Abstract
The arrival of eggs in the liver during Schistosoma mansoni infection initiates a protective granulomatous response; however, as the infection progresses, this response results in chronic liver fibrosis. To better understand the impact of schistosomiasis on liver function, we used a proteomic approach to identify proteins whose expression was significantly altered in schistosome-infected mice 8 weeks postinfection. Identification of differentially expressed proteins by mass fingerprinting revealed that schistosome infection markedly reduced the abundance of proteins associated with several normal liver functions (i.e., citric acid cycle, fatty acid cycle, and urea cycle), while proteins associated with stress responses, acute phase reactants, and structural components were all significantly more abundant. The expression patterns of several immunity-related proteins (peroxiredoxin 1, arginase 1, and galectin 1) suggested that different protein forms are associated with schistosome infection. These findings indicate that acute schistosomiasis has a significant impact on specific liver functions and, moreover, that the alterations in specific protein isoforms and upregulation of unique proteins may be valuable as new markers of disease.
Schistosomiasis is a parasitic disease caused by infection with the helminth Schistosoma spp. (7). Two hundred million people are currently infected worldwide, primarily in the equatorial areas (19). Eggs laid by Schistosoma mansoni adult females in the mesenteric veins pass through the intestinal wall and then exit the host through the feces, or they are swept into the liver and trapped in the sinusoids, where they induce granulomatous lesions (19). The granulomas protect the liver from hepatotoxins produced by the schistosome eggs (16); however, the accumulation of fibrotic tissue also obstructs blood flow through the liver, resulting in portal hypertension, extended periportal fibrosis, and portal shunting (9, 19).
The schistosome eggs induce a strong Th2-dominated immune response (28), and this response has been shown to be both protective and pathogenic (8, 13). Interleukin-4 (IL-4) plays an important role in preventing oxidative damage to the liver mediated by the overproduction of reactive oxygen and nitrogen species (39), and mice with an IL-4 deficiency have a fatal acute disease (8). Another Th2 cytokine, IL-13, mediates the fibrotic process (21), and expression of its decoy receptor, IL-13R2α, has been shown to be essential in the resolution of fibrosis (13). These results emphasize the conclusion that a finely balanced response is a key aspect of surviving schistosome-mediated liver inflammation and minimizing the effect of tissue remodeling on normal liver processes.
The impact of schistosomiasis on liver function has been studied using targeted approaches to determine the effect on specific liver-derived proteins and enzymes. Such studies have shown that schistosomiasis alters the expression of enzymes involved in the urea cycle, glycolysis, energy metabolism, and other essential metabolic processes (1, 17, 41), and the alterations have some similarities with other liver-damaging insults (i.e., liver cancer and hepatotoxicity) (22, 66). However, it is clear that some changes may be unique to schistosomiasis. To more fully explore the global impact of schistosomiasis on liver protein expression and to gain insight into the pathogenesis of schistosome-mediated liver damage, we used a proteomic approach to identify the major changes in protein abundance that occurred during schistosome infection.
MATERIALS AND METHODS
Mice and experimental treatments.
C57BL/6 female mice were bred at the Wellington School of Medicine animal facility and were utilized when they were 8 to 12 weeks old. For experimental infections, mice were exposed percutaneously to approximately 200 S. mansoni cercariae (NIMR Puerto Rican strain) as previously described (47). Liver samples were collected from uninfected mice and mice infected for 8 weeks and snap frozen at −80°C. All experimental procedures used in this study were approved by the Victoria University of Wellington Animal Ethics Committees.
Sample preparation and two-dimensional gel separation.
Liver samples were homogenized in a solution containing 8 M urea, 2% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 0.15% (wt/vol) dithiothreitol, and the appropriate IPG buffer (pH 4 to 7 or 6 to 11; Amersham Biosciences, Uppsala, Sweden) at a concentration of 0.5%, vortexed for 10 min, and centrifuged at 10,000 × g for 10 min. After further dilution in sample buffer, the samples were revortexed and centrifuged before they were loaded onto 7-cm Immobiline DryStrips (pH 4 to 7 or 6 to 11; Amersham Biosciences) by passive rehydration overnight. Approximately 400 μg of protein was loaded, as assessed by the Bradford assay.
Samples were focused in the first dimension with an Ettan IPGphor (Amersham Biosciences) using a program that increased the voltage to 500 V over 45 min and then to 4,000 V over 3 h, followed by 5,000 V for 45 min. Strips were either stored at −80°C or used immediately. Proteins on focused strips were reduced using LDS sample buffer (Invitrogen Life Technologies, Carlsbad, CA) and a reducing agent (Invitrogen Life Technologies) for 10 min and then alkylated for 10 min with 116 μg iodoacetamide (Sigma, St. Louis, MO) per strip in LDS buffer (Invitrogen Life Technologies).
Electrophoresis was carried out immediately on 4 to 12% gradient Novex NuPAGE Bis-Tris precast gels (Invitrogen Life Technologies) at 200 V using morpholinepropanesulfonic acid (MOPS) buffer (Invitrogen Life Technologies). NuPAGE antioxidant (Invitrogen Life Technologies) was included for reducing conditions. After completion of electrophoresis, the gels were fixed overnight in 50% (vol/vol) ethanol containing 2% (vol/vol) ortho-phosphoric acid, washed three times in double-distilled water, and then immersed in a solution containing 0.075% (wt/vol) Coomassie brilliant blue G-250 in 17% (wt/vol) ammonium sulfate, 2% (vol/vol) ortho-phosphoric acid, and 34% (vol/vol) methanol with gentle agitation for 4 days to achieve staining to equilibrium.
Gels were scanned using a Molecular Dynamics (Sunnyvale, CA) SI personal densitometer, and protein spot abundance was analyzed using the ImageQuant gel analysis software (version 5.2; Molecular Dynamics). Spots were manually detected on triplicate gels, and background values were subtracted from spot values before normalization; these values gave the average spot volumes for individual animals. The average percent volume of each spot was then calculated for all animals in a group (uninfected or infected), and these values were used to calculate the fold change due to schistosome infection (percent spot volume in infected samples/average percent spot volume in uninfected samples).
MALDI-TOF mass spectrometry and protein identification.
Protein spots for matrix-assisted laser desorption ionization—time of flight (MALDI-TOF) analysis were prepared as described previously (36). Excised gel spots (approximately 1 to 2 mm3) were destained for 45 min in 100 μl of 50% (vol/vol) acetonitrile (ACN)-50 mM NH4HCO3 and then dehydrated with two washes in 100% ACN and dried by vacuum centrifugation. Dried gel pieces were then reswollen in 12 μl digestion buffer containing 50 mM NH4HCO3 and 0.2 μg trypsin (modified sequencing grade; Roche, Mannheim, Germany) and incubated overnight at 37°C.
Enzyme activity was then quenched with 0.2% trifluoroacetic acid (TFA) (Perkin-Elmer DNA synthesis grade); the aqueous extract was collected and added to successive extracts of the gel pieces with 10 μl of 50% (vol/vol) ACN-0.2% TFA and then with 10 μl ACN. Lyophilized extracts were dissolved in 10 μl of 0.2% TFA and then concentrated and partially purified using C18 Ziptips (Millipore, Bedford, MA) according to the manufacturer's instructions (using 0.2% TFA). Purified peptides were then mixed 1:1 with a saturated solution of α-cyano-4-hydroxycinnamic acid prepared in 0.2% TFA-ACN (40:60).
