Abstract
Toll-like receptors (TLRs) are involved in the sensing of microbially derived compounds. We analyzed the contribution of these receptors to cytokine production by macrophages following stimulation with whole bacteria. Using knockout mice, we confirmed that the TLR4 and TLR2 contribution was predominant in the induction of tumor necrosis factor alpha and interleukin-10 by gram-negative bacteria. In contrast, the absence of TLR2 and/or TLR4 or TLR9 did not affect the response to gram-positive bacteria. In the absence of TLR2, phagocytosis was essential for cytokine production in response to heat-killed Staphylococcus aureus (HKSA). Because intracellular sensing was important in the absence of TLR2, we evaluated the contribution of Nod1 and Nod2, intracytoplasmic sensors of peptidoglycan-derived muropeptides, to the response to HKSA. By transfecting RAW 264.7 macrophages with dominant negative (DN) forms of Nod1 and Nod2, we showed that both molecules inhibited NF-κB activation in response to HKSA. The unexpected interference of DN Nod1 in the response of macrophages to gram-positive bacteria was confirmed with a Nod2 agonist (muramyl dipeptide) in transfection experiments with HEK293T cell. Taken together, these results show the contribution of phagocytosis and Nod molecules to the response to HKSA in macrophages and also identify possible cross talk between Nod1 and Nod2.
Since Elie Metchnikoff's studies one century ago, the study of innate immunity has reemerged following the identification of the molecules used as sensors of danger signals from invading microorganisms. The Toll-like receptors (TLRs) are one such family of molecules with highly conserved structures, including an extracellular sensing leucine-rich repeat (LRR) domain and a cytoplasmic Toll/interleukin-1 (IL-1) receptor involved in intracellular signaling. TLRs are transmembrane molecules that can be found on the cell surface, like TLR2 and TLR4, or within endosomal vesicles, like TLR9 (15). Microbially derived compounds specifically recognized by these receptors are called pathogen-associated molecular patterns (PAMPs), although they are not specific to pathogenic microorganisms (1). In bacteria, PAMPs include surface compounds such as endotoxin of gram-negative bacteria (lipopolysaccharide [LPS]), peptidoglycan (PGN), and lipoproteins.
In addition to TLRs, a family of intracellular sensors has been described. These receptors, termed nucleotide oligodimerization domains 1 and 2 (Nod1 and Nod2), also possess an LRR domain in addition to two other domains: a caspase recruitment domain and a nucleotide binding domain (12). Unlike TLRs, Nod1 and Nod2 proteins are located in the cytosol and recognize distinct PGN-derived muropeptides (4, 7, 8, 13).
The sensing of PGN motifs by Nod1 or Nod2 induces, through receptor-interacting protein 2, the activation of NF-κB (11) as well as c-Jun N-terminal kinase (29). Nod2 has also been shown to interact with other molecules such as transforming growth factor β-activated kinase (5), gene associated with retinoid interferon-induced mortality (GRIM-19), an inhibitor of transcription factor STAT3 (2), or Erbin (17, 21). Mutations in the Nod2 LRR domain are associated with the development of Crohn's disease (10, 25), a chronic inflammatory disease of the intestinal tract. Because of this association, most of the first studies of Nod molecules were carried out using epithelial cells. However, more recent studies of cytokine production in response to Nod1 and Nod2 agonists have also been performed using human monocytes and dendritic cells (6, 23).
In the past few years, insights into the mechanisms of leukocyte activation leading to cytokine production using isolated PAMPs have been gained, whereas few studies have addressed the question of whether these sensors trigger similar responses when stimulated with whole microorganisms. Monocytes/macrophages possess phagocytic properties. Thus, when triggered with whole bacteria, the signaling cascades leading to cytokine production can be initiated both by TLRs at the cell surface and intracellularly by Nod proteins. Accordingly, we compared the respective roles of surface TLR versus intracellular Nod proteins in the initiation of the signaling cascade following the interaction of whole bacteria with macrophages and analyzed how this may lead either to pro- or anti-inflammatory cytokine production. To do so, we evaluated the production of cytokines by macrophages from TLR2 and TLR2/TLR4 knockout mice in response to gram-positive and gram-negative bacteria. The impact of phagocytosis on cytokine production was determined by using cytochalasin D. The role of Nod1 and Nod2 in the activation of NF-κB in response to heat-killed Staphylococcus aureus (HKSA) was studied in the macrophage cell line RAW 264.7 by using dominant negative (DN) forms of these molecules. In addition, the interference of Nod1 in Nod2-dependent NF-κB activation was further confirmed in transfection experiments in HEK293T cells.
MATERIALS AND METHODS
Mice.
Eight- to 12-week-old male C57BL/6 (from Janvier, Le Genest-St.-Isle, France), TLR2 and TLR4 double knockout, TLR2−/−, TLR9−/−, MyD88−/−, and Nod1−/− mice were used. All knockout mice were in the C57BL/6 genetic background. All animal care and experimentation were conducted in accordance with the Pasteur Institute animal care and use committee guidelines.
