Abstract
Hemolytic-uremic syndrome (HUS), the life-threatening complication following infection by the intestinal pathogen Escherichia coli O157:H7, is due to the ability of the pathogen to produce toxins in the Shiga toxin (Stx) family. Activated neutrophils are observed in HUS patients, yet it is unclear whether Stx exerts a direct effect on neutrophils or whether the toxin acts indirectly. The effect of Stx1 and Stx2 on human neutrophils was examined. Neither Stx1 nor Stx2 altered the rate of neutrophil apoptosis. Minimal binding of either toxin to neutrophils was observed, and the toxin was easily eluted from the cells. Stx1 and Stx2 were found to circulate in the plasma of mice following intravenous injection, and both toxins were cleared rapidly from the blood. Together these results suggest that neither Stx1 nor Stx2 interacts directly with neutrophils.
The association of Escherichia coli O157:H7 with disease outbreaks around the world has caused the pathogen to become a global public health concern. In the United States alone, the food-borne pathogen accounts for approximately 70,000 cases of disease each year (28). Disease caused by E. coli O157:H7 is characterized by diarrhea and can progress to hemorrhagic colitis and hemolytic-uremic syndrome (HUS) (3). The severe sequela resulting from E. coli O157:H7 infection is due in large part to the ability of the pathogen to produce a virulence factor known as Shiga toxin (Stx). Stx is an AB5 toxin comprised of a single A subunit and a homopentameric B subunit (5). The A subunit is responsible for the enzymatic activity of the toxin, functioning as an N-glycosidase (6). It cleaves a single adenine residue from the 28S rRNA, rendering the ribosome incapable of protein synthesis (6). The B subunit binds to the glycosphingolipid receptor globotriaosylceramide (Gb3) and delivers the A subunit into the host cell cytoplasm (22).
There are two antigenic variants of Stx, Stx1 and Stx2, which share approximately 60% amino acid sequence homology (33). Strains of E. coli O157:H7 can produce Stx1, Stx2, or both (40). However, epidemiological studies (2) and animal model data (39, 45) suggest that Stx2 is more often associated with fatal disease than Stx1. The molecular basis for the difference in toxicity between the two structurally similar toxins has not been elucidated. The most severe clinical manifestation of E. coli O157:H7 infection is HUS, a potentially fatal sequela characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal failure (3). The administration of purified Stx in animal models replicates much of the pathology associated with HUS (39).
Interactions of Stx with neutrophils have been proposed to promote Stx production and dissemination. Neutrophils are recruited to the initial site of E. coli O157:H7 infection (15). Hydrogen peroxide and other neutrophil products are able to induce the bacterial stress response, which increases Stx production (47). In vitro data indicate that the transmigration of neutrophils across polarized intestinal epithelial cells enhances the movement of Stx in the opposite direction, presenting a possible role for neutrophils in the entry of Stx into the bloodstream (18).
Neutrophils are also thought to play a role in the progression of E. coli O157:H7 disease to HUS. Clinical data indicate that neutrophil levels are elevated during HUS (19), and elevated peripheral blood neutrophil counts correlate positively with an adverse outcome (30). Renal histopathological analysis revealed that HUS cases have significantly greater numbers of neutrophils than controls (19). Stx1 and Stx2 stimulate endothelial cells to release chemokines and express leukocyte adhesion molecules (27, 31, 49), events that would increase direct neutrophil-mediated endothelial injury. Serum levels of elastase, a major lysosomal protease released by neutrophils that has been shown to cause injury to endothelial cells in vessels (1), were found to be significantly elevated in HUS patients (17). In addition, the neutrophils of HUS patients were shown to induce endothelial injury in vitro (10). Recent studies have shown that neutrophil depletion in mice results in a reduction in Stx2-induced lethality and renal damage (8).
While neutrophils are clearly involved in the development of HUS, it is unclear whether Stx exerts a direct effect on neutrophils or whether it acts indirectly. Glycolipid analyses of human neutrophils did not demonstrate the expression of Gb3 (11, 25), the receptor for Stx, and studies examining the influence of Stx on neutrophil apoptosis have been inconsistent. Stx has been reported to bind to the surface of human neutrophils (4, 41-44) and circulate in the blood bound to neutrophils until encountering renal endothelial cells, when the transfer of the toxin to the endothelial cells occurs (43). However, others have been unable to reproduce this phenomenon (7). Liu et al. previously reported that Stx2 significantly inhibits the rate of neutrophil apoptosis (23, 24). However, in vivo injection of Stx2 in mice was reported to increase the rate of neutrophil apoptosis after about 72 h (14), leading those authors to hypothesize that the enhancement occurred by an indirect mechanism rather than a direct effect of Stx2 on neutrophils. King et al. previously reported that Stx1 exhibited no effect on neutrophil apoptosis (20). Given that Stx2 is approximately 400-fold more toxic than Stx1 in mice (45), it is possible that the two toxins could exhibit different toxicities with regard to neutrophils.
