Abstract
Infection with the protozoan parasite Trypanosoma cruzi results in a robust and multifaceted immune response that controls parasite load but is unable to completely clear infection, resulting in parasite persistence and a chronic illness known as Chagas' disease in humans. The severity of Chagas' disease is correlated with persistent parasitism of muscle, neuronal, and gut tissues. The natural immunomodulatory function of endogenous CD4+ CD25+ regulatory T cells (Treg cells) to limit hyperactive immune responses may be exploited by microbes to persist despite host responses. In this study, we show that Treg cells are not necessary for T. cruzi evasion of immune responses during acute or chronic infection. In vivo anti-CD25 monoclonal antibody-mediated depletion of Treg cells from mice prior to challenge with a lethal strain or prior to and during acute infection with a nonlethal strain of parasite neither improved nor worsened the outcome of immune responses: differences in parasitemia, kinetics of antigen-specific CD8+ T-cell expansion, and CD8+ T-cell effector function (both in vivo and ex vivo) were of similar magnitudes for both depleted and control groups. Furthermore, depletion of CD25+ cells from chronically infected mice did not enhance immune responses of muscle-derived CD8+ T cells, nor could FoxP3 mRNA/scurfin-expressing leukocytes be isolated from muscle tissue. Based on the results of this study, it is concluded that Treg cells do not appear to play a major role in regulating CD8+ T-cell effector responses during the acute phase of infection or in the muscles of mice during chronic T. cruzi infection.
Infection with the parasite Trypanosoma cruzi leads to repeated cycles of obligatory intracellular parasite replication by the amastigote form followed by eventual dissemination throughout the host and infection of various cell types by the morphologically distinct trypomastigote. As this process iterates, the number of parasites circulating in the bloodstream increases. This allows for systemic infection and widespread tissue parasitism. Infection is rarely lethal in immunocompetent hosts and, after a characteristic period of acute parasitemia, is usually controlled by a potent immune response. However, sterile cure of infection seldom occurs. Instead, parasites persist in muscle and nerve tissues, leading to chronic inflammation and the formation of Chagas' disease in humans (24). Of the estimated 18 to 20 million people who are infected, 30 to 40% will develop severe chronic disease symptoms (9, 41), and 50,000 will die annually. Currently, chemotherapy is problematic, and no effective vaccine exists (23, 31).
Control of infection requires both humoral and cell-mediated immunity (20, 44) directed by a type 1 cytokine response (45). CD8+ T cells are essential for the control of the intracellular amastigote stages, as CD8+ T cells survey class I major histocompatibility complex (MHC) molecules displaying antigens derived from the cytosolic environment of infected nucleated host cells (33, 44, 46, 47, 53) and secrete gamma interferon (IFN-γ) in response to (and/or kill) target cells presenting antigen in the appropriate context (52).
In murine models of T. cruzi infection, the inability to produce IFN-γ is lethal (28, 50), and clinical studies demonstrate an inverse correlation between chronic disease severity and the ability of CD8+ T cells to secrete IFN-γ (21). Thus, a functional CD8+ T-cell compartment and the ability to produce IFN-γ are required for increased resistance to T. cruzi infection.
Despite a strong antiparasite immune response, T. cruzi is able to persist in the majority of hosts. Various groups have reported potential mechanisms of escape from immunity (8, 25, 30, 42, 43), although the causes of initial T. cruzi immune escape and ultimate persistence are unknown and debatable. In several infectious disease models, one demonstrated mechanism of parasite escape from host immune responses involves the induction or exploitation of at least one component of the profound regulatory network known as “peripheral tolerance.” Peripheral tolerance is actively sustained by CD4+ CD25+ regulatory T cells (Treg cells), which express the regulatory lineage factor Foxp3, comprise 5 to 10% of peripheral CD4+ T cells in healthy individuals, and are thus known as “natural” or “endogenous” regulatory T cells (36). Treg cells modulate immune responses not only to self and tumor antigens (29, 35, 37) but also to exogenous antigens (19) and infectious agents (3, 13, 26, 40). Treg cells have the ability to suppress proliferation and IFN-γ production of both CD4+ and CD8+ T-cell subsets (48), downregulate the activation and cytolytic ability of CD8+ T cells (32), and modulate humoral immune responses (54). In suppressing potentially pathological effector T-cell responses during infection, Treg cells serve to limit collateral tissue damage but may also ultimately prevent the clearance of infection by suppressing crucial immune responses (5).
To investigate the role that endogenous Treg cells play during T. cruzi infection, we used depleting anti-CD25 monoclonal antibodies (MAbs) prior to infection and during different phases of infection to deplete Treg cells and then subsequently monitored immune responses as a function of depletion. We used immunophenotypical as well as functional approaches to characterize the effects of depletion on immune responses and looked for well-characterized regulatory molecular markers to describe the regulatory potential of muscle-infiltrating leukocytes.
Here it is reported that in a rodent model where CD25+ cells were depleted prior to and after challenge with T. cruzi, parasitemia, host survival, and T. cruzi antigen-specific immune responses were not significantly different from those of nondepleted control mice. Furthermore, regulatory cells were not detected in the muscle tissue of chronically infected mice, and depletion of Treg cells during chronic infection had no therapeutic effect. This suggests that CD4+ CD25+ Treg cells are not required for T. cruzi evasion of initial or eventual immune responses, nor are Treg cells responsible for CD8+ T-cell dysfunction in a site of parasite persistence.
MATERIALS AND METHODS
Mice, parasites, and infections.
C57BL/6 (Ly 5.2+), B6.SJL (Ly 5.1+), and C3H/HeSnJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were bred and maintained in the AAALAC-approved University of Georgia animal facility in microisolator cages. At 6 to 12 weeks of age, female mice were infected intraperitoneally with blood-form or tissue culture-derived trypomastigotes of the Brazil strain or with blood-form strain Tulahuen parasites (the parasite strain and quantities used are indicated for each experiment). Blood-form Tulahuen trypomastigotes were maintained via serial passage through C3H/HeSnJ mice, and tissue culture-derived Brazil trypomastigotes were created by passage through Vero cell monolayers cultured in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT). Chronically infected mice were used at 5 to 15 months postinfection.
Hybridomas and in vivo depletion with anti-CD25 antibodies.
