurtis, J. D., D. E. Lanar, M. Opollo, and P. E. Duffy. 1999. Interleukin-10 responses to liver-stage antigen 1 predict human resistance to Plasmodium falciparum. Infect. Immun. 67:3424-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalvani, A., N. Hurt, M. Aidoo, P. Kibatala, M. Tanner, and A. V. Hill. 1996. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur. J. Immunol. 26:773-779. [DOI] [PubMed] [Google Scholar]
- 26.Langermans, J. A., T. M. Doherty, R. A. Vervenne, T. van der Laan, K. Lyashchenko, R. Greenwald, E. M. Agger, C. Aagaard, H. Weiler, D. van Soolingen, W. Dalemans, A. W. Thomas, and P. Anderson. 2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23:2740-2750. [DOI] [PubMed]
- 27.Lau, A. O., J. B. Sacci, Jr., and A. F. Azad. 2001. Host responses to Plasmodium yoelii hepatic stages: a paradigm in host-parasite interaction. J. Immunol. 166:1945-1950. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., K. Svehla, N. L. Mathy, G. Voss, J. R. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, F. Migot-Nabias, P. Deloron, R. S. Nussenzweig, and P. G. Kremsner. 1999. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179:980-988. [DOI] [PubMed] [Google Scholar]
- 30.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, S. Ulbert, F. Migot-Nabias, B. Dubois, P. Deloron, and P. G. Kremsner. 1998. Parasite antigen-specific interleukin-10 and antibody responses predict accelerated parasite clearance in Plasmodium falciparum malaria. Eur. Cytokine Netw. 9:639-646. [PubMed] [Google Scholar]
- 31.May, J., B. Lell, A. J. Luty, C. G. Meyer, and P. G. Kremsner. 2001. HLA-DQB1*0501-restricted Th1 type immune responses to Plasmodium falciparum liver stage antigen 1 protect against malaria anemia and reinfections. J. Infect. Dis. 183:168-172. [DOI] [PubMed] [Google Scholar]
- 32.Ockenhouse, C. F., E. Angov, K. E. Kester, C. Diggs, L. Soisson, J. F. Cummings, A. V. Stewart, D. R. Palmer, B. Mahajan, U. Krzych, N. Tornieporth, M. Delchambre, M. Vanhandenhove, O. Ofori-Anyinam, J. Cohen, J. A. Lyon, and D. G. Heppner. 2005. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine 24:3009-3017. [DOI] [PubMed] [Google Scholar]
- 33.Palmer, D. R., and U. Krzych. 2002. Cellular and molecular requirements for the recall of IL-4-producing memory CD4(+)CD45RO(+)CD27(-) T cells during protection induced by attenuated Plasmodium falciparum sporozoites. Eur. J. Immunol. 32:652-661. [DOI] [PubMed] [Google Scholar]
- 34.Pichyangkul, S., M. Gettayacamin, R. S. Miller, J. A. Lyon, E. Angov, P. Tongtawe, D. L. Ruble, D. G. Heppner, Jr., K. E. Kester, W. R. Ballou, C. L. Diggs, G. Voss, J. D. Cohen, and D. S. Walsh. 2004. Pre-clinical evaluation of the malaria vaccine candidate P. falciparum MSP1(42) formulated with novel adjuvants or with alum. Vaccine 22:3831-3840. [DOI] [PubMed] [Google Scholar]
- 35.Plebanski, M., M. Aidoo, H. C. Whittle, and A. V. Hill. 1997. Precursor frequency analysis of cytotoxic T lymphocytes to pre-erythrocytic antigens of Plasmodium falciparum in West Africa. J. Immunol. 158:2849-2855. [PubMed] [Google Scholar]
- 36.Renia, L., D. Grillot, M. Marussig, G. Corradin, F. Miltgen, P. H. Lambert, D. Mazier, and G. Del Giudice. 1993. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J. Immunol. 150:1471-1478. [PubMed] [Google Scholar]
- 37.Renia, L., M. S. Marussig, D. Grillot, S. Pied, G. Corradin, F. Miltgen, G. Del Giudice, and D. Mazier. 1991. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc. Natl. Acad. Sci. USA 88:7963-7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouse, B. T., and S. Suvas. 2004. Regulatory cells and infectious agents: detentes cordiale and contraire. J. Immunol. 173:2211-2215. [DOI] [PubMed] [Google Scholar]
- 39.Steers, N., R. Schwenk, D. J. Bacon, D. Berenzon, J. Williams, and U. Krzych. 2005. The immune status of Kupffer cells profoundly influences their responses to infectious Plasmodium berghei sporozoites. Eur. J. Immunol. 35:2335-2346. [DOI] [PubMed] [Google Scholar]
- 40.Stoute, J. A., J. Gombe, M. R. Withers, J. Siangla, D. McKinney, M. Onyango, J. F. Cummings, J. Milman, K. Tucker, L. Soisson, V. A. Stewart, J. A. Lyon, E. Angov, A. Leach, J. Cohen, K. E. Kester, C. F. Ockenhouse, C. A. Holland, C. L. Diggs, J. Wittes, and D. Gray Heppner, Jr. 7. December 2005. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine [Epub ahead of print.] [DOI] [PubMed]
- 41.Tobery, T. W., S. Wang, X. M. Wang, M. P. Neeper, K. U. Jansen, W. L. McClements, and M. J. Caulfield. 2001. A simple and efficient method for the monitoring of antigen-specific T cell responses using peptide pool arrays in a modified ELISpot assay. J. Immunol. Methods 254:59-66. [DOI] [PubMed] [Google Scholar]
- 42.Vandepapeliere, P., B. Rehermann, M. Koutsoukos, P. Moris, N. Garcon, M. Wettendorff, and G. Leroux-Roels. 2005. Potent enhancement of cellular and humoral immune responses against recombinant hepatitis B antigens using AS02A adjuvant in healthy adults. Vaccine 23:2591-2601. [DOI] [PubMed] [Google Scholar]
- 43.Walsh, D. S., S. Pichyangkul, M. Gettayacamin, P. Tongtawe, C. A. Siegrist, P. Hansukjariya, K. E. Kester, C. A. Holland, G. Voss, J. Cohen, A. V. Stewart, R. S. Miller, W. R. Ballou, and D. G. Heppner, Jr. 2004. Safety and immunogenicity of rts,s+trap malaria vaccine, formulated in the as02a adjuvant system, in infant rhesus monkeys. Am. J. Trop. Med. Hyg. 70:499-509. [PubMed] [Google Scholar]
- 44.Wang, R., Y. Charoenvit, G. Corradin, P. De La Vega, E. D. Franke, and S. L. Hoffman. 1996. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J. Immunol. 157:4061-4067. [PubMed] [Google Scholar]
- 45.Weiss, W. R., M. Sedegah, J. A. Berzofsky, and S. L. Hoffman. 1993. The role of CD4+ T cells in immunity to malaria sporozoites. J. Immunol. 151:2690-2698. [PubMed] [Google Scholar]
- 46.Wizel, B., R. A. Houghten, K. C. Parker, J. E. Coligan, P. Church, D. M. Gordon, W. R. Ballou, and S. L. Hoffman. 1995. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2. J. Exp. Med. 182:1435-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]