Peritoneal macrophage isolation and stimulation.
Mice were injected intraperitoneally with 2 ml of thioglycolate (Bio-Rad, Marnes-la-Coquette, France). Four days later, peritoneal exudate cells were isolated from the peritoneal cavity by washing with ice-cold RPMI 1640 (Glutamax; Cambrex, Rockland, ME). Cells were counted and plated at 0.5 × 106 cells/ml for 2 h and washed with RPMI to remove nonadherent cells. Adherent cells were used as peritoneal macrophages and cultured in RPMI supplemented with 1% heat-inactivated fetal calf serum (FCS). Macrophages were stimulated for 24 h with 100 ng/ml of highly purified Escherichia coli O111:B4 LPS (a kind gift from Martine Caroff, Orsay, France), 1 μg/ml Pam3CysSK4 (EMC Microcollection, Tübingen, Germany), 6 μg/ml of CpG oligonucleotide (completely phosphorothioate modified) (9), 30 ng/ml of tumor necrosis factor alpha (TNF-α) (R&D Systems, Abington, United Kingdom), 50 μg/ml of poly(I:C) (Invivogen, San Diego, CA), or 1 × 107 heat-killed bacteria/ml (Escherichia coli O7, Neisseria meningitidis group C, Staphylococcus aureus Cowan I, and Streptococcus pyogenes A78). In some experiments, macrophages were incubated for 20 min with 3 μM cytochalasin D (BioMol, Plymouth Meeting, PA) before stimulation with heat-killed bacteria. Cytochalasin D was maintained during the stimulation period. After 24 h, supernatants were collected and stored at −20°C.
All our non-LPS PAMPs, as well as cytochalasin D, were negative for endotoxin contamination according to tests using Limulus amebocyte lysate (QCL-1000; Cambrex). Furthermore, the absence of contamination by peptidoglycan in LPS and cytochalasin D was assessed using HEK293T cells transfected with Nod1 or Nod2 as described previously (6).
ELISA.
The concentrations of TNF-α, IL-6, and IL-10 in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) (DuoSet; R&D systems, Minneapolis, MN) as specified by the manufacturer.
Phagocytosis assay.
Mouse peritoneal macrophages were isolated, counted, plated at 1 × 106 cells/ml in RPMI containing 1% FCS for 2 h, and washed with RPMI to remove nonadherent cells. Macrophages were pretreated for 20 min with 3 μM cytochalasin D and then incubated with 10 μg/ml Alexa 488-labeled S. aureus cells (Invitrogen, Carlsbad, CA) (equivalent to 107 bacteria/ml; ratio of 10:1 bacteria per cell). After 1 h, the medium was removed, and the cells were washed on ice with phosphate-buffered saline (PBS) containing 1.3 μM EDTA. The cells were detached from plates using a scraper and washed again in PBS-EDTA, and the pellet was resuspended in PBS-EDTA-1% bovine serum albumin. Phagocytosis was analyzed by using FACScan and CellQuest software (Becton Dickinson). The fluorescence of extracellular bacteria that were adherent to macrophages was quenched using 0.12% trypan blue (24).
Transient transfection and luciferase assay.
RAW 264.7 cells (ATCC, Manassas, VA) were plated the day before the assay at 500,000 cells/well in Dulbecco's modified Eagle's medium supplemented with 10% FCS. Cells were transfected with 250 ng of a DN form of MyD88, a DN form of Nod1, a DN form of Nod2, or an expression vector for β-galactosidase (14) and 250 ng of an NF-κB-dependent luciferase reporter plasmid (Promega) using Lipofectamine 2000 and Opti-MEM (Invitrogen). As a negative control, the cells were transfected with NF-κB reporter plasmid and 250 ng of the empty vector (pcDNA3.1). After 6 h, the cells were stimulated with 107 heat-killed S. aureus cells/ml for 3 h, lysed, and assayed for luciferase activity. Human embryonic kidney HEK293T cells (ATCC) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FCS. Cells were transiently transfected with 75 ng of NF-κB-dependent luciferase reporter plasmid, 15 ng Nod2 or Nod1 expression plasmid, and 125 ng of DN Nod1 or TLR2 expression plasmid (Invivogen, San Diego, CA) using Fugene 6 (Roche, Milano, Italy). The total plasmid concentration was 250 ng and was balanced by the addition of the pcDNA3.1 vector. At the same time, muramyl dipeptide (MDP) (100 nM) or Pam3CysSK4 (1 μg/ml) was added, and the luciferase activity was measured after 16 h of incubation. In addition, using a dual luciferase reporter assay (Promega) and Renilla luciferase, we verified that the transfection efficiency was not altered by the addition of DN Nod1 or TLR2. One nanogram of Renilla luciferase vector (pRL-TK; Promega) was cotransfected with the other plasmids, and firefly luciferase values (NF-κB-dependent luciferase reporter) were normalized for differences in transfection efficiencies on the basis of Renilla luciferase activity in the same extracts. Results were expressed as induction (n-fold) compared to the unstimulated empty vector control.
