Abstract
Despite technological advances, no vaccine to prevent serogroup B meningococcal disease is available. The failure to develop a vaccine has shifted the focus to an alternative outer membrane structure, lipooligosaccharide (LOS), because disseminated disease induces bactericidal immunoglobulin G (IgG) that binds LOS. The purpose of this study was to identify the LOS structure(s) that induces human bactericidal IgG by purification and characterization of these antibodies. Human LOS IgG antibodies were affinity purified by passage of intravenous immunoglobulin through purified, type-specific LOS having a known structure coupled to epoxy-activated Sepharose 6B. Pathogenic group B strains representing the major LOS serotypes were used to examine the binding and bactericidal activities of four LOS-specific IgG preparations. All four LOS-specific IgG preparations bound to strains expressing homologous, as well as heterologous, LOS serotypes as determined by flow cytometry and an enzyme-linked immunosorbent assay. With human complement, IgG that was purified with L7 LOS was bactericidal for strains expressing L3,7 and L2,4 LOS, serotypes expressed by the majority of disease-associated group B and C meningococci. In conclusion, we purified human LOS-specific IgG that binds meningococci across LOS glycose-specific serotypes. An antigen that is dependent on the glycose lacto-N-neotetraose induces IgG in humans that is bactericidal for L2, L3, L4, and L7 strains. A vaccine containing this antigen would have the potential to protect against the vast majority of group B meningococcal strains.
Meningococcal disease can be rapidly fatal despite prompt antibiotic treatment, and survivors are often left with brain damage, deafness, or amputated limbs. Currently licensed meningococcal vaccines are composed of capsular polysaccharides A, C, Y, and W135, recently formulated as protein conjugates. They induce serogroup-specific antibodies, but they do not contain the capsular polysaccharide of serogroup B Neisseria meningitidis that causes much of the meningococcal disease in industrialized nations (36). Polysialyl structures identical to the group B capsular polysaccharide are found on human tissue, including neural cells (14, 21), which has diminished enthusiasm for a group B conjugate vaccine.
The lack of a group B polysaccharide vaccine led to the development of vaccines composed of outer membrane proteins (OMP) in the form of outer membrane vesicles. Efficacy trials (3, 4, 7, 31, 32, 42, 48) have shown that these vaccines induce a short-lived immunoglobulin G (IgG) response in older children and adults but that they are poorly immunogenic in younger children, who are at greatest risk of disease. The protective antibody response is subtype specific, which limits the use of these vaccines because of the large antigenic variability of OMP among meningococci (36).
Outer membrane lipooligosaccharides (LOS) are alternative vaccine candidates. Anti-LOS antibodies have been detected in normal human serum (NHS) and sera from patients convalescing from disease (8, 12, 18, 28, 33). Although the LOS in the outer membrane vesicle vaccines was depleted, about 8% LOS relative to protein remained and induced IgG antibodies in humans (35). This has led to an interest in the development of LOS vaccines (6, 24).
Meningococcal LOS are short, surface-exposed glycolipids (17, 20, 24). They all contain lipid A that is integrated into the bacterial outer membrane, a proximal core segment that is conserved irrespective of the capsular serogroup or LOS serotype, and three variable, short, distal oligosaccharide chains, designated the α, β, and γ chains. The core region consists of a highly conserved structure that is composed of two heptoses (Hep1 and Hep2) and two 3-deoxy-d-manno-2-octulosonic acid (KDO) residues that are attached to the lipoidal moiety. Glucose is added to Hep1 to initiate the α chain, which is usually elongated further. Hep2 may be substituted with phosphoethanolamine (PEA) at C-3 (cyclic) or at C-6 and/or C-7 (exocyclic). Structural studies have shown that LOS variability, reflected in the LOS serotyping system (serotypes L1 to L12), is due to differences in the extension of the three chains and in the PEA substituents of the core region (23). L types are related, and strains often express more than one type. The vast majority of disease-associated group B and C meningococci express LOS serotypes (L2, L3, L4, and L7) that have an α chain terminating in the paraglobosyl structure lacto-N-neotetraose (LNnT) Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep1→KDO. L3 and L7 LOS molecules are identical except that L3 LNnT is capped with sialic acid. A minority of group B and C strains express L1 and/or L8, serotypes that do not contain LNnT (17, 20, 24).
An LOS-based vaccine that would prevent group B disease in infants and young children would have to contain the LOS structure(s) that induces bactericidal (i.e., protective) IgG in the target population. To this end, we used purified LOS of defined L types to affinity purify human IgG. The binding of the resulting LOS-specific IgG populations to encapsulated human case isolates was characterized, as was the bactericidal activity of these human antibodies.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are described in Table 1. Strain 8532V was a gift from B. Brandt, Walter Reed Army Institute of Research (WRAIR), Silver Spring, MD. Organisms were cultured as described previously (44).
TABLE 1.
N. meningitidis and N. gonorrhoeae strains used
| Strain | Species | Serogroup | LOS type | Reference(s) |
|---|---|---|---|---|
| 6940 | N. meningitidis | B | L1 | 17 |
| 35E | N. meningitidis | C | L2 | 29 |
| 8532V | N. meningitidis | B | L8 variant | 53a |
| 7993 | N. meningitidis | B | L2,4 | 17 |
| 7994 | N. meningitidis | B | L1,8 | 17 |
| 7946 | N. meningitidis | B | L3,7 | 17 |
| 7948 | N. meningitidis | B | L3,7 | 17 |
| 7949 | N. meningitidis | B | L3,7,8 | 17 |
| 7971 | N. meningitidis | B | L2 | 17 |
| 8003 | N. meningitidis | B | L3,7 | 17 |
| 1291 | N. gonorrhoeae | LNnTa | 1, 22, 44 |
N. gonorrhoeae does not have designated L types, but strain 1291 expresses LNnT paraglobosyl LOS that is identical to the L7 LOS made by meningococci.
