Abstract
A genetic subpopulation of Enterococcus faecium, called clonal complex 17 (CC-17), is strongly associated with hospital outbreaks and invasive infections. Most CC-17 strains contain a putative pathogenicity island encoding the E. faecium variant of enterococcal surface protein (Esp). Western blotting, flow cytometric analyses, and electron microscopy showed that Esp is expressed and exposed on the surface of E. faecium, though Esp expression and surface exposure are highly varied among different strains. Furthermore, Esp expression depends on growth conditions like temperature and anaerobioses. When grown at 37°C, five of six esp-positive E. faecium strains showed significantly increased levels of surface-exposed Esp compared to bacteria grown at 21°C, which was confirmed at the transcriptional level by real-time PCR. In addition, a significant increase in surface-exposed Esp was found in half of these strains when grown at 37°C under anaerobic conditions compared to the level in bacteria grown under aerobic conditions. Finally, amounts of surface-exposed Esp correlated with initial adherence to polystyrene (R2 = 0.7146) and biofilm formation (R2 = 0.7535). Polystyrene adherence was competitively inhibited by soluble recombinant N-terminal Esp. This study demonstrates that Esp expression on the surface of E. faecium (i) varies consistently between strains, (ii) is growth condition dependent, and (iii) is quantitatively correlated with initial adherence and biofilm formation. These data indicate that E. faecium senses and responds to changing environmental conditions, which might play a role in the early stages of infection when bacteria transit from oxygen-rich conditions at room temperature to anaerobic conditions at body temperature. In addition, variation of surface exposure may explain the contrasting findings reported on the role of Esp in biofilm formation.
Over the last 2 decades, enterococci have emerged as important nosocomial pathogens resistant to virtually all antibiotics, including vancomycin (1, 35). Resistance to clinically relevant antibiotics, such as ampicillin and vancomycin, is increasing and found primarily in E. faecium. This may explain its rapid emergence as an etiological agent of nosocomial infections, noticed first in the United States and, more recently, in Europe and Asia (24, 39; see also the European Antimicrobial Resistance Surveillance System at http://www.earss.rivm.nl). Molecular epidemiological studies of E. faecium isolates, resistant and susceptible to vancomycin and derived from different ecological niches, identified a specific clonal complex, designated CC-17, strongly associated with nosocomial outbreaks in five continents (45). Almost all CC-17 isolates are resistant to β-lactam antibiotics, and a substantial proportion contains a putative pathogenicity island, which carries the E. faecium variant of the enterococcal surface protein (esp) gene (17, 45). Esp of E. faecium shares a homology of up to 90% with the previously described Esp protein of E. faecalis, also located on a pathogenicity island (30) and expressed on the surface of the bacterium (31). Little is known about the role of Esp in the pathogenesis of enterococcal infections. For E. faecalis, esp-positive stains were more frequently found among isolates associated with invasive infections than among isolates colonizing the gut (32). Furthermore, insertional inactivation of the esp gene attenuated E. faecalis virulence in an ascending urinary tract infection mouse model (31), but no role of Esp could be demonstrated in a mouse intestinal colonization model (26). Conflicting results have also been reported about the role of esp in biofilm formation and adherence to polystyrene. In several studies, Esp of E. faecalis appeared to be important in the initial adherence to polystyrene and biofilm development (20, 36, 41). In a recent study, the N-terminal region of Esp, covering approximately half of the protein in E. faecalis, was identified as the region of the protein involved in biofilm formation (38). In other studies, however, biofilm development appeared to be independent of Esp (6, 12, 16, 27). In E. faecalis, several other factors have been associated with biofilm development, like the sugar-binding transcriptional regulator BopD (13), the quorum-sensing locus fsr (12), heterogeneity in surface charge (43), the bee locus (37), and the secreted metalloprotease GelE (12, 16, 25). Yet absence of a correlation between gelatinase and biofilm development has also been found (6, 21, 43).
Currently, little is known about esp of E. faecium. As mentioned, esp of E. faecium is predominantly present in clinical isolates. Di Rosa et al. (6) report that, although some esp-negative strains developed biofilm, esp-positive strains produced thicker biofilms. Furthermore, the presence of both esp and biofilm development was found only in strains from clinical settings.
To explore the role of Esp in the pathogenesis of E. faecium infections, we studied Esp expression using Western blotting and flow cytometry under different growth conditions, like temperature and anaerobioses. We also assessed the association between the amount of cell surface-associated Esp and the ability to adhere to polystyrene and biofilm development.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Eight E. faecium strains— E135, E155, E300, E470, E745, E1165, E1172, and E1176 (2, 9, 40, 42, 44, 45)—were used in this study. All bacteria were of human origin, and except for E135, all were esp positive. E135, E300, E155, E470, and E745 were vancomycin resistant. E135 was a community surveillance isolate. E155, E300, E470, and E745 were isolates from hospital outbreaks in the United States and The Netherlands. Finally, E1165, E1172, and E1176 were all clinical isolates recovered from wounds, urine, and the respiratory tract, respectively (Table 1). For the cloning and expression of Esp, Escherichia coli TOP10F′ and BL21(DE3)pLysS were used. E. faecium was grown on sheep red blood agar (SRA) containing tryptic soy agar with 5% sheep red blood cells (BD, Alphen aan den Rijn, The Netherlands), brain heart infusion, brain heart infusion agar, tryptic soy broth (TSB), and TSB supplemented with 0.25% glucose. Bacteria grown on SRA were incubated for 72 h at 21°C or 18 h at 37°C under aerobic conditions or in a container with an anaerobic atmosphere (5% H2, 10% CO2, and 85% N2). Before each experiment, bacteria were initially grown at 21°C on SRA. E. coli was grown either on Luria-Bertani agar or on Luria-Bertani agar supplemented, if necessary, with 50 μg/ml ampicillin.