Statistical analysis.
Data are given as means ± standard errors of the means (SEM). Statistical analyses were performed using the nonparametric Mann-Whitney U test or the Wilcoxon signed-rank test. A P value of less than 0.05 was considered significant.
RESULTS
TLR2 and TLR4 are dispensable for cytokine production in response to gram-positive bacteria.
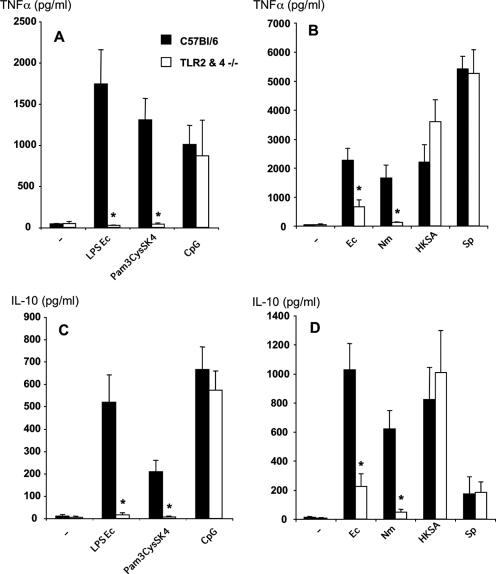
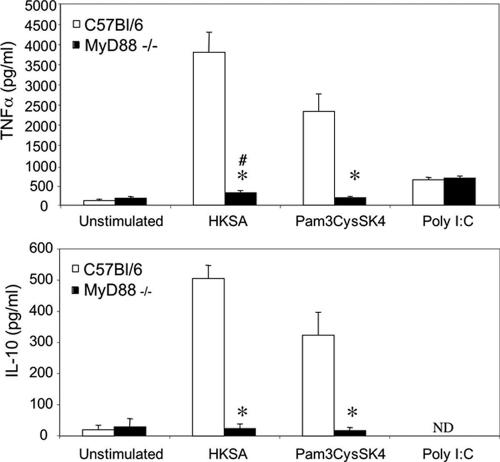
Peritoneal macrophages from TLR2 and TLR4 double knockout mice were stimulated with various heat-killed bacteria in order to analyze the contribution of these receptors to the cellular response in terms of pro- and anti-inflammatory cytokine production. As expected, macrophages from these mice did not produce TNF-α or IL-10 in response to highly purified LPS (a TLR4 agonist) or Pam3CysSK4 (a TLR2 agonist), whereas wild-type macrophages were fully responsive (Fig. 1A and C). In addition, the production of TNF-α and IL-10 in response to CpG (a TLR9 agonist) was comparable to that of macrophages from wild-type mice. When we analyzed the response to gram-negative heat-killed bacteria (Escherichia coli and Neisseria meningitidis), we found that TNF-α as well as IL-10 production was profoundly reduced in the absence of TLR4 and TLR2 (Fig. 1B and D). These data suggest that these receptors play a major role in the induction of cytokines by gram-negative bacteria. In addition, using macrophages from TLR2 or TLR4 knockout mice, we could determine that the TLR4 contribution was predominant in the induction of TNF-α and IL-10 after stimulation with gram-negative bacteria (data not shown). In contrast, the absence of TLR2 and TLR4 did not affect TNF-α and IL-10 production in response to gram-positive bacteria (Staphylococcus aureus and Streptococcus pyogenes), as similar amounts of these cytokines were produced compared to those produced by C57BL/6 mice. Similar results were obtained with macrophages from TLR2−/− mice (data not shown). Our data suggest that in addition to the sensing of lipoproteins by TLR2, another receptor(s) contributes to the macrophage response to gram-positive bacteria. However, while TLR2 was dispensable for cytokine production in response to HKSA, the same was not true for MyD88. Macrophages from MyD88−/− mice showed no TNF-α or IL-10 production in response to a purified TLR2 agonist, Pam3CysSK4, and dramatically decreased cytokine production in response to HKSA (Fig. 2). Although profoundly diminished, TNF-α production in response to HKSA remained, however, significantly higher than that of unstimulated cells. Stimulation with poly(I:C), a MyD88-independent TLR3 agonist, was done as a positive control and gave similar TNF-α production in wild-type and MyD88−/− macrophages. IL-10 production in response to poly(I:C) was below the detection limit.
FIG. 1.
TNF-α and IL-10 production by peritoneal macrophages from C57BL/6 and TLR2/TLR4−/− mice. Macrophages were stimulated with LPS from E. coli (100 ng/ml), Pam3CysSK4 (1 μg/ml), or an oligonucleotide containing the CpG motif (6 μg/ml) or with various heat-killed bacteria at 1 × 107 bacteria/ml. Ec, Escherichia coli; Nm, Neisseria meningitidis; Sp, Streptococcus pyogenes. The presence of TNF-α (A and B) or IL-10 (C and D) in culture supernatants was assessed by ELISA. Results represent the means ± SEM of five independent experiments. *, P < 0.05 for TLR2 and TLR4 double knockout mice versus C57BL/6 mice (Mann-Whitney U test).