In addition, an isogenic LOS mutant of strain 7994 (L1,8) was prepared. Meningococcal strain NMB-SS3 (a gift from M. Apicella, University of Iowa) is an LOS mutant that lacks galactose because of inactivation of galE, which encodes the UDP-glucose-4-epimerase that converts glucose to galactose and is required for the incorporation of galactose into LOS (26, 43). DNA from this strain was used to transform strain 7994. Single colonies of 7994 were mixed with 5 μl of NMB-SS3 DNA and grown for 6 h before transfer to GC agar plates containing tetracycline. The transformation was confirmed by loss of binding of an L1 monoclonal antibody (MAb) (17) and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Strain 7994 (L1,8) expresses two major LOS molecules, L1 (α chain structure, Galα1→4Galβ1→4Glcβ1→4Hep1→KDO) and L8 (Galβ1→4Glcβ1→4Hep1→KDO). The mutant expresses LOS with an α chain structure that is truncated to Glcβ1→4Hep1→KDO.
LOS and OMC purification.
H. Schneider (WRAIR) kindly provided purified LOS from strain 1291. LOS from strains 35E, 6940, and 8532V were a gift from B. Brandt (WRAIR). Outer membrane complex (OMC) containing proteins and LOS was purified from whole organisms as previously described (40, 53).
MALDI-TOF MS.
Negative-ion matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) was performed with purified, O-deacylated LOS as recently described (44). The compositions of LOS made by strains 6940, 35E, and 8532V were determined by MALDI-TOF MS; structures were assigned to molecular ions on the basis of previously published structures (17, 20, 22, 25), L types (29), MAb binding, and the genetic basis of LOS biosynthesis (24).
Affinity purification of human LOS antibodies.
Purified meningococcal LOS was disaggregated into monomers in 3% deoxycholic acid in 0.2 M carbonate buffer (pH 10.2) (2) and then coupled to expoxy-activated Sepharose 6B (Amersham Biosciences, Uppsala, Sweden) through the epoxy linkage according to the manufacturer's instructions. Immune globulin intravenous (human) Carimune NF (IGIV) (ZLB Behring AG, Berne, Switzerland) was used as the source of antibody (96% IgG according to the manufacturer's package insert). This concentrated, purified IgG pharmaceutical product is produced from pooled blood donations and contains all of the IgG antibodies that regularly occur in the blood donor population (16). One gram diluted in 50 ml phosphate-buffered saline (PBS) was loaded onto a column and allowed to bind to LOS. After washing, the bound IgG was eluted from the affinity gel with glycine-HCl (pH 2.3), and the pH was immediately adjusted to pH 7 to 8 with 1 M Tris; this was followed by dialysis against PBS. The elution step regenerated the gel, which then was washed three times with alternating 0.1 M Tris-0.5 M NaCl (pH 8.0) and 0.1 M sodium acetate-0.5 M NaCl (pH 4) solutions. The regenerated gel was used multiple times.
SDS-PAGE and immunoblot analysis.
Whole-cell lysates of bacteria were prepared by a modification of the method of Mason et al. (30). After overnight growth, organisms were harvested into PBS, washed, and suspended in PBS to an absorbance at 650 nm of 1.0. One milliliter of the suspension was pelleted, resuspended in 100 μl Laemmli sample buffer (Bio-Rad, Hercules, CA) with 2.5% (vol/vol) 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), and boiled. Proteins and LOS were separated by 12% SDS-PAGE using a previously described modification of the method of Laemmli (11, 41) with a Mini-Protean 3 cell (Bio-Rad). For optimal LOS resolution, some samples of purified LOS or OMC were electrophoresed through 27 cm of a 13.1% polyacrylamide gel (17) with an SE600 series vertical slab gel unit (Hoefer, San Francisco, CA). The LOS molecules were visualized with silver stain, and the proteins were stained with Coomassie brilliant blue G-250 (Sigma).
The SDS-PAGE-separated proteins and LOS were electroblotted onto nitrocellulose, blocked with DIG block buffer (Roche, Mannheim, Germany), and then incubated with 3 to 5 μg/ml of the LOS-specific IgG, followed by secondary goat anti-human IgG antibody conjugated to alkaline phosphatase (Sigma-Aldrich). The blots were developed as recently described (11).
ELISA analyses.
An enzyme-linked immunosorbent assay (ELISA) was used to measure the binding of the LOS-specific IgG to purified homologous LOS. Microtiter wells were coated with 0.1 or 0.2 μg/ml purified LOS diluted in PBS with 0.1% deoxycholic acid (11). The wells were blocked with DIG block buffer and then incubated with serial dilutions of the LOS-specific IgG. The wells were developed as described above for immunoblotting, except that the substrate was p-nitrophenyl phosphate (2 mg/ml in 0.1 M bicarbonate buffer [pH 9.5] with 0.01 M MgCl2; Sigma-Aldrich). The absorbance at 405 nm was determined with a MAXline microplate reader (Molecular Devices, Sunnyvale, CA). Negative control wells received everything except IgG.
A whole-cell ELISA (10) was used to measure the binding of the LOS-specific IgG preparations to five meningococcal group B cerebrospinal fluid (CSF) isolates. Briefly, organisms were harvested from agar plates, washed, and suspended in PBS to an optical density at 640 nm of 0.1. The suspension was used to coat microtiter wells, which were then reacted with 10 μg/ml LOS-specific IgG. The wells were developed as described above.
A whole-cell ELISA was also used to compare the binding of the LOS-specific IgG to strain 7994 and to a ΔgalE mutant of this strain. Microtiter wells were coated with strain 7994 and 7994-ΔgalE and then were reacted with 10 μg/ml LOS-specific IgG and developed. Positive control wells coated with organisms were reacted with MAb B33 (provided by G. Brooks, University of California at San Francisco), which binds to all outer membrane Opa proteins (19). The assay was repeated using purified OMC.
Flow cytometry analyses. (i) N. meningitidis.
Various intact, sialylated, and encapsulated meningococcal strains were reacted with the different LOS IgG preparations (100 μg/ml) and analyzed by flow cytometry, as described previously (45), using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Alexa Fluor 488 fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (Molecular Probes, Eugene, OR) was used as the secondary antibody.
(ii) Human neutrophils.
Flow cytometry was used to determine whether the paraglobosyl 1291 LOS-specific IgG bound human cells. Neutrophils known to express LNnT (46) were isolated from a healthy adult as described previously (9, 13). MAb 1B2 (IgM) induced by human paragloboside (5, 52) was used as a positive control, as it binds neutrophils (46) as well as neisserial LOS that express LNnT (27). IgG from the neutrophil donor was used as a negative control and was isolated by passage of serum through protein G Sepharose 4 Fast Flow (Amersham, Piscataway, NJ) according to the manufacturer's instructions. Control IgG and 1291 IgG F(ab′)2 fragments were prepared with an ImmunoPure F(ab′)2 preparation kit (Pierce, Rockford, IL) used according to the manufacturer's instructions. The binding of 1291 IgG F(ab′)2 to 1291 bacteria was confirmed by flow cytometry.