TABLE 1.
Bacteria used in this study
| Strain | esp | vanA | Source of isolation | Reference(s) |
|---|---|---|---|---|
| E135 | − | + | Community surveillance | 44, 45 |
| E155 | + | + | Hospital outbreak | 3, 45 |
| E300 | + | + | Hospital outbreak | 9, 45 |
| E470 | + | + | Hospital outbreak | 40, 45 |
| E745 | + | + | Hospital outbreak | 44, 45 |
| E1165 | + | − | Clinical isolate, wound | 45 |
| E1172 | + | − | Clinical isolate, urine | 45 |
| E1176 | + | − | Clinical isolate, respiratory tract | 45 |
Cloning N-terminal Esp.
A fragment of the N-terminal domain of Esp was cloned using the primer set Esp-1 (5′-ATGGGAACGCCTTGGTATG-3′) and Esp-2 (5′-TACTGCTAAATCGGTCGTG-3′). Both Esp-1 and Esp-2 are based on esp of E. faecalis (AF034779) and start at positions 999 and 2295, respectively. In Esp-1, an additional 5′ ATG was added for the in-frame expression of Esp. PCRs were performed with 25 μl using HotStarTaq DNA polymerase (QIAGEN Benelux B.V., Venlo, The Netherlands), with 10 pmol of each primer and 10 nmol of E300 chromosomal DNA. DNA was isolated as described previously (30). PCR conditions were as follows: initial denaturation at 95°C for 15 min followed by 30 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 1 min, followed by an extension at 72°C for 7 min. PCR fragments were directly cloned in the pCRT7/CT-TOPO TA expression vector (Invitrogen, Breda, The Netherlands) and were used to transform One Shot TOP10F′ chemically competent E. coli (Invitrogen, Breda, The Netherlands) according to the manufacturer's instructions. Plasmids were isolated with a plasmid purification kit (QIAGEN Benelux B.V., Venlo, The Netherlands) and checked by sequencing using the BigDye Terminator reaction kit and an ABI PRISM 3700 DNA analyzer (both from Applied Biosystems, Foster City, CA). Plasmid DNA containing the right constructs was used to transform One Shot BL21(DE3)pLysS chemically competent E. coli (Invitrogen, Breda, The Netherlands). The recombinant N-terminal Esp domain with a His tag at its C-terminal end (rN-Esp) was induced by isopropyl-β-d-thiogalactopyranoside (IPTG) and isolated using a nickel column (Probond, Invitrogen, Breda, The Netherlands), all according to the manufacturer's instructions.
Raising polyclonal antibodies and isolation of immunoglobulin G.
Polyclonal antibodies to E. faecium rN-Esp were raised by immunizing two rabbits. For the initial doses, 150 μg rN-Esp in incomplete Freund's adjuvant was injected subcutaneously. Subsequently, a booster dose of 150 μg was administered at day 28. Rabbits were exsanguinated at day 42, and their sera were collected and stored at −20°C. All experiments were performed according to Dutch regulations on animal experiments.
Western blotting.
After growth at 21°C or 37°C on SRA plates (bacteria grown at 37°C were stored under both aerobic and anaerobic conditions), bacteria were collected from the plates and resuspended in phosphate-buffered saline (PBS), generating an optical density at 660 nm (OD660) of 1.0 (1 × 109 bacteria/ml). For each sample, 1 ml (1 × 109 bacteria) was harvested at 6,500 × g for 1 min, and the bacteria were lysed by incubating the cell pellet in 200 μl buffer containing 50 μg/ml linezolid, 0.5 mM phenylmethylsulfonyl fluoride, 1 mg/ml lysozyme, and 20% sucrose in PBS at 37°C. After 2 h, samples were centrifuged (10,000 × g, 5 min), and total protein content was quantified by OD280 measurements. Samples were normalized for protein content, and Western blotting was performed as described previously (28). Esp was detected with anti-Esp rabbit immune serum followed by horseradish peroxidase-conjugated goat anti-rabbit (Santa Cruz Biotechnology, Santa Cruz, CA).
Real-time quantitative PCR.