MALDI-TOF mass fingerprint spectra were acquired using an Voyager-DE PRO mass spectrometer (Applied Biosystems, Foster City, CA). Spectra were acquired in the positive ion reflector mode with an accelerating voltage of 20,000 V, a grid voltage of 75%, a guide wire voltage of 0.002%, and a 180-ns delay time. Monoisotopic masses were calculated after internal calibration with autolytic tryptic peaks. Peptide mass fingerprints were searched using ProFound (version 4.10.5; http://prowl.rockefeller.edu/profound_bin/WebProfound.exe) and/or XProteo (version 1.2.2; http://xproteo.com:2698/) algorithms using protonated monoisotopic masses, with one missed trypsin cleavage, complete modification of cysteine by carboxyamidation, and partial modification of methionine by oxidation in the search settings to search the NCBI database. The criteria for a match included the number of peptides matched, the sequence coverage, the use of Z scores in ProFound (sensitivity scores in XProteo), and the difference in probability between first and second matches. The Z score in a ProFound search is a measure of the probability of the match in a database search. Z scores of 1.28, 1.65, and 2.33 indicate that the searches are in the 90th, 95th, and 99th percentiles, respectively (http://prowl.rockefeller.edu/profound/help.html#ZSCORE).
Statistical analysis.
Data were analyzed using unpaired Student's t test with all replicate gels and animals per group.
RESULTS AND DISCUSSION
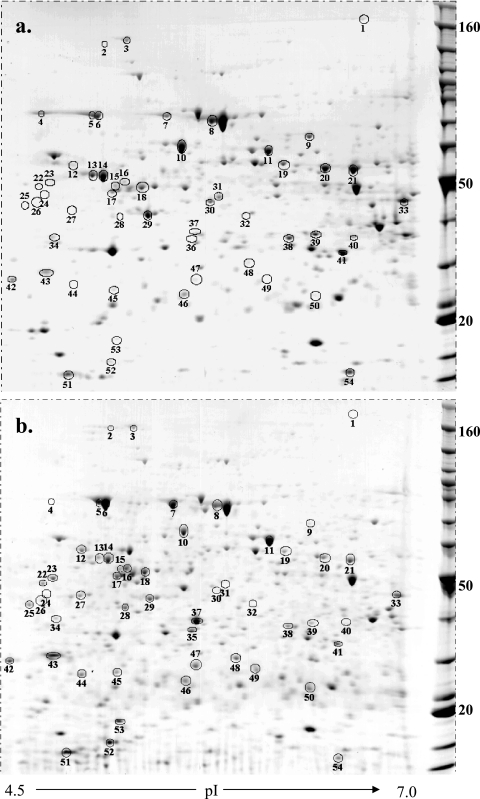
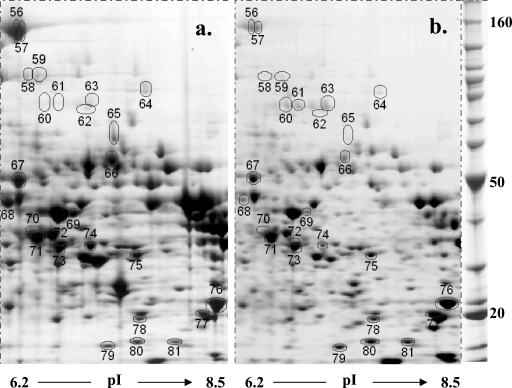
Changes in liver protein expression in female C57BL/6 mice were examined 8 weeks after infection with S. mansoni. All infected mice had enlarged, fibrotic livers containing visible granulomatous lesions, as expected. Liver lysates were separated by two-dimensional electrophoresis (Fig. 1 and 2), and proteins that were consistently more or less abundant in schistosome-infected livers were then selected for mass fingerprint analysis. A total of 211 medium- to high-abundance protein spots were analyzed quantitatively for each sample. Of these, 83 were subjected to MALDI-TOF analysis, and 81 were successfully matched to sequence databases. The results are shown in Table 1 (also see Tables S1 and S2 in the supplemental material). Twenty-nine of the 81 protein spots were matched for both infected and uninfected liver samples (see Tables S1 and S2 in the supplemental material). Particular emphasis was placed on determining whether the spots were parasite or host derived through comparative matching to known schistosome and mouse proteins; however, none of the selected spots were matched to known schistosome proteins. This finding is not surprising as only high- to medium-abundance proteins were selected for analysis and parasite-derived proteins were unlikely to comprise more than 1% of the soluble proteins in our samples. Finally, many of the matched spots had molecular weights and pIs comparable to those of previously identified spots from mouse liver protein lysates (Swiss-Pro 2D) (53), providing further support for their identification (see Tables S1 and S2 in the supplemental material).
FIG. 1.
Differential patterns of protein abundance in liver samples from uninfected (a) and schistosome-infected (b) mice for proteins with pIs in the range from 4.5 to 7. C57BL/6 mice were percutaneously infected with 200 S. mansoni cercariae, and liver samples were isolated 8 weeks later. Four hundred micrograms of protein was loaded on pH 4 to 7 IPG strips, separated by molecular weight on a 12% polyacrylamide gel, and visualized with Coomassie blue G250. Molecular masses (in kDa) are indicated on the right. The circled spots are described in Table 1. The gels are representative gels for one of six mice per group.
FIG. 2.
Differential patterns of protein abundance in liver samples from uninfected (a) and schistosome-infected (b) mice for proteins with pIs in the range from 6.2 to 8.5. C57BL/6 mice were infected and liver samples were prepared and analyzed as described in the legend to Fig. 1. Molecular masses (in kDa) are indicated on the right. The circled spots are described in Table 1. The gels are representative gels for one of three mice per group.
TABLE 1.