In the present study, a comparative analysis of the effects of Stx1 and Stx2 on human neutrophils was performed. Treatment with either Stx1 or Stx2 was unable to alter the apoptotic program of neutrophils. Minimal binding of Stx to neutrophils was observed in vitro and in vivo. Together, these results suggest that neither Stx1 nor Stx2 interacts directly with neutrophils, and the neutrophil activation observed in patients with HUS is likely mediated by cytokines produced by other cells damaged by Stx.
MATERIALS AND METHODS
Purification of Stx1 and Stx2.
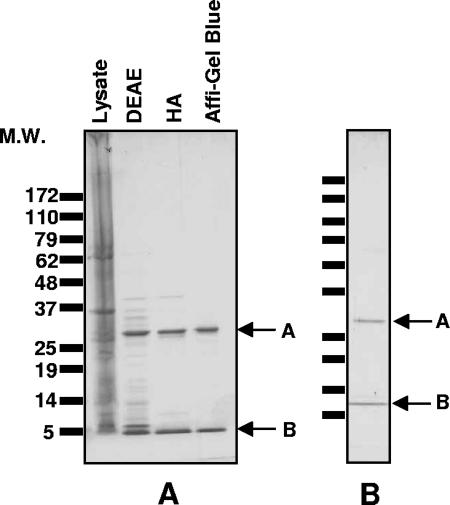
E. coli strain C600, lysogenized with the Stx1-encoding phage H19B or the Stx2-encoding phage 933W (32) and harboring a kanamycin resistance-encoding plasmid (pBBR1-MCS-2), was used to express the two variants of Stx. The expression of toxin was induced with ciprofloxacin as previously described (13). The supernatant was concentrated by ammonium sulfate, and Stx was recovered from the 40 to 70% fraction. The toxin was purified by DEAE ion-exchange, hydroxyapatite affinity, and Affi-Gel Blue (Bio-Rad, Hercules, CA) chromatography, followed by size exclusion chromatography (Stx1) or phenyl-Sepharose chromatography (Stx2). The purity of toxin preparations was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by Coomassie blue staining (Fig. 1), and toxicity was measured by Vero cell assay, as described previously (12). Lipopolysaccharide (LPS) content in purified toxin preparations was determined using the Limulus amoebocyte lysate assay (Cambrex, Walkersville, MD). To heat inactivate the toxin, Stx1 was incubated in a boiling water bath for 2 h. No Vero cell death was observed following heat treatment.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of Shiga toxin. One-microgram samples of toxin at various steps in the purification process were loaded onto 8 to 16% acrylamide gels, separated by electrophoresis, and stained with Coomassie. (A) Stx1 purification. Samples of cell lysate from the ciprofloxacin-induced culture following DEAE ion-exchange, hydroxyapatite (HA), and Affi-Gel Blue chromatography are shown. (B) Purified Stx2. M.W., molecular weight (in thousands).
Isolation of human neutrophils.
Neutrophils were purified from human peripheral blood by dextran sedimentation and Ficoll-Paque centrifugation as previously described (48). Cells were washed three times in Hanks' HEPES-bovine serum albumin and brought up in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum. In instances specifically noted in the text, a negative-selection antibody cocktail (StemSep granulocyte enrichment cocktail; StemCell Technologies) was used as an additional purification step according to the manufacturer's instructions. Cell viability was measured by trypan blue exclusion, and viable neutrophils were counted using a hemocytometer.
Neutrophil apoptosis.
Approximately 2 × 106 neutrophils were treated with 1 μg/ml of Stx1 or Stx2 or an equivalent volume of phosphate-buffered saline (PBS) and incubated for 20 h at 37°C in 5% CO2. A compilation of neutrophil apoptosis rates from various sources indicated that the majority of previously published reports examined apoptosis at 20-h or 24-h time points (37); therefore, 20 h was chosen as the time point of analysis in our experiments. As controls, neutrophils were treated with 1 μg/ml of heat-inactivated Stx1 or 0.58 ng/ml of LPS serotype O25 purified from E. coli strain FI-4 (12). Neutrophil apoptosis was measured using the Annexin V-PE Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions and analyzed by flow cytometry using a Becton Dickinson FACSCalibur. Fluorescence parameters were gated using untreated cells that were either left unstained or single stained with Annexin V-PE or 7-amino-actinomycin D (7-AAD). Annexin V-positive cells were classified as early apoptotic cells, while Annexin V- and 7-AAD-double-positive cells were classified as late apoptotic cells. Total apoptotic cells were determined by adding together the percentages of early and late apoptotic cells. Three independent experiments were performed, and the data were analyzed by Student's t test.
