Abstract
Mutans streptococcal glucosyltransferases (GTF) have been demonstrated to be effective components of dental caries vaccines. We had previously selected peptide subunits of GTF for vaccine development based on putative functional significance and conservation of GTF primary structure among enzyme isoforms. In this study, 20 20-mer linear GTF peptides were synthesized, 17 identified on the basis of the highest potential major histocompatibility complex (MHC) class II-binding activity using computer-generated algorithms (Epimatrix and ProPred) and 3 with previously demonstrated functional significance. The immunoreactivities of these peptides were explored with rodent systems. Sera from GTF-immunized rats, assessed for binding to linear peptides by enzyme-linked immunosorbent assay, demonstrated immunoglobulin G antibody reactivity with peptides 6 and 11 and a T-cell proliferation response to peptides 6, 9, 11, and 16. Multiple antigenic peptide (MAP) constructs were synthesized from promising linear sequences. Rats that were immunized with MAP 7, 11, or 16, respectively, responded well to the immunizing MAP. Most importantly, a robust immune response (antibody and T-cell proliferation) was observed to native GTF following MAP 11 (amino acids 847 to 866; VVINNDKFVSWGITDFEM) immunization. This response inhibited GTF enzyme function. Two dental caries pathogenesis experiments were performed wherein rats were immunized with MAP constructs 11, 16, and/or 11 plus 16, followed by infection with cariogenic Streptococcus sobrinus. In both experiments cariogenic bacterial recoveries were reduced relative to total streptococci in the MAP 11- and MAP 11 plus 16-immunized groups, and the extent of dental caries was also significantly reduced in these groups. Thus, we have identified a peptide with projected avid MHC-binding activity that elicited immunoreactivity with native GTF and demonstrated protection against dental caries infection after immunization, implying that this peptide may be important in a subunit dental caries vaccine.
Dental decay is a major public health challenge and the cause of considerable social and economic burdens (3, 6, 14). A causative relationship between mutans streptococci and dental caries has been elucidated (5, 10) and has provided a rationale for successful reduction of caries by vaccination in animal models (9, 11, 31). Mutans streptococcal accumulation on the teeth, crucial to the pathogenesis of caries, is facilitated by extracellular glucan, synthesized from sucrose by glucosyltransferases (GTF) (5). Functional investigation of GTF, by site-directed mutagenesis and sequence alignment with catalytically similar enzymes (33), demonstrated a catalytic domain contained within a region of α-amylase homology and a glucan-binding domain (12). The latter contains differing numbers of highly conserved, structurally similar repeat regions which have been associated with carbohydrate binding (13).
Compared to vaccines formulated with whole organisms or proteins, vaccines containing subunit peptides provide the advantage of focusing the immune response exclusively on protective epitopes and not on irrelevant or even potentially harmful antigenic determinants. Previously, selection of such peptides was guided principally by GTF function (29). Some of these peptides induced GTF-inhibitory, caries-protective immune responses after injection into rodents (1, 8, 32).
T-cell responses are limited by and restricted to allelic forms of a set of highly polymorphic glycoproteins encoded in the major histocompatibility complex (MHC) (4). The immunogenicity of GTF is based, in part, on the presentation of processed GTF peptides on the surfaces of antigen-presenting cells in the context of MHC class II molecules to T lymphocytes involved in the process leading to antibody formation (4, 7). Matrix-based algorithms have been used in T-cell epitope prediction to prospectively identify conserved class II-restricted MHC ligands in the protein sequence (15, 19). Application of this approach to GTF sequences in order to identify such peptides could suggest peptide constructs which might be used to focus the dental caries-protective responses seen with the intact protein. GTF peptides associated with these regions could then be synthesized and evaluated for immunogenicity, reactivity with the parent protein, and ultimately induction of caries-protective immunity. Based on the potential of peptides to serve as MHC class II ligands, it is possible to seek sequences which could be associated with the inherent immunogenicity of GTF. Therefore, subunit vaccines selected by this approach have an additional advantage in that, if properly selected, they would not induce immunity to irrelevant or unwanted epitopes.
In this study, peptide selection from GTF was principally based on predicted immunogenicity rather than enzymatic function. Peptide immunogenicity was predicted using peptide-MHC interaction analyses. The objectives of this study were to synthesize candidate peptides identified by potential for MHC binding, experimentally determine their immunologic characteristics, and evaluate the most promising peptide candidates for their ability to induce protective immune responses in an experimental dental caries rat model.