Functional effects on the liver and known disease associations
| Spot | Protein match | Functional group | Schistosome association (reference[s])a | Liver disease association (reference[s])a | Fold increase in spot densityb |
|---|---|---|---|---|---|
| 66 | Catalase 1 | Acute phase | − (26, 39) | −T (66), −C (22, 40) | −1.9 (0.3)c |
| 60 | Transferrin | Acute phase | + (4) | 4.2 (1.7)d | |
| 61 | Transferrin | Acute phase | + (4) | 4.0 (1.8) | |
| 63 | Transferrin | Acute phase | + (4) | 3.6 (1.1)c | |
| 41 | 3-Hydroxyanthranilate 3,4-dioxygenase | Amino acid catabolism/metabolism | −C (22), −C/H (5) | −2.2 (0.1)e | |
| 69 | 4-Hydroxyphenyl pyruvate dioxygenase | Amino acid catabolism/metabolism | −C (22) | −4.4 (0.7)e | |
| 68 | Adenosylhomocysteinase | Amino acid catabolism/metabolism | −C (22, 40), −C/H (5) | −2.6 (0.3)e | |
| 58 | Dimethylglycine dehydrogenase | Amino acid catabolism/metabolism | −C (22) | −10 (3.3)e | |
| 59 | Dimethylglycine dehydrogenase | Amino acid catabolism/metabolism | −C (22) | −4.2 (0.9)e | |
| 33 | Isovaleryl coenzyme A dehydrogenase | Amino acid catabolism/metabolism | −C (22) | −3.2 (0.04)e | |
| 31 | Methionine adenosyl transferase | Amino acid catabolism/metabolism | −C (40), −C/H (5) | −4.0 (1.1)e | |
| 76 | Glutathione S-transferase mu 1 | Antioxidant | − and NC (26, 54, 58, 59) | +T (2), +C (30, 51) | 2.2 (0.2)e |
| 77 | Glutathione S-transferase Pi class chain A | Antioxidant | − and NC (26, 54, 58, 59) | +C (30, 32, 51) | 2.7 (0.1)e |
| 51 | Thioredoxin 1 | Antioxidant | +C/H (5) | 2.2 (0.4)e | |
| 8 | Albumin 1 | Blood volume | − (29, 49, 71, 74) | −C (22) | −2.1 (0.1)e |
| 46 | Apolipoprotein A-I precursor | Cholesterol transport | ±C (22) | 1.1 (0.3) | |
| 64 | Aconitase 2, mitochondrial | Citric acid cycle | −3.0 (0.4)e | ||
| 65 | Mod1 (malic enzyme) | Citric acid cycle | − (20) | −15 (2.5)e | |
| 30 | Succinate-coenzyme A ligase β subunit | Citric acid cycle | −C/H (5) | −2.9 (0.3)e | |
| 20 | Aldehyde dehydrogenase class 2 | Energy | +T (45, 64, 68), ±C (22, 40, 46, 61) | −4.2 (0.2)e | |
| 21 | Aldehyde dehydrogenase class 2 | Energy | +T (45, 64, 68), ±C (22, 40, 46, 61) | −2.2 (0.1)e | |
| 67 | Aldehyde dehydrogenase class 2 mitochondrial precursor | Energy | +T (45, 64, 68), ±C (22, 40, 46, 61) | −1.6 (0.1)c | |
| 14 | ATP synthase beta chain | Energy | −C (14, 22), −C/H (5) | −1.8 (0.1)e | |
| 13 | ATP synthase F1 complex β chain | Energy | −C (14, 22), −C/H (5) | −3.0 (0.3)e | |
| 39 | Fructose bisphosphatase | Energy | −C (22) | −5.5 (0.7)e | |
| 70 | l-Iditol 2 (sorbitol)-dehydrogenase | Energy | + (52) | +T (2) | −2.0 (0.7)d |
| 75 | l-Lactate dehydrogenase A chain | Energy | + (1, 17, 41) | +T 33), change in isozymes C (48) | 2.0 (0.3)c |
| 3 | 170-kDa glucose-related protein | Endoplasmic reticulum/stress | 1.1 (0.2) | ||
| 5 | 78-kDa glucose-regulated protein (Hsp70, 5 kDa) | Endoplasmic reticulum/stress | 3.9 (0.5)e | ||
| 6 | 78-kDa glucose-regulated protein (Hsp70, 5 kDa) | Endoplasmic reticulum/stress | 3.0 (0.1)e | ||
| 4 | 78-kDa glucose-regulated protein (Hsp70, 5 kDa) | Endoplasmic reticulum/stress | −10 (5.0)c | ||
| NS | Calreticulin | Endoplasmic reticulum/stress | 2.9 (0.7)c | ||
| 10 | Chaperonin (60-kDa Hsp) | Endoplasmic reticulum/stress | ±C (22) | −2.1 (0.03)e | |
| 49 | Endoplasmic reticulum protein 29 | Endoplasmic reticulum/stress | 2.8 (0.5)e | ||
| 7 | Heat shock protein 8 (71 kDa) | Endoplasmic reticulum/stress | 4.6 (0.6)e | ||
| 80 | Peptidyl-prolyl isomerase | Endoplasmic reticulum/stress | 2.8 (0.2)e | ||
| 81 | Peptidyl-prolyl isomerase | Endoplasmic reticulum/stress | 2.5 (0.1)e | ||
| 28 | Protein disulfide isomerase | Endoplasmic reticulum/stress | −C (22) | 5.4 (0.7)e | |
| 44 | Protein disulfide isomerase | Endoplasmic reticulum/stress | −C (22) | 1.8 (0.1)e | |
| 11 | Protein disulfide isomerase A3 ERp60, glucose-related protein | Endoplasmic reticulum/stress | −C (22) | 2.6 (0.2)e | |
| 12 | Protein disulfide isomerase precursor | Endoplasmic reticulum/stress | −C (22) | 7.5 (2.5)e | |
| 16 | Protein disulfide isomerase-related protein | Endoplasmic reticulum/stress | −C (22) | 3.6 (1.2)c | |
| 48 | Proteosome 28 subunit | Endoplasmic reticulum/stress | 2.8 (0.3)e | ||
| 25 | Reticulocalbin 3 | Endoplasmic reticulum/stress | + (34) | +C (72) | 26 (10)e |
| 15 | Suppressor of tumorogenesis 13 (Hsc-70 interacting protein) | Endoplasmic reticulum/stress | 1.8 (0.9)c | ||
| 18 | Thioredoxin domain-containing protein 5 (ERp46) | Endoplasmic reticulum/stress | 3.9 (0.6)e | ||
| 9 | 2-Hydroxyphytanoyl coenzyme A lyase | Fatty acid cycle | −6.8 (0.3)e | ||
| 40 | Acetyl coenzyme A acyltransferase | Fatty acid cycle | −C (40) | −5.2 (1.0)e | |
| 71 | Arginase-1 | Immune/urea cycle | NC and + (31, 54) | −T (63, 66), −C (40) | 1.1 (0.1) |
| 72 | Arginase-1 | Immune/urea cycle | NC and + (31, 54) | −T (63, 66), −C (40) | NDf |
| 73 | Arginase-1 | Immune/urea cycle | NC and + (31, 54) | −T (63, 66), −C (40) | 5.0 (0.6)e |
| 54 | d-Dopachrome tautomerase | Immune | −3.1 (0.3)e | ||
| 52 | Galectin-1 | Immune | ±C (25, 60) | 18 (3.4)e | |
| 50 | Peroxiredoxin-1 | Immune | −C (44), −C/H (5) | 15 (14) | |
| 78 | Peroxiredoxin-1 | Immune | −C (44), −C/H (5) | 2.2 (0.1)e | |
| 79 | Nucleoside diphosphate kinase B | Nucleotide synthesis | +C (42, 70) | 2.3 (0.2)e | |
| 42 | Elongation factor 1 beta | Protein synthesis | 2.4 (0.4)e | ||
| 53 | Eukaryotic initiation factor 5A | Protein synthesis | +C (25), −C/H (5) | 9.8 (2.6)e | |
| 29 | Actin (β or γ) | Structural | ± (3, 15, 54, 71) | −4.9 (0.8)e | |
| 32 | Actin (β or γ) | Structural | ± (3, 15, 54, 71) | 12 (2.4)e | |
| 37 | Actin (β or γ) | Structural | ± (3, 15, 54, 71) | 20 (2.1)e | |
| 47 | A-X actin (fragment) | Structural | +C (50) | 11 (2.1)e | |
| 17 | Cytokeratin | Structural | + (6) | 14 (11)d | |
| 27 | Cytokeratin | Structural | + (6) | 18 (7.3)e | |
| 34 | Cytokeratin | Structural | + (6) | 2.2 (0.6)c | |
| 62 | Keratin complex 2 basic, gene 6A | Structural | 4.4 (1.7)c | ||
| 2 | Procollagen VI α1 | Structural | + (11, 49, 54, 56, 71, 74) | 11 (1.8)e | |
| 45 | Rho GDP dissociation inhibitor 1 | Structural | +C (22), −C/H (5) | 3.8 (0.2)e | |
| 43 | Tropomyosin 5 | Structural | 5.1 (1.0)e | ||
| 22 | Vimentin | Structural | + (6, 18) | +C (22) | 15 (2.7)e |
| 23 | Vimentin | Structural | + (6, 18) | +C (22) | 18 (3.0)e |
| 24 | Vimentin | Structural | + (6, 18) | +C (22) | 4.3 (1.2)e |
| 26 | Vimentin | Structural | + (6, 18) | +C (22) | 2.2 (0.4)e |
| 38 | RIKEN cDNA 4931406C07 | Unknown | −3.6 (0.1)e | ||
| 19 | Selenium-binding protein | Unknown | −C/H (5) | −2.2 (0.3)e | |
| 1 | Carbamoyl phosphate synthase | Urea cycle | − (65) | ±T (66), +C (38) | −2.6 (0.6)c |
| 56 | Carbamoyl phosphate synthetase | Urea cycle | − (65) | ±T (66), +C (38) | −8.1 (1.8)e |
| 57 | Carbamoyl phosphate synthetase | Urea cycle | − (65) | ±T (66), +C (38) | −7.7 (1.4)e |
| 74 | Ornithine carbamoyl transferase mitochondrial precursor (fragment) | Urea cycle | − (65) | −3.2 (0.8)e |
−, decreased expression; +, increased expression; ±, both increased and decreased expression; NC, no change. For liver associations: T, drug toxicity; C, liver cancer; C/H, hepatitis C-induced liver cancer.