Stx-treated whole blood.
Blood drawn from healthy human donors was treated with 0.11 ml of 3.8% sodium citrate per ml of blood. Approximately 5 μg of Stx1 or Stx2 was added to 3.5 ml of blood and incubated at 37°C in 5% CO2 for 1 h. Toxin-treated blood samples were transferred to 11.5-ml Sorvall Ultracrimp tubes (Kendro Laboratory Products, Newtown, CT), and 5 ml of Mono-Poly resolving medium (MP Biomedicals, Solon, OH) was carefully layered over each sample. Samples were centrifuged for 30 min at 300 × g. Fractions containing plasma, peripheral blood mononuclear cells (PBMCs), neutrophils, and red blood cells (RBCs) were collected. The concentration and purity of PBMCs and neutrophils were determined by flow cytometry. Some of the purified cells were subjected to an additional wash. To wash the cells, 0.1 ml of cells was diluted to 1 ml in PBS, centrifuged for 10 min at 250 × g, and suspended to the starting volume in PBS. Toxin levels for all samples were quantified by enzyme-linked immunosorbent assay (ELISA) using the Premier EHEC test (Meridian Bioscience, Cincinnati, OH). To ensure that toxin internalized by neutrophils or PBMCs was detected, aliquots of cells were lysed by freeze-thaw. Cell-associated toxin levels did not differ significantly between lysed and unlysed cells (data not shown).
Stx in mice.
Mice received 320 ng of Stx1 or 476 ng Stx2 in a 100-μl volume by intravenous injection. At 5 and 30 min after injection, mice were bled from the retro-orbital sinus. Cells were separated from the plasma by centrifugation, and Stx levels were determined by ELISA. The amount of toxin in the circulation was calculated based on the weight of the mice by using a value of 0.0785 ml of blood per gram of mouse weight and an average weight of 15 g per mouse. The limits of detection for Stx1 and Stx2 by ELISA are approximately 0.07 ng/ml and 0.15 ng/ml, respectively. The means and standard deviations of circulating Stx1 and Stx2 were calculated for 5- and 30-min time points, and values below the limit of detection were treated as 0.
RESULTS
Stx1 and Stx2 do not alter neutrophil apoptosis.
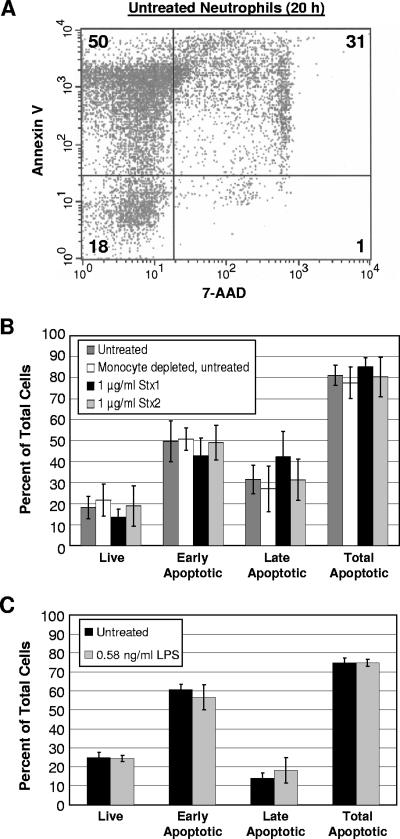
To determine the effects of Stx1 and Stx2 on neutrophil apoptosis, neutrophils were isolated from the peripheral blood of healthy human donors and treated with either Stx1 or Stx2. Apoptosis was measured at 20 h posttreatment by staining the cells with both Annexin V-PE and the vital dye 7-AAD. Annexin V binds to the membrane phospholipid phosphatidylserine (34), which is externalized during the early stages of apoptosis (26). The percentage of live cells, early apoptotic cells, late apoptotic cells, and total apoptotic cells was measured by flow cytometry. A representative experiment for untreated neutrophils is shown in Fig. 2A. Treatment with neither Stx1 nor Stx2 resulted in a statistically significant change (P > 0.05 for all samples) in any of the apoptosis stages measured relative to untreated cells (Fig. 2B).
FIG. 2.