Neutrophils (1 × 106 cells/tube) were incubated with MAb 1B2 hybridoma supernatant, a negative isotype control (mouse IgM; Chemicon International, Temecula, CA), 1291 IgG F(ab′)2, or control IgG F(ab′)2, followed by Alexa Fluor 488 FITC-conjugated goat anti-mouse IgM or FITC-conjugated goat anti-human IgG (Fab specific; Sigma) as appropriate. The IgG F(ab′)2 was used at a concentration of 5 μg/tube. Samples were analyzed with a FACSCalibur or FACScan (BD Biosciences) flow cytometer.
(iii) HEC-1-B cells.
A human endometrial adenocarcinoma cell line (ATCC HTB 113; American Type Culture Collection, Rockville, MD) was also used to determine if 1291 LOS IgG bound to human cells because HEC-1-B cells bind MAb 1B2. HEC-1-B cells (5 × 105 cells/tube) were analyzed as described above for neutrophils.
Confocal fluorescence microscopy.
Confocal microscopy with HEC-1-B cells was also used to determine whether the paraglobosyl 1291 LOS IgG bound human structures. HEC-1-B cells were grown on Lab-Tek II chamber slides (Nalge Nunc International, Naperville, IL) overnight and then incubated for 6 h with gonococcal strain MS11mkC (39) (1 × 108 to 2 × 108 CFU/ml), which adheres to cells (19) and binds MAb 1B2. The cells were fixed with Fix & Perm Medium A (Caltag, Burlingame, CA) and then incubated with 1291 F(ab′)2 fragments (200 μg/ml) and MAb 1B2, followed by Alexa Fluor 568-conjugated goat anti-human IgG and Alexa Fluor 488-conjugated goat anti-mouse IgM. Mounted slides were observed with a Leica confocal laser scanning microscope.
Bactericidal activity of LOS-specific IgG.
A modification of the method described previously (10) was used to assess the functional activity of the LOS-specific IgG. Briefly, after overnight growth, bacteria were harvested and grown to the mid-log phase in GC broth with 1% IsoVitaleX. Bacteria were washed in gonococcal buffer as described previously (38) and then suspended in gonococcal buffer at a concentration of 108 organisms per ml. The reaction mixture contained 1 × 104 to 2 × 104 organisms per ml, 15 to 25% NHS depending on the strain, and LOS-specific IgG at a concentration of 100 μg/ml. The control tubes included (i) tubes containing bacteria plus NHS and (ii) tubes containing bacteria plus heat-inactivated NHS. Some assays included a tube containing bacteria, IgG, and heat-inactivated NHS. The tubes were incubated with end-over-end rotation at 37°C. At time zero and 60 min, triplicate aliquots were removed and plated. After overnight growth in the presence of 5% CO2, the number of CFU was determined. Survival was expressed as the percentage of organisms that were present at time zero that survived for 60 min. The NHS was obtained from two adults with no history of meningococcal infection, and the concentration was adjusted to maximize the complement concentration while minimizing any intrinsic bactericidal activity of the sera against each strain tested. In preliminary experiments we determined the maximum NHS concentration for each strain that allowed at least ∼100% survival in the control tube containing bacteria plus NHS. Each assay was repeated at least three times.
RESULTS
MALDI-TOF MS.
The structures of LOS molecules of the strains used to affinity purify human IgG (6940, 35E, 1291, and 8532V) are shown in Table 2. Strain 6940 LOS yielded a single major molecular ion at −(H) m/z 2,588.56; this Mr is the Mr of the (Hex)3PEA1 structure of L1 LOS. The L1 type and partial structures of LOS made by meningococcal strain 6940 also have been described previously (17).
TABLE 2.
MALDI-TOF MS molecular ions and structures of LOS moleculesa
| Strain | L type | Ion (m/z)b | Calculated Mr | LOS |
|---|---|---|---|---|
| 6940 | L1 | 2,588.56 | 2,589.25 | Galα1→4Galβ1→4Glcβ1→4Hep1 |
| PEA→3Hep2 | ||||
| 35E | L2 | 2,954.71 | 2,955.47 | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep1c |
| Glcα1→3Hep2 | ||||
| ↑ | ||||
| Exocyclic (C-6 and/or C-7)PEA | ||||
| 1291 | L7 | —d | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep1 | |
| PEA→3Hep2 | ||||
| 8532V | L8v | 2,465.51 | 2,466.21 | Galβ1→4Glcβ1→4Hep1 |
| Glcα1→3Hep2 |
Based on MALDI-TOF MS, known structures, and genetic considerations.
MALDI-TOF MS was performed in the negative-ion mode; the observed m/z values are −(H).
A sialylated molecular ion (m/z 291.26) of this species is also present.
See reference 22.
Strain 35E is the L2 prototype strain; the structures of LOS made by a different L2 strain have been described previously (15). The MALDI-TOF molecular ions of 35E were consistent with the previously described structures. The Mr of the major ion at −(H) m/z 2,954.71 is the Mr of an LOS molecule with an LNnT α chain, a β chain glucose substitution at C-3 of Hep2, and a single exocyclic (C-6 or C-7 of Hep2) PEA substitution. Ions that represented two exocyclic PEA substitutions and terminal sialylation of the m/z 2,954.71 molecule (m/z 3,245.95) also were present.
Strain 8532V is an LOS variant of the L8 strain 8532 that does not bind the L8-specific MAb 2-1-L8 (53a); its LOS yielded a single −(H) molecular ion at m/z 2,465.51. The Mr of this molecule is the Mr of an LOS that has three hexoses and no PEA substitutions. The provenance of this variant and biosynthetic considerations strongly suggest that the structure of this molecule is consistent with an L8 molecule with a β chain glucose substitution at C-3 of Hep2; this would require only a single gene mutation.
The structure of the LOS made by gonococcal strain 1291 has been reported previously; this LOS has an LNnT α chain and a single basal PEA substitution at C-3 of Hep2 (22). This is the structure of the L7 meningococcal LOS (25).
Affinity purification of human LOS antibodies.