Anti-interleukin-2 (IL-2) receptor alpha chain-specific B-cell hybridomas (PC61.5.3 and 7D4) were grown in protein-free hybridoma medium II (Invitrogen, Carlsbad, CA). Supernatants were routinely harvested, spun at 400 × g to remove cells and debris, filtered through a 0.45-μm filter, and stored for less than 2 months at 4°C. To precipitate antibodies, an equal amount of ice-cold saturated ammonium sulfate solution was added to the hybridoma supernatant, mixed at 4°C with a stir bar for 12 h, and then centrifuged at 1,500 × g for 30 min at 4°C. Protein pellets were resuspended and dialyzed until isotonic with phosphate-buffered saline (PBS), filter sterilized with a 0.2-μm filter, and stored at −20°C at a concentration of >1.0 mg/ml until use. Mice were depleted of CD25+ cells by intraperitoneal (i.p.) injections of 250 μg to 1,000 μg anti-CD25 monoclonal antibodies (MAbs) prior to or at different intervals during infection (dose frequency and quantity are indicated for each experiment). Flow cytometric assessment of PC61.5.3 MAb-mediated depletion was conducted using fluorescein isothiocyanate (FITC)-conjugated anti-CD25 MAb (7D4 clone; BD Pharmingen, San Diego, CA), and assessment of 7D4 MAb-mediated depletion was conducted using allophycocyanin (APC)-conjugated anti-CD25 MAb (PC61 clone; Caltag, Burlingame, CA).
Extra- and intracellular immunophenotyping, tetramer staining, and flow cytometry.
For direct ex vivo phenotyping, 100 μl of whole blood obtained from retro-orbital puncture using heparinized Natelson blood collection tubes (Fisher, Pittsburgh, PA) or 1 × 106 red blood cell (RBC)-depleted single-cell suspensions from spleens, lymph nodes (LN), or muscles were first washed using PAB buffer (1× PBS, pH 7.4, 0.05% azide, and 1% bovine serum albumin [wt/vol]) and then centrifuged at 400 × g for 10 min (as for all additional washing steps). In the case of MHC tetramer staining, cells were first incubated for 30 min at 37°C with MHC class I (H2Kb) tetramers synthesized at the Tetramer Core Facility (Emory University, Atlanta, GA) loaded with TSKb18 (peptide sequence, ANYDFTLV) or TSKb20 (sequence, ANYKFTLV) peptide (25) conjugated to APC or phycoerythrin (PE), respectively. Cells were ultimately stained for surface markers in a final volume of 100 μl PAB containing a 1/50 dilution of FcBlock (BD Pharmingen) and appropriate combinations of vendor-purchased fluorescently labeled monoclonal antibodies (diluted 1/100) specific for the murine surface markers CD3, CD4, CD8, CD11b, CD25, CD45, CD45.1, CD45.2, and CD45R (BD Pharmingen, Caltag) for 20 min on ice in the dark, followed by two washes in PAB and fixation in 2% formaldehyde for at least 15 min and up to 20 h at 4°C. Erythrocytic whole blood was lysed in 500 ml lysis buffer (10 mM HEPES, 0.83% ammonium chloride) for 5 min, followed by two washes in PAB prior to fixation. In some cases, following surface staining, intracellular scurfin was detected using PE-conjugated anti-scurfin antibodies (1/50 dilution) and a staining kit according to the manufacturer's instructions (eBioscience, San Diego, CA). A Cyan (Dako Cytomation, Fort Collins, CO) or FACSCalibur (BD Pharmingen) flow cytometer and FlowJo software (Tree Star, Inc., Ashland, OR) were used for cytometric data collection and analysis.
Lymphocyte isolation from secondary and nonlymphoid tissues.
In most experiments, animals were exsanguinated and, in some cases (as noted), perfused with 10 ml sterile Alsever's buffered salt solution (pH 7.2). Spleens and lymph nodes (superficial inguinal, popliteal, and mesenteric LN) were removed, and sterile, single-cell, RBC-depleted suspensions were prepared as described previously (22). To aid in the isolation of lymphocytes from muscle tissue after mechanical disruption with forceps and mincing with blades, in most cases muscle tissue was then prepared as stated previously (26), with minor modifications. Briefly, muscle tissue was incubated in serum-free RPMI medium containing Liberase Blendzyme II (70 μg/ml; Roche, Indianapolis, IN) for 1 h at 37°C. Muscle tissue was dissociated in RPMI medium containing 10% FBS and 80 U/ml DNase I (Roche), and supernatants were washed through 40-μm nylon cell strainers (BD Biosciences, Bedford, MA) to filter debris, followed by staining with PE-conjugated anti-CD45 antibodies (BD Pharmingen) (15 min on ice, followed by two washes with RPMI medium). CD45+ cells (white blood cells) were enriched to as least 95% purity by fluorescence-activated cell sorting (FACS) using a MoFlo FACS flow cytometer (Dako Cytomation, Fort Collins, CO).
Cell stimulation, intracellular cytokine staining, and analysis.
A previously described method (22) was used for IFN-γ detection. Briefly, 1 × 106 RBC-depleted splenocytes were cultured overnight in 0.2 ml RPMI 1640 supplemented with 10% FBS in 96-well flat-bottomed plates that had been coated previously with 30 μg/ml anti-CD3 (145-2C11). In the case of peptide stimulations, splenocytes were incubated for 5 h in 96-well round-bottomed plates in a total volume of 0.2 ml RPMI medium plus 10% FBS containing a 1:1,000 dilution of Golgi Plug (BD Pharmingen) and a 2.5 μM concentration of the indicated peptide, phorbol myristate acetate (PMA)-ionophore (50 ng/ml and 500 ng/ml, respectively), or medium only. In vitro-cultured splenocytes were assayed for intracellular IFN-γ, using a Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions. Briefly, FcRγIII and -II were blocked using FcBlock (BD Pharmingen), and cells were stained for surface expression of CD8, using PE-conjugated anti-CD8α diluted 1/100 (BD Pharmingen) for 30 min on ice. Cells were fixed using Cytofix/Cytoperm (BD Pharmingen) on ice for 15 min, washed twice with PermWash (BD Pharmingen), and stained with anti-IFN-γ-APC for 20 min on ice in the dark. In some cases, surface and intracellular staining was performed simultaneously. Cells were fixed and analyzed as described above.
In vivo cytotoxicity assay.