FIG. 2.
TNF-α and IL-10 production by peritoneal macrophages from C57BL/6 and MyD88−/− mice. Macrophages were stimulated with Pam3CysSK4 (1 μg/ml), HKSA at 1 × 107 bacteria/ml, or poly(I:C) (50 μg/ml). The presence of TNF-α or IL-10 in culture supernatants was assessed by ELISA. Data are the means ± SEM of eight independent experiments. *, P < 0.05 for MyD88−/− mice versus C57BL/6 mice (Mann-Whitney U test); #, P < 0.05 for unstimulated cells versus stimulated HKSA cells in MyD88−/− macrophages (Wilcoxon signed-rank test). ND, not detectable.
In the absence of TLR2 and TLR4, gram-positive bacterium signaling depends on phagocytosis.
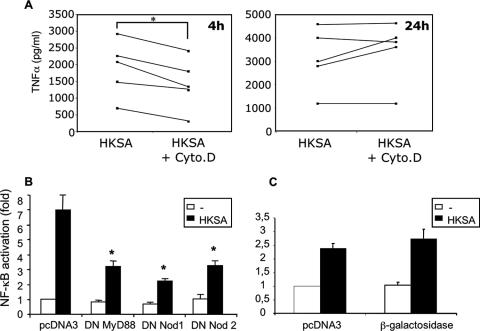
Macrophages possess phagocytic properties that may contribute to the presentation of microbial molecular patterns to intracellular sensors. To examine the impact of phagocytosis on cytokine production, peritoneal macrophages were incubated with cytochalasin D before stimulation with HKSA. The inhibitory effect of cytochalasin D on phagocytosis was confirmed by flow cytometry on macrophages from C57BL/6 mice using Alexa Fluor 488-labeled S. aureus cells (Fig. 3A). It can be seen that cytochalasin D inhibited S. aureus uptake both for the number of ingested bacteria (84.3% inhibition of mean fluorescence intensity) and for the percentage of positive cells (56.5% inhibition). Phagocytosis was inhibited at similar levels for TLR2−/− macrophages (data not shown). As expected, unstimulated cells did not produce any TNF-α and IL-10, and cytochalasin D did not alter cell viability (data not shown). In the case of macrophages from wild-type animals, cytochalasin D had no effect on TNF-α and IL-10 production after 24 h of stimulation with HKSA. In contrast, a strong inhibitory effect was observed for macrophages lacking TLR2 and TLR4 (Fig. 3B and C). Thus, in the absence of TLR2, bacterial entry into the cells is important for both pro- and anti-inflammatory cytokine production.
FIG. 3.
Role of phagocytosis in cytokine production by peritoneal macrophages from C57BL/6 and TLR2/TLR4−/− mice. The inhibition of phagocytosis by cytochalasin D was checked by flow cytometry (A). Cytochalasin D (Cyto.D) (3 μM) was added to macrophages from C57BL/6 mice 20 min before incubation with Alexa 488-labeled S. aureus for 1 h. In other experiments, macrophages were stimulated for 24 h with heat-killed Staphylococcus aureus with protein A (HKSA) or without protein A (− prot A) (10 μg/ml, equivalent to 1 × 107 bacteria/ml). The presence of TNF-α (B), IL-10 (C), or IL-6 (D) in culture supernatants was assessed by ELISA. Results represent the means ± SEM of four independent experiments. *, P < 0.05 versus cells without the addition of cytochalasin D (Wilcoxon signed-rank test). MFI, mean fluorescence intensity.
Protein A is a major component of the surface of S. aureus and plays a role in the induction of cytokines by monocytes and lymphocytes (30). To test whether this protein was playing a role in our model, we used a protein A-deficient strain. Similar results were found for IL-6 production by macrophages from TLR2−/− and wild-type mice stimulated with HKSA or a mutant strain of S. aureus lacking protein A (Fig. 3D). We can conclude that the impact of phagocytosis is not dependent on the expression of protein A on HKSA.
Reactivity of macrophages from TLR9−/− mice to HKSA.
As bacterial entry into macrophages was important for cytokine production in the absence of TLR2, we were interested in the possible role of TLR9 in HKSA detection. Similarly to TLR2−/− and TLR2/TLR4−/− mice, macrophages from TLR9−/− mice were reactive to HKSA and showed no difference compared to wild-type animals (Fig. 4). This was true for both TNF-α and IL-10, whereas the response of TLR9−/− macrophages to CpG was considerably decreased. As expected, the reactivity towards Pam3CysSK4 was not modified. These results led us to examine the contribution of Nod2, an intracytoplasmic pattern recognition receptor that has been shown to detect muramyl (MDP), the minimal motif present in all PGNs (8, 13).
FIG. 4.