After growth at 21°C or 37°C under aerobic conditions on SRA plates, bacteria were collected from the plates and resuspended in PBS to an OD660 of 1.0 (1 × 109 bacteria/ml). For each sample, 3 ml (3 × 109 bacteria) was taken and pelleted by centrifugation (6,500 × g, 1 min). RNA species were isolated according to procedures outlined by Cheung et al. (4). RNA purification and cDNA generation were done as described by Nallapareddy et al. (22) with some modifications. Total RNA was treated three times with 20 U of RQ1 DNase (Promega Corp., Madison, WI) for 30 min at 37°C, and RNA was isolated using an RNeasy minikit (QIAGEN). From 1.5 μg total RNA, cDNA was synthesized with the SuperScript II first-stand synthesis system (Invitrogen Corp., Carlsbad, CA) using random primers according to the instructions of the manufacturer. Three primer-probe sets were used in this study, one to detect cDNA of 23S rRNA, one to detect esp of E470, and one to detect Esp cDNA of the other esp-positive strains. The DNA region encoding the Esp N-terminal domain is too diverse to use one primer-probe combination for all esp-positive strains. The sequences of the primer-probe combinations are as follows: for 23S rRNA-F, 5′-CCAGGTTGAAGGTGCGGT-3′; for 23S rRNA-R, 5′-CCTCATCCCCGCACTTTTC-3′; for 23S rRNA-probe, (6-carboxyfluorescein [6-FAM]-CACTGGAGGACCGAACCCACGG-6-carboxytetramethylrhodamine [TAMRA]); for EspE470-F, 5′-TTGGTCTTATCTTTGGAGCAACTG-3′; for EspE470-R, 5′-TTCGTAGCTGTTGCCAATATTTTG-3′; for EspE470-probe, 6-FAM-AGCTGTTAATGCACAAGGCAACTTTTCTTCAA-TAMRA; for EspE300-F, 5′-GGTGATGGAAACCCTGACGA-3′; for EspE300-R, 5′-CTTTCCCCTTAACTGTTGTGTCAAC-3′; and for EspE300-probe, 6-FAM-AAGAAGAGAGCGGAGACACGAATCCATATATCG-TAMRA.
Real-time PCR was performed using TaqMan Universal PCR master mix (Applied Biosystems). The concentrations of the three primer-probe combinations were optimized and were 300 μM for 23S rRNA-F, 900 μM for 23S rRNA-R, 100 μM for 23S rRNA-probe, 300 μM for EspE470-F, 300 μM for EspE470-R, 50 μM for EspE470-probe, 300 μM for EspE300-F, 900 μM for EspE300-R, and 50 μM for EspE300-probe. The chosen PCR conditions were 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 94°C for 15 s and 60°C for 1 min on an ABI PRISM 7700 sequence detector (Applied Biosystems). The ΔΔCT method of Livak et al. (18) was used to calculate the difference in Esp mRNA present in bacteria grown at 37°C and at 21°C using 23S rRNA as an internal control. Analyses were performed in triplicate.
Detection of Esp on the surface of E. faecium.
After the appropriate incubation, bacteria were collected from the plates and resuspended in RPMI 1640 (Cambrax Bioscience) containing 0.05% human serum albumin (HSA) to an OD660 of 1.0 (1 × 109 CFU/ml). For each sample, 100 μl (1 × 107 CFU) was taken and pelleted by centrifugation (6,500 × g, 1 min) and 50 μl RPMI 1640-HSA containing 1/100 anti-Esp rabbit immune serum was added. Bacteria were resuspended and incubated on ice. After 30 min, 1 ml cold RPMI 1640-HSA was added, and the bacteria were isolated by centrifugation (6,500 × g, 1 min). Bacteria were resuspended in 50 μl RPMI 1640-HSA containing a 1/50 dilution of goat anti-rabbit fluorescein isothiocyanate (Sigma-Aldrich, Saint Louis, MO) and left for 30 min on ice. Bacteria were washed with 1 ml cold RPMI 1640-HSA, and after centrifugation (6,500 × g, 1 min), bacteria were resuspended in 50 μl RPMI 1640-HSA. Before analyses in a FACSCalibur (BD, Alphen aan den Rijn, The Netherlands), bacteria were resuspended in 300 μl RPMI 1640-HSA. All measurements were performed with the same machine using the same parameters. The data were normalized for bacterial size, and experiments were performed at least three times. The mean fluorescence (mean fluorescence channel 1) was used as a measure for cell surface-associated Esp. Pooled rabbit preimmune serum and bacteria incubated without anti-Esp rabbit immune serum were used as negative controls. The specificity of the anti-Esp rabbit immune serum was demonstrated by blocking the binding of serum to Esp on the bacterial cell surface after preincubating 1/300-diluted anti-Esp serum in RPMI 1640-HSA with serial dilutions of rN-Esp, starting with 100 μg/ml, for 15 min prior to incubation with the bacteria.
Electron microscopy.
Bacteria were grown on SRA at 37°C and treated as follows. A drop of 1 × 109 CFU/ml in PBS was placed on Parafilm, and a 200-mesh Formvar-carbon-coated copper grid was floated on the surface for 10 min. Grids were washed three times by floatation for 5 min on drops of 0.02 M glycine in PBS and blocked by floatation for 15 min on drops of 1% bovine serum albumin in PBS (PBSb). Esp was labeled by floating the grids for 1 h on drops containing a 1/100 dilution of anti-Esp rabbit immune serum in PBSb. Grids were washed four times by floating them for 2 min on drops of 0.1% bovine serum albumin in PBS. Antibodies were labeled by floating the grids for 20 min on drops of proteinA-Gold (15 nm) (34) in PBSb. Grids were washed by floatation four times for 2 min on drops of PBS, fixed by floatation on drops of 1% glutaraldehyde, and washed again by floatation eight times for 2 min on drops of H2O. Bacteria were stained by floating the grids for 5 min on drops containing 1.8% methylcellulose (25 centipoises; Sigma-Aldrich, Saint Louis, MO) and 0.4% uranyl acetate (pH 4) and subsequently air dried for 10 min. Bacteria used as negative controls were treated in a similar way except that the incubation with anti-Esp rabbit immune serum was left out. Grids were examined for bacteria by using a Jeol 1010 transmission electron microscope (Jeol-Europe, Amsterdam, The Netherlands) at a magnification of ×30,000.
Initial adherence assay.