Average fold increase in spot density compared to the spot intensity for uninfected livers. The numbers in parentheses are standard errors of the means, and the data are data for three to six animals per group.
P < 0.01 for a comparison of normal and schistosome-infected livers (three to six animals per group), as determined by Student's t test.
P < 0.05 for a comparison of normal and schistosome-infected livers (three to six animals per group), as determined by Student's t test.
P < 0.001 for a comparison of normal and schistosome-infected livers (three to six animals per group), as determined by Student's t test.
ND, not detected in schistosome-infected livers.
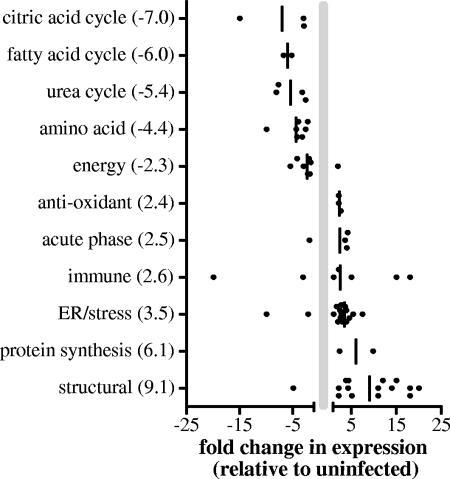
To better understand the impact of schistosome infection on the liver, the matched proteins were classified based on their functions (Table 1), and the overall changes in expression of the proteins in the functional groups were compared (Fig. 3). These analyses revealed that by 8 weeks postinfection schistosomiasis caused a >5-fold decrease in the expression of enzymes associated with the citric acid cycle, fatty acid cycle, and urea cycle and a 4.4-fold decrease in the expression of enzymes associated with amino acid metabolism and catabolism (Fig. 3). These findings are supported by the results of previous studies that showed that there were significant decreases in carbamoyl phosphate synthetase and ornithine carbamoyltrasferase activities in the livers of schistosome-infected mice (65). In contrast to our studies, where the expression of malic enzyme was decreased 15-fold by infection, Fahim et al. reported an increase in malic enzyme activity in the plasma of schistosome-infected hamsters (20). However, no difference was found in the electrophoretic pattern (20). A recent metabolomic study of urine samples from mice infected for 7 to 8 weeks revealed that infection resulted in disturbance of carbohydrate metabolism, amino acid metabolism, and the tricarboxylic acid cycle (69), and these findings supported our results indicating that multiple functional pathways in the liver are impaired during schistosomiasis.
FIG. 3.
Changes in protein abundance for functional groups.
In another recent study workers used microarray analysis to understand how polarizing the immune response during schistosome infection affected gene expression in the liver (34). However, while some comparisons are shown in Table 1, relatively few direct comparisons could be made as the focus of the study of Hoffmann et al. was on the immune response and downstream effects, whereas in this study we concentrated on the medium- to high-abundance proteins and the impact on liver function. One additional issue affecting comparisons of the microarray and proteomics data is the lack of correlation between transcript abundance and protein abundance due to the effects of posttranslational modification that generates protein complexity that is not detected at the RNA level (27). We therefore did not expect the microarray results to show potential changes in protein modifications, such as the changes possible for arginase 1 protein spots 72 and 73, whose expression decreased and increased, respectively, in schistosome-infected livers. While in this instance these two analytical tools may not be directly comparable, as more data are collected by these methods, they will certainly prove to be complementary.
The greatest increases in expression based on functional groups were the increases in expression of proteins associated with structural components, protein synthesis, the stress response, or the endoplasmic reticulum. The increased abundance of structural components is consistent with the deposition of fibrosis molecules induced by the schistosome eggs. Many studies have demonstrated that there are increases in deposition of procollagens I, III, and IV and collagen isoforms I and III to VI (49, 54, 56, 71, 74), and these increases in collagen deposition have been shown to be positively regulated by IL-13 (12) and negatively regulated by gamma interferon (15).
In addition to increased procollagen VI deposition, our study also showed that the expression of other cytoskeletal components (i.e., vimentin, keratin, and actin) was significantly increased. β-Actin expression has previously been shown to be elevated during schistosomiasis (3, 54, 71); however, this study is the first study to show that the pattern of expression for different actin isoforms or modified actin proteins is altered during infection, with changes ranging from a 5-fold reduction to a 20-fold increase. The significance of this altered expression pattern is unknown, but the changes may be due to alterations in the cellular composition in the liver or protein phosphorylation during schistosomiasis. Interestingly, higher levels of autoantibodies to the intermediate filaments, vimentin and keratin, have been detected in patients with S. mansoni infections (6). As our study showed that there was substantially increased abundance of multiple isoforms of these proteins (14- to 18-fold), it is possible that immunorecognition of specific isoforms is a unique marker of schistosome infection.
Another group of proteins which were markedly more abundant in the livers of infected animals was the stress or endoplasmic reticulum proteins. This group contained heat shock proteins (e.g., Hsp60), chaperones (e.g., chaperonin), and protein-modifying or folding enzymes (e.g., protein disulfide isomerase). The increased abundance may have reflected either an increase in protein synthesis in general or enhanced activity in the misfolded protein pathway due to increased oxidative or cellular stress. This is a novel finding as there have been no previous analyses of the effects of schistosomiasis on proteins associated with these cellular functions.