TNF-α and IL-10 production by peritoneal macrophages from C57BL/6 and TLR9−/− mice. Macrophages were stimulated with Pam3CysSK4 (1 μg/ml), an oligonucleotide containing the CpG motif (6 μg/ml), or HKSA at 1 × 107 bacteria/ml. The presence of TNF-α or IL-10 in culture supernatants was assessed by ELISA. Results represent the means ± SEM of five independent experiments. *, P < 0.05 for TLR9−/− mice versus C57BL/6 mice (Mann-Whitney U test).
Contribution of Nod1 and Nod2 to the macrophage response to HKSA.
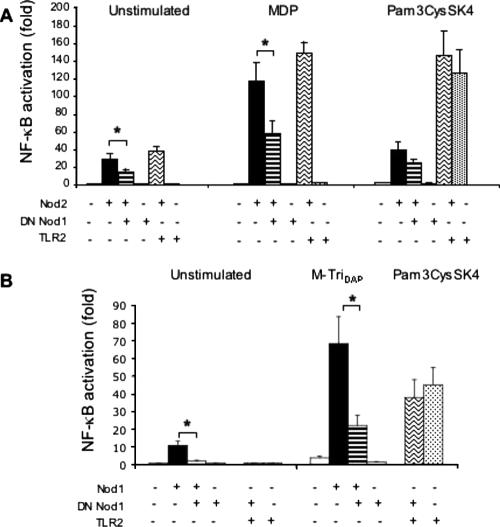
As shown in Fig. 3, after 24 h of stimulation, intracellular sensing is important for macrophages from TLR2-deficient mice but not for those from wild-type animals. However, we noticed that during a shorter period of stimulation (4 h), cytochalasin D also had an inhibitory effect on TNF-α production by wild-type macrophages that was no longer observed after 24 h (Fig. 5A). Thus, the possible contribution of the Nod2 protein to macrophage activation by HKSA could be examined in a 4-h experimental model of NF-κB-dependent luciferase activation. This assay was performed in the macrophage cell line RAW 264.7 expressing TLR2. As shown in Fig. 5B, the transfection of DN MyD88 as well as DN Nod2 inhibited the activation of NF-κB induced by HKSA. More surprisingly, DN Nod1 also showed an inhibitory effect on NF-κB activation in response to HKSA. This was an unexpected result since Nod1 has been shown to sense muropeptides almost exclusively found in the peptidoglycan of gram-negative bacteria (7). This effect seemed to be specific, as the NF-κB activation in response to HKSA was not significantly modified when overexpressing an irrelevant protein such as β-galactosidase (Fig. 5C). To confirm that the effect of DN Nod1 seen on HKSA was specific, we addressed its influence on Nod2 signaling by overexpression experiments using HEK293T cells (Fig. 6A). As reported previously (12), the transfection of low amounts of Nod2 by itself induced a moderate activation of NF-κB (Fig. 6A, unstimulated cells). This effect was not seen with DN Nod1 or with TLR2. The addition of MDP, a Nod2 agonist, potentiated the level of Nod2-dependent NF-κB activation. Similarly to what was observed for macrophages, the overexpression of DN Nod1 significantly inhibited this Nod2-dependent NF-κB activation. Moreover, the effect was seen for unstimulated cells or after challenge with MDP. This inhibitory effect was specific and was not seen when TLR2 was overexpressed with Nod2. The coexpression of Nod2 with TLR2 had no effect on TLR2-dependent NF-κB activation induced by Pam3CysSK4. In addition, using a dual luciferase reporter assay and Renilla luciferase, we verified that the transfection efficiency was not altered by the addition of DN Nod1. We also tested the specificity of DN Nod1 on the Nod1 response when stimulated with its ligand MurNAc-l-Ala-d-Glu-meso-diaminopimelic acid (M-TriDAP) (Fig. 6B). DN Nod1 inhibited Nod1-dependent NF-κB activation following stimulation with M-TriDAP but did not alter the response to Pam3CysSK4 when cotransfected with TLR2. The direct contribution of Nod1 to cytokine production in response to HKSA (after 24 h of stimulation) was also tested by measuring the production of TNF-α and IL-10 by peritoneal macrophages from Nod1−/− mice. However, similar to the results obtained with TLR2 and TLR2/TLR4 double knockout mice, we did not see any difference compared to macrophages from wild-type animals (data not shown). Thus, the absence of Nod1 in these cells is likely compensated for by the presence of other receptors including TLRs and also Nod2, which can respond to both gram-negative and gram-positive bacteria.
FIG. 5.
Role of phagocytosis and intracellular Nod proteins in the recognition of Staphylococcus aureus (HKSA). (A) Effect of phagocytosis inhibition by cytochalasin D (Cyto.D) on TNF-α production by peritoneal macrophages from C57BL/6 mice after 4 h or 24 h of stimulation by HKSA. Each line represents an individual experiment. *, P < 0.05 compared to cells without cytochalasin D (Wilcoxon signed-rank test). The macrophage cell line RAW 264.7 was transfected with an NF-κB-dependent luciferase reporter and (B) 250 ng of DN forms of MyD88, Nod1, or Nod2 or (C) 250 ng of a plasmid coding for β-galactosidase. The luciferase activity was measured after stimulation for 4 h with 107 bacteria/ml of HKSA. The activation (n-fold) was determined and compared to that of unstimulated cells transfected with an equivalent amount of empty vector (pcDNA3). The results are the means ± SEM of five independent experiments. *, P < 0.05 compared to cells transfected with pcDNA3 and stimulated with HKSA (Wilcoxon signed-rank test).