Red blood cell-depleted spleen cells from naïve mice were pulsed separately with 10 μM cruzipain-9 (CZKb9; amino acid sequence, VPLNKCNRL) or TSKb20 peptide or with no peptide for 1 h at 37°C. Cells were washed twice in PBS and labeled (1 × 107 cells/ml) with 5 μM, 1 μM, or 0.2 μM (CZKb9-treated, TSKb20-treated, and unpulsed cells, respectively) carboxyfluorescein diacetate succinimidyl ester (CFSE) for 3 min at room temperature. CFSE labeling was quenched with an equal volume of cold FBS, and cells were washed thrice with RPMI medium containing 10% FBS. The 5 μM (CZKb9), 1 μM (TSKb20), and 0.2 μM (unpulsed) CFSE-labeled cells were combined in equal quantities and transferred intravenously to naïve and acutely infected CD25-depleted or control mice. After 12 h, recipient mice were sacrificed, and single-cell suspensions were prepared from spleens and fixed. CFSE-labeled cells were detected using a Cyan flow cytometer and analyzed using FlowJo software. The percentage of specific killing for each peptide-condition combination was calculated using the following formula: {1 − [(% CFSENO PEPTIDE labeling for naïve mice/% CFSEPEPTIDE labeling for naïve mice)/(% CFSENO PEPTIDE labeling for acutely infected mice/% CFSEPEPTIDE labeling for acutely infected mice)]} × 100.
RNA isolation, reverse transcriptase PCR, and qualitative PCR with cDNA.
Total RNAs were isolated from 1 × 105 to 1 × 106 FACS-enriched CD45+ splenocytes and muscle-derived lymphocytes and also from directly ex vivo-isolated lymph node-derived lymphocytes, using a HighPure RNA isolation kit, and were treated with DNase I according to the manufacturer's protocol (Roche). First-round cDNA products were obtained from 500 ng purified total RNA primed with a mixture of oligo(dT)VN (5′-T24VN-3′) and a cDNA primer specific to murine 18S rRNA (5′-TAATGATCCTTCCGCAGGTTC-3′). Briefly, 0.5 μg oligo(dT)VN and 5 pmol of 18S rRNA primer were added to RNA samples, and the primer-template mixes were heated to 70°C for 10 min to denature. The samples were then cooled to 42°C, and 20-μl reaction mixes were created which contained 1× first-strand buffer, 10 mM dithiothreitol, a 0.5 mM concentration of each deoxynucleoside triphosphate, 40 U RNasin, and 200 U Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). The first-round cDNA reaction mixes were incubated for 1.5 h at 42°C. For each RNA sample, parallel control cDNA reaction mixes were prepared in which the reverse transcriptase was omitted. After first-strand synthesis, the reactions were inactivated by incubation for 10 min at 70°C and then treated with 2 U RNase H (Invitrogen) for 45 min at 37°C to degrade RNA.
The following specific primers and conditions were then used to perform separate qualitative PCRs, using equal amounts of first-round cDNA as a template: 18S forward (5′-GATGGTTTAGTGAGGCCCTCGG-3′) and reverse (5′-ACCTACGGAAACCTTGTTACGACTTTTA-3′) primers, with a 60°C annealing temperature; Foxp3 primers and conditions, as previously described (14); and CD8α and CD4 primers and conditions, as previously described (1). PCR amplifications were carried out separately in 50-μl reaction mixtures containing 2 μl cDNA and final concentrations of 2.5 U Jumpstart Taq (Sigma), 200 μM deoxynucleoside triphosphates, and 0.5 μM specific primer mixes, utilizing the following program: 2.5 min of denaturation at 94°C, followed by 34 cycles of 30 s at 94°C, 30 s at the indicated annealing temperature, and 30 s at 72°C. Ten microliters of each reaction mix and a 1-kb DNA ladder were loaded in a 1.4% agarose-Tris-borate-EDTA gel, electrophoresed at 100 V for 30 min, and stained using ethidium bromide.
Statistical analysis.
Student's t test was used to determine statistical significance between appropriate groups.
RESULTS
Depletion of natural Treg cells prior to lethal challenge does not improve survival.
For some infectious disease models, depletion of CD4+ CD25+ T cells prior to infection has demonstrated that Treg cells are necessary to allow parasite escape from the adaptive immune response (13). To examine whether CD4+ CD25+ Treg cells modulate acute-phase immune responses and contribute to the inability of C57BL/6 mice to survive a lethal parasite challenge, anti-CD25 MAbs (7D4 clone) were administered prior to infection with Tulahuen strain blood-form trypomastigotes. Flow cytometric analysis of peripheral blood lymphocytes (PBLs), using an antibody (PC61 clone) that recognizes an epitope different from that for the depleting MAb, demonstrated that CD4+ CD25+ T cells were significantly reduced, by over 90%, compared to those in PBS-treated controls on the day of infection (Fig. 1A and B). By day 6 postinfection, frequencies of CD4+ CD25+ T cells remained partially reduced, by approximately 25% and 20%, in depleted versus control mice (groups receiving 102 and 103 Tulahuen parasites, respectively) (Fig. 1B).
FIG. 1.
Depletion of CD4+ CD25+ cells prior to lethal challenge with Tulahuen strain parasites does not lead to increased survival. Mice were given 400 μg anti-CD25 MAb (7D4 clone) or PBS at 7 days and 1 day prior to infection, then infected with either 100 or 1000 Tulahuen strain parasites. (A) Representative flow cytometric analysis of PBLs obtained from one mouse of a PBS-treated group (control) and one mouse of an anti-CD25 MAb-treated group (depleted) immediately prior to infection. Numbers in upper-right corner represent the percentage of CD4+ T cells that express CD25. (B) Cumulative data representing the mean frequencies of CD4+ CD25+ T cells in control and depleted groups prior to depletion, immediately prior to infection, and 6 days after infection with either 100 or 1,000 Tulahuen strain parasites. Each group consisted of 5 mice. Error bars represent standard deviation. (C) On day 15 postinfection, the frequency of TSKb20-specific CD8+ T cells was determined by staining PBLs with a FITC-conjugated anti-CD8α antibody and a PE-conjugated class I MHC H2-kb tetramer loaded with TSKb20. A statistically significant increase was observed in the frequency of TSKb20-specific CD8+ T cells in the depleted, compared to the control group, of those mice infected with 1,000 Tulahuen strain parasites (P = 0.013, n = 5 per group). Error bars represent standard deviation. (D) The survival of mice treated with anti-CD25 MAb (filled) or control-treated with PBS (open), prior to infection with 100 (squares) or 1,000 (triangles) Tulahuen strain T. cruzi blood form trypomastigotes, was monitored on a daily basis. Each group was comprised of 5 mice at the start of the experiment.