Stx1 and Stx2 do not influence neutrophil apoptosis. Approximately 2 × 106 neutrophils isolated from human peripheral blood were incubated with 1 μg of Stx1 or Stx2 or with LPS. Twenty hours after treatment, cells were stained with Annexin V-PE and 7-AAD and analyzed by flow cytometry. (A) Distribution of live (lower left quadrant), early apoptotic (upper left quadrant), and late apoptotic (upper right quadrant) neutrophils. Values refer to the percentages of total cells in each quadrant. (B) Distribution of live, early apoptotic, late apoptotic, and total apoptotic cells in untreated neutrophils, neutrophils depleted of monocytes, and toxin-treated neutrophils after 20 h of incubation. Results are expressed as the means ± standard deviations of three independent experiments. (C) Distribution of neutrophils following treatment with LPS. Means and standard deviations of the means are displayed. None of the values in a similar stage of apoptosis were found to be different by Student's t test (P > 0.05).
The presence of monocytes and other cells can influence apoptosis (37). Our neutrophil purification strategy typically produces neutrophils that are greater than 95% pure, but to verify that monocyte contamination did not influence the results, a negative-selection antibody cocktail was used to remove residual PBMCs from the purified neutrophil preparations. The cocktail contained monoclonal antibodies directed against cell surface antigens on human hematopoietic cells, including CD2, CD3, CD14, CD19, CD56, and glycophorin A. Apoptosis rates for neutrophils that had undergone the additional purification step did not differ from those for neutrophils purified by our standard protocol (Fig. 2B), indicating that residual PBMCs did not influence the measured apoptosis levels.
The presence of contaminating endotoxin has been shown to influence neutrophil apoptosis (21) indirectly by inducing other cells to produce cytokines (38). LPS is heat stable, and neutrophils treated with heat-inactivated Stx1, which was no longer cytotoxic to Vero cells, did not result in a statistically significant change in neutrophil apoptosis (data not shown). The toxin preparations used in these experiments possessed less than 0.011 ng of LPS per μg of toxin. Treatment with 0.58 ng/ml of LPS, a concentration that exceeded those present in 1 μg/ml of purified Stx1 and Stx2, did not alter the rate of apoptosis (Fig. 2C).
Stx binding in whole blood.
We also examined the ability of Stx1 and Stx2 to bind to neutrophils and other cells in whole blood. Toxin was added to human blood, and samples were then separated over Mono-Poly resolving medium, which resolves mononuclear and polymorphonuclear leukocytes into two distinct bands and yields a plasma fraction and an RBC pellet. The highest concentration of Stx1 and Stx2 was found in the cell-free plasma fraction (Table 1), and some toxin was detected in the cellular fractions. The amount of neutrophil- and PBMC-associated toxin was calculated on a per-cell basis, and more toxin was bound to the PBMCs than to the neutrophils. The purified PBMCs and neutrophils were washed with PBS. The amount of Stx2 associated with the PBMCs was reduced about 100-fold following the wash, while Stx1 was below the limit of detection. After the wash, neither toxin was found to be associated with the neutrophils. Similarly, Stx1 and Stx2 were easily eluted from purified human neutrophils incubated with toxin for 1 h and then washed (data not shown).
TABLE 1.
Analysis of Stx binding in blood
| Treatment (trial) | Fractiona | Amt of Stx (ng/ml) | Stx binding (ng of Stx/cell)
|
|
|---|---|---|---|---|
| Before washb | After washc | |||
| Stx2 (1) | Plasma | 523 | ||
| PBMC | 317 | 1.0 × 10−4 | 2.5 × 10−6 | |
| Neutrophil | 39 | 1.8 × 10−5 | BDLd | |
| RBC | 13 | Not determined | Not determined | |
| Stx2 (2) | Plasma | 643 | ||
| PBMC | 310 | 1.2 × 10−4 | 9.8 × 10−7 | |
| Neutrophil | 127 | 4.7 × 10−5 | BDL | |
| RBC | 7 | Not determined | Not determined | |
| Stx1 (1) | Plasma | 1,022 | ||
| PBMC | 589 | 4.0 × 10−4 | BDL | |
| Neutrophil | 168 | 5.3 × 10−5 | BDL | |
| RBC | 51 | Not determined | Not determined | |
Approximately 5 μg of Stx1 or Stx2 was added to 3.5 ml of blood, incubated at 37°C for 1 h, and fractionated over Mono-Poly resolving medium.
The numbers of cells in PBMC and neutrophil fractions were determined by flow cytometry and used to calculate the ng of Stx per cell.
Cells were washed once in PBS prior to ELISA.