Two proteins in this group are of particular interest, reticulocalbin 3 and Hsp70. Reticulocalbin is remarkable in that its level increased 26-fold during infection. Hoffmann et al. also reported upregulation of a reticulocalbin-like precursor (34). Reticulocalbin, a member of the CREC family, is a Ca2+-binding protein which has multiple EF-hand motifs (35). While the exact function of reticulcalbin is unknown, it is believed to be essential for protein processing (35), and the Small eye Harwell mutation, believed to be a deletion of the reticulocalbin gene, leads to early lethality (37). Moreover, overexpression of reticulocalbin has been linked to the invasiveness of breast cancer cell lines, yet downregulation of reticulocalbin appears to correlate with the acquisition of drug resistance in non-small-cell lung cancer (32). Together, these findings show that reticulocalbin is centrally involved in normal cellular function and that its expression is significantly and distinctly altered in various pathogenic states, including schistosomiasis.
In contrast to reticulocalbin, there was only a modest total increase (2.75-fold) in the abundance of Hsp70 during schistosome infection based on the total spot volume of spots 4 to 6 (2.64 and 7.26 for normal and schistosome-infected mice, respectively). However, a distinct change in the pattern of isoforms of Hsp70 expressed during infection was observed, as there appeared to be a complete loss of expression of the most acidic form in infected livers (spot 4) (Fig. 1). Moreover, the expression of two other basic forms (spots 5 and 6) (Fig. 1) was elevated concurrently. Currently, we cannot distinguish between differences among Hsp forms due to differences in posttranslational modification or differences in gene expression, although a tandem mass spectrometry analysis might provide further structure-based evidence. These changes in Hsp forms may provide insight into the impact of schistosomiasis on the liver because, previously, the expression of Hsp70 isoforms with different pIs was shown to be altered after heat shock of rat myocardium and the accumulation of these different isoforms was linked to the time after physiological insult (73). Additionally in chondrocytes, Hsp70 appears to be involved in the molecular chaperoning of collagen molecules (24). Our results showed that there was a distinct increase in the abundance of Hsp70, which may have been due to its role in collagen processing, but whether the alteration in pI dictated an alteration in function or was a result of changes in cellular composition of the liver is unclear.
The functional group with the widest range of abundance was the immune-related proteins, whose abundance ranged from undetectable to an 18-fold increase during infection. This group contained the proteins galectin-1, arginase-1, and peroxiredoxin-1. Galectin-1 is a galactose-binding protein whose expression increases 18-fold during schistosomiasis. It has been shown to have immunomodulatory effects, to induce Fas-independent T-cell apoptosis, to protect animals from concanavalin-induced hepatitis, and to promote tissue fibrosis (23, 55, 62). Recent work has also shown that another galectin family member, galectin-3, binds to soluble schistosome egg antigens and thereby mediates recognition of these antigens by macrophages (67). The substantial increase in galectin-1 abundance during schistosomiasis supports the hypothesis that this protein is involved in parasite recognition and suggests that it may play a role in regulating the schistosome-induced immune response and subsequent effects on liver pathology and fibrosis.
Peroxiredoxin-1 is an antioxidant enzyme whose expression is upregulated during oxidative stress in the liver (57), and it has been shown play a role in suppressing cancer development (44). This enzyme can become “overoxidized” and thus inactivated in certain disease states, and this oxidation can be detected by a change in pI (10). We found two protein spots that matched peroxiredoxin-1, spots 50 (Fig. 1) and 78 (Fig. 2). Spot 50 had an experimental pI of approximately 6.3 (well below the theoretical pI, 8.6), and thus, this alteration raises the possibility that this spot, whose expression increased 15-fold during schistosomiasis, is indicative of an oxidized, phosphorylated, or otherwise altered form of peroxiredoxin. Therefore, although peroxiredoxin-1 is upregulated during several liver diseases (44, 57), the presence of the more acidic isoform may be unique to schistosomiasis and result in a reduced or modified function of the enzyme.
Finally, arginase-1 is an essential enzyme in the urea cycle and is important in regulating NO production by controlling the availability of arginine, the substrate for nitric oxide synthase (43). Arginase-1 is normally expressed by hepatocytes, but it is also expressed by alternatively activated macrophages (i.e., macrophages exposed to IL-4 or helminth products) (43). Our results indicate that the overall abundance of arginase-1 protein in schistosome-infected livers was similar to that in normal livers (the total spot density was 4.29, compared with 3.75 in uninfected livers), and this finding is consistent with the finding of previous studies, in which workers assayed the total enzymatic activity in the liver and found no difference between infected and uninfected mice (31). However, while we found no difference in the total abundance of arginase-1, there were significant differences in the forms that were expressed, as shown by the three arginase-1-identified spots (spots 71 to 73) (Table 1). Other studies have indicated that this enzyme is upregulated in macrophages during schistosome infection (31), and the increased expression occurs in the granuloma and not the liver parenchyma (31). Therefore, it is possible that the three arginase-1 spots represent transcriptional variants or posttranslational modifications that are expressed in different cell types or under different environmental conditions and that the preferential expression of one of these forms may be indicative of a distinct liver pathology. Because in previous nonproteomic approaches workers have primarily assessed total enzyme activity or abundance, they may have overlooked this potentially important distinction.
In addition to altered expression during schistosome infection, many of the identified proteins are also differentially expressed during hepatic cancer or other liver diseases or during exposure to toxins. The associations between cancer and liver disease or toxicity are summarized in Table 1. From the data it is clear that many of the effects on liver protein abundance may be due to a generalized response to liver injury and not necessarily schistosome-specific changes. However, some proteins or protein expression patterns appear to be unique to schistosomiasis (calreticulin, protein disulfide isomerase, peptidylprolyl isomerase, peroxiredoxin-1, arginase-1, and β- and γ-actins). In addition, it is interesting that several proteins which are used as diagnostic markers for hepatic cancers are also strongly associated with schistosome infection (F0F1-ATP synthase, nucleoside diphosphate kinase B, glutathione S-transferase Pi class, and actin Ax). Although more suggestive than conclusive, given the connection between Schistosoma haematobium infection and bladder cancer, these results support the hypothesis that the early processes in hepatic cancer development and schistosome-induced liver disease may have similar pathways and/or responses.
Taken together, the results of our study indicate that by 8 weeks postinfection, distinct and unique alterations in protein expression are apparent in the livers of infected mice. These alterations include reductions in the expression of proteins associated with the citric acid cycle, the fatty acid cycle, the urea cycle, and amino acid metabolism, increases in the levels of stress and structural components, and several unique changes in the pattern of isoforms expressed for Hsp70, actin, peroxiredoxin-1, and arginase-1. These results suggest that in the acute stage of infection, liver function may be subsequently impaired by these changes. Additionally, our study revealed several distinct and unique changes specific for schistosomiasis that may be valuable as potential biomarkers or may be useful in understanding the pathology of schistosome-mediated liver damage and its contributions to the more subtle morbidity observed in patients with gastrointestinal schistosomiasis.
Supplementary Material
Acknowledgments
This work was supported by grants from the School of Biological Sciences Strategic Research Fund to A.C.L.F. and T.W.J. Schistosome life cycle stages for this work were supplied through NIH-NIAID contract N01-A1-55270.
