Abstract
We isolated a rough variant of Mycobacterium abscessus CIP 104536T during experimental infection of mice. We show that this variant has lost the ability to produce glycopeptidolipids, is hyperlethal for C57BL/6 mice infected intravenously, and induces a strong tumor necrosis factor-alpha response by murine monocyte-derived macrophages.
Mycobacterium abscessus (formerly Mycobacterium chelonae subsp. abscessus) is an emerging, rapidly growing mycobacterium that causes a wide spectrum of human infections, including skin and soft tissue infections (3, 26), lung infections (11, 24), and disseminated infections in patients either under immunosuppressive therapy (3, 23) or with a Mendelian syndrome conferring susceptibility to mycobacteria (5). Extended lung infections and disseminated infections raise serious therapeutic issues because M. abscessus strains are resistant to most antibiotics and are associated with a particular high fatality rate (3, 23).
M. abscessus organisms may be isolated with a smooth (S) or a rough (R) morphotype from clinical samples (23). Recently, Byrd and Lyons described an R strain of M. abscessus from an ileal granuloma in a patient with Crohn's disease, and they reported the spontaneous in vitro dissociation of this isolate into an S variant (4). Interestingly, these authors showed that the S variant was severely attenuated in its ability to infect the murine host and to persist in human monocytes. M. abscessus may thus undergo an S/R phase variation linked to pathogenicity. The molecular basis for the S or R appearance of M. abscessus, as well as the reason for the difference in pathogenicity of S and R variants, is presently unknown (12).
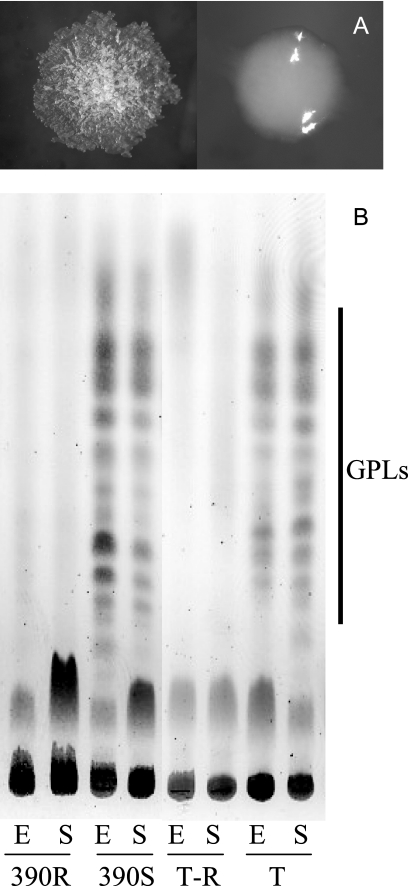
We have recently used M. abscessus CIP 104536T (=ATCC 19977T) (19) to study the immune control of M. abscessus infection in the murine host. This strain, which was provided by the Laboratoire de Référence des Mycobactéries (Institut Pasteur, Paris, France) has an S morphotype on ordinary solid media such as Trypticase soy agar (BioMérieux, Marcy l'Etoile, France) (Fig. 1A). Following the intravenous (i.v.) injection of 107 CFU of CIP 104536T into immunoglobulin μ chain knockout mice (15), we obtained mycobacterial colonies of the R morphotype from deep organs of several animals on day 90 following infection. We verified that these colonies were truly M. abscessus by partial hsp65 sequencing (21). One isolated colony (CIP 104536T-R) was selected, reisolated, and cryopreserved at −80°C using cryobeads (Mast Diagnostics, Reinfeld, Germany). The R morphotype of this variant was confirmed to be stable after 10 subcultures on solid media and three iterative in vivo passages. Thus, in contrast with previous studies reporting the switch of an R M. abscessus strain into an S morphotype (4), we show here that R variants may be selected in vivo from an M. abscessus strain with an S morphotype.
FIG. 1.
Lack of GPLs in the rough variants of M. abscessus. (A) Colonial morphotypes of CIP 104536T (right) and CIP 104536T-R (left) after 4 days of incubation at 37°C on Trypticase soy agar; (B) thin-layer chromatography analysis of the crude lipid extracts of CIP 104536T, CIP 104536T-R, 390S, and 390R. Sugar-containing lipids were visualized by anthrone staining. The positions of the GPLs are indicated on the right. Abbreviations: E, exponential phase; S, stationary phase; T, CIP 104536T; T-R, CIP 104536T-R.