The purified LOS of four strains, 6940 (L1), 35E (L2), 1291 (L7 equivalent), and 8532V (L8 variant) (Fig. 1), were used to purify human IgG from IGIV. The yield from 1 g of IGIV ranged from 0.04% to 0.1%. The LOS-specific IgG was immunoblotted onto the SDS-PAGE-separated proteins and LOS of the homologous strain. Each of the LOS-specific IgG preparations bound to LOS but not to protein, as shown by the representative example in Fig. 2. The LOS-specific IgG also bound to the purified homologous LOS in a dose-dependent manner, as determined by ELISA (data not shown).
FIG. 1.
Silver staining of the SDS-PAGE-separated LOS molecules of the four strains used to affinity purify human IgG, 35E (L2), 1291 (L7 equivalent), 6940 (L1), and 8532V (L8 variant). The LOS were electrophoresed through 27 cm of a 13.1% polyacrylamide gel. The asterisks indicate the position of lacto-N-neotetraose, and the arrowhead indicates the position of L1.
FIG. 2.
SDS-PAGE-separated proteins and LOS from a whole-cell lysate (WCL) of strain 35E were immunoblotted with 5 μg/ml of 35E LOS-specific IgG. Binding occurred to LOS but not to proteins. The binding of 35E LOS IgG to purified 35E LOS is also shown to indicate the location of LOS molecules when they were electrophoresed on a minigel.
Flow cytometry analysis of the binding of LOS-specific IgG.
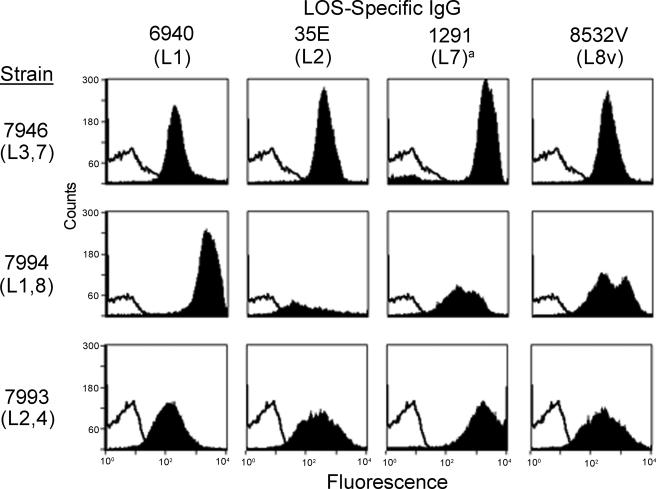
Flow cytometry was used to assess the binding of the four LOS-specific IgG preparations to three clinically relevant group B meningococcal strains that represented the major LOS types. Strains 7946 (L3,7), 7993 (L2,4), and 7994 (L1,8) were isolated from the blood or CSF of children (17). As Fig. 3 shows, all four LOS-specific IgG preparations bound to encapsulated organisms expressing homologous as well as heterologous LOS serotypes. 6940 LOS IgG (L1) bound with greatest intensity to the strain with the homologous L1 type (7994) but also bound to the two LNnT-bearing strains (7946 and 7993) that do not express L1 (17). 35E LOS IgG (L2; LNnT) bound best to the two LNnT strains (7946 and 7993), as did 1291 LOS IgG (L7; LNnT), but both preparations also bound to the L1,8 strain (7994) that does not express LNnT. It is interesting that the 8532V LOS IgG (L8 variant) bound to all three strains with equal intensity, but a second population of bacteria with higher-intensity binding was also apparent for the L1,8 strain (7994).
FIG. 3.
Binding of four LOS-specific IgG preparations (6940, 35E, 1291, and 8532V) to group B N. meningitidis strains 7946, 7994, and 7993 as determined by flow cytometry. The open curves indicate the binding of the secondary antibody alone, and the solid curves indicate the binding of IgG. N. gonorrhoeae does not have designated L types, but strain 1291 expresses LNnT paraglobosyl LOS that is identical to that of the L7 LOS made by meningococci.
Whole-cell ELISA.
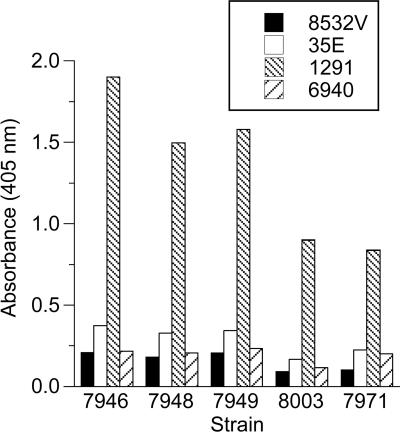
Whole-cell ELISA was used to confirm the binding of the 1291 LOS IgG observed by flow cytometry to additional group B case isolates expressing LNnT LOS. Strains 7948 (L3,7), 7949 (L3,7,8), 8003 (L3,7), and 7971 (L2), as well as strain 7946 (L3, 7), bound the 1291 LOS IgG (L7 equivalent) very well (Fig. 4). The other three IgG preparations (35E [L2] 6940 [L1], and 8532V [L8 variant]) also bound to these LNnT-bearing strains, but the amount of binding was much less than that of 1291 IgG, consistent with the results of flow cytometry.
FIG. 4.
Binding of four LOS-specific IgG preparations (8532V, 35E, 1291, and 6940) to group B N. meningitidis strains as determined by whole-cell ELISA. Microtiter wells were coated with the bacteria and reacted with 10 μg/ml of the IgG. Strains 7946, 7948, and 8003 are L3,7 strains. Strain 7949 is an L3,7,8 strain, and strain 7971 is an L2 strain.
Binding of LOS-specific IgG to 7994 and 7994-ΔgalE.
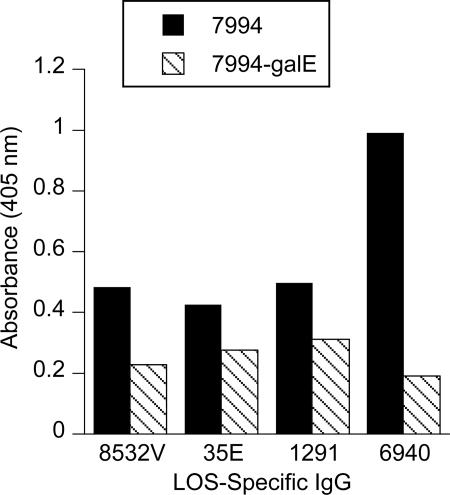
An isogenic LOS mutant (Fig. 5) was used to explore the optimal LOS structure for IgG binding. All four of the LOS-specific IgG preparations bound more, as determined by ELISA, to whole bacteria of strain 7994 (L1,8) than to the LOS mutant 7994-ΔgalE (Fig. 6), indicating that an α chain glucose by itself was not sufficient for optimal antibody binding. The truncated LOS of a ΔgalE mutant would not be an optimal immunogen for induction of the IgG preparations that were affinity purified for this study. The assay was repeated with purified OMC, and similar results were obtained.