Depletion of CD4+ CD25+ T cells had a modest effect on the frequency of circulating CD8+ T cells specific for the immunodominant T. cruzi epitope TSKb20, as determined by MHC class I H2-Kb-TSKb20 tetramer staining for one group of treated mice (Fig. 1C) (P = 0.013), indicating that Treg cells may influence the immunodominance of certain antigen-specific CD8+ T-cell responses during acute infection with the Tulahuen strain of parasite. However, the Treg-cell-depleted and control groups exhibited similar mortality rates with both challenge doses (Fig. 1D).
Similarly, there were no significant differences in parasitemia on day 16 postinfection between treated and control mice within each infectious dose group (for group receiving 102 Tulahuen parasites, parasitemia was 8.55 ± 4.77 [control] versus 7.20 ± 4.59 [treated]; and for group receiving 103 Tulahuen parasites, parasitemia was 31.4 ± 30.6 [control] versus 23.5 ± 23.7 [treated] [all values are numbers of parasites {105}/ml of blood]). Thus, while we did observe a significant, albeit modest, increase in the frequency of H2-Kb-TSKb20-specific CD8+ T cells as a function of Treg-cell depletion, this was not sufficient to improve control of infection and did not affect the final outcome.
Depletion of natural Treg cells prior to or during nonlethal infection neither improves nor worsens acute immune responses.
To address the possibility that the partial recovery of CD4+ CD25+ Treg cells during the early time points in infection might account for the lack of an effect of anti-CD25 treatment on acute T. cruzi infection, mice were administered anti-CD25 MAbs both prior to and throughout the acute phase of infection with the lower-virulence Brazil strain of T. cruzi. Using this approach, frequencies of circulating CD4+ CD25+ T cells could be reduced for a longer period, allowing for investigation of the effects of Treg-cell depletion throughout the acute phase. This protocol resulted in a 70 to 90% reduction in CD4+ CD25+ T-cell frequencies throughout the course of the experiment (Fig. 2A). As in the previous experiment, no difference was observed during the course of infection with regards to the longevity of the mice. In addition, the general health of the mice, as assessed by weight measurement (6), was not different in the anti-CD25-treated and control mice (Fig. 2B).
FIG. 2.
Kinetics of CD4+ CD25+ T-cell frequencies and body weight during acute infection with Brazil strain T. cruzi in mice untreated or treated with anti-CD25 MAbs. 2 days prior to infection, and on the day of infection, 2 groups of mice were i.p. injected with 400 μg anti-CD25 depleting MAb (PC61 clone). As a control, another group of mice was left untreated. These 3 groups of mice were then inoculated i.p. with 1 × 103 Brazil strain tissue culture trypomastigotes. Additionally, 400 μg anti-CD25 MAb injections were continuously administered to one group of previously treated mice (deplete-infect-deplete, filled triangles, n = 7) on days 7, 11, 14, 19, 24, and 29 post infection. After the day of infection, neither treated mice (deplete-infect, filled squares, n = 7) nor untreated mice (infect only, filled circles, n = 7) received subsequent anti-CD25 MAb injections. As an additional control, one group of age-matched mice remained untreated and uninfected (“naïve,” open circles, n = 3). (A) Frequencies of circulating CD4+ CD25+ T cells on days indicated. PBLs were stained with CD4-PE and CD25 (7D4 clone)-FITC, and analyzed on a Cyan (Dako Cytomation) flow cytometer. (B) Body weights were measured on days indicated. For both graphs, error bars represent standard deviation.
Previous studies have indicated that Treg cells could influence clonal expansion and the formation of immunodominance hierarchies (12). In order to determine if depletion of Treg cells influenced the T. cruzi-specific CD8+ T-cell clonal expansion, frequencies of peripheral blood-circulating CD8+ T cells specific for the TSKb18 and TSKb20 epitopes were determined by class I MHC H2-kb tetramer staining. The kinetics of the expansion and contraction of CD8+ T cells specific for these epitopes were very similar for all three infected groups, as measured on days 16, 20, and 28 postinfection (Fig. 3A). Thus, removal of Treg cells did not appear to modulate the magnitude of the acute-phase immune response, as measured by clonal expansion of TSKb20- or TSKb18-specific CD8+ T cells.
FIG. 3.
Depletion of CD4+ CD25+ T cells prior to or during nonlethal acute infection with Brazil strain T. cruzi has no effect on the magnitude of antigen-specific immune responses. Following infection with 1 × 103 Brazil strain T. cruzi tissue culture trypomastigotes, peripheral blood samples were taken from mice depleted only prior to infection (deplete-infect, n = 7), depleted both prior to and during infection (deplete-infect-deplete, n = 7), infected only (infect, n = 7), and noninfected (“naïve,” n = 3). (A) PBLs were simultaneously stained with anti-CD8α FITC-conjugated antibodies and class I MHC H2-kb tetramers loaded with either TSKb18 or TSKb20, conjugated to the fluorophores APC or PE, respectively. Naïve mice were included to determine nonspecific background of tetramer-staining. PBLs were fixed and analyzed on a Cyan (Dako Cytomation) flow cytometer. Error bars represent standard deviation. (B) Thirty-two days after infection, an in vivo CTL assay was performed. Spleen cells from naïve B6 mice were separately pulsed with TSKb20 or CZKb9 peptides, or no peptide (unpulsed), then labeled with CFSE, and adoptively transferred to one mouse of each indicated group, as described in the Material and Methods. Splenocytes were recovered from naïve and acutely infected recipient mice 12 h posttransfer, fixed, and analyzed using a flow cytometer (Dako Cytomation). Numbers at the lower left and upper right indicate specific killing of TSKb20 and CZKb9-pulsed target cells, respectively. (C) Splenocytes from naïve, infected only, deplete-infect-depleted, and deplete-infected mice (n = 1, each) 32 days postinfection were cultured in the presence of indicated peptide, media only (“no stim,” negative control), or PMA/ionomycin (“PMA,” positive control) for 5 h in media containing Brefeldin-A. To determine the frequency of CD8+ T cells that produced IFN-γ, cells were stained with fluorescently labeled anti-CD8α and anti-IFN-γ antibodies, as stated in the Material and Methods.