BDL, below the detection limit.
Circulation of Stx1 and Stx2 in mouse blood.
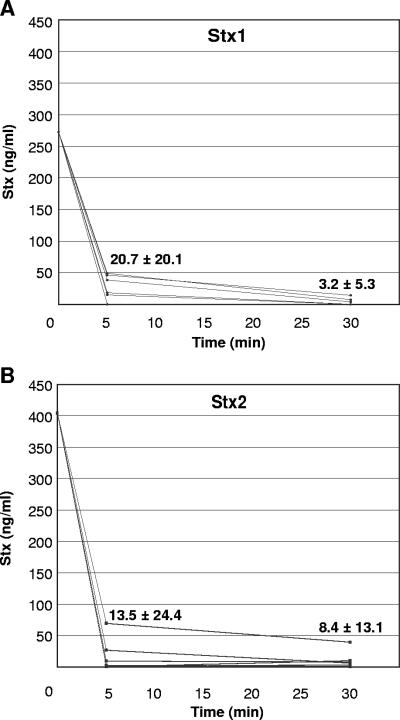
To examine how Stx circulates in vivo, mice were injected intravenously with Stx1 or Stx2 and bled from the retro-orbital sinus at 5 and 30 min after injection. The cells were separated from the plasma by centrifugation, and Stx levels were determined by ELISA. Toxin was detected exclusively in the serum, and no cell-bound toxin was detected at any time points. Interestingly, within 5 min, 93% of the injected Stx1 and 97% of the injected Stx2 had been cleared from the blood (Fig. 3). By 30 min after injection, only 1% of the injected Stx1 and 2% of the injected Stx2 were detected in the plasma.
FIG. 3.
Stx1 and Stx2 circulate in the plasma in vivo. Groups of eight mice were injected intravenously in the tail with either Stx1 (A) or Stx2 (B) and bled from the retro-orbital sinus at 5 and 30 min after injection. Cells were separated from the plasma by centrifugation, and Stx levels were determined by ELISA. Values refer to the means ± standard deviations for values at 5 and 30 min after injection.
DISCUSSION
Neutrophils play an important role in the pathogenesis of HUS. Neutrophils are activated during HUS (10), resulting in the release of factors capable of causing tissue damage. Furthermore, recent studies of a murine model of disease suggest that Stx2-induced lethality and renal damage are reduced in neutrophil-depleted mice (8). However, there have been conflicting reports regarding whether Stx directly promotes neutrophil activation or whether Stx indirectly promotes neutrophil activation by inducing an inflammatory response in other cells. Developing postexposure therapeutics for Stx will require an understanding of the molecular basis for Stx-mediated pathology. In this study, we examined the ability of Stx to bind to and influence neutrophil apoptosis.
Neutrophils are short-lived cells that naturally undergo apoptosis as a mechanism to regulate their number in the circulation and to resolve inflammation (16). Liu et al. previously reported that Stx2 significantly inhibits neutrophil apoptosis (23, 24). In their studies, neutrophils were incubated with Stx2 for 24 h, treated with a hypotonic fluorochrome solution containing 100 μg per ml of propidium iodide, and then stored overnight at 4°C prior to analysis (23, 24). During this long incubation, cellular alteration that was not directly due to apoptosis could have occurred (9). In our experiments, both early (binding of the membrane stain Annexin V-PE) and later (accumulation of 7-ADD) markers were used to measure apoptosis immediately following incubation with Stx. Our data indicate that 1 μg/ml of neither Stx1 nor Stx2 is able to alter the rate of neutrophil apoptosis. In addition, Toll-like receptor 4 activation of monocytes is associated with the release of neutrophil survival factors (38), and LPS, an activator of Toll-like receptor 4 signaling, has been shown to prolong neutrophil survival in a monocyte-dependent manner (36). We demonstrated that neither LPS nor monocyte contamination was a factor in our studies of neutrophil apoptosis. Liu et al. (23, 24) previously reported low endotoxin values for Stx2 but did not comment on the degree of monocyte contamination in their neutrophil preparations.