FIG. 5.
Silver staining of SDS-PAGE-separated LOS of strain 7994 and the LOS mutant 7994-ΔgalE. Purified OMC of the two strains was electrophoresed through 27 cm of a 13.1% polyacrylamide gel for optimal LOS resolution.
FIG. 6.
Comparison of the binding of the LOS-specific IgG preparations (8532V, 35E, 1291, and 6940) to meningococcal strain 7994 (L1,8) and to the LOS mutant 7994-ΔgalE as determined by ELISA. Microtiter wells were coated with whole bacteria and reacted with 10 μg/ml of the IgG. 7994 expresses two major LOS molecules, L1 (α chain structure, Galα1→4Galβ1→4Glcβ1→4Hep1→KDO) and L8 (Galβ1→4Glcβ1→4Hep1→KDO). The mutant expresses LOS with an α chain structure that is truncated to Glcβ1→4Hep1→KDO.
Bactericidal activity of the LOS-specific IgG.
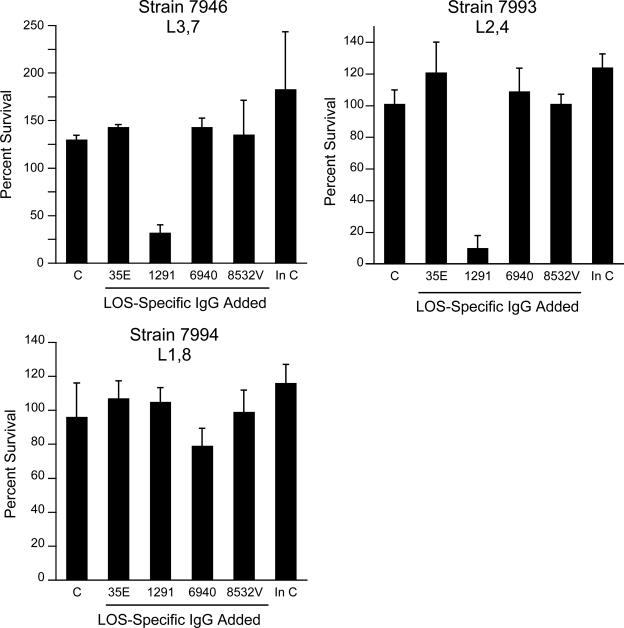
The abilities of the four LOS-specific IgG populations to kill the group B meningococcal strains that bound them as determined by flow cytometry were determined. As Fig. 7 shows, the 1291 LOS IgG was bactericidal for the two strains that expressed LNnT, the L3,7 strain (7946) and the L2,4 strain (7993). It was not bactericidal for the L1,8 strain (7994). The other three IgG preparations were not bactericidal, although 6940 IgG inhibited the growth of strain 7994 slightly.
FIG. 7.
Bactericidal activities of four LOS-specific IgG preparations (35E, 1291, 6940, and 8532V; 100 μg/ml) against group B N. meningitidis strains 7946 (L3,7), 7993 (L2,4), and 7994 (L1,8). Survival was expressed as the percentage of the organisms present at time zero that survived for 60 min. C, complement alone; In C, heat-inactivated complement alone. The amount of NHS used as a complement source was 25% for strain 7946, 15% for strain 7993, and 20% for strain 7994. The error bars indicate standard deviations.
Lack of binding of 1291 LOS IgG to human paragloboside structures.
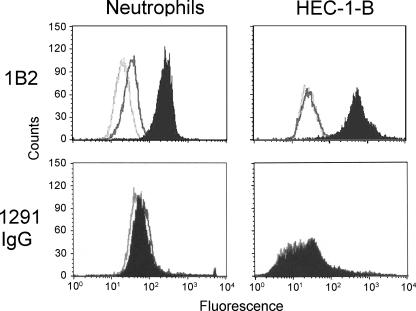
The 1291 LOS-specific IgG was bactericidal, making 1291 LOS a potential vaccine candidate, but this LOS shares the LNnT structure with human paraglobosyl glycosphingolipids expressed on cells such as neutrophils. This was confirmed by the binding of MAb 1B2 specific for paraglobosyl structures (Fig. 8). Neutrophils did not bind 1291 IgG F(ab′)2, nor did human HEC-1-B cells that also stained brightly with MAb 1B2 (Fig. 8).
FIG. 8.
Flow cytometry analysis of the binding of MAb 1B2 and 1291 LOS-specific IgG F(ab′)2 to human neutrophils and HEC-1-B cells. For MAb 1B2, the light gray open curve indicates the binding of the secondary antibody alone, the dark gray open curve indicates the binding of the isotype-matched negative control, and the solid curve indicates the binding of MAb 1B2. For 1291 IgG, the light gray open curve indicates the binding of the secondary antibody alone, the dark gray open curve indicates the binding of the negative control IgG F(ab′)2, and the solid curve indicates the binding of 1291 IgG F(ab′)2. The three curves were nearly identical for HEC-1-B with 1291 IgG.
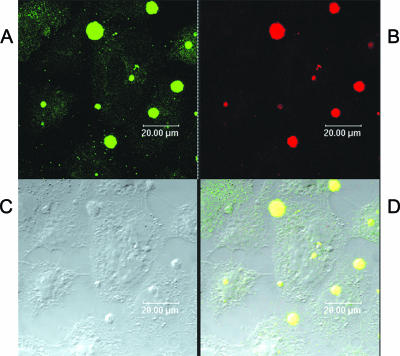
Confocal microscopy of HEC-1-B cells was used to confirm that 1291 LOS IgG did not bind LNnT structures on human cells. As Fig. 9 shows, the 1291 LOS IgG F(ab′)2 fragments did not bind human HEC-1-B cells but brightly stained the gonococci. The positive control, MAb 1B2, bound HEC-1-B cells and the gonococcal microcolonies growing on and invading them. These data demonstrate that the F(ab′)2 fragments of 1291 LOS IgG avidly bind neisserial LOS but do not bind human paragloboside structures.