FIG. 6.
Inhibition of NF-κB activation via Nod2 by overexpression of DN Nod1. (A) HEK293T cells were cotransfected with an NF-κB-dependent luciferase reporter, Nod2, and DN Nod1 or TLR2 expression plasmids. Simultaneously, the cells were stimulated with 100 nM MDP or 1 μg/ml Pam3CysSK4. The luciferase activity was measured, and NF-κB activation compared to that of unstimulated cells transfected with empty vector was determined. (B) HEK293T cells were cotransfected with an NF-κB-dependent luciferase reporter, Nod1, and DN Nod1 or TLR2 expression plasmids to check the specificity of DN Nod1. Simultaneously, the cells were stimulated with 100 nM M-TriDAP or 1 μg/ml Pam3CysSK4. The luciferase activity was measured, and NF-κB activation compared to that unstimulated cells transfected with empty vector was determined. The results are the means ± SEM of five independent experiments. *, P < 0.05 (Wilcoxon signed-rank test).
DISCUSSION
In this study, we analyzed the contribution of phagocytosis as well as Nod1 and Nod2 to the sensing of whole bacteria by mouse macrophages. Our aim was to determine the relative role of TLRs versus these intracytoplasmic receptors in the induction and regulation of cytokine production. The specific roles of TLR4 in LPS detection and that of TLR2 in lipoprotein sensing have been widely described (19, 26). However, besides highly purified PAMPs, it is important to understand the response to whole bacteria, since they are the real actors in the infectious insult. In agreement with a study performed previously by Lembo et al., we found that TLR2 and TLR4 were dispensable for TNF-α production in response to gram-positive bacteria (18). In contrast, in the absence of TLR2 and TLR4, the macrophage response was dramatically decreased in response to gram-negative bacteria. Indeed, it was previously reported that TNF-α production induced by gram-negative bacteria was due mainly to its interaction with TLR4 (18). Our results were independent of the bacterial strain used, as similar results were obtained with S. aureus and S. pyogenes on one hand and E. coli and N. meningitidis on the other. Furthermore, we show that TLR2 and TLR4 were also dispensable for the induction of an anti-inflammatory cytokine, such as IL-10, by gram-positive bacteria. The role of TLR2 and MyD88 in the host defense against S. aureus infection remains controversial. Indeed, one study showed that mice deficient for either molecule are highly susceptible to S. aureus infection (28), whereas another study showed that MyD88-deficient mice control the infection (27). The discrepancy between these experiments may be due to the route of infection (intravenous versus pulmonary infection) as well as to the bacterial inoculum, which was higher when the mice were found to be susceptible. Here, we found that in contrast to TLR2, the presence of MyD88 was crucial for cytokine production by mouse macrophages in response to HKSA. This effect may be due to the blockade of the signaling pathway through several MyD88-dependent TLRs. We cannot rule out a role of an unidentified TLR other than TLR2, TLR4, or TLR9 in the response to HKSA. However, MyD88 is also an adaptor molecule for the IL-1 receptor, and macrophages stimulated with HKSA produce IL-1 (data not shown). Thus, the dramatic decrease in cytokine production by macrophages from MyD88−/− mice in response to HKSA may also be due to the absence of a paracrine/autocrine loop of IL-1 in these cells. To address this hypothesis, we performed experiments in the presence of IL-1 receptor antagonist (IL-1Ra). The bioactivity of IL-1Ra (1 μg/ml) was ensured on HEK293T cells, where it fully abolished IL-8 production and NF-κB activation (monitored by transfecting an NF-κB-dependent luciferase gene), in response to mouse recombinant IL-1α (10 ng/ml) or human IL-1β (10 ng/ml). IL-1Ra failed to alter TNF-α production by macrophages stimulated with HKSA. Furthermore, mouse macrophages did not produce TNF-α in response to exogenous IL-1 (data not shown). These results strongly suggest that IL-1 is not involved in this experimental model.
Macrophages from MyD88−/− mice show an impairment of phagocytosis (3). This defect in conjunction with a marked depression in phagosome maturation (34) could contribute to the impairment of bacterial detection by intracellular sensors, such as Nod proteins, in MyD88−/− macrophages.