Expression of the Stx receptor Gb3 has not been demonstrated by mature human neutrophils (11, 25). te Loo et al. previously reported binding to neutrophils, although Stx1 exhibited a 100-fold-lower affinity for neutrophils than Gb3 (43). We attempted to demonstrate binding of Stx to neutrophils using flow cytometry, as performed previously by te Loo et al., but did not detect binding above background levels (data not shown). Similarly, Fernandez et al. were unable to demonstrate the binding of either toxin to human neutrophils (7). In previous studies, Stx binding to neutrophils was detected indirectly by using flow cytometry and labeled antibody to Stx (4, 7, 41, 42, 44) or direct detection of fluorescein isothiocyanate-modified Stx (43). In the present study, we directly analyzed the binding of native Stx using ELISA. Stx was detected primarily in the plasma fraction of human blood, not associated with cells. The small amount of cell-bound toxin associated with neutrophil and PBMC fractions was easily removed when cells were washed. The interaction is likely nonspecific, which explains the easy dissociation of the toxin from neutrophils when the cells are washed. Slightly more Stx1 and Stx2 were associated with PBMCs than with neutrophils, consistent with the observation that unstimulated human monocytes express small amounts of Gb3 (46), while neutrophils do not. A recent study demonstrated that Stx1 is able to bind to and increase apoptosis in ovine granulocytes, which express Gb3, yet is unable to bind to or induce apoptosis in bovine granulocytes, which do not express Gb3 (29). Expression of Gb3 likely determines the ability of neutrophils to bind to and initiate a direct response to Stx.
In vivo studies of mice corroborated our in vitro results with human blood, showing that both Stx1 and Stx2 circulate in the blood free in the plasma. Both Stx1 and Stx2 were cleared rapidly from the blood, as greater than 90% of toxin had been cleared by 5 min after injection. Interestingly, no statistically significant difference between the rate of clearance of Stx1 and that of Stx2 was observed. Rutjes et al. previously reported that within 5 min of intravenous injection of 50 ng Stx1 and Stx2 into mice, 90% of injected Stx1 but only 40% of injected Stx2 had been cleared (35). In the study by Rutjes et al., however, the mice were injected with iodinated Stx, and it is possible that this covalent modification could have altered the physical properties of the toxins and thus affected their circulation in the blood.
The inability to demonstrate a direct interaction between Stx and neutrophils in this study suggests that neutrophil activation occurs by an indirect mechanism, likely as a result of the activation of the inflammatory response in toxin-susceptible cells. A previous study reported that the incubation of human vascular endothelial cells with Stx1 and Stx2 resulted in a dramatic increase in interleukin-8 expression levels (27). Since interleukin-8 is a strong neutrophil chemoattractant, the recruitment of neutrophils to the renal endothelium and subsequent neutrophil-mediated endothelial injury could be a major event in the progression of disease toward HUS, regardless of whether or not Stx binds to neutrophils.
Acknowledgments
We thank Scott Millen for purification of Stx1 and Stx2 used in apoptosis experiments.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Aoki, Y., and R. Machinami. 1983. Role of medullasin in granulocytes in the development of inflammation. I. Phlogistic activity and the effect on functions of macrophages and granulocytes. Arthritis Rheum. 26:1002-1010. [DOI] [PubMed] [Google Scholar]
- 2.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce, T. G., D. L. Swerdlow, and P. M. Griffin. 1995. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N. Engl. J. Med. 333:364-368. [DOI] [PubMed] [Google Scholar]