During the acute phase of murine T. cruzi infection, CD8+ T cells kill target cells pulsed with the immunodominant peptide TSKb20 more efficiently than target cells pulsed with the subdominant peptide CZKb9. To investigate the role of Treg cells in modulating T. cruzi-specific cytotoxicity, an in vivo cytotoxic T-lymphocyte assay was performed to determine if the lytic activity of CD8+ T cells against CZKb9-pulsed target cells was increased when Treg cells were depleted during the acute immune response. For mice at 32 days postinfection, 96 to 97% killing of target cells pulsed with the immunodominant TSKb20 epitope was observed after 12 h, whether mice were previously depleted of Treg cells or not. Only 50 to 57% of target cells pulsed with the subdominant CZKb9 epitope were killed in the same infected and depleted mice. Thus, no significant differences were observed in the specific killing of TSKb20- or CZKb9-pulsed targets as a function of depletion of Treg cells (Fig. 3B), suggesting that Treg cells do not appear to suppress CD8+ T-cell cytolytic activity directed against the subdominant CZKb9 epitope.
To further investigate the effects of Treg-cell depletion on the immune responses of acutely infected mice, the ability of CD8+ T cells to produce IFN-γ in response to T. cruzi antigen-specific stimuli was assessed in one mouse. IFN-γ production is crucial for control of T. cruzi infection (50), and in some infections, it is correlated with cytotoxicity (15). Splenocytes from a naïve mouse and from infected, depleted-infected-depleted, and depleted-infected mice were cultured separately in the presence of single T. cruzi-derived immunogenic peptides and brefeldin A for 5 h and then stained for intracellular IFN-γ production. In contrast to the case for the naïve and medium-only controls, significant frequencies of CD8+ T cells from all infected groups produced IFN-γ in response to T. cruzi-derived peptides and PMA-ionomycin, but these frequencies did not differ between depleted and control infected mice (Fig. 3C).
Thus, no apparent differences were observed regarding clonal expansion or immunodominance hierarchies of antigen-specific CD8+ T cells as a function of depletion prior to or during infection.
CD4+ CD25+ T cells are detected in lymphoid and nonlymphoid tissues of chronically infected mice.
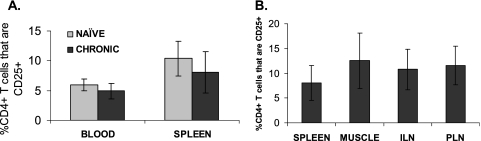
We previously reported that effector memory CD8+ T cells obtained from the muscle tissues of mice with chronic T. cruzi infection are defective in the generation of effector functions (22). To determine if CD4+ CD25+ natural Treg cells play a role in regulating CD8+ T-cell effector function in muscle tissues of chronically infected hosts—the sites of T. cruzi parasite persistence—we examined muscle tissue for the presence of Treg cells. The frequencies of CD4+ CD25+ T cells were determined as percentages of the whole CD4+ T-cell compartment in the peripheral blood, muscle tissue, spleens, and lymph nodes of naïve and chronically infected mice. CD4+ CD25+ T cells were detected in all tissues analyzed, except naïve muscle tissue, as lymphocytes are rarely found in muscles of uninfected mice. No significant differences in the mean frequencies of CD4+ CD25+ T cells were observed with relation to infection status (e.g., naïve versus chronically infected mice), as determined for either the peripheral blood or the spleen (Fig. 4A). Likewise, no significant differences in the mean frequencies of CD4+ CD25+ T cells were observed across different tissues from chronically infected mice, comparing blood, spleens, lymph nodes, and muscles (Fig. 4B).
FIG. 4.
CD4+ CD25+ T cells are detected in peripheral tissues of naïve and chronically infected mice. Blood and tissue lymphocytes were stained with anti-CD4 and anti-CD25 antibodies, and analyzed by flow cytometry. Mean frequencies of CD4+ CD25+ T cells as a percentage of CD4+ T cells are depicted from (A) blood and spleen of naïve and chronically infected mice (n = 102 naïve blood; n = 23 chronic blood; n = 6, naïve spleen; n = 16, chronic spleen) and (B) tissues of chronically infected mice (n = 16, spleen; n = 14, muscle; n = 10, inguinal lymph nodes; n = 6, popliteal lymph nodes). Error bars represent standard deviations.
Foxp3 expression in lymphocytes from muscles and spleens of chronically infected mice.
To date, the expression of Foxp3 is the most widely accepted marker for natural Treg cells (10, 18, 36). Foxp3 mRNA was detected in all spleen- and lymph node-derived total RNA samples from mice with chronic T. cruzi infection, as well as in naïve splenocytes (Fig. 5A). However, CD45+ FACS-enriched (95% purity) cells from muscle tissues of three chronically infected mice revealed relatively low to undetectable levels of Foxp3-specific product, in contrast to their splenic counterparts (Fig. 5A).
FIG. 5.
Analysis of Foxp3 mRNA and scurfin expression. (A) Mice were perfused, total RNA was extracted from spleen, muscle, or lymph node-derived lymphocytes, reverse transcribed, and amplified as described in Materials and Methods. “Sp” and “Mu” refer to FACS-enriched CD45+ lymphocytes from the spleen and muscle, respectively, of mice chronically infected with Brazil-strain T. cruzi. “NSp” refers to splenocytes from a naïve mouse, while “CILN” and “CPLN”, respectively, refer to lymphocyte preparations from the inguinal and popliteal lymph nodes of one mouse chronically infected with Brazil-strain T. cruzi. (B) Lymphocytes were isolated from tissues of naïve or chronically infected mice and stained for extracellular CD4 and intracellular scurfin. Mean data from at least 2 mice are depicted here.
Low Foxp3 expression in lymphocytes from the muscles of mice with chronic T. cruzi infection was confirmed by staining for the protein product of the Foxp3 gene, scurfin. Scurfin expression was detected in lymphocytes derived from all tissues analyzed, with the majority being within the CD4+ T-cell subset (data not shown). However, relatively little scurfin was detected in CD4+ T cells derived from chronically infected muscle, complementing the above findings regarding the relative reduction of Foxp3 mRNA (Fig. 5B).
Treatment of chronically infected mice with anti-CD25 antibodies does not increase the frequency of IFN-γ-producing CD8+ T cells in lymphoid or nonlymphoid compartments.