The initial adherence assay was performed according to Hufnagel et al. (13). Briefly, plate-grown bacteria were resuspended in TSB at a concentration of 5 × 107 CFU/ml. To the wells of a polystyrene 96-well plate (Corning Inc., Corning, NY), 100-μl bacterial suspensions were added in triplicate, and the plate was incubated at 37°C. In inhibition studies, inhibitors were added directly to the bacterial suspension. After incubation for 1 h, bacteria were removed, and the wells were gently washed three times with 200 μl PBS. The plates were dried by incubating them for 1 h at 60°C. To each well, 50 μl Gram's crystal violet solution (Merck, Darmstadt, Germany) was added. After 2 min, the stain was taken off and the plates were washed in tap water. Finally, the plates were dried for 10 min at 60°C, and the OD595 was measured with an enzyme-linked immunosorbent assay reader. Experiments were performed three times in triplicate.
Blocking of adherence by rN-Esp.
Strains were grown for 72 h on SRA at 21°C and for 18 h at 37°C. Subsequently, strains were suspended at different bacterial concentrations (5 × 107, 2.5 × 107, and 1.25 × 107 CFU) in TSB containing different concentrations of rN-Esp (0.01 to 100 μg/ml) and assayed for primary attachment.
Biofilm assay.
Biofilm development was assayed in a way similar to the way initial adherence was assayed, except that the test was performed with TSB supplemented with 0.25% glucose, and 1 × 105 CFU of bacteria/ml were left for 24 h at 37°C in 96-well polystyrene plates.
Statistics.
Student's t test was used to assess statistically significant differences.
RESULTS
Growth conditions influence Esp expression.
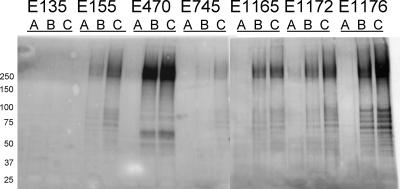
To determine the influence of growth conditions on Esp expression, strains were grown on SRA at 21°C (72 h) or 37°C (18 h) under aerobic conditions and at 37°C (18 h) under anaerobic conditions and analyzed by Western blotting using the anti-Esp polyclonal rabbit serum. Esp expression at 37°C increased relative to that at 21°C in all esp-positive strains, though expression differed substantially among strains. In five of six esp-positive strains, Esp expression increased when they were grown under anaerobic conditions at 37°C, relative to what occurred under aerobic conditions at 37°C (Fig. 1).
FIG. 1.
Western blot analyses of a 10% sodium dodecyl sulfate-polyacrylamide gel using the rabbit polyclonal anti-Esp serum. The Western blot shows growth culture-dependent expression of Esp in one esp-negative E. faecium strain (E135) and six esp+ E. faecium strains (E155, E470, E745, E1165, E1172, and E1176). Bacteria listed were grown on SRA for 72 h at 21°C under aerobic conditions (lanes A), 18 h at 37°C under aerobic conditions (lanes B), or 18 h at 37°C under anaerobic conditions (lanes C). Numbers to the left of the gel are molecular weights (in thousands).
Growth condition-dependent Esp exposure on the surface of E. faecium.
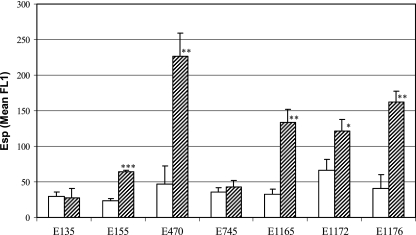
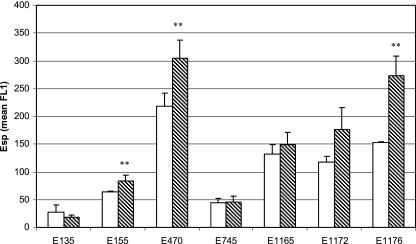
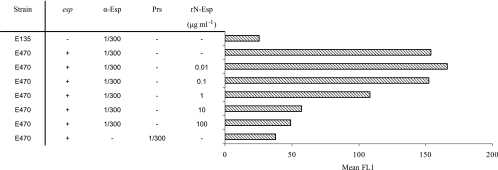
To show that Esp of E. faecium is exposed on the surface of the cell, bacteria were grown overnight at 37°C, incubated with anti-Esp polyclonal rabbit serum, and assayed by flow cytometry. Surface exposure of Esp was demonstrated in all six esp-positive strains (data not shown). This was confirmed for the esp-positive strain E470 and the esp-negative strain E135 by transmission electron microscopy on negatively stained immunogold-labeled bacteria (Fig. 2). On strain E470, gold particles are clearly associated with the bacterial cell wall, demonstrating the association of Esp with the bacterial cell wall (Fig. 2A). On the surface of strain E135 (Fig. 2C), gold particles were found in small amounts comparable to those found in the negative controls without the anti-Esp polyclonal rabbit serum (Fig. 2B and D). To quantify the influence of growth conditions on surface-associated Esp, all seven strains were grown at two different temperatures, 21°C and 37°C, and at 37°C under aerobic and anaerobic conditions, before they were assayed by flow cytometry. Five of six esp-positive strains had significantly increased levels of surface-associated Esp when grown at 37°C compared to bacteria grown at 21°C (Fig. 3). Only E745 had low-level surface-associated Esp at 21°C and 37°C. A similar effect of temperature-dependent, elevated, surface-associated Esp expression was obtained when bacteria were grown on brain heart infusion agar medium (data not shown), indicating that the level of surface-associated Esp on E. faecium is growth temperature dependent but independent of the growth media tested. Three of the six esp-positive strains (E155, E470, E1176) had significantly elevated levels of surface-associated Esp when grown under anaerobic conditions (Fig. 4). Binding of anti-Esp antiserum could be blocked by adding an excess of the N-terminal domain of Esp (Fig. 5), indicating that the anti-Esp antiserum specifically recognized Esp.