Indeed, macrophages possess phagocytic properties, and when triggered with whole bacteria, intracellular pattern recognition receptors may also contribute to the signaling cascades leading to cytokine production. Our results show that in the absence of TLR2, cytokine production was impaired in response to HKSA if phagocytosis was inhibited. Phagocytosis was inhibited by using cytochalasin D, a drug that prevents actin polymerization and that, in contrast to cytochalasin B, has no effect on glucose transportation (20). The strong inhibition of phagocytosis by cytochalasin D was confirmed by flow cytometry. In addition, similar results were obtained by using latrunculin A, another drug known to inhibit phagocytosis (data not shown). We checked the production of cytokines in the absence of TLR9, the receptor for bacterial DNA that is expressed intracellularly. The TLR9−/− macrophage response to HKSA did not differ from that of wild-type macrophages. Furthermore, the absence of this receptor, specific for bacterial DNA, would not explain the difference seen between gram-positive and gram-negative bacteria. Thus, we evaluated the contribution of the intracellular sensor Nod2. Nod1 and Nod2 belong to a family of cytosolic pattern recognition receptors that are involved in the recognition of pathogens and sense different peptidoglycan motifs. The ligand of Nod1 is a PGN fragment that is found mainly in gram-negative bacteria (4, 7). Nod2 also detects a PGN fragment, although it is distinct from that of Nod1. Nod2 is a general sensor of bacteria through its recognition of MDP, which is the minimal bioactive fragment common to all PGNs (8, 13). Using dominant negative forms of these receptors, we showed that both DN Nod1 and DN Nod2 interfere with the activation of NF-κB by S. aureus in RAW 264.7 macrophages. Nod2 is likely involved in the sensing of PGN motifs of S. aureus, after its phagocytosis. While Nod1 does not sense PGN from gram-positive bacteria directly, it appears to interfere with Nod2 signaling. We confirmed these results in overexpression experiments in HEK293T cells using purified Nod1 and Nod2 agonists. Our results show that DN Nod1 could alter the Nod2 signaling pathway and that this effect was specific and was not observed with another receptor such as TLR2.
Nod1 has been shown to form homodimers upon activation by interactions via its nucleotide binding domain (11). Since Nod1 and Nod2 molecules are highly homologous, we tested the hypothesis that Nod1 could bind to Nod2. This could not be seen in primary macrophages, where the concentrations of endogenous Nod1 and Nod2 molecules are very low. We tested this possibility by overexpressing Nod1 and Nod2 in HEK293T cells. The immunoprecipitation experiments were not conclusive, because Nod1 and Nod2 were not coprecipitated in all the experiments (data not shown). However, even without a direct interaction, Nod1 may modulate Nod2 signaling. This result could seem odd at first, but recently, Netea et al. (23) showed that a frameshift mutation in Nod2 also resulted in unresponsiveness to a Nod1 agonist. In addition, an increasing number of studies have shown the existence of cross talk between TLR and Nod signaling pathways (16, 22). A synergistic effect of Nod1 or Nod2 agonists with endotoxin (6, 31, 33) as well as an interaction between Nod2 and Tak1 (a molecule involved in TLR2 signaling) have been described (5). More recently, a study demonstrated that a Nod2 deficiency could dysregulate TLR2 signaling (32). Thus, the blunting of TLR signaling pathway, as in MyD88 knockout mice, may have unexpected consequences for the Nod pathway.
Our results illustrate that in addition to the signaling initiated by TLRs, bacterial phagocytosis and Nod proteins are also important for macrophage responsiveness to S. aureus. Furthermore, similar to the cross talk that has been shown between the TLR pathway and Nod proteins (16), it also appears that Nod1 can have a functional impact on Nod2-mediated signaling pathways.
Acknowledgments
We thank Martine Caroff for the kind gift of highly purified LPS, Gabriel Nunez for the kind gift of the Nod2 expression plasmid, and Sylvie Memet for the kind gift of the β-galactosidase expression plasmid. We are grateful to Shizuo Akira and Michel Chignard, who kindly provided us with the TLR2, TLR2/TLR4, and MyD88 knockout mice. We also thank Shizuo Akira and Noëlle Doyen for the TLR9 knockout mice and John Bertin for the Nod1 knockout mice. We thank Thomas Kufer for helpful discussions, fruitful advice for immunoprecipitation experiments, and the kind gift of the Nod1-Flag plasmid.
R.K. is supported by a studentship from the Ministère de la Recherche et de la Technologie. This work was partially supported by a grant from Institut Pasteur (Programme Transversal de Recherche no. 94) and by a grant from the association “Vaincre la Mucoviscidose.”
The authors have no financial conflict of interest.