To test the hypothesis that the CD4+ CD25+ T cells in the muscles of chronically infected mice were regulating CD8+ T-cell function there, monoclonal antibodies to CD25 were used to systemically deplete Treg cells (26, 40, 49). After administration of 1 mg/day of anti-CD25 MAbs (PC61 clone) or PBS (mock treatment) every other day for 6 days to chronically infected mice, CD4+ CD25+ T-cell frequencies were reduced by 67% to 79% in peripheral blood, spleens, lymph nodes, and muscles compared to those in the matching tissues of mock-treated control mice (Fig. 6A). Ex vivo polyclonal stimulation of CD45+ FACS-enriched lymphocytes derived from the spleens and muscle tissues of depleted and mock-treated chronically infected animals indicated no significant differences in the frequencies of IFN-γ-producing CD8+ T cells in antibody-depleted mice compared to mock-treated controls for either tissue (Fig. 6B). Extending the anti-CD25 treatment for 11 or 24 days also failed to alter the frequencies of IFN-γ-producing CD8+ T cells after ex vivo polyclonal stimulation (data not shown). These results demonstrate that either short-term (6 days) or long-term (24 days) depletion of Treg cells does not alter the dysfunctional status of muscle-resident CD8+ T cells in mice with chronic T. cruzi infection.
FIG. 6.
Treatment of chronically infected mice with anti-CD25 monoclonal antibodies does not increase the frequency of IFN-γ producing CD8+ T cells. Mice chronically infected with Brazil strain T. cruzi were given intraperitoneal injections of anti-CD25 MAbs (PC61 clone, n = 2), or mock treated with PBS as a control (n = 2). Mice were perfused and lymphocytes were then isolated from the spleen and muscle tissue. CD45+ cells from muscle tissue were FACS-enriched to >97% purity. (A) Analysis of CD4 and CD25 (7D4 clone) expression. (B) Intracellular IFN-γ responses of spleen and muscle-derived CD8+ lymphocytes cultured for 15 h in the presence of plate-bound anti-CD3. Figure depicts one of three similar independent experiments.
DISCUSSION
While much literature regarding Treg cells and infectious disease has focused on their suppressive effects against effector CD4+ T cells (4, 26, 35), accumulating evidence demonstrates that Treg cells also have the ability to suppress CD8+ T-cell cytokine production and proliferation (7, 32) and that depletion of Treg cells before or during infection can lead to increased CD8+ T-cell cytotoxicity, IFN-γ secretion, and clonal expansion and to enhanced formation of immunological memory (12, 40, 49). Since induction of Treg-cell function can sometimes favor the microbe rather than the host, this may be a mechanism of immune evasion by some parasites, or in some cases, when bystander tissue damage is prevented, both the host and the parasite may experience a mutual benefit (5, 34).
In vivo injection of anti-IL-2 receptor alpha chain-specific monoclonal antibodies depletes Treg cells and thus abrogates their suppressive effects. Apparent roles for Treg cells in allowing parasite escape from host immunity have been demonstrated in various acute and chronic infection models by depleting CD4+ CD25+ Treg cells with anti-CD25 MAbs (for a review, see reference 27). This approach has been used to demonstrate a role for Treg cells in allowing Leishmania (4) to escape from host immunity, as well as uncovering Treg-cell roles in shaping the immunodominance of CD8+ T-cell responses (12). Other studies have demonstrated that CD8+ T-cell-mediated primary immune responses are enhanced when CD4+ CD25+ regulatory T cells are depleted prior to infection with Plasmodium (13) or prior to vaccination and then infection with hepatitis B virus or herpes simplex virus (11, 40).
In this study, similar experiments were performed to determine the role of Treg cells in the acute immune response to T. cruzi infection. While depletion of Treg cells prior to a lethal parasite challenge did not enhance the survival of depleted versus nondepleted control animals, a modest difference in the magnitude of antigen-specific CD8+ T-cell clonal expansion was observed during a period of high parasitemia. While Treg cells may play a role in limiting the magnitude of CD8+ T-cell immune responses in some situations, as suggested by the increased magnitude of TSKb20-specific CD8+ T cells in the Treg-cell-depleted animals, this was not sufficient to improve the outcome of the infection. This indicates that while Treg cells may play a part in modulating immune responses during infection with T. cruzi, Treg cells do not play a pivotal role in suppressing either beneficial or detrimental immune responses, which could benefit the parasite or host, respectively.
To further investigate the role of Treg cells in modulating the acute immune response to T. cruzi, Treg cells were depleted prior to and during acute infection with a nonlethal dose/strain of parasite. In our hands, depletion of CD4+ CD25+ T cells had neither beneficial nor detrimental effects on the outcome of the acute immune response. Interestingly, while anti-CD25 treatments delivered throughout the course of infection did deplete CD4+ CD25+ T cells, this did not appear to reduce the overall magnitudes of antigen-specific CD8+ effector T cells compared to those in nondepleted infected animals, suggesting that anti-CD25 MAb treatments preferentially target CD4+ CD25+ T cells. Had Treg cells been suppressing the priming, activation, or effector function of CD8+ T cells during acute infection, it was hypothesized that their removal would lead to enhanced antigen-specific immune responses, perhaps followed by decreased parasitemia and increased longevity. These outcomes were not observed. Conversely, if Treg cells were playing an overall beneficial role during the immune response by dampening immune hyperactivity, it was hypothesized that depletion of Treg cells would lead to earlier mortality or cachexia, such as occurs during acute T. cruzi infection in the absence of the immunomodulatory cytokine IL-10 (17). This was not the case, as differential mortality and morbidity were not observed for depleted versus nondepleted animals. Therefore, T. cruzi has adopted a mechanism of immune evasion that appears to be independent of Treg-cell-mediated suppression of T. cruzi-specific immune responses.