- 4.Brigotti, M., A. Caprioli, A. E. Tozzi, P. L. Tazzari, F. Ricci, R. Conte, D. Carnicelli, M. A. Procaccino, F. Minelli, A. V. Ferretti, F. Paglialonga, A. Edefonti, and G. Rizzoni. 2006. Shiga toxins present in the gut and in the polymorphonuclear leukocytes circulating in the blood of children with hemolytic-uremic syndrome. J. Clin. Microbiol. 44:313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donohue-Rolfe, A., G. T. Keusch, C. Edson, D. Thorley-Lawson, and M. Jacewicz. 1984. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J. Exp. Med. 160:1767-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez, G. C., S. A. Gomez, C. J. Rubel, L. V. Bentancor, P. Barrionuevo, M. Alduncin, I. Grimoldi, R. Exeni, M. A. Isturiz, and M. S. Palermo. 2005. Impaired neutrophils in children with the typical form of hemolytic uremic syndrome. Pediatr. Nephrol. 20:1306-1314. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez, G. C., M. F. Lopez, S. A. Gomez, M. V. Ramos, L. V. Bentancor, R. J. Fernandez-Brando, V. I. Landoni, G. I. Dran, R. Meiss, M. A. Isturiz, and M. S. Palermo. 2006. Relevance of neutrophils in the murine model of haemolytic uraemic syndrome: mechanisms involved in Shiga toxin type 2-induced neutrophilia. Clin. Exp. Immunol. 146:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink, S. L., and B. T. Cookson. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsyth, K. D., A. C. Simpson, M. M. Fitzpatrick, T. M. Barratt, and R. J. Levinsky. 1989. Neutrophil-mediated endothelial injury in haemolytic uraemic syndrome. Lancet ii:411-414. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda, M. N., A. Dell, J. E. Oates, P. Wu, J. C. Klock, and M. Fukuda. 1985. Structures of glycosphingolipids isolated from human granulocytes. The presence of a series of linear poly-N-acetyllactosaminylceramide and its significance in glycolipids of whole blood cells. J. Biol. Chem. 260:1067-1082. [PubMed] [Google Scholar]
- 12.Gamage, S. D., C. M. McGannon, and A. A. Weiss. 2004. Escherichia coli serogroup O107/O117 lipopolysaccharide binds and neutralizes Shiga toxin 2. J. Bacteriol. 186:5506-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamage, S. D., J. E. Strasser, C. L. Chalk, and A. A. Weiss. 2003. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infect. Immun. 71:3107-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez, S. A., G. C. Fernandez, G. Camerano, G. Dran, F. A. Rosa, P. Barrionuevo, M. A. Isturiz, and M. S. Palermo. 2005. Endogenous glucocorticoids modulate neutrophil function in a murine model of haemolytic uraemic syndrome. Clin. Exp. Immunol. 139:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin, P. M., L. C. Olmstead, and R. E. Petras. 1990. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 99:142-149. [DOI] [PubMed] [Google Scholar]
- 16.Haslett, C., J. S. Savill, M. K. Whyte, M. Stern, I. Dransfield, and L. C. Meagher. 1994. Granulocyte apoptosis and the control of inflammation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 345:327-333. [DOI] [PubMed] [Google Scholar]
- 17.Hughes, D. A., G. C. Smith, J. E. Davidson, A. V. Murphy, and T. J. Beattie. 1996. The neutrophil oxidative burst in diarrhoea-associated haemolytic uraemic syndrome. Pediatr. Nephrol. 10:445-447. [DOI] [PubMed] [Google Scholar]
- 18.Hurley, B. P., C. M. Thorpe, and D. W. Acheson. 2001. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun. 69:6148-6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inward, C. D., A. J. Howie, M. M. Fitzpatrick, F. Rafaat, D. V. Milford, C. M. Taylor, et al. 1997. Renal histopathology in fatal cases of diarrhoea-associated haemolytic uraemic syndrome. Pediatr. Nephrol. 11:556-559. [DOI] [PubMed] [Google Scholar]
- 20.King, A. J., S. Sundaram, M. Cendoroglo, D. W. Acheson, and G. T. Keusch. 1999. Shiga toxin induces superoxide production in polymorphonuclear cells with subsequent impairment of phagocytosis and responsiveness to phorbol esters. J. Infect. Dis. 179:503-507. [DOI] [PubMed] [Google Scholar]
- 21.Lee, A., M. K. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 22.Lingwood, C. A., H. Law, S. Richardson, M. Petric, J. L. Brunton, S. De Grandis, and M. Karmali. 1987. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J. Biol. Chem. 262:8834-8839. [PubMed] [Google Scholar]
- 23.Liu, J., T. Akahoshi, T. Sasahana, H. Kitasato, R. Namai, T. Sasaki, M. Inoue, and H. Kondo. 1999. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect. Immun. 67:6203-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, J., T. He, Y. He, Z. Zhang, T. Akahoshi, H. Kondo, and S. Zhong. 2002. Prolongation of functional life-span of neutrophils by recombinant verotoxin 2. Chin. Med. J. 115:900-903. [PubMed] [Google Scholar]
- 25.Macher, B. A., and J. C. Klock. 1980. Isolation and chemical characterization of neutral glycosphingolipids of human neutrophils. J. Biol. Chem. 255:2092-2096. [PubMed] [Google Scholar]
- 26.Martin, S. J., C. P. Reutelingsperger, A. J. McGahon, J. A. Rader, R. C. van Schie, D. M. LaFace, and D. R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182:1545-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matussek, A., J. Lauber, A. Bergau, W. Hansen, M. Rohde, K. E. Dittmar, M. Gunzer, M. Mengel, P. Gatzlaff, M. Hartmann, J. Buer, and F. Gunzer. 2003. Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood 102:1323-1332. [DOI] [PubMed] [Google Scholar]