Glycopeptidolipids (GPLs), also called C-mycosides or J-substances, represent up to 85% of the surface-exposed lipids in M. abscessus and a number of other nontuberculous mycobacterial species, such as M. chelonae, M. smegmatis, and M. avium (8). Studies have shown that the lack of GPLs at the mycobacterial surface is often associated with rough colony morphology in M. smegmatis and M. avium mutants obtained spontaneously or by insertional mutagenesis (1, 2, 8). We thus studied whether CIP 104536T-R was affected in its ability to express GPLs. We also tested the 390S and 390R M. abscessus variants described by Byrd and Lyons (4), which were kindly provided by Blaine Beaman (University of California Davis, Davis, CA). Mycobacteria were grown in Middlebrook 7H9-0.05% Tween 80 medium (Difco-Becton Dickinson, Le Pont-de-Claix, France) at 37°C. Lipids were extracted from mycobacterial cells with a mixture of chloroform and methanol and further partitioned by methanol precipitation as previously described (27). The GPLs (250 μg lipid for each deposit) were identified, by comparison with standard GPLs purified from M. smegmatis (27), by thin-layer chromatography on silica gel Durasil 25-precoated plates (Macherey-Nagel, Düren, Germany) run in chloroform-methanol-water (60:16:2 [vol/vol/vol]). The sugar-containing GPL-like compounds were identified by spraying plates with 0.2% anthrone in concentrated sulfuric acid, followed by heating at 110°C (8). As shown in Fig. 1B, the S strains CIP 104536T and 390S both produced GPL-like compounds of comparable thin-layer chromatography mobilities and in similar quantities. In contrast, no GPL-like compounds were detectable at any growth phase in cell extracts of CIP 104536T-R and 390R. The precise nature of these GPL-like compounds was resolved by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. All of the GPL-associated pseudomolecular ions ([M + Na]+) detected in the smooth strains perfectly matched the GPLs previously described for M. abscessus in calculated molecular weight (16). In contrast, these GPL-associated pseudomolecular ions were nondetectable in the rough strains (data not shown). Thus, both CIP 104536T-R and 390R appear to have lost the ability to produce GPLs.
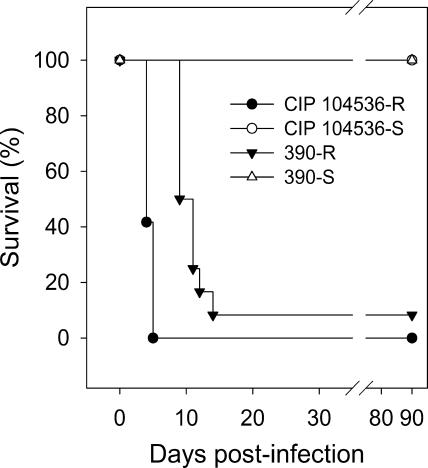
The virulence of M. abscessus CIP 104536T and CIP 104536T-R was evaluated in C57BL/6 mice injected intravenously. C57BL/6 mice were purchased from Elevage Janvier (Le-Genest-Saint-Ile, France). All animals were 8 to 9 weeks old when infected and were hosted in a confinement class II facility under filter covers, placed in single-use cages with irradiated litter, and fed irradiated chow and autoclaved water ad libitum. Experiments were conducted in accordance with the guidelines of the animal welfare committee of the Université de Versailles-Saint Quentin. Mycobacterial strains were grown at 37°C in Middlebrook 7H9-0.05% Tween 80 medium, harvested by centrifugation, and frozen with 10% glycerol. Aliquots of 1 ml were stored at −80°C until required and then titrated. Challenge inocula were prepared from rapidly thawed frozen aliquots. Bacterial clumps were eliminated by iterative passages through a 29.5-gauge needle, and suspensions were then diluted appropriately in phosphate-buffered saline (PBS). Mice were inoculated by i.v. injection with 107 CFU via the lateral tail vein in a volume of 0.2 ml. This challenge was lethal to all 12 mice injected with CIP 104536T-R as early as 5 days postinfection, whereas no mortality was observed for 90 days in the group of mice injected with CIP 104536T (Fig. 2), consistent with the results of our previous experiments. Similar experiments were conducted with the 390S and 390R variants. All 12 mice injected with 107 CFU of 390S were still alive 90 days postinfection, whereas 11 out of 12 mice injected with the 390R variant died within 15 days of infection (Fig. 2). Thus, the R-morphotype variants CIP 104536T-R and 390R were clearly hyperlethal in the i.v. murine model. The spleens and livers had similar bacterial counts for both the S and R morphotypes of CIP 104536T from days 1 to 4 (data not shown). Mice challenged with 107 CFU and 108 CFU of heat-inactivated CIP 104536T-R (30 min at 95°C; yielding >99.99% inactivation) were asymptomatic, with 100% survival on day 90. Thus, the hypervirulence of the R variants requires live mycobacterial cells or, alternatively, may depend on the expression of thermolabile determinants.