MATERIALS AND METHODS
Identification of regions of GTF binding to MHC class II alleles.
Two methods were used to search the primary sequence of GTF-I of Streptococcus sobrinus strain 6715 (accession number P27470) for binding motifs associated with MHC class II alleles. The first method applied a matrix-based algorithm for epitope prediction (Epimatrix; Epivax, Inc., Providence, RI) (15) to search the primary amino acid sequence for 17 known MHC class II binding motifs based on a set of alleles at the MHC class II DRB1 locus. These motif-matching algorithms analyze consecutive GTF peptide sequences against each MHC class II allele to indicate regions of sequence that contain clusters of putative avidly binding motifs. The sequences with high estimated binding probabilities predict potential MHC ligands.
The second method was derived from published algorithms (ProPred [19]), allowing identification of promiscuous binding regions in proteins. Fifty-one alleles were assessed at the DRB1 or DRB5 locus. Alleles were assessed for binding to GTF using these quantitative matrices of 20 20-mer linear candidate peptides. Seventeen were selected based on the highest binding scores in Epivax and ProPred analyses, and three were selected based on previously demonstrated function (see Table 1).
TABLE 1.
Summary of sequences reacting with rat sera and cells
| Assigned no. | Peptide position | Peptide sequence | ProPred scorea | Epivax scoreb | Peptide with greatest homologyc |
|---|---|---|---|---|---|
| 5 | 438-457 | DANFDSIRVDAEDNVDADQL | 6 | 0 | CAT27 |
| 6 | 478-498 | NNHVSIVEAWSDNDTPYLHD | 0 | 0 | EAW21 |
| 7 | 502-521 | LMNMDNKFRLSMLWSLAKPT | 43 | 31 | |
| 9 | 548-567 | VPSYSFARAHDSEVQDIIRD | 5 | 0 | HDS21 |
| 11 | 847-866 | VVIANNDKFVSWGITDFEM | 43 | 22 | |
| 16 | 1376-1395 | SGALRFYNLKGQLVTGSGWY | 44 | 14 |
ProPred scores (19) describe the number of MHC class II alleles predicted to bind with the sequences out of a possible total of 51.
Epimatrix scores (15) provided as “hits,” with higher hits having greater probability of binding.
Previously identified peptide to which sequence shows greatest homology.
Peptide synthesis.
Linear peptides for initial immunological evaluation were synthesized by SynPep (Dublin, CA) with greater than 70% purity. Prior to use, they were resuspended in sterile phosphate-buffered saline (PBS) and stored at −20°C.
Multiple antigenic peptide (MAP) constructs from peptides 7, 11, and 16 (see Table 1) were synthesized (AnaSpec, Inc., San Jose, CA) for use in pathogenesis experiments. Each peptide contained 20 amino acids and was synthesized as a quadruplicate peptide on a three-lysine backbone with greater than 90% purity.
Immune reactivity with linear peptides.
Ten-week-old Sprague-Dawley (Charles River Laboratories, Raleigh, NC) rats were immunized subcutaneously (s.c.) in multiple sites with a total of 15 μg S. sobrinus strain 6715 GTF (n = 3), purified as previously described (23), or with PBS (n = 2) in complete Freund adjuvant (CFA). Sera and all macroscopically visible lymph nodes were harvested 10 days postimmunization. Sera from each group were pooled and assessed for antibody to GTF and the linear peptides (enzyme-linked immunosorbent assay [ELISA]). Lymph node mononuclear cells were prepared and tested for proliferation with peptides or GTF.
ELISA.
Serum antibody binding to peptides was assessed by ELISA as previously described (24). Briefly, polystyrene microtiter plates (ICN Biomedicals) were coated with 5 μg/ml of peptide or 0.15 μg/ml of S. sobrinus GTF (prepared as previously described [23]). Antibody activity was measured by addition of duplicate 1:100 dilutions of sera. Plates were then developed for immunoglobulin G (IgG) antibody with rabbit antirat IgG, followed in sequence by alkaline phosphatase-labeled goat antirabbit IgG (Biosource, Inc.) and p-nitrophenylphosphate (Sigma Chemical Co., St. Louis, MO). Reactivity was recorded as the A405 value in a microplate reader (Biotek Instruments, Winooski, Vt.). To confirm binding specificity, immune serum was added to peptide-coated plates, incubated for 2 h, and then transferred to identically coated plates; incubated and transferred plates were developed as described above. In all cases, IgG reactivity to the peptide was removed after the first incubation.