FIG. 2.
Transmission electron microscope picture at a magnification of ×30,000 of E470 and E135 E. faecium strains negatively stained by methylcellulose uranyl acetate and labeled with immunogold (15 μm) using anti-Esp antiserum. The inserted bar indicates a length of 200 nm. E470 incubated with anti-Esp polyclonal rabbit serum and proteinA-Gold (A), E470 incubated with proteinA-Gold (B), E135 incubated with anti-Esp polyclonal rabbit serum and proteinA-Gold (C), and E135 incubated with proteinA-Gold (D).
FIG. 3.
Temperature-dependent expression of cell wall-associated Esp of E. faecium. Shown are the means of the mean FL1 from three independent experiments. Bacteria listed were grown on SRA for 72 h at 21°C (open bars) or 18 h at 37°C (dashed bars). Error bars denote standard deviations. *, P < 0.05; **, P < 0.005; and ***, P < 0.0001.
FIG. 4.
Effect of anaerobiosis on the expression of cell wall-associated Esp of E. faecium. Shown are the means of the mean FL1 from three independent experiments. Bacteria listed were grown on SRA for 18 h at 37°C under aerobic (open bars) or anaerobic (dashed bars) conditions. Error bars denote standard deviations. **, P < 0.005.
FIG. 5.
Histograms showing the Esp-specific binding of the rabbit polyclonal anti-Esp serum. Strains were grown for 18 h on SRA at 37°C. E470 was incubated with anti-Esp (α-Esp) serum pretreated with increasing amounts of rN-Esp. As negative controls, E135 (esp negative) incubated with the anti-Esp serum and E470 incubated with pooled rabbit preimmune serum (Prs) were used. On the x axis, the mean fluorescence (mean FL1) is indicated.
Temperature-dependent transcription of esp.
Growth temperature-dependent expression of Esp was confirmed at the transcriptional level using real-time PCR. All esp-positive strains showed increased Esp mRNA levels when grown at 37°C compared to bacteria grown at 21°C. Based on the ΔΔCT values, 6.63-, 1.03-, 3.14-, 2.51-, 1.40-, and 5.35-fold increases of Esp mRNA were found for strains E470, E155, E745, E1165, E1172, and E1176, respectively. These data also show that strains E470 and E1176, which demonstrated the largest differences in levels of surface-exposed Esp upon the growth temperature shift (Fig. 3), also showed the highest increases of Esp mRNA, namely, 6.63- and 5.35-fold increases, respectively. As expected for the esp-negative strain E135, no Esp mRNA could be detected. These data indicate that growth temperature-dependent surface expression of Esp is regulated at the transcriptional level.
Surface-exposed Esp expression and binding to polystyrene.
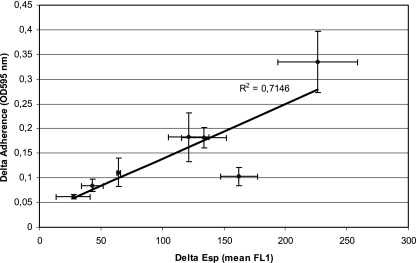
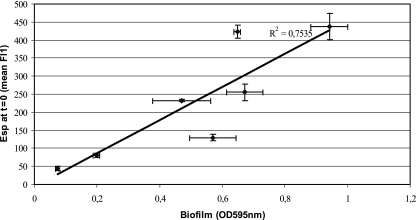
To investigate whether surface exposure of Esp correlates with initial adherence and biofilm formation, E. faecium strains were grown at 21°C (72 h) and 37°C (18 h) under aerobic conditions on SRA and subsequently exposed to polystyrene. Surface expression of Esp, as determined by flow cytometry, and initial adherence to polystyrene were clearly associated (R2 = 0.7146) (Fig. 6). Strain E470 had the highest increase in adherence and Esp expression upon shifting the temperature from 21°C to 37°C. Correlation was close to linear (R2 = 0.9887) if the results for E1176, which had a relatively small increase in initial adherence when grown at 37°C compared to 21°C, were not included. The amount of surface-exposed Esp at the start of biofilm development (0 h), determined by flow cytometry, positively correlated with biofilm development, determined at 24 h (R2 = 0.7535) (Fig. 7). This strongly suggests that, under the conditions tested, the amount of Esp on the surface of E. faecium is indicative of the binding of polystyrene and the ability to develop biofilms.
FIG. 6.
Correlation between Esp expression and initial adherence to polystyrene. Difference in Esp expression and adherence between bacteria grown at 21°C and 37°C are indicated at the x and y axes, respectively. Error bars denote standard deviations.
FIG. 7.
Correlation between Esp expression and biofilm formation. The x axis indicates the Esp expression at 0 h (t = 0), and the y axis indicates the biofilm development measured after 24 h. Error bars denote standard deviations.
The N-terminal domain of Esp blocks the adherence of E. faecium to polystyrene.