FIG. 9.
Confocal microscopy of gonococcal microcolonies growing on HEC-1-B cells (C) and stained with human paraglobosyl LOS (1291) IgG F(ab′)2 fragments (red) (B) or stained with MAb 1B2 (green) (A). Panel D is a composite of panels A and B; overlapping binding of the two antibodies is indicated by yellow dots. MAb 1B2, which was induced by human paraglobosyl structures and binds human and neisserial paraglobosyl glycolipids, bound to HEC-1-B cells and bacteria (A and D), while 1291 LOS IgG F(ab′)2 fragments bound to the microcolonies but not to the HEC-1-B cells (B and D).
DISCUSSION
Serum bactericidal antibody is critical in host defense against N. meningitidis; by the time that children are 10 years old, 45 to 75% of them have developed this antibody (37). We used purified LOS whose structure was known to affinity purify human IgG from a large donor pool that should have contained these bactericidal antibodies. Three encapsulated group B pathogenic strains that represented the major L types of group B and C meningococci were used to examine the binding and bactericidal activity of the LOS-specific IgG preparations.
As determined by flow cytometry, the four LOS-specific IgG preparations bound to the three diverse strains. Each preparation bound the strain expressing the homologous L type, while heterologous L-type binding occurred as well. In L2, L3, L4, and L7 LOS molecules the α chain terminates in LNnT, but the core adornments vary; e.g., the position of PEA, the presence of β glucose on Hep2, and O acetylation of the γ chain N-acetylglucosamine are variable (23, 25). The L2- and L7-specific IgG bound to strains expressing LNnT irrespective of these differences. The L7-specific IgG (1291) bound, but less well, to the L1,8 strain (7994), which does not express LNnT. Likewise, the L1- and L8v-specific IgG bound to the LNnT-bearing strains, but with lower intensity than the homologous binding. These data suggest that human IgG binds to a conserved LOS structure regardless of the L type. IgG might also bind to L-type-specific structures as well.
With human complement, L7-specific IgG (1291) not only bound to but also was bactericidal for group B meningococcal strains expressing LNnT irrespective of basal adornments (L3,7 and L2,4 strains). When we determined the LOS serotypes of 34 consecutive blood and CSF meningococcal isolates (76% group B and 21% group C) from epidemiologically unrelated children in Houston, 88% expressed LNnT as L2, L3, L4, or L7 or a combination of these types (17). Bactericidal antibodies that are affinity purified by using L7 LNnT LOS are likely to be pauciclonal and recognize slightly different epitopes with different affinities.
Although the 1291 L7 LOS IgG killed the L2,4 test strain, 7993, the 35E L2 LOS IgG did not, despite being partially L type homologous. The 1291 LOS IgG appears to be specific for the LNnT structure that is shared by L3,7 and L2,4 strains. L4 differs from L7 in having the PEA residue on C-6 rather than C-3 of Hep2, and in L2 the PEA residue is on C-6 or C-7 and there is a β-glucose on C-3. These differences may not influence the antigenicity of LNnT. The 35E LOS IgG, on the other hand, appears to be specific for the β chain structure rather than LNnT (preliminary data not shown), and β chain-specific IgG may not be bactericidal. Why the 35E LOS affinity gel did not also purify LNnT-specific IgG is not clear.
An additional IgG preparation that decreased survival (albeit minimally) was the 6940 LOS IgG for the strain with the homologous L1 type, 7994. The IgG used in these assays was potentially less efficient for complement activation than native IgG because the IGIV purification process alters IgG aggregation (Carimune manufacturer's package insert [34]) to make the product safe for human infusion. It is expected that native IgG having the same specificity in whole serum would be much more active. We might also have failed to detect other bactericidal IgG populations that were present at lower concentrations or had lower binding affinities and that might be bactericidal with a higher complement concentration. It is noteworthy that bactericidal activity correlated with the highest-intensity binding observed by flow cytometry (Fig. 3), specifically 1291 LOS IgG for strains 7946 and 7993 and 6940 LOS IgG for strain 7994.
Importantly, the bactericidal 1291 LOS IgG did not bind human cells that express paragloboside but avidly bound neisserial organisms. The apparent conundrum that humans respond to the paraglobosyl structure on Neisseria with IgG that does not appear to bind to the same structure on human cells can be explained by the observation that whereas the MAb 1B2 epitope is composed of the LOS paraglobosyl tetrasaccharide, its conformation is driven by interactions between the glycose and lipoidal moieties (50, 51). The structure of the LOS lipoidal moiety is quite different from that of the human ceramide lipid of glycosphingolipids (47). Thus, the induction of high levels of antibodies having the specificity of 1291 LOS IgG by vaccination should not result in autoimmune binding to human tissues.
Recent studies have supported the hypothesis that there are immunogenic LOS structures. Adults immunized with meningococcal OMP and detoxified LOS from the L8 variant strain 8532V used in the current study developed increased levels of antibody to both OMP and LOS as determined by ELISA. A solid-phase antibody depletion assay showed that 50 to 79% of the bactericidal activity of vaccine recipients' sera against strain 8532V was removed with purified 8532V LOS (53a). While we found in preliminary studies that the 8532V LOS IgG was bactericidal against strain 8532V, this antibody was not bactericidal for the three diverse pathogenic strains tested.
Plested et al. (33a) used LOS from a mutant strain (α chain truncated after Hep1 and PEA negative) coupled to lysine-activated Sepharose to purify antibodies from adult NHS. These antibodies were bactericidal against the homologous mutant strain. Recently, Yamasaki et al. (49) affinity purified gonococcal LOS antibodies from NHS that were bactericidal for only the homologous strain. Our work differs from these studies in that we showed that LOS-specific IgG populations representing the major L types of group B and C meningococci bind to strains with heterologous serotypes and that one IgG preparation is bactericidal for fully encapsulated, heterologous, human case isolates rather than mutants or laboratory-derived strains whose pathogenic potential is uncertain.
In conclusion, we purified human LOS-specific IgG that bind meningococci across LOS glycose-specific serotypes. An antigen that is dependent on the glycose lacto-N-neotetraose induces IgG in humans that is bactericidal for L2, L3, L4, and L7 strains. A vaccine containing this antigen would have the potential to protect against the vast majority of group B meningococcal strains.