FIG. 2.
Hyperlethality of R variants. Survival analysis was done with C57/BL6 mice injected i.v. with 107 CFU per animal (12 mice per group).
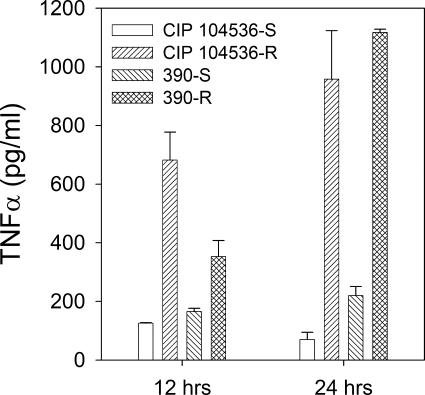
GPLs have recently been shown to interfere with the production of tumor necrosis factor-alpha (TNF-α) by murine macrophages (13). We thus studied the production of TNF-α by murine macrophages infected with S and R variants. Bone-marrow-derived macrophages (BMMs) were prepared from 6- to 8-week-old C57BL/6 mice (Elevage Janvier, Le-Genest-Saint-Ile, France). Mice were killed by carbon dioxide narcosis, and their femurs and tibias were aseptically removed. Bone marrow was flushed with PBS. Cells were washed once in PBS and resuspended in Dulbecco modified Eagle medium (DMEM)-Glutamax (Invitrogen GIBCO, Cergy-Pontoise, France) supplemented with 20% L-cell conditioned medium (LCCM) and 10% fetal calf serum (FCS), and 5 × 104 cells per well were inoculated onto 24-well plates (TPP, Trasadingen, Switzerland) in a volume of 1 ml. After 6 days of incubation (37°C, 5% CO2 atmosphere), nonadherent cells were eliminated with two washes, and fresh DMEM containing 10% FCS-10% LCCM was added. BMMs were used for infection 24 h later. Mycobacteria were prepared as described above for virulence studies. BMMs were infected for 1 h at 37°C at a multiplicity of infection of 5:1. BMMs were then washed twice in PBS to remove extracellular bacteria, and fresh DMEM containing 10% FCS-10% LCCM and 40 mg/liter amikacin was added. Macrophages were lysed with 1% Triton at 12 and 24 h postinfection to assess bacterial survival. TNF-α was measured in culture supernatants at 12 and 24 h postinfection using an enzyme-linked immunosorbent assay duoset kit (R&D Systems, Lille, France). The mean result for duplicate wells was determined by following the manufacturer's recommendations. Student's t test was used for statistical analysis of data. As shown in Fig. 3, CIP 104536T-R induced a significantly higher level of TNF-α production than CIP 104536T at both 12 and 24 h postinfection (P = 0.014 and 0.017, respectively). Similar results were observed with 390R and 390S (Fig. 3). A study of bacterial survival in BMMs done in parallel showed no significant differences between S and R isogenic strains (not shown).
FIG. 3.
Increased TNF-α release by BMMs infected with R variants. BMMs were infected for 1 h with S and R strains at a multiplicity of infection of 5:1, washed twice, and further incubated for 24 h in fresh medium containing 40 mg/liter of amikacin. TNF-α was measured in culture supernatants at 12 and 24 h postinfection. CIP 104536T-R versus CIP 104536T at 12 and 24 h, P = 0.014 and 0.017, respectively. 390R versus 390S at 12 and 24 h, P = 0.041 and 0.0043, respectively.