We thank Evan Secor, Pisana Rawson, and Kylie Hellier for excellent advice and assistance.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 13 November 2006.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ahmed, S. A., and M. Z. Gad. 1996. Diagnostic value of serum lactate dehydrogenase isoenzyme and amino acid patterns in several schistosomal and non-schistosomal disorders as compared to other biochemical parameters. Dis. Markers 13:19-29. [DOI] [PubMed] [Google Scholar]
- 2.Amacher, D. E. 2002. A toxicologist's guide to biomarkers of hepatic response. Hum. Exp. Toxicol. 21:253-262. [DOI] [PubMed] [Google Scholar]
- 3.Andrade, Z. A., E. Peixoto, S. Guerret, and J. A. Grimaud. 1992. Hepatic connective tissue changes in hepatosplenic schistosomiasis. Hum. Pathol. 23:566-573. [DOI] [PubMed] [Google Scholar]
- 4.Arinola, O. G., L. Salawu, and O. Ojurongbe. 2005. Immunoglobulin classes (IgG, IgA and IgM) and acute phase proteins in pregnant women with urinary schistosomiasis. West Afr. J. Med. 24:44-48. [DOI] [PubMed] [Google Scholar]
- 5.Blanc, J. F., C. Lalanne, C. Plomion, J. M. Schmitter, K. Bathany, J. M. Gion, P. Bioulac-Sage, C. Balabaud, M. Bonneu, and J. Rosenbaum. 2005. Proteomic analysis of differentially expressed proteins in hepatocellular carcinoma developed in patients with chronic viral hepatitis C. Proteomics 5:3778-3789. [DOI] [PubMed] [Google Scholar]
- 6.Boehme, M. W., P. K. Kataaha, and E. J. Holborow. 1989. Autoantibodies to intermediate filaments in sera of patients with Schistosoma mansoni infection. Clin. Exp. Immunol. 77:230-233. [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, H. W. 1975. Basic clinical parasitology, 4th ed., p. 239-252. Appleton-Century-Crofts, New York, NY.
- 8.Brunet, L. R., F. D. Finkelman, A. W. Cheever, M. A. Kopf, and E. J. Pearce. 1997. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol. 159:777-785. [PubMed] [Google Scholar]
- 9.Butterworth, A. E., A. J. Curry, D. W. Dunne, A. J. Fulford, G. Kimani, H. C. Kariuki, R. Klumpp, D. Koech, G. Mbugua, J. H. Ouma, et al. 1994. Immunity and morbidity in human schistosomiasis mansoni. Trop. Geogr. Med. 46:197-208. [PubMed] [Google Scholar]
- 10.Cesaratto, L., C. Vascotto, C. D'Ambrosio, A. Scaloni, U. Baccarani, I. Paron, G. Damante, S. Calligaris, F. Quadrifoglio, C. Tiribelli, and G. Tell. 2005. Overoxidation of peroxiredoxins as an immediate and sensitive marker of oxidative stress in HepG2 cells and its application to the redox effects induced by ischemia/reperfusion in human liver. Free Radic. Res. 39:255-268. [DOI] [PubMed] [Google Scholar]
- 11.Chen, F., W. Cai, Z. Chen, X. Chen, and R. Liu. 2002. Dynamic changes in the collagen metabolism of liver fibrosis at the transcription level in rabbits with Schistosomiasis japonica. Chin. Med. J. (Engl. Ed.). 115:1637-1640. [PubMed] [Google Scholar]
- 12.Chiaramonte, M. G., D. D. Donaldson, A. W. Cheever, and T. A. Wynn. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 104:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiaramonte, M. G., M. Mentink-Kane, B. A. Jacobson, A. W. Cheever, M. J. Whitters, M. E. Goad, A. Wong, M. Collins, D. D. Donaldson, M. J. Grusby, and T. A. Wynn. 2003. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J. Exp. Med. 197:687-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuezva, J. M., M. Krajewska, M. L. de Heredia, S. Krajewski, G. Santamaria, H. Kim, J. M. Zapata, H. Marusawa, M. Chamorro, and J. C. Reed. 2002. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 62:6674-6681. [PubMed] [Google Scholar]
- 15.Czaja, M. J., F. R. Weiner, S. Takahashi, M. A. Giambrone, P. H. van der Meide, H. Schellekens, L. Biempica, and M. A. Zern. 1989. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology 10:795-800. [DOI] [PubMed] [Google Scholar]
- 16.Doenhoff, M. J., S. Pearson, D. W. Dunne, Q. Bickle, S. Lucas, J. Bain, R. Musallam, and O. Hassounah. 1981. Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans. R. Soc. Trop. Med. Hyg. 75:41-53. [DOI] [PubMed] [Google Scholar]
- 17.el-Haieg, M. O., M. M. Enein, M. A. Mustafa, M. I. el-Khodary, M. A. Refaat, I. A. Ibrahim, and M. A. Abul-Fadl. 1978. Studies on certain serum enzymatic activities in hepatosplenic bilharziasis. Egypt. J. Bilharz. 5:19-28. [PubMed] [Google Scholar]
- 18.El-Koraie, A. F., N. M. Baddour, A. G. Adam, E. H. El-Kashef, and A. M. El Nahas. 2002. Cytoskeletal protein expression and regenerative markers in schistosomal nephropathy. Nephrol. Dial. Transplant. 17:803-812. [DOI] [PubMed] [Google Scholar]
- 19.Elliott, D. E. 1996. Schistosomiasis. Pathophysiology, diagnosis, and treatment. Gastroenterol. Clin. N. Am. 25:599-625. [DOI] [PubMed] [Google Scholar]
- 20.Fahim, F. A., E. W. Mohareb, N. S. Mansour, and A. M. Nour. 1988. Effect of immature Schistosoma mansoni worms on hamsters' plasma enzymes. Comp. Biochem. Physiol. B 90:851-854. [DOI] [PubMed] [Google Scholar]
- 21.Fallon, P. G., E. J. Richardson, G. J. McKenzie, and A. N. McKenzie. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164:2585-2591. [DOI] [PubMed] [Google Scholar]
- 22.Fella, K., M. Gluckmann, J. Hellmann, M. Karas, P. J. Kramer, and M. Kroger. 2005. Use of two-dimensional gel electrophoresis in predictive toxicology: identification of potential early protein biomarkers in chemically induced hepatocarcinogenesis. Proteomics 5:1914-1927. [DOI] [PubMed] [Google Scholar]
- 23.Fitzner, B., H. Walzel, G. Sparmann, J. Emmrich, S. Liebe, and R. Jaster. 2005. Galectin-1 is an inductor of pancreatic stellate cell activation. Cell. Signal. 17:1240-1247. [DOI] [PubMed] [Google Scholar]
- 24.Freyria, A. M., M. C. Ronziere, M. M. Boutillon, and D. Herbage. 1995. Effect of retinoic acid on protein synthesis by foetal bovine chondrocytes in high-density culture: down-regulation of the glucose-regulated protein, GRP-78, and type II collagen. Biochem. J. 305:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii, K., T. Kondo, H. Yokoo, T. Yamada, K. Iwatsuki, and S. Hirohashi. 2005. Proteomic study of human hepatocellular carcinoma using two-dimensional difference gel electrophoresis with saturation cysteine dye. Proteomics 5:1411-1422. [DOI] [PubMed] [Google Scholar]