Acknowledgments
This work was supported by Public Health Service grants AI053728 (to J.M.G.) and AI063927 (to G.A.J.) from the National Institute of Allergy and Infectious Diseases and by the Research Service of the U.S. Department of Veterans Affairs.
We thank Hui Cheng and David Quan for their excellent technical assistance.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 13 November 2006.
Paper 102 from the Center for Immunochemistry.
REFERENCES
- 1.Apicella, M. A., J. F. Breen, and N. C. Gagliardi. 1978. Degradation of the polysaccharide component of gonococcal lipopolysaccharide by gonococcal and meningococcal sonic extracts. Infect. Immun. 20:228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertram, M. A., J. M. Griffiss, and D. D. Broud. 1976. Response to antigenic determinants of Neisseria meningitidis lipopolysaccharide investigated with a new radioactive antigen-binding assay. J. Immunol. 116:842-846. [PubMed] [Google Scholar]
- 3.Bjune, G., E. A. Holby, J. K. Gronnesby, O. Arenesen, J. Fredriksen, A. Halstensen, E. Holten, and A. Lindbak. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 4.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, E. Moran, W. Hankins, J. Gilly, and J. Mays. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Chilean National Committee for Meningococcal Disease. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 5.Campos, L., J. Portoukalian, S. Bonnier, Z. Shi, P. Calmard-Oriol, D. Treille, and D. Guyotat. 1992. Specific binding of anti-N-acetyllactosamine monoclonal antibody 1B2 to acute myeloid leukemia cells. Eur. J. Cancer 28:37-41. [DOI] [PubMed] [Google Scholar]
- 6.Cox, A. D., W. Zou, M. A. Gidney, S. Lacelle, J. S. Plested, K. Makepeace, J. C. Wright, P. A. Coull, E. R. Moxon, and J. C. Richards. 2005. Candidacy of LPS-based glycoconjugates to prevent invasive meningococcal disease: developmental chemistry and investigation of immunological responses following immunization of mice and rabbits. Vaccine 23:5045-5054. [DOI] [PubMed] [Google Scholar]
- 7.De Moraes, J., B. A. Perkins, M. Camargo, N. Hildago, H. Barbosa, C. Sacchi, I. Landgraf, V. Gattas, H. Vasconcelos, J. Wenger, and C. V. Broome. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 8.Estabrook, M. M., C. J. Baker, and J. M. Griffiss. 1993. The immune response of children to meningococcal lipooligosaccharides during disseminated disease is directed primarily against two monoclonal antibody-defined epitopes. J. Infect. Dis. 167:966-970. [DOI] [PubMed] [Google Scholar]
- 9.Estabrook, M. M., N. C. Christopher, J. M. Griffiss, C. J. Baker, and R. E. Mandrell. 1992. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J. Infect. Dis. 166:1079-1088. [DOI] [PubMed] [Google Scholar]
- 10.Estabrook, M. M., J. M. Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 65:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estabrook, M. M., D. L. Jack, N. J. Klein, and G. A. Jarvis. 2004. Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J. Immunol. 172:3784-3792. [DOI] [PubMed] [Google Scholar]
- 12.Estabrook, M. M., R. E. Mandrell, M. A. Apicella, and J. M. Griffiss. 1990. Measurement of the human immune response to meningococcal lipooligosaccharide antigens by using serum to inhibit monoclonal antibody binding to purified lipooligosaccharide. Infect. Immun. 58:2204-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estabrook, M. M., D. Zhou, and M. A. Apicella. 1998. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect. Immun. 66:1028-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finne, M., A. Dell, J. Oates, P. Wu, J. Klock, and M. Fukuda. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 15.Gamian, A., M. Beurret, F. Michon, J. R. Brisson, and H. J. Jennings. 1992. Structure of the L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J. Biol. Chem. 267:922-925. [PubMed] [Google Scholar]
- 16.Gardi, A. 1984. Quality control in the production of an immunoglobulin for intravenous use. Blut 48:337-344. [DOI] [PubMed] [Google Scholar]
- 17.Griffiss, J. M., B. L. Brandt, N. B. Saunders, and W. Zollinger. 2000. Structural relationships and sialylation among meningococcal L1, L8, and L3,7 lipooligosaccharide serotypes. J. Biol. Chem. 275:9716-9724. [DOI] [PubMed] [Google Scholar]
- 18.Griffiss, J. M., B. L. Brandt, D. D. Broud, D. K. Goroff, and C. J. Baker. 1984. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J. Infect. Dis. 150:71-79. [DOI] [PubMed] [Google Scholar]
- 19.Griffiss, J. M., C. J. Lammel, J. Wang, N. P. Dekker, and G. F. Brooks. 1999. Neisseria gonorrhoeae coordinately uses pili and Opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci. Infect. Immun. 67:3469-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiss, J. M., and H. Schneider. 1999. The chemistry and biology of lipooligosaccharides: the endotoxins of bacteria of the respiratory and genital mucosae, p. 179-194. In H. Brade, D. C. Morrison, S. Opal, and S. Vogel (ed.), Endotoxin in health and disease. Marcel Dekker, New York, NY.