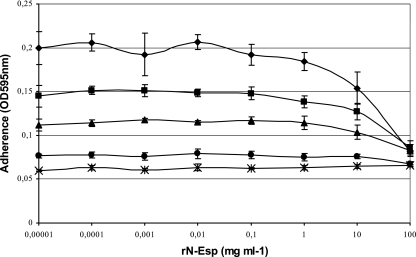
The esp-negative strain (E135) and the strain with the largest amounts of surface-associated Esp (E470) were used to determine whether adherence could be blocked by rN-Esp. Primary attachment of E470 grown at 37°C was blocked by rN-Esp in a concentration-dependent manner (Fig. 8). Although maximal adherence was bacterial concentration dependent, the concentrations of rN-Esp needed to block attachment were similar for all bacterial concentrations. This suggests that the N-terminal domain of Esp is sufficient for binding to polystyrene.
FIG. 8.
Inhibition of initial adherence of E. faecium to polystyrene by the N-terminal Esp domain. The ability to block initial adherence by N-terminal Esp was studied using two different strains. Prior to the assay, strains were grown at different temperatures, and in the assay different bacterial concentrations were used. Diamonds, 5 × 107 CFU of E470 grown at 37°C; squares, 2.5 × 107 CFU of E470 grown at 37°C; triangles, 1.25 × 107 CFU of E470 grown at 37°C; circles, 5 × 107 CFU of E470 grown at 21°C; asterisks, 5 × 107 CFU of E135 grown at 37°C.
DISCUSSION
Using Western blotting and FACscan analysis, we have shown that the amounts of Esp expressed on the cell surfaces of esp-positive E. faecium strains varied considerably from strain to strain. Furthermore, in almost all strains, the amount of Esp on the surface of E. faecium correlated with the ability to adhere to polystyrene, which could be blocked by exogenous N-terminal Esp, suggesting a role for the N-terminal domain of Esp in initial adherence to polystyrene. This is in line with recent observations by Tendolkar and coworkers, who showed that, by constructing in-frame-deletion mutants, the minimal region contributing to Esp-mediated biofilm enhancement in E. faecalis was confined to the N-terminal domain (38). The observed dose dependency further supports a role for Esp in initial adherence and the biofilm formation of E. faecium.
With both E. faecalis and E. faecium, there is controversy about the role of Esp in biofilm development. Although Esp appeared to be important for biofilm development (20, 36, 41) in some studies, E. faecalis isolates lacking the esp gene did produce biofilm in vitro (6, 12, 16, 27). Furthermore, for both E. faecium and E. faecalis, isolates were found that carried the esp gene but that failed to produce biofilms. Esp-independent biofilm formation by E. faecalis is probably mediated by multiple additional factors, like GelE (12, 16, 25), BopD (13), the fsr locus (12), the bee locus (37), and heterogeneity in surface charge (43). The observations that biofilms produced by E. faecalis are much thicker than those produced by E. faecium and that the biofilm development of E. faecalis is much more sensitive to the growth medium used (29) may indicate that in E. faecalis, additional factors aside from Esp are involved.
From data presented in the current study, the lack of biofilm formation in esp-positive strains can be explained by absent or only low-level expression of functional Esp on the cell surface despite the presence of its gene. Furthermore, detectable levels of Esp on the surface of E. faecium does not indicate in all cases availability for adherence to polystyrene, as was illustrated for strain E1176. This could be the result of shielding of Esp by other surface structures, like polysaccharide capsules. In general, however, the amount of surface-exposed Esp correlates well with the ability to adhere to polystyrene and develop biofilm.
Temperature-dependent regulation of expression, as shown for Esp in E. faecium, is a characteristic feature of pathogens alternating between a vector or an environmental reservoir and a mammalian host. Pathogens like Bordetella, Borrelia, Clostridium, Escherichia, Salmonella, Shigella, Vibrio, and Yersinia spp. (14, 15) respond to temperature transitions by inducing the expression of temperature-regulated genes that often encode virulence factors. E. faecium shares these ecological features. Besides being a common colonizer of the gastrointestinal tracts of humans and animals, it is also able to survive, for prolonged periods of time, in environmental reservoirs, both outside hospitals (in soil and sewage) and inside hospitals (on thermometers, bed rails, over-the-bed tables, bed linen, urinals, bedpans, hands of health care workers, and patients' skin) (3). We postulate that growth temperature-dependent expression of Esp is a niche-dependent adaptation mechanism of E. faecium. As such, Esp may contribute to the early stages of colonization and subsequent infection.
Furthermore, in five of six esp-positive strains, Esp expression increased under anaerobic conditions. E. faecium is a facultative anaerobic bacterium, which allows the bacterium to alternate between environmental reservoirs and its host. In E. faecalis, up-regulation of household genes and the virulence factor cytolysin were found under anaerobic conditions (5, 33). Our observation that Esp expression is up-regulated under anaerobic conditions once more suggests a role for Esp in the early stages of E. faecium colonization and infection.
Within hospital settings, enterococci have been described as triple-threat pathogens combining characteristics of Enterobactericeae (i.e., gut colonization), Staphylococcus aureus (i.e., skin colonization), and Clostridium difficile (prolonged survival on inanimate environments) (3). The combination of skin colonization and survival on inanimate environments has probably been instrumental in the rapid nosocomial spread of these bacteria. Furthermore, extensive colonization of the skin is an important risk for the development of intravascular-device-related infections and subsequent bacteremia, which are among the most frequently occurring enterococcal infections (7). Migration of bacteria from the skin along the catheter into the bloodstream, accompanied by a shift in temperature from 21°C to 37°C, might be the signal for E. faecium to up-regulate Esp expression, which will result in initial adherence to the catheter and subsequent biofilm development. Once part of a biofilm on the surface of an invasive medical device, bacterial cells are shielded against the detrimental activities of the host immune response and antibiotics (8, 10, 11).