The impetus for the second part of this investigation was our recent report that effector/memory CD8+ T cells isolated from the muscles of mice with chronic T. cruzi infection have little cytolytic ability and are poor producers of IFN-γ compared to effector/memory CD8+ T cells from the spleens of the same chronically infected animals (22). Since dysfunctional CD8+ T cells reside in muscle, a site of parasite persistence in chronically infected mice, investigating the cause or maintenance of the observed CD8+ T-cell dysfunction was of great interest. Thus, we asked if Treg-cell-mediated suppression was an explanation for the poor effector function of CD8+ T cells in muscle tissue. Since the infiltration and retention of Treg cells in chronically inflamed tissues are a requirement for Treg-cell-mediated suppression of tissue-resident effector cells (16, 38, 39), it was hypothesized that if Treg cells were suppressing CD8+ T-cell responses in chronically infected muscle tissue, then Treg cells should be detectable in this tissue. While CD4+ CD25+ T cells were detected in chronically infected muscle tissue, as well as other tissues, one caveat of this phenotypical approach is that CD25 is not expressed exclusively by natural Treg cells but is also transiently expressed by other lymphoid cells upon activation during autocrine IL-2 signaling. Thus, we measured the expression of the Foxp3 gene (and its protein product, scurfin), which has been identified as the best-defined marker of natural Treg cells (36). It was hypothesized that if Treg cells were playing a dominant suppressive role in chronically infected muscle, we would expect to see a relative increase in the frequency of CD4+ Foxp3+ T cells in the muscle, in contrast to the case for the spleen, where CD8+ effector T-cell responses appear to be robust. However, the CD4+ T-cell expression of scurfin and total lymphocyte Foxp3 mRNA expression were relatively lower in the muscle tissues than in the spleens of chronically infected mice.
The observed expression of CD25 by CD4+ T cells derived from chronically infected muscle tissue may indicate that these CD4+ T cells are activated effector cells. Class II MHC molecules are expressed in inflamed muscle (51), in chronically T. cruzi-infected muscle (53), and on professional antigen-presenting cells that are found in the inflamed muscles of T. cruzi-infected mammals (2). Therefore, MHC class II-positive antigen-presenting cells capable of activating CD4+ T cells are found in chronically infected muscle tissue.
In at least three separate experiments administering depleting anti-CD25 monoclonal antibodies to chronically infected mice for continuous periods ranging from 6 to 24 days, we observed relative reductions of CD4+ CD25+ T cells of approximately 70 to 90%, comparing depleted versus PBS-treated mice. Ex vivo polyclonal stimulation of lymphocytes isolated from the muscles and spleens of chronically infected-depleted and control treated mice revealed that the frequency of IFN-γ-secreting CD8+ T cells was not increased as a result of depletion, further suggesting that natural Treg cells do not play a role in the suppression of CD8+ T-cell function in muscle tissue. However, the relative reduction of CD4+ CD25+ T cells varied according to the target tissue analyzed. In our hands, the greatest reduction of CD4+ CD25+ T cells achieved in muscle tissue was 76%. While the inability to completely deplete CD4+ CD25+ T cells from the muscle tissue of chronically infected mice might confound interpretation of the resultant functional effects of depletion, the finding that this degree of depletion did not increase the frequency of IFN-γ-producing CD8+ T cells, taken together with the finding that CD4+ Foxp3+ Treg cells are not enriched in muscle tissue, supports our conclusion that in the Brazil strain/B6 mouse model, Treg cells do not play a major role in the regulation of muscle-resident CD8+ T cells of chronically infected mice.
These results rule out one possibility but offer little insight into other possibilities in determining the mechanism of T. cruzi evasion of the immune response during the acute phase of infection or the cause of CD8+ T-cell dysfunction in the muscle tissue of mice with chronic T. cruzi infection.
Acknowledgments
We thank Julie Nelson of the Center for Tropical and Emerging Global Diseases at the University of Georgia for assistance with flow cytometry and cell sorting. We also thank Diana Martin and Todd Minning for advice and technical help as well as Daniel Colley, Boris Striepen, and Yasmine Belkaid for helpful conversations.
This work was supported by NIH grant R01 AI22070 to R.L.T.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Akiba, H., J. Kehren, M. T. Ducluzeau, M. Krasteva, F. Horand, D. Kaiserlian, F. Kaneko, and J. F. Nicolas. 2002. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J. Immunol. 168:3079-3087. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, S. G., A. R. Pimentel, M. M. de Souza, and Z. A. Andrade. 2000. Interstitial dendritic cells of the heart harbor Trypanosoma cruzi antigens in experimentally infected dogs: importance for the pathogenesis of chagasic myocarditis. Am. J. Trop. Med. Hyg. 63:64-70. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y. 2003. The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert Opin. Biol. Ther. 3:875-885. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 6.Chamekh, M., V. Vercruysse, M. Habib, M. Lorent, M. Goldman, A. Allaoui, and B. Vray. 2005. Transfection of Trypanosoma cruzi with host CD40 ligand results in improved control of parasite infection. Infect. Immun. 73:6552-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieckmann, D., H. Plottner, S. Dotterweich, and G. Schuler. 2005. Activated CD4+ CD25+ T cells suppress antigen-specific CD4+ and CD8+ T cells but induce a suppressive phenotype only in CD4+ T cells. Immunology 115:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DosReis, G. A., C. G. Freire-de-Lima, M. P. Nunes, and M. F. Lopes. 2005. The importance of aberrant T-cell responses in Chagas disease. Trends Parasitol. 21:237-243. [DOI] [PubMed] [Google Scholar]
- 9.Dutra, W. O., M. O. Rocha, and M. M. Teixeira. 2005. The clinical immunology of human Chagas disease. Trends Parasitol. 21:581-587. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot, J. D., J. P. Rasmussen, L. M. Williams, J. L. Dooley, A. G. Farr, and A. Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22:329-341. [DOI] [PubMed] [Google Scholar]
- 11.Furuichi, Y., H. Tokuyama, S. Ueha, M. Kurachi, F. Moriyasu, and K. Kakimi. 2005. Depletion of CD25+CD4+ T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J. Gastroenterol. 11:3772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeryfar, S. M., R. J. DiPaolo, D. C. Tscharke, J. R. Bennink, and J. W. Yewdell. 2005. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 174:3344-3351. [DOI] [PubMed] [Google Scholar]