Cell culture and proliferative stimulation.
Rodent cells were obtained from all macroscopically visible peripheral lymph nodes, removed 10 days postimmunization from immunized or naive rats. Lymph nodes were expressed through a sterile stainless steel mesh to obtain a single-cell suspension. Cells were washed three times in RPMI 1640 medium (Invitrogen, Carlsbad, CA) and resuspended in complete medium (containing penicillin-streptomycin, 12.5 mM HEPES, 3 × 10−6 M 2-mercaptoethanol), which was supplemented with 10% fetal bovine serum (or autologous human serum) and plated into triplicate wells of 96-well tissue culture plates (2 × 105 cells per well). Cells were simulated with peptides or GTF. Proliferation was measured by the addition of 3[H]thymidine (0.5 mCi/well) for the final 18 h of culture. Thymidine incorporation was assessed by liquid scintillography. A proliferation index was calculated by dividing each rat's responding cell mean number of cpm by its medium control mean number of cpm.
Immune response to MAPs.
The inbred Forsyth strain heterozygous male Rowett rats aged 4.5 months (devoid of mutans streptococci [28]) were injected s.c. in the vicinity of the major salivary glands (sgv) with 50 μg of each MAP construct in CFA (n = 6 per group) for comparison of peptide immunogenicity. Groups were injected with MAP 7, 11, or 16 or with PBS in CFA as a control. Immunization was repeated 29 days later with peptides in incomplete Freund adjuvant (IFA). Separate groups (n = 5 per group) of 4- to 5-month-old female Rowett rats devoid of mutans streptococci were immunized intranasally (i.n.) on day 1 and 29 and 30 days later with 50 μg of each MAP construct with 5 μg cholera toxin (Sigma). The 30-μl dose was divided between nostrils. Serum and saliva were collected 7 weeks after the initial immunization.
GTF inhibition assay.
Rat sera from control or immunized rats were evaluated for the ability to inhibit glucan synthesis by GTF in a modified filter assay described previously (30). Serum (1 μl) was incubated with GTF in a final volume of 100 μl in 0.02 M sodium phosphate-buffered saline and 0.02% sodium azide (PBSA) (pH 6.5) for 2 h at 37°C, after which 100 μl of PBSA containing 0.85 mg of sucrose and 22 nCi of [14C]glucose-sucrose was added. This mixture was incubated further for 2 h at 37°C. Insoluble glucan was collected on Whatman GF/F glass fiber filters and washed with PBSA and the radioactivity determined by liquid scintillography.
Dental caries pathogenesis experiment 1. Rats.
Pregnant female Sprague-Dawley rats (Charles River Laboratory, Raleigh, NC) were cured of mutans streptococcal infection by amoxicillin (Henry Schein, Port Washington, NY) s.c. injection (150 mg/kg) twice a day for 1 week, followed directly by administration of sulfamethoxazole-trimethoprim (Sulfatrim; Hi Tech Pharmacal Co., Amityville, NY) in the drinking water for 1 week (6.75 ml Sulfatrim/200 ml drinking water). Swabbing of the mother's oral cavities and plating on mitis-salivarius agar (MS) (total streptococci) and on MS with 0.2 mg streptomycin sulfate (Sigma)/ml (MSS) (S. sobrinus strain 6715) 3 days after cessation of Sulfatrim indicated the complete absence of any mutans streptococci. The progeny, swabbed at 29 days of age (Diet 2000 present at all times [32]), were plated on MS, and no mutans streptococci were detected. The protocol was as follows. Rat progeny were removed from maternal cages at 28 days of age and randomly divided into four groups. Rats were immunized in the sgv in the following manner: sham immunized (n = 8), immunized with MAP construct 11 (n = 11), immunized with MAP 16 (n = 9), or immunized with both MAPs 11 and 16 (n = 9). Booster injections in IFA were given at 35 days of age, and blood and saliva were collected. Streptomycin-sulfate, 4 mg/100 ml, was administered in the drinking water for five consecutive days, followed by oral infection with streptomycin-resistant S. sobrinus 6715 (109/rat/day), initiated for the last three consecutive days of streptomycin administration. All rats were found to be infected with streptomycin-resistant S. sobrinus, the only mutans streptococcus by oral swabbing and plating on MS and MSS agar 2 days after discontinuance of the streptomycin. Infection was confirmed by swabbing 13 days later. The experiment was terminated after 38 days of infection, at which time rats were swabbed for oral bacterial enumeration, blood and saliva were collected for immunologic assay, and dental caries was evaluated on defleshed jaws.