Whether Esp in E. faecium serves as an adhesin to abiotic materials or is involved in colonization remains to be determined. Esp-positive blood isolates adhered well to Caco-2 human colon cancer cells (19), suggesting a role for Esp in gut colonization. In contrast, in E. faecalis, Esp appeared not to be instrumental in a mouse intestinal colonization model (26).
In this study, we have shown that Esp is expressed on the surface of E. faecium and that Esp expression may vary considerably between esp-positive isolates, which may explain previous conflicting results on the relation between esp and adherence or biofilm formation. For the first time, we demonstrated, quantitatively, that Esp expression levels correlate with initial adherence to polystyrene and biofilm formation. Furthermore, it was shown for the first time that E. faecium senses and responds to environmental changes. These findings support the idea of a role for Esp in the early stages of E. faecium colonization. Naturally, definitive claims of Esp functions await conformation of our findings with esp knockout strains. Until now, we and others have not been successful in constructing an esp knockout strain of E. faecium. Recent findings reported by Nallapareddy et al. (23) on improved temperature-sensitive vectors with which acm mutations were constructed in poorly transformable clinical E. faecium strains might be instrumental in overcoming this problem.
Acknowledgments
We thank Rob Schuurman, Mei Ling Chu, and Mignon Vughs for technical assistance and Ad Fluit for fruitful discussions.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 21 November 2006.
REFERENCES
- 1.Aarestrup, F. M., P. Butaye, and W. Witte. 2002. Nonhuman reservoirs of enterococci, p. 55-100. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 2.Bonten, M. J., M. K. Hayden, C. Nathan, T. W. Rice, and R. A. Weinstein. 1998. Stability of vancomycin-resistant enterococcal genotypes isolated from long-term-colonized patients. J. Infect. Dis. 177:378-382. [DOI] [PubMed] [Google Scholar]
- 3.Bonten, M. J., M. K. Hayden, C. Nathan, J. van Voorhis, M. Matushek, S. Slaughter, T. Rice, and R. A. Weinstein. 1996. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 348:1615-1619. [DOI] [PubMed] [Google Scholar]
- 4.Cheung, A. L., K. J. Eberhardt, and V. A. Fischetti. 1994. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal. Biochem. 222:511-514. [DOI] [PubMed] [Google Scholar]
- 5.Day, A. M., J. H. Cove, and M. K. Phillips-Jones. 2003. Cytolysin gene expression in Enterococcus faecalis is regulated in response to aerobiosis conditions. Mol. Genet. Genomics 269:31-39. [DOI] [PubMed] [Google Scholar]
- 6.Di Rosa, R., R. Creti, M. Venditti, R. D'Amelio, C. R. Arciola, L. Montanaro, and L. Baldassarri. 2006. Relationship between biofilm formation, the enterococcal surface protein (Esp) and gelatinase in clinical isolates of Enterococcus faecalis and Enterococcus faecium. FEMS Microbiol. Lett. 256:145-150. [DOI] [PubMed] [Google Scholar]
- 7.Donelli, G., and E. Guaglianone. 2004. Emerging role of Enterococcus spp in catheter-related infections: biofilm formation and novel mechanisms of antibiotic resistance. J. Vasc. Access 5:3-9. [DOI] [PubMed] [Google Scholar]
- 8.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunne, W. M., Jr., and W. Wang. 1997. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J. Clin. Microbiol. 35:388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 11.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, L. E., and M. Perego. 2004. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 186:5629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hufnagel, M., S. Koch, R. Creti, L. Baldassarri, and J. Huebner. 2004. A putative sugar-binding transcriptional regulator in a novel gene locus in Enterococcus faecalis contributes to production of biofilm and prolonged bacteremia in mice. J. Infect. Dis. 189:420-430. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson, S., B. Dupuy, K. Mukherjee, E. Norin, L. G. Burman, and T. Åkerlund. 2003. Expression of Clostridium difficile toxins A and B and their sigma factor TcdD is controlled by temperature. Infect. Immun. 71:1784-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konkel, M. E., and K. Tilly. 2000. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2:157-166. [DOI] [PubMed] [Google Scholar]
- 16.Kristich, C. J., Y.-H. Li, D. G. Cvitkovitch, and G. M. Dunny. 2004. Esp-independent biofilm formation by Enterococcus faecalis. J. Bacteriol. 186:154-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leavis, H., J. Top, N. Shankar, K. Borgen, M. Bonten, J. van Embden, and R. J. L. Willems. 2004. A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J. Bacteriol. 186:672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 19.Lund, B., and C. Edlund. 2003. Bloodstream isolates of Enterococcus faecium enriched with the enterococcal surface protein gene, esp, show increased adhesion to eukaryotic cells. J. Clin. Microbiol. 41:5183-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed, J. A., W. Huang, S. R. Nallapareddy, F. Teng, and B. E. Murray. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed, J. A., and B. E. Murray. 2005. Lack of correlation of gelatinase production and biofilm formation in a large collection of Enterococcus faecalis isolates. J. Clin. Microbiol. 