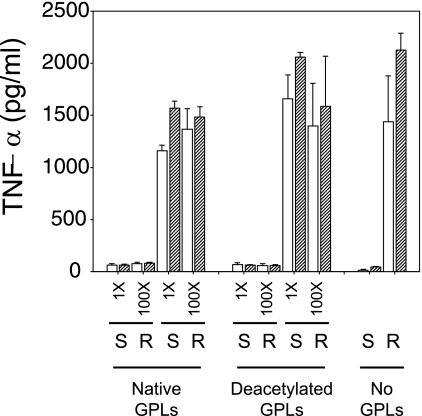
The lack of activation of BMMs by the smooth variant could result from an inhibitory effect of the GPLs that it synthesizes. In order to test this hypothesis, we incubated CIP 104536T and CIP 104536T-R in PBS with purified GPLs for 60 min prior to infection of BMMs and measurement of TNF-α release. Two GPL extracts were used: “native GPLs” (80 to 90% purity) were obtained by methanol precipitation of the lipid extract (23) and “deacetylated GPLs” (>98% purity) were obtained by mild alkaline hydrolysis of the methanol supernatant (18). GPLs were used at 1- and 100-fold concentrations by incubating 108 CFU with GPLs extracted from 108 and 1010 CFU, respectively. Infecting BMMs in the presence of either amount of GPLs did not significantly alter the TNF-α response to either the rough or the smooth variant (Fig. 4), suggesting that GPLs were not inhibitory components per se.
FIG. 4.
TNF-α release by BMMs infected with R variants is unaffected by GPL supplementation. CIP 104536T-R and CIP 104536T were incubated for 1 h with low (1-fold) or high (100-fold) concentrations of “native” or “deacetylated” GPLs purified from CIP 104536T prior to infection. Infection of BMMs was carried out as described in the legend to Fig. 3, and TNF-α was measured in culture supernatants at 12 (open bars) and 24 (hatched bars) h postinfection. The addition of native or deacetylated GPL did not significantly alter TNF-α production.
One of the trademarks of mycobacteria is the presence of a complex array of lipidic molecules exposed at the cell surface (6). The most superficial layer is made up of glycolipids which, depending on the species, can be either GPLs (e.g., M. abscessus, M. smegmatis, M. avium) or phenolglycolipids (e.g., M. tuberculosis). There is an increasing number of studies showing that mycobacterial glycolipids play a major role in pathogenicity by interfering with the host immune system. GPLs have been shown to inhibit the phagocytic properties of human macrophages (27) and to modulate the induction of TNF-α synthesis in murine macrophages (13). Recently, the hypervirulent phenotype of M. tuberculosis strains belonging to the W-Beijing family was shown to be associated with the production of a particular glycolipid that inhibits the release of TNF-α and other key proinflammatory cytokines (20). The results of our study are consistent with these data because we showed that surface-exposed GPLs interfered with the release of inflammatory effector molecules from murine macrophages after interaction with M. abscessus.
We cannot exclude the possibility that the hyperlethality observed with R variants of M. abscessus is related to mediators of the host innate response other than TNF-α. Indeed, production of TNF-α in response to mycobacterial infection orchestrates the early induction of chemokines and is required for the organization of the leukocytes recruited into granulomas (22). As such, it is essential to the control of pathogenic mycobacteria in the murine model (7, 10, 14). However, hyperproduction of TNF-α could directly participate in a lethal “toxic-shock”-like syndrome (9) or be reflective of a dysregulated immune response. The mechanisms by which mycobacterial glycolipids interfere with host cell responsiveness also remain to be elucidated. Mycobacterial GPLs can insert into phospholipid monolayers and alter their biological properties (17, 25). However, our experiments using purified GPLs favor the hypothesis that some proinflammatory components, possibly underlying lipids, are overexpressed or unmasked in rough variants. This hypothesis is currently under investigation by our group.
Acknowledgments
We thank Veronique Vincent and Cristina Gutierrez (Institut Pasteur, Paris, France) and Blaine Beaman, (UC-Davis, Davis, CA) for kindly providing the strains used in this study.
This work received financial support from the association “Vaincre la Mucoviscidose.” E. Catherinot was supported by the Legs Poix (Chancellerie des Universités de Paris).