Dental caries pathogenesis experiment 2.
The progeny of pregnant female Sprague-Dawley rats (devoid of mutans streptococci), originally from Taconic Farms (breeding unit IBU21) and raised in our facility, were weaned at approximately 21 days of age and placed on high-sucrose diet 2000 (32). Male and female rats (four or five of each sex/group) were sham immunized (control) or injected with MAP 11 or with MAP 11 and 16 at age 24 days (n = 9 to 10 rats/group). Animals received CFA with PBS alone (control) or 50 μg MAP in PBS plus CFA. Rats were immunized in the sgv and boosted with the same antigen in IFA 7 days later. Blood and saliva were collected 7 days later at 38 days of age, and all animals were infected at 39 days of age with approximately 2 × 108 CFU of streptomycin-resistant S. sobrinus strain 6715 on each of three consecutive days. Infection was verified in rats by systematic tooth swabbing and plating on MS and MSS agar 1, 8, and 30 days after infection. Infection proceeded for 33 days, at which time the experiment was terminated, blood and saliva were collected, and dental caries evaluated.
Bacterial recoveries.
The presence of mutans streptococcal infection was assessed during and at the end of pathogenesis experiments. After systematic swabbing of teeth, sonication, and plating appropriate dilutions on MS and MSS agar, plates were incubated for 48 h at 37°C in 80% N2-10% CO2-10% H2. Total streptococci (on MS agar) and streptomycin-resistant S. sobrinus strain 6715 CFU (on MSS agar) were then enumerated microscopically.
Caries assessment.
The extent and depth of carious lesions on all rat molar teeth (caries score) were microscopically evaluated by a modified Keyes method as described previously (30). The caries scores were determined for smooth and sulcal dental surfaces and then combined to obtain a total caries score.
Statistical analyses.
Proliferative responses and antibody ratios were analyzed by a one-sample t test with values above the 95% confidence interval considered significant (P < 0.05). The difference in group mean values among in vivo-treated groups were analyzed by one-way analysis of variance, followed by the Student Newman Keuls (SNK) multiple-comparisons test. In some cases the paired t test was used for comparisons.
RESULTS
GTF peptide sequence analyses.
Twenty regions within the S. sobrinus GTF-I sequence formed the basis for the synthesis of 20 20-mer linear peptides to be screened for reactivity with antibody to GTF. Three of these peptides (peptide 5 [position 438 to 457], peptide 6 [position 478 to 498], and peptide 9 [position 548 to 567]) contained residues associated with aspects of the catalytic functional activity of GTF (21, 24). However, putative MHC class II binding potential was low. Seventeen other peptides were selected on the basis of high binding probability with Epimatrix (15) and ProPred (19) algorithms. Of these, peptides 7, 11, and 16 had the highest binding probabilities based on both algorithms; their binding scores and sequences are indicated in Table 1.
Reactivity with linear GTF peptides with antibody to GTF.
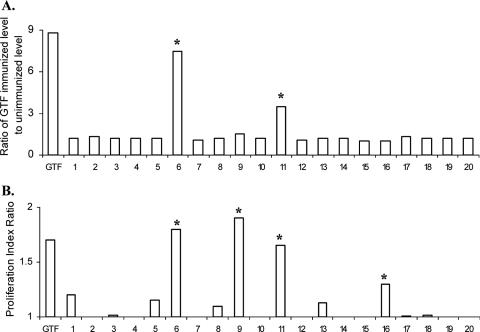
As a surrogate for natural infection, Rowett rats were immunized with intact GTF protein. Pooled sera from sham-immunized or GTF-immunized rats were investigated for IgG antibody binding to GTF and the 20 linear peptides. Sera demonstrated strong IgG reactivity with peptides 6 and 11 (Fig. 1A). The cellular responses to peptides were assessed by lymphocytes obtained from GTF-immunized rats. Significant proliferation was observed when lymph node mononuclear cells were incubated with GTF and with peptides 6, 9, 11, and 16 (Fig. 1B).