43:5405-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nallapareddy, S. R., and B. E. Murray. 2006. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect. Immun. 74:4982-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nallapareddy, S. R., K. V. Singh, and B. E. Murray. 2006. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl. Environ. Microbiol. 72:334-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh, W. S., K. S. Ko, J.-H. Song, M. Y. Lee, S. Park, K. R. Peck, N. Y. Lee, C.-K. Kim, H. Lee, S.-W. Kim, H.-H. Chang, Y.-S. Kim, S.-I. Jung, J. S. Son, J.-S. Yeom, H. K. Ki, and G.-J. Woo. 2005. High rate of resistance to quinupristin-dalfopristin in Enterococcus faecium clinical isolates from Korea. Antimicrob. Agents Chemother. 49:5176-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillai, S. K., G. Sakoulas, G. M. Eliopoulos, R. C. Moellering, Jr., B. E. Murray, and R. T. Inouye. 2004. Effects of glucose on fsr-mediated biofilm formation in Enterococcus faecalis. J. Infect. Dis. 190:967-970. [DOI] [PubMed] [Google Scholar]
- 26.Pultz, N. J., N. Shankar, A. S. Baghdayan, and C. J. Donskey. 2005. Enterococcal surface protein Esp does not facilitate intestinal colonization or translocation of Enterococcus faecalis in clindamycin-treated mice. FEMS Microbiol. Lett. 242:217-219. [DOI] [PubMed] [Google Scholar]
- 27.Raad, I. I., H. A. Hanna, M. Boktour, G. Chaiban, R. Y. Hachem, T. Dvorak, R. Lewis, and B. E. Murray. 2005. Vancomycin-resistant Enterococcus faecium: catheter colonization, esp gene, and decreased susceptibility to antibiotics in biofilm. Antimicrob. Agents Chemother 49:5046-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rooijakkers, S. H., W. J. Van Wamel, M. Ruyken, K. P. Van Kessel, and J. A. van Strijp. 2005. Anti-opsonic properties of staphylokinase. Microbes Infect. 7:476-484. [DOI] [PubMed] [Google Scholar]
- 29.Sandoe, J. A., I. R. Witherden, J. H. Cove, J. Heritage, and M. H. Wilcox. 2003. Correlation between enterococcal biofilm formation in vitro and medical-device-related infection potential in vivo. J. Med. Microbiol. 52:547-550. [DOI] [PubMed] [Google Scholar]
- 30.Shankar, N., A. S. Baghdayan, and M. S. Gilmore. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746-750. [DOI] [PubMed] [Google Scholar]
- 31.Shankar, N., C. V. Lockatell, A. S. Baghdayan, C. Drachenberg, M. S. Gilmore, and D. E. Johnson. 2001. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect. Immun. 69:4366-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shepard, B. D., and M. S. Gilmore. 1999. Identification of aerobically and anaerobically induced genes in Enterococcus faecalis by random arbitrarily primed PCR. Appl. Environ. Microbiol. 65:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slot, J. W., and H. J. Geuze. 1985. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur. J. Cell Biol. 38:87-93. [PubMed] [Google Scholar]
- 35.Tannock, G. W., and G. Cook. 2002. Enterococci as members of the intestinal microflora of humans, p. 101-132. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 36.Tendolkar, P. M., A. S. Baghdayan, M. S. Gilmore, and N. Shankar. 2004. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 72:6032-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tendolkar, P. M., A. S. Baghdayan, and N. Shankar. 2006. Putative surface proteins encoded within a novel transferable locus confer a high-biofilm phenotype to Enterococcus faecalis. J. Bacteriol. 188:2063-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tendolkar, P. M., A. S. Baghdayan, and N. Shankar. 2005. The N-terminal domain of enterococcal surface protein, Esp, is sufficient for Esp-mediated biofilm enhancement in Enterococcus faecalis. J. Bacteriol. 187:6213-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenover, F. C., and L. C. McDonald. 2005. Vancomycin-resistant staphylococci and enterococci: epidemiology and control. Curr. Opin. Infect. Dis. 18:300-305. [DOI] [PubMed] [Google Scholar]
- 40.Timmers, G. J., W. C. van der Zwet, I. M. Simoons-Smit, P. H. Savelkoul, H. H. Meester, C. M. Vandenbroucke-Grauls, and P. C. Huijgens. 2002. Outbreak of vancomycin-resistant Enterococcus faecium in a haematology unit: risk factor assessment and successful control of the epidemic. Br. J. Haematol. 116:826-833. [DOI] [PubMed] [Google Scholar]
- 41.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penadés, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Bogaard, A. E., P. Mertens, N. H. London, and E. E. Stobberingh. 1997. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J. Antimicrob. Chemother. 40:454-456. [DOI] [PubMed] [Google Scholar]
- 43.van Merode, A. E. J., H. C. van der Mei, H. J. Busscher, and B. P. Krom. 2006. Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis. J. Bacteriol. 188:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willems, R. J. L., W. Homan, J. Top, M. Santen-Verheuvel, D. Tribe, X. Manzioros, C. Gaillard, C. M. J. E. Vandenbroucke-Grauls, E. M. Mascini, E. van Kregten, J. D. A. van Embden, and M. J. M. Bonten. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853-855. [DOI] [PubMed]
- 45.Willems, R. J. L., J. Top, M. van Santen, D. A. Robinson, T. M. Coque, F. Baquero, H. Grundmann, and M. J. M. Bonten. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg. Infect. Dis. 11:821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]