- 28.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menge, C., T. Eisenberg, I. Stamm, and G. Baljer. 2006. Comparison of binding and effects of Escherichia coli Shiga toxin 1 on bovine and ovine granulocytes. Vet. Immunol. Immunopathol. 113:392-403. [DOI] [PubMed] [Google Scholar]
- 30.Milford, D. V., C. M. Taylor, B. Guttridge, S. M. Hall, B. Rowe, and H. Kleanthous. 1990. Haemolytic uraemic syndromes in the British Isles 1985-8: association with verocytotoxin producing Escherichia coli. Part 1. Clinical and epidemiological aspects. Arch. Dis. Child. 65:716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morigi, M., G. Micheletti, M. Figliuzzi, B. Imberti, M. A. Karmali, A. Remuzzi, G. Remuzzi, and C. Zoja. 1995. Verotoxin-1 promotes leukocyte adhesion to cultured endothelial cells under physiologic flow conditions. Blood 86:4553-4558. [PubMed] [Google Scholar]
- 32.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694-696. [DOI] [PubMed] [Google Scholar]
- 33.Proulx, F., E. G. Seidman, and D. Karpman. 2001. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50:163-171. [DOI] [PubMed] [Google Scholar]
- 34.Raynal, P., and H. B. Pollard. 1994. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1197:63-93. [DOI] [PubMed] [Google Scholar]
- 35.Rutjes, N. W., B. A. Binnington, C. R. Smith, M. D. Maloney, and C. A. Lingwood. 2002. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney Int. 62:832-845. [DOI] [PubMed] [Google Scholar]
- 36.Sabroe, I., E. C. Jones, L. R. Usher, M. K. Whyte, and S. K. Dower. 2002. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 168:4701-4710. [DOI] [PubMed] [Google Scholar]
- 37.Sabroe, I., L. R. Prince, S. K. Dower, S. R. Walmsley, E. R. Chilvers, and M. K. Whyte. 2004. What can we learn from highly purified neutrophils? Biochem. Soc. Trans. 32:468-469. [DOI] [PubMed] [Google Scholar]
- 38.Sabroe, I., L. R. Prince, E. C. Jones, M. J. Horsburgh, S. J. Foster, S. N. Vogel, S. K. Dower, and M. K. Whyte. 2003. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 170:5268-5275. [DOI] [PubMed] [Google Scholar]
- 39.Siegler, R. L., T. G. Obrig, T. J. Pysher, V. L. Tesh, N. D. Denkers, and F. B. Taylor. 2003. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr. Nephrol. 18:92-96. [DOI] [PubMed] [Google Scholar]
- 40.Slutsker, L., A. A. Ries, K. D. Greene, J. G. Wells, L. Hutwagner, and P. M. Griffin. 1997. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann. Intern. Med. 126:505-513. [DOI] [PubMed] [Google Scholar]
- 41.Tazzari, P. L., F. Ricci, D. Carnicelli, A. Caprioli, A. E. Tozzi, G. Rizzoni, R. Conte, and M. Brigotti. 2004. Flow cytometry detection of Shiga toxins in the blood from children with hemolytic uremic syndrome. Cytom. B Clin. Cytom. 61:40-44. [DOI] [PubMed] [Google Scholar]
- 42.te Loo, D. M., A. E. Heuvelink, E. de Boer, J. Nauta, J. van der Walle, C. Schroder, V. W. van Hinsbergh, H. Chart, N. C. van de Kar, and L. P. van den Heuvel. 2001. Vero cytotoxin binding to polymorphonuclear leukocytes among households with children with hemolytic uremic syndrome. J. Infect. Dis. 184:446-450. [DOI] [PubMed] [Google Scholar]
- 43.te Loo, D. M., L. A. Monnens, T. J. van Der Velden, M. A. Vermeer, F. Preyers, P. N. Demacker, L. P. van Den Heuvel, and V. W. van Hinsbergh. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 95:3396-3402. [PubMed] [Google Scholar]
- 44.te Loo, D. M., V. W. van Hinsbergh, L. P. van den Heuvel, and L. A. Monnens. 2001. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:800-806. [DOI] [PubMed] [Google Scholar]
- 45.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Setten, P. A., L. A. Monnens, R. G. Verstraten, L. P. van den Heuvel, and V. W. van Hinsbergh. 1996. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood 88:174-183. [PubMed] [Google Scholar]
- 47.Wagner, P. L., D. W. Acheson, and M. K. Waldor. 2001. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infect. Immun. 69:1934-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weingart, C. L., G. Broitman-Maduro, G. Dean, S. Newman, M. Peppler, and A. A. Weiss. 1999. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 67:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoja, C., S. Angioletti, R. Donadelli, C. Zanchi, S. Tomasoni, E. Binda, B. Imberti, M. te Loo, L. Monnens, G. Remuzzi, and M. Morigi. 2002. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 62:846-856. [DOI] [PubMed] [Google Scholar]