FIG. 1.
Antibody and cellular response of GTF-immunized rats to linear GTF sequence peptides. (A) Pooled rat sera from three immunized rats or from two control (ctl) rats were assessed for antibody binding to linear 20-mer peptides derived from S. sobrinus GTF-I sequence by ELISA. Data bars represent the ratio of the immunized level of IgG to the IgG level without immunization (optical density at 405 nm for each). Asterisks indicate ratio levels that were different from those for the other peptides when tested by one sample t test; statistically significant, P < 0.05. (B) Proliferation of lymph node cells of two GTF-immunized rats and one control rat 10 days after immunization with GTF. Cultured cells were stimulated with GTF or the respective peptide and [3H]thymidine (added for the last 18 h of culture). Data presented as bars are the average ratios of immunized proliferation indices/unimmunized proliferation indices. Asterisks indicate ratios that were higher than those for the other peptides when tested by one sample t test; statistically significant, P < 0.05.
Immunogenicity of MAP constructs.
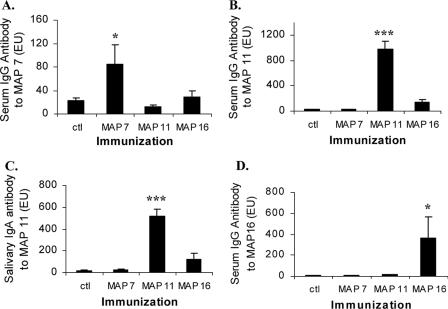
Three peptides were selected for further analysis on the basis of high MHC class II binding probability or reactivity with rat antibody and/or T-cell reactivity (peptides 7, 11, and 16). These peptides were synthesized as branched MAP constructs and used to immunize rats. Sera from immunized rats were assayed for IgG antibody reactivity with each of the MAP constructs and GTF. All immunized groups demonstrated significant levels of IgG antibody to the homologous immunizing MAP construct but not to the heterologous MAPs (Fig. 2A, B, and D). Salivas of rats immunized with MAP 11 contained IgA antibody to the homologous MAP 11 but not to MAP 7 or 16 (Fig. 2C). Salivas from rats immunized with MAP 7 or 16 did not contain significant levels of IgA antibody (data not shown).
FIG. 2.
Immune reactivity of MAP construct-immunized rats with MAPs (from linear peptides). Rats (five or six/group) were injected with buffer control (ctl) or 50 μg MAP construct in CFA s.c. in the salivary gland vicinity and in IFA 4 weeks apart. Serum was harvested 3 weeks later. (A) Serum IgG mean antibody levels (ELISA units [EU]) to MAP 7 when MAP 7, 11, or 16 was used for immunization (bars and standard errors of the mean). *, levels different from control and other MAPs by Student-Newman Keuls multiple-comparisons test (SNK); statistically significant, P < 0.05. (B) Level of IgG antibody to MAP 11 in serum of animals injected with ctl or MAP 7, 11, or 16. ***, levels different from those of control and other MAPs by SNK multiple comparison test; statistically significant, P < 0.001. (C) Salivary IgA antibody (EU) to MAP 11 after immunization with MAP 7, 11, or 16. (D) Mean serum IgG antibody to MAP 16 after immunization of groups of rats with MAP 7, 11, or 16. (Bars indicate mean level and standard errors of the mean). *, levels different from those of control and other MAP immunizations by SNK; statistically significant, P < 0.05.
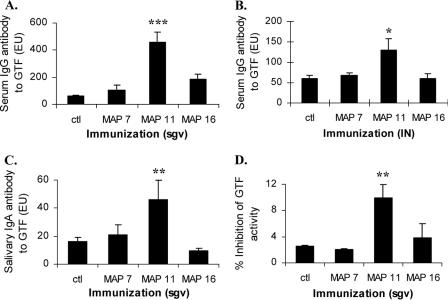
Reactivity of antipeptide IgG with S. sobrinus GTF.