- 21.Hayrinen, J., H. Jennings, H. V. Raff, G. Rougon, N. Hanai, R. Gerardy-Schahn, and J. Finne. 1995. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J. Infect. Dis. 171:1481-1490. [DOI] [PubMed] [Google Scholar]
- 22.John, C. M., J. M. Griffiss, M. A. Apicella, R. E. Mandrell, and B. W. Gibson. 1991. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J. Biol. Chem. 266:19303-19311. [PubMed] [Google Scholar]
- 23.Kahler, C. M., S. Lyons-Schindler, B. Choudhury, J. Glushka, R. W. Carlson, and D. S. Stephens. 2006. O-Acetylation of the terminal N-acetylglucosamine of the lipooligosaccharide inner core in Neisseria meningitidis: influence on inner core structure and assembly. J. Biol. Chem. 281:19939-19948. [DOI] [PubMed] [Google Scholar]
- 24.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 25.Kogan, G., D. Uhrin, J. R. Brisson, and H. J. Jennings. 1997. Structural basis of the Neisseria meningitidis immunotypes including L4 and L7. Carbohydr. Res. 298:191-199. [DOI] [PubMed] [Google Scholar]
- 26.Lee, F. K., D. S. Stephens, B. W. Gibson, J. J. Engstrom, D. Zhou, and M. A. Apicella. 1995. Microheterogeneity of Neisseria lipooligosaccharide: analysis of a UDP-glucose 4-epimerase mutant of Neisseria meningitidis NMB. Infect. Immun. 63:2508-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandrell, R., J. Griffiss, and B. Macher. 1988. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens: carbohydrate specificity of the mouse monoclonal antibodies that recognize cross-reacting antigens on LOS and human erythrocytes. J. Exp. Med. 168:107-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandrell, R. E., and W. D. Zollinger. 1989. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect. Immun. 57:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandrell, R. E., and W. D. Zollinger. 1977. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect. Immun. 16:471-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason, E. O., Jr., S. L. Kaplan, B. L. Wiedermann, E. P. Norrod, and W. A. Stenback. 1985. Frequency and properties of naturally occurring adherent piliated strains of Haemophilus influenzae type b. Infect. Immun. 49:98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 33.Plested, J. S., M. Gidney, P. Coull, H. Griffiths, M. Herbert, A. G. Bird, J. Richards, and E. R. Moxon. 2000. Enzyme linked immunosorbent assay (ELISA) for the detection of serum antibodies to the core lipopolysaccharide of Neisseria meningitidis group B. J. Immunol. Methods 237:73-84. [DOI] [PubMed] [Google Scholar]
- 33a.Plested, J. S., A. Jakel, J. C. Wright, K. Makepeace, M. A. J. Gidney, S. Lacelle, F. St. Michael, W. Zou, A. D. Cox, J. C. Richards, and E. R. Moxon. 2004. Abstr. 14th Int. Pathog. Neisseria Conf., abstr. 187.
- 34.Romer, J., P. J. Spath, F. Skvaril, and U. E. Nydegger. 1982. Characterization of various immunoglobulin preparations for intravenous application. II. Complement activation and binding to staphylococcus protein A. Vox Sang. 42:74-80. [DOI] [PubMed] [Google Scholar]
- 35.Rosenqvist, E., E. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 37.Ross, S. C., and P. Densen. 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine 63:243-273. [PubMed] [Google Scholar]
- 38.Ross, S. C., and P. Densen. 1985. Opsonophagocytosis of Neisseria gonorrhoeae: interaction of local and disseminated isolates with complement and neutrophils. J. Infect. Dis. 151:33-41. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, H., A. S. Cross, R. A. Kuschner, D. N. Taylor, J. C. Sadoff, J. W. Boslego, and C. D. Deal. 1995. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J. Infect. Dis. 172:180-185. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, H., J. M. Griffiss, G. D. Williams, and G. B. Pier. 1982. Immunological basis of serum resistance of Neisseria gonorrhoeae. J. Gen. Microbiol. 128:13-22. [DOI] [PubMed] [Google Scholar]
- 41.Schneider, H., T. L. Hale, W. D. Zollinger, R. Seid, Jr., C. A. Hammack, and J. M. Griffiss. 1984. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 45:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sierra, G., H. Campa, N. Varacel, I. Garcia, P. Izquierdo, P. Sotolongo, G. Casanueva, C. Rico, C. Rodriquez, and M. Terry. 1991. Vaccine against group B Neisseria meningitis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-207. [PubMed] [Google Scholar]
- 43.Stephens, D. S., C. F. McAllister, D. Zhou, F. K. Lee, and M. A. Apicella. 1994. Tn916-generated lipooligosaccharide mutants of Neisseria meningitidis and Neisseria gonorrhoeae. Infect. Immun. 62:2947-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson, K. V., and J. M. Griffiss. 2006. Separation and identification of neisserial lipooligosaccharide oligosaccharides using high-performance anion-exchange chromatography with pulsed amperometric detection. Carbohydr. Res. 341:388-396. [DOI] [PubMed] [Google Scholar]
- 45.Swanson, K. V., G. A. Jarvis, G. F. Brooks, B. J. Barham, M. D. Cooper, and J. M. Griffiss. 2001. CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells. Cell. Microbiol. 3:681-691. [DOI] [PubMed] [Google Scholar]
- 46.Symington, F. W., D. L. Hedges, and S. Hakomori. 1985. Glycolipid antigens of human polymorphonuclear neutrophils and the inducible HL-60 myeloid leukemia line. J. Immunol. 134:2498-2506. [PubMed] [Google Scholar]
- 47.Takayama, K., N. Qureshi, K. Hyver, J. Honovich, R. Cotter, P. Mascagni, and H. Schneider. 1986. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. J. Biol. Chem. 261:10624-10631. [PubMed] [Google Scholar]
- 48.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 49.Yamasaki, R., T. Maruyama, U. Yabe, and S. Asuka. 2005. Normal human sera contain bactericidal IgG that binds to the oligosaccharide epitope expressed within lipooligosaccharides of Neisseria gonorrhoeae. J. Biochem. 137:487-494. [DOI] [PubMed] [Google Scholar]
- 50.Yamasaki, R., W. Nasholds, H. Schneider, and M. A. Apicella. 1991. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae: IgM monoclonal antibodies (3F11 and 1-1-M) recognize non-reducing termini of the LOS components. Mol. Immunol. 28:1233-1242. [DOI] [PubMed] [Google Scholar]
- 51.Yamasaki, R., H. Schneider, J. M. Griffiss, and R. Mandrell. 1988. Epitope expression of gonococcal lipooligosaccharide (LOS). Importance of the lipoidal moiety for expression of an epitope that exists in the oligosaccharide moiety of LOS. Mol. Immunol. 25:799-809. [DOI] [PubMed] [Google Scholar]
- 52.Young, W. W. J., J. Portoukalian, and S. Hakomori. 1981. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J. Biol. Chem. 21:10967-10972. [PubMed] [Google Scholar]
- 53.Zollinger, W. D., R. E. Mandrell, J. M. Griffiss, P. Altieri, and S. Berman. 1979. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J. Clin. Investig. 63:836-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Zollinger, W. D., J. G. Babcock, J. B. Berman, B. L. Brandt, E. E. Moran, N. M. Wassif, and C. R. Alving. 2004. Abstr. 14th Int. Pathog. Neisseria Conf., abstr. 200.