- 26.Gharib, B., O. M. Abdallahi, H. Dessein, and M. De Reggi. 1999. Development of eosinophil peroxidase activity and concomitant alteration of the antioxidant defenses in the liver of mice infected with Schistosoma mansoni. J. Hepatol. 30:594-602. [DOI] [PubMed] [Google Scholar]
- 27.Griffin, T. J., S. P. Gygi, T. Ideker, B. Rist, J. Eng, L. Hood, and R. Aebersold. 2002. Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Mol. Cell. Proteomics 1:323-333. [DOI] [PubMed] [Google Scholar]
- 28.Grzych, J. M., E. Pearce, A. Cheever, Z. A. Caulada, P. Caspar, S. Heiny, F. Lewis, and A. Sher. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146:1322-1327. [PubMed] [Google Scholar]
- 29.Guangjin, S., J. Mingdao, L. Qiyang, X. Hui, H. Jiangming, and Y. Xiaomei. 2002. Study on histopathology, ultrasonography and some special serum enzymes and collagens for 38 advanced patients of schistosomiasis japonica. Acta Trop. 82:235-246. [DOI] [PubMed] [Google Scholar]
- 30.Harrison, D. J., L. May, J. D. Hayes, and G. E. Neal. 1990. Glutathione S-transferase localization in aflatoxin B1-treated rat livers. Carcinogenesis 11:927-931. [DOI] [PubMed] [Google Scholar]
- 31.Hesse, M., M. Modolell, A. C. La Flamme, M. Schito, J. M. Fuentes, A. W. Cheever, E. J. Pearce, and T. A. Wynn. 2001. Differential regulation of NOS-2 and arginase-1 by type-1/type-2 cytokines in vivo. Granuloma pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 167:6533-6544. [DOI] [PubMed] [Google Scholar]
- 32.Hirano, T., H. Kato, M. Maeda, Y. Gong, Y. Shou, M. Nakamura, J. Maeda, K. Yashima, Y. Kato, S. Akimoto, T. Ohira, M. Tsuboi, and N. Ikeda. 2005. Identification of postoperative adjuvant chemotherapy responders in non-small cell lung cancer by novel biomarker. Int. J. Cancer 117:460-468. [DOI] [PubMed] [Google Scholar]
- 33.Ho, D. W., S. T. Fan, J. To, Y. H. Woo, Z. Zhang, C. Lau, and J. Wong. 2002. Selective plasma filtration for treatment of fulminant hepatic failure induced by d-galactosamine in a pig model. Gut 50:869-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann, K. F., T. C. McCarty, D. H. Segal, M. Chiaramonte, M. Hesse, E. M. Davis, A. W. Cheever, P. S. Meltzer, H. C. Morse III, and T. A. Wynn. 2001. Disease fingerprinting with cDNA microarrays reveals distinct gene expression profiles in lethal type 1 and type 2 cytokine-mediated inflammatory reactions. FASEB J. 15:2545-2547. [DOI] [PubMed] [Google Scholar]
- 35.Honore, B., and H. Vorum. 2000. The CREC family, a novel family of multiple EF-hand, low-affinity Ca2+-binding proteins localised to the secretory pathway of mammalian cells. FEBS Lett. 466:11-18. [DOI] [PubMed] [Google Scholar]
- 36.Hughes, S. M., P. Moroni-Rawson, R. D. Jolly, and T. W. Jordan. 2001. Submitochondrial distribution and delayed proteolysis of subunit c of the H+-transporting ATP-synthase in ovine ceroid-lipofuscinosis. Electrophoresis 22:1785-1794. [DOI] [PubMed] [Google Scholar]
- 37.Kent, J., M. Lee, A. Schedl, S. Boyle, J. Fantes, M. Powell, N. Rushmere, C. Abbott, V. van Heyningen, and W. A. Bickmore. 1997. The reticulocalbin gene maps to the WAGR region in human and to the Small eye Harwell deletion in mouse. Genomics 42:260-267. [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita, M., and M. Miyata. 2002. Underexpression of mRNA in human hepatocellular carcinoma focusing on eight loci. Hepatology 36:433-438. [DOI] [PubMed] [Google Scholar]
- 39.La Flamme, A. C., E. A. Patton, B. Bauman, and E. J. Pearce. 2001. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J. Immunol. 166:1903-1911. [DOI] [PubMed] [Google Scholar]
- 40.Liang, C. R., C. K. Leow, J. C. Neo, G. S. Tan, S. L. Lo, J. W. Lim, T. K. Seow, P. B. Lai, and M. C. Chung. 2005. Proteome analysis of human hepatocellular carcinoma tissues by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics 5:2258-2271. [DOI] [PubMed] [Google Scholar]
- 41.Mahmoud, O. M., F. Elsamani, A. A. Gameel, and M. G. Taylor. 1987. Serum enzyme changes in calves experimentally infected with Schistosoma bovis. J. Comp. Pathol. 97:335-339. [DOI] [PubMed] [Google Scholar]
- 42.Martinez, J. A., S. Prevot, B. Nordlinger, T. M. Nguyen, Y. Lacarriere, A. Munier, I. Lascu, J. C. Vaillant, J. Capeau, and M. L. Lacombe. 1995. Overexpression of nm23-H1 and nm23-H2 genes in colorectal carcinomas and loss of nm23-H1 expression in advanced tumour stages. Gut 37:712-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munder, M., K. Eichmann, and M. Modolell. 1998. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 160:5347-5354. [PubMed] [Google Scholar]
- 44.Neumann, C. A., D. S. Krause, C. V. Carman, S. Das, D. P. Dubey, J. L. Abraham, R. T. Bronson, Y. Fujiwara, S. H. Orkin, and R. A. Van Etten. 2003. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424:561-565. [DOI] [PubMed] [Google Scholar]
- 45.Panes, J., X. Soler, A. Pares, J. Caballeria, J. Farres, J. Rodes, and X. Pares. 1989. Influence of liver disease on hepatic alcohol and aldehyde dehydrogenases. Gastroenterology 97:708-714. [DOI] [PubMed] [Google Scholar]
- 46.Park, K. S., S. Y. Cho, H. Kim, and Y. K. Paik. 2002. Proteomic alterations of the variants of human aldehyde dehydrogenase isozymes correlate with hepatocellular carcinoma. Int. J. Cancer 97:261-265. [DOI] [PubMed] [Google Scholar]
- 47.Pearce, E. J., A. Cheever, S. Leonard, M. Covalesky, R. Fernandez-Botran, G. Kohler, and M. Kopf. 1996. Schistosoma mansoni in IL-4-deficient mice. Int. Immunol. 8:435-444. [DOI] [PubMed] [Google Scholar]
- 48.Rotenberg, Z., I. Weinberger, E. Davidson, J. Fuchs, D. Harell, and J. Agmon. 1989. Lactate dehydrogenase isoenzyme patterns in serum of patients with metastatic liver disease. Clin. Chem. 35:871-873. [PubMed] [Google Scholar]
- 49.Saber, M. A., D. A. Shafritz, and M. A. Zern. 1983. Changes in collagen and albumin mRNA in liver tissue of mice infected with Schistosoma mansoni as determined by in situ hybridization. J. Cell Biol. 97:986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadano, H., S. Taniguchi, T. Kakunaga, and T. Baba. 1988. cDNA cloning and sequence of a new type of actin in mouse B16 melanoma. J. Biol. Chem. 263:15868-15871. [PubMed] [Google Scholar]