- 13.Hisaeda, H., Y. Maekawa, D. Iwakawa, H. Okada, K. Himeno, K. Kishihara, S. Tsukumo, and K. Yasutomo. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 10:29-30. [DOI] [PubMed] [Google Scholar]
- 14.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 15.Horton, H., N. Russell, E. Moore, I. Frank, R. Baydo, C. Havenar-Daughton, D. Lee, M. Deers, M. Hudgens, K. Weinhold, and M. J. McElrath. 2004. Correlation between interferon-gamma secretion and cytotoxicity, in virus-specific memory T cells. J. Infect. Dis. 190:1692-1696. [DOI] [PubMed] [Google Scholar]
- 16.Huehn, J., K. Siegmund, and A. Hamann. 2005. Migration rules: functional properties of naive and effector/memory-like regulatory T cell subsets. Curr. Top. Microbiol. Immunol. 293:89-114. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, C. A., L. A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311-3316. [PubMed] [Google Scholar]
- 18.Khattri, R., T. Cox, S. A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337-342. [DOI] [PubMed] [Google Scholar]
- 19.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M. C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219-1227. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., and R. L. Tarleton. 1998. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 20:207-216. [DOI] [PubMed] [Google Scholar]
- 21.Laucella, S. A., M. Postan, D. Martin, B. Hubby Fralish, M. C. Albareda, M. G. Alvarez, B. Lococo, G. Barbieri, R. J. Viotti, and R. L. Tarleton. 2004. Frequency of interferon-gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J. Infect. Dis. 189:909-918. [DOI] [PubMed] [Google Scholar]
- 22.Leavey, J. K., and R. L. Tarleton. 2003. Cutting edge: dysfunctional CD8+ T cells reside in nonlymphoid tissues during chronic Trypanosoma cruzi infection. J. Immunol. 170:2264-2268. [DOI] [PubMed] [Google Scholar]
- 23.Martin, D., and R. Tarleton. 2004. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol. Rev. 201:304-317. [DOI] [PubMed] [Google Scholar]
- 24.Martin, D. L., and R. L. Tarleton. 2005. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J. Immunol. 174:1594-1601. [DOI] [PubMed] [Google Scholar]
- 25.Martin, D. L., D. B. Weatherly, S. A. Laucella, M. A. Cabinian, M. T. Crim, S. Sullivan, M. Heiges, S. H. Craven, C. S. Rosenberg, M. H. Collins, A. Sette, M. Postan, and R. L. Tarleton. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez, S., S. K. Reckling, C. A. Piccirillo, D. Sacks, and Y. Belkaid. 2004. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J. Exp. Med. 200:201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills, K. H. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4:841-855. [DOI] [PubMed] [Google Scholar]
- 28.Nabors, G. S., and R. L. Tarleton. 1991. Differential control of IFN-gamma and IL-2 production during Trypanosoma cruzi infection. J. Immunol. 146:3591-3598. [PubMed] [Google Scholar]
- 29.Onizuka, S., I. Tawara, J. Shimizu, S. Sakaguchi, T. Fujita, and E. Nakayama. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128-3133. [PubMed] [Google Scholar]
- 30.Ouaissi, A., and M. Ouaissi. 2005. Molecular basis of Trypanosoma cruzi and Leishmania interaction with their host(s): exploitation of immune and defense mechanisms by the parasite leading to persistence and chronicity, features reminiscent of immune system evasion strategies in cancer diseases. Arch. Immunol. Ther. Exp. (Warsaw) 53:102-114. [PubMed] [Google Scholar]
- 31.Paulino, M., F. Iribarne, M. Dubin, S. Aguilera-Morales, O. Tapia, and A. O. Stoppani. 2005. The chemotherapy of Chagas' disease: an overview. Mini Rev. Med. Chem. 5:499-519. [DOI] [PubMed] [Google Scholar]
- 32.Piccirillo, C. A., and E. M. Shevach. 2001. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 167:1137-1140. [DOI] [PubMed] [Google Scholar]
- 33.Reis, D. D., E. M. Jones, S. Tostes, Jr., E. R. Lopes, G. Gazzinelli, D. G. Colley, and T. L. McCurley. 1993. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am. J. Trop. Med. Hyg. 48:637-644. [DOI] [PubMed] [Google Scholar]
- 34.Rouse, B. T., and S. Suvas. 2004. Regulatory cells and infectious agents: detentes cordiale and contraire. J. Immunol. 173:2211-2215. [DOI] [PubMed] [Google Scholar]
- 35.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531-562. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi, S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6:345-352. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455-458. [DOI] [PubMed] [Google Scholar]
- 38.Siegmund, K., M. Feuerer, C. Siewert, S. Ghani, U. Haubold, A. Dankof, V. Krenn, M. P. Schon, A. Scheffold, J. B. Lowe, A. Hamann, U. Syrbe, and J. Huehn. 2005. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 106:3097-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suffia, I., S. K. Reckling, G. Salay, and Y. Belkaid. 2005. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 174:5444-5455. [DOI] [PubMed] [Google Scholar]
- 40.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarleton, R. L. 2003. Chagas disease: a role for autoimmunity? Trends Parasitol. 19:447-451. [DOI] [PubMed] [Google Scholar]
- 42.Tarleton, R. L. 1988. Trypanosoma cruzi-induced suppression of IL-2 production. I. Evidence for the presence of IL-2-producing cells. J. Immunol. 140:2763-2768. [PubMed] [Google Scholar]
- 43.Tarleton, R. L. 1988. Trypanosoma cruzi-induced suppression of IL-2 production. II. Evidence for a role for suppressor cells. J. Immunol. 140:2769-2773. [PubMed] [Google Scholar]
- 44.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 45.Tarleton, R. L., M. J. Grusby, and L. Zhang. 2000. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J. Immunol. 165:1520-1525. [DOI] [PubMed] [Google Scholar]
- 46.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 47.Tarleton, R. L., J. Sun, L. Zhang, and M. Postan. 1994. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas' disease. Infect. Immun. 62:1820-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornton, A. M., C. A. Piccirillo, and E. M. Shevach. 2004. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur. J. Immunol. 34:366-376. [DOI] [PubMed] [Google Scholar]
- 49.Toka, F. N., S. Suvas, and B. T. Rouse. 2004. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J. Virol. 78:13082-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torrico, F., H. Heremans, M. T. Rivera, E. Van Marck, A. Billiau, and Y. Carlier. 1991. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 146:3626-3632. [PubMed] [Google Scholar]
- 51.Wiendl, H., R. Hohlfeld, and B. C. Kieseier. 2005. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol. 26:373-380. [DOI] [PubMed] [Google Scholar]
- 52.Wizel, B., M. Nunes, and R. L. Tarleton. 1997. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J. Immunol. 159:6120-6130. [PubMed] [Google Scholar]
- 53.Zhang, L., and R. L. Tarleton. 1996. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp. Parasitol. 84:203-213. [DOI] [PubMed] [Google Scholar]
- 54.Zhao, D. M., A. M. Thornton, R. J. Dipaolo, and E. M. Shevach. 2006. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 107:3925-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]