Editor: J. L. Flynn
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Barrow, W. W., and P. J. Brennan. 1982. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J. Bacteriol. 150:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belisle, J. T., M. R. McNeil, D. Chatterjee, J. M. Inamine, and P. J. Brennan. 1993. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J. Biol. Chem. 268:10510-10516. [PubMed] [Google Scholar]
- 3.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd, T. F., and C. R. Lyons. 1999. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 67:4700-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 6.Daffé, M., and P. Draper. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131-203. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers, S., J. Benini, S. Kutsch, R. Endres, E. T. Rietschel, and K. Pfeffer. 1999. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect. Immun. 67:3571-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etienne, G., C. Villeneuve, H. Billman-Jacobe, C. Astarie-Dequeker, M. A. Dupont, and M. Daffe. 2002. The impact of the absence of glycopeptidolipids on the ultrastructure, cell surface and cell wall properties, and phagocytosis of Mycobacterium smegmatis. Microbiology 148:3089-3100. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner, L., A. Cooper, C. Fantino, D. M. Altmann, and S. Sriskandan. 2005. The mechanism of superantigen-mediated toxic shock: not a simple Th1 cytokine storm. J. Immunol. 175:6870-6877. [DOI] [PubMed] [Google Scholar]
- 10.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 11.Griffith, D. E., W. M. Girard, and R. J. Wallace, Jr. 1993. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am. Rev. Respir. Dis. 147:1271-1278. [DOI] [PubMed] [Google Scholar]
- 12.Howard, S. T., T. F. Byrd, and C. R. Lyons. 2002. A polymorphic region in Mycobacterium abscessus contains a novel insertion sequence element. Microbiology 148:2987-2996. [DOI] [PubMed] [Google Scholar]
- 13.Irani, V. R., and J. N. Maslow. 2005. Induction of murine macrophage TNF-alpha synthesis by Mycobacterium avium is modulated through complement-dependent interaction via complement receptors 3 and 4 in relation to M. avium glycopeptidolipid. FEMS Microbiol. Lett. 246:221-228. [DOI] [PubMed] [Google Scholar]
- 14.Kamijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 16.López-Marin, L. M., N. Gautier, M. A. Laneelle, G. Silve, and M. Daffe. 1994. Structures of the glycopeptidolipid antigens of Mycobacterium abscessus and Mycobacterium chelonae and possible chemical basis of the serological cross-reactions in the Mycobacterium fortuitum complex. Microbiology 140:1109-1118. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Marin, L. M., D. Quesada, F. Lakhdar-Ghazal, J. F. Tocanne, and G. Laneelle. 1994. Interactions of mycobacterial glycopeptidolipids with membranes: influence of carbohydrate on induced alterations. Biochemistry 33:7056-7061. [DOI] [PubMed] [Google Scholar]
- 18.McNeil, M., D. Chatterjee, S. W. Hunter, and P. J. Brennan. 1989. Mycobacterial glycolipids: isolation, structures, antigenicity, and synthesis of neoantigens. Methods Enzymol. 179:215-242. [DOI] [PubMed] [Google Scholar]
- 19.Moore, M., and J. B. Frerichs. 1953. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J. Investig. Dermatol. 20:133-169. [DOI] [PubMed] [Google Scholar]
- 20.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 21.Ringuet, H., C. Akoua-Koffi, S. Honore, A. Varnerot, V. Vincent, P. Berche, J. L. Gaillard, and C. Pierre-Audigier. 1999. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 37:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 23.Sanguinetti, M., F. Ardito, E. Fiscarelli, M. La Sorda, P. D'Argenio, G. Ricciotti, and G. Fadda. 2001. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 39:816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sermet-Gaudelus, I., M. Le Bourgeois, C. Pierre-Audigier, C. Offredo, D. Guillemot, S. Halley, C. Akoua-Koffi, V. Vincent, V. Sivadon-Tardy, A. Ferroni, P. Berche, P. Scheinmann, G. Lenoir, and J. L. Gaillard. 2003. Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect. Dis. 9:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergne, I., M. Prats, J. F. Tocanne, and G. Laneelle. 1995. Mycobacterial glycopeptidolipid interactions with membranes: a monolayer study. FEBS Lett. 375:254-258. [DOI] [PubMed] [Google Scholar]
- 26.Villanueva, A., R. V. Calderon, B. A. Vargas, F. Ruiz, S. Aguero, Y. Zhang, B. A. Brown, and R. J. Wallace, Jr. 1997. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin. Infect. Dis. 24:1147-1153. [DOI] [PubMed] [Google Scholar]
- 27.Villeneuve, C., G. Etienne, V. Abadie, H. Montrozier, C. Bordier, F. Laval, M. Daffe, I. Maridonneau-Parini, and C. Astarie-Dequeker. 2003. Surface-exposed glycopeptidolipids of Mycobacterium smegmatis specifically inhibit the phagocytosis of mycobacteria by human macrophages. Identification of a novel family of glycopeptidolipids. J. Biol. Chem. 278:51291-51300. [DOI] [PubMed] [Google Scholar]