- 51.Sakai, H., T. Tsukamoto, M. Yamamoto, K. Kobayashi, H. Yuasa, T. Imai, T. Yanai, T. Masegi, and M. Tatematsu. 2002. Distinction of carcinogens from mutagens by induction of liver cell foci in a model for detection of initiation activity. Cancer Lett. 188:33-38. [DOI] [PubMed] [Google Scholar]
- 52.Salah, L. A., A. A. Kheireldin, M. M. Mansour, and F. Hussein. 1976. Levels of some serum enzymes in patients with schistosomiasis. J Trop. Med. Hyg. 79:270-274. [PubMed] [Google Scholar]
- 53.Sanchez, J. C., D. Chiappe, V. Converset, C. Hoogland, P. A. Binz, S. Paesano, R. D. Appel, S. Wang, M. Sennitt, A. Nolan, M. A. Cawthorne, and D. F. Hochstrasser. 2001. The mouse SWISS-2D PAGE database: a tool for proteomics study of diabetes and obesity. Proteomics 1:136-163. [DOI] [PubMed] [Google Scholar]
- 54.Sandler, N. G., M. M. Mentink-Kane, A. W. Cheever, and T. A. Wynn. 2003. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 171:3655-3667. [DOI] [PubMed] [Google Scholar]
- 55.Santucci, L., S. Fiorucci, F. Cammilleri, G. Servillo, B. Federici, and A. Morelli. 2000. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology 31:399-406. [DOI] [PubMed] [Google Scholar]
- 56.Shahin, M., D. Schuppan, R. Waldherr, J. Risteli, L. Risteli, E. R. Savolainen, C. Oesterling, H. M. Abdel Rahman, A. M. el Sahly, S. M. Abdel Razek, et al. 1992. Serum procollagen peptides and collagen type VI for the assessment of activity and degree of hepatic fibrosis in schistosomiasis and alcoholic liver disease. Hepatology 15:637-644. [DOI] [PubMed] [Google Scholar]
- 57.Shau, H., A. Merino, L. Chen, C. C. Shih, and S. D. Colquhoun. 2000. Induction of peroxiredoxins in transplanted livers and demonstration of their in vitro cytoprotection activity. Antioxid. Redox. Signal. 2:347-354. [DOI] [PubMed] [Google Scholar]
- 58.Sheweita, S. A., F. G. El-Shahat, M. A. Bazeed, M. R. Abu El-Maati, and P. J. O'Connor. 2004. Effects of Schistosoma haematobium infection on drug-metabolizing enzymes in human bladder cancer tissues. Cancer Lett. 205:15-21. [DOI] [PubMed] [Google Scholar]
- 59.Sheweita, S. A., M. H. Mostafa, F. Ebid, and W. El-Sayed. 2003. Changes in expression and activity of glutathione S-transferase in different organs of schistosoma haematobium-infected hamster. J. Biochem. Mol. Toxicol. 17:138-145. [DOI] [PubMed] [Google Scholar]
- 60.Shimonishi, T., K. Miyazaki, N. Kono, H. Sabit, K. Tuneyama, K. Harada, J. Hirabayashi, K. Kasai, and Y. Nakanuma. 2001. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum. Pathol. 32:302-310. [DOI] [PubMed] [Google Scholar]
- 61.Stickel, F., D. Schuppan, E. G. Hahn, and H. K. Seitz. 2002. Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut 51:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stillman, B. N., D. K. Hsu, M. Pang, C. F. Brewer, P. Johnson, F. T. Liu, and L. G. Baum. 2006. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176:778-789. [DOI] [PubMed] [Google Scholar]
- 63.Tabuchi, S., T. Gotoh, K. Miyanaka, K. Tomita, and M. Mori. 2000. Regulation of genes for inducible nitric oxide synthase and urea cycle enzymes in rat liver in endotoxin shock. Biochem. Biophys. Res. Commun. 268:221-224. [DOI] [PubMed] [Google Scholar]
- 64.Takase, S., A. Takada, M. Yasuhara, and M. Tsutsumi. 1989. Hepatic aldehyde dehydrogenase activity in liver diseases, with particular emphasis on alcoholic liver disease. Hepatology 9:704-709. [DOI] [PubMed] [Google Scholar]
- 65.Tanabe, M., N. Kaneko, and T. Takeuchi. 1989. Schistosoma mansoni: suppression of carbamoyl phosphate synthetase (ammonia) and ornithine carbamoyltransferase activities in the liver of infected mice. Exp. Parasitol. 68:432-442. [DOI] [PubMed] [Google Scholar]
- 66.Thome-Kromer, B., I. Bonk, M. Klatt, G. Nebrich, M. Taufmann, S. Bryant, U. Wacker, and A. Kopke. 2003. Toward the identification of liver toxicity markers: a proteome study in human cell culture and rats. Proteomics 3:1835-1862. [DOI] [PubMed] [Google Scholar]
- 67.van den Berg, T. K., H. Honing, N. Franke, A. van Remoortere, W. E. Schiphorst, F. T. Liu, A. M. Deelder, R. D. Cummings, C. H. Hokke, and I. van Die. 2004. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 173:1902-1907. [DOI] [PubMed] [Google Scholar]
- 68.Vidal, F., R. Toda, C. Gutierrez, M. Broch, F. Fernandez-Muixi, A. Lorenzo, and C. Richart. 1998. Influence of chronic alcohol abuse and liver disease on hepatic aldehyde dehydrogenase activity. Alcohol 15:3-8. [DOI] [PubMed] [Google Scholar]
- 69.Wang, Y., E. Holmes, J. K. Nicholson, O. Cloarec, J. Chollet, M. Tanner, B. H. Singer, and J. Utzinger. 2004. Metabonomic investigations in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc. Natl. Acad. Sci. USA 101:12676-12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei, S. J., C. S. Trempus, R. C. Ali, L. A. Hansen, and R. W. Tennant. 2004. 12-O-tetradecanoylphorbol-13-acetate and UV radiation-induced nucleoside diphosphate protein kinase B mediates neoplastic transformation of epidermal cells. J. Biol. Chem. 279:5993-6004. [DOI] [PubMed] [Google Scholar]
- 71.Weiner, F. R., M. J. Czaja, M. A. Giambrone, S. Takahashi, L. Biempica, and M. A. Zern. 1987. Transcriptional and posttranscriptional effects of dexamethasone on albumin and procollagen messenger RNAs in murine schistosomiasis. Biochemistry 26:1557-1562. [DOI] [PubMed] [Google Scholar]
- 72.Yu, L. R., R. Zeng, X. X. Shao, N. Wang, Y. H. Xu, and Q. C. Xia. 2000. Identification of differentially expressed proteins between human hepatoma and normal liver cell lines by two-dimensional electrophoresis and liquid chromatography-ion trap mass spectrometry. Electrophoresis 21:3058-3068. [DOI] [PubMed] [Google Scholar]
- 73.Zamotrinskii, A. V., I. Malyshev, and F. Z. Meerson. 1992. Isoform pattern of inducible HSP 70 in the rat myocardium after heat shock. Biull. Eksp. Biol. Med. 113:586-587. [In Russian.] [PubMed] [Google Scholar]
- 74.Zern, M. A., M. A. Saber, and D. A. Shafritz. 1983. Molecular mechanisms for changes in hepatic protein synthesis induced by schistosomiasis infection in mice. Biochemistry 22:6072-6077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.