Editor: F. C. Fang
Footnotes
Published ahead of print on 21 November 2006.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Barnich, N., T. Hisamatsu, J. Aguirre, R. Xavier, H. Reinecker, and D. Podolsky. 2005. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J. Biol. Chem. 280:19021-19026. [DOI] [PubMed] [Google Scholar]
- 3.Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304:1014-1018. [DOI] [PubMed] [Google Scholar]
- 4.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. Valvano, S. Foster, T. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:652-654. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C., Y. Gong, M. Zhang, and J. Chen. 2004. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J. Biol. Chem. 279:25876-25882. [DOI] [PubMed] [Google Scholar]
- 6.Fritz, J. H., S. E. Girardin, C. Fitting, C. Werts, D. Mengin-Lecreulx, M. Caroff, J. M. Cavaillon, D. J. Philpott, and M. Adib-Conquy. 2005. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 35:2459-2470. [DOI] [PubMed] [Google Scholar]
- 7.Girardin, S. E., I. G. Boneca, L. A. M. Carneiro, A. Antignac, M. Jéhanno, J. Viala, K. Tedin, M.-K. Taha, A. Labigne, U. Zähringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 8.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Hugot, J.-P., M. Chamaillard, H. Zouali, S. Lesage, J.-P. Cézard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J.-F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 11.Inohara, N., T. Koseki, J. Lin, L. del Peso, P. C. Lucas, F. F. Chen, Y. Ogura, and G. Nunez. 2000. An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 275:27823-27831. [DOI] [PubMed] [Google Scholar]
- 12.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nunez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551-2554. [DOI] [PubMed] [Google Scholar]
- 13.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. Foster, A. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 14.Kaltschmidt, B., D. Ndiaye, M. Korte, S. Pothion, L. Arbibe, M. Prüllage, J. Pfeiffer, A. Lindecke, V. Staiger, A. Israël, C. Kaltschmidt, and S. Mémet. 2006. NF-κB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol. Cell. Biol. 26:2936-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai, T., and S. Akira. 2006. TLR signaling. Cell Death Differ. 13:816-825. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194-199. [DOI] [PubMed] [Google Scholar]
- 17.Kufer, T. A., E. Kremmer, D. J. Banks, and D. J. Philpott. 2006. Role for Erbin in bacterial activation of Nod2. Infect. Immun. 74:3115-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lembo, A., C. Kalis, C. J. Kirschning, V. Mitolo, E. Jirillo, H. Wagner, C. Galanos, and M. A. Freudenberg. 2003. Differential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in mice. Infect. Immun. 71:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Caroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel, M., C. Roth, J. Fink, G. Fyfe, and P. Lacy. 1975. Effects of cytochalasins B and D on alloxan inhibition of insulin release. Biochem. Biophys. Res. Commun. 66:1089-1096. [DOI] [PubMed] [Google Scholar]
- 21.McDonald, C., F. F. Chen, V. Ollendorff, Y. Ogura, S. Marchetto, P. Lecine, J. P. Borg, and G. Nunez. 2005. A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J. Biol. Chem. 280:40301-40309. [DOI] [PubMed] [Google Scholar]
- 22.Netea, M. G., G. Ferwerda, D. J. de Jong, T. Jansen, L. Jacobs, M. Kramer, T. H. Naber, J. P. Drenth, S. E. Girardin, B. J. Kullberg, G. J. Adema, and J. W. Van der Meer. 2005. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J. Immunol. 174:6518-6523. [DOI] [PubMed] [Google Scholar]
- 23.Netea, M. G., G. Ferwerda, D. J. de Jong, C. Werts, I. G. Boneca, M. Jéhanno, J. W. M. Van Der Meer, D. Mengin-Lecreulx, P. J. Sansonetti, D. J. Philpott, S. Dharancy, and S. E. Girardin. 2005. The frameshift mutation in Nod2 results in unresponsiveness not only to Nod2- but also Nod1-activating peptidoglycan agonists. J. Biol. Chem. 280:35859-35867. [DOI] [PubMed] [Google Scholar]
- 24.Nuutila, J., and E.-M. Lilius. 2005. Flow cytometric quantitative determination of ingestion by phagocytes needs the distinguishing of overlapping populations of binding and ingesting cells. Cytometry 65A:93-102. [DOI] [PubMed] [Google Scholar]
- 25.Ogura, Y., D. K. Bonen, N. Inohara, D. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J.-P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 26.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutation in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 27.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172:3377-3381. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 29.Thome, M., K. Hofmann, K. Burns, F. Martinon, J.-L. Bodmer, C. Mattmann, and J. Tschopp. 1998. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 8:885-888. [DOI] [PubMed] [Google Scholar]
- 30.Tufano, M. A., G. Cipollaro de l'Ero, R. Ianniello, M. Galdiero, and F. Galdiero. 1991. Protein A and other surface components of Staphylococcus aureus stimulate production of IL-1 alpha, IL-4, IL-6, TNF and IFN-gamma. Eur. Cytok. Netw. 2:361-366. [PubMed] [Google Scholar]
- 31.Uehara, A., S. Yang, Y. Fujimoto, K. Fukase, S. Kusumoto, K. Shibata, S. Sugawara, and H. Takada. 2005. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell. Microbiol. 7:53-61. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe, T., A. Kitani, P. J. Murray, Y. Wakatsuki, I. J. Fuss, and W. Strober. 2006. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473-485. [DOI] [PubMed] [Google Scholar]
- 33.Wolfert, M. A., T. F. Murray, G.-J. Boons, and J. N. Moore. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J. Biol. Chem. 277:39179-39186. [DOI] [PubMed] [Google Scholar]
- 34.Yates, R. M., and D. G. Russell. 2005. Phagosome maturation proceeds independently of stimulation of Toll-like receptors 2 and 4. Immunity 23:409-417. [DOI] [PubMed] [Google Scholar]