Animals immunized with MAP 7 or MAP 16 showed little or no serum IgG or salivary IgA antibody reactivity with intact GTF, whereas serum IgG and salivary IgA antibody from MAP 11-immunized rats were significantly reactive with GTF regardless of whether the animals had been immunized s.c. or i.n. (Fig. 3A and B). Also, immunization with MAP 11 resulted in salivary IgA antibody to GTF, but not after immunization with MAP 7 or 16 (Fig. 3C). Importantly, the anti-GTF IgG antibody from MAP 11-immunized rats also significantly inhibited glucan synthesis mediated by the GTF enzyme, whereas sera from rats immunized with MAP 7 or 16 were not inhibitory (Fig. 3D).
FIG. 3.
Immune reactivity of MAP construct-immunized rats with GTF. Rats (five or six/group) were immunized with 50 μg MAP construct 7, 11, or 16 either s.c. in the salivary gland vicinity (sgv) with CFA (A, C, and D) or i.n. (IN) with cholera toxin (B). Serum IgG antibody to GTF (ELISA units [EU]), group means, and standard errors of the means are indicated by bars. Serum was harvested 3 weeks post-booster immunization. ctl, control. ***, levels higher than those of other groups by SNK multiple-comparisons test; statistically significant, P < 0.001. *, levels higher than those of other groups by SNK multiple comparison test; P < 0.05. (C) Saliva harvested 3 weeks post-s.c. booster immunization contained mean IgA (EU) levels to GTF, shown as bars and standard errors, after MAP 7, 11, or 16 immunization. **, SNK, multiple comparison test; P < 0.01. (D) Serum inhibited GTF-mediated incorporation of [14C]glucose from sucrose into water-insoluble glucan. Mean percent inhibition of GTF activity by serum is indicated by bars with standard errors. **, SNK multiple comparison test, statistically significant (P < 0.01).
Effects of MAP immunization on dental caries.
Since antibody to MAP 11 showed profound effects on GTF function, we chose to investigate the effect of MAP construct vaccines on the pathogenesis of dental caries initiated by S. sobrinus infection. Two of the three MAPs (peptides 7 and 16), although immunogenic in rats, indicated little or no antibody reactivity to GTF and little or no effects of antibody on GTF functions (Fig. 3A to D). Therefore, MAP 7 was eliminated as a candidate for vaccine testing, and peptide 16 was used as a possible negative control in these studies. Two experiments were performed in which weanling rats were immunized with MAP 11, alone or mixed with MAP 16, and then infected with cariogenic S. sobrinus to evaluate the effect of immunization on infection and disease.
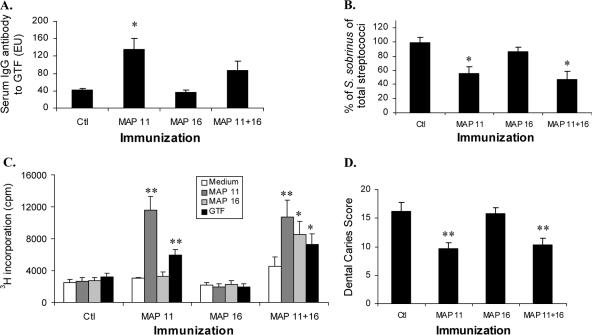
Experiment 1.
Levels of antibody to GTF, measured in sera harvested at termination (Fig. 4A), confirmed the ability of MAP 11 alone to induce serum IgG antibody reactive with native GTF, but MAP 16 and the control showed no reactivity. Immunization with MAP 11 antigens also had a significant effect on the recovery of S. sobrinus (on MSS agar) as a percentage of total streptococci recovered (on MS agar). After 38 days of infection, both MAP 11-containing immunogen groups were associated with significant reductions in the S. sobrinus percentage of the total streptococci recovered, compared with sham-immunized control rats (Fig. 4B). Significant reductions in infection were not observed after immunization with MAP 16 alone.
FIG. 4.
Immune response to MAP constructs and pathogenesis of dental caries (pathogenesis experiment 1). Rats (8 to 11/group) injected with buffer (control [Ctl]) or MAP construct 11, 16, or 11 plus 16 were infected with S. sobrinus for 38 days. (A) Serum IgG antibody (ELISA units [EU]) at termination boost is shown as bars depicting group mean and standard errors. *, different from control and MAP 16-immunized animal groups by SNK multiple comparison test; statistically significant, P < 0.05. (B) Recovery of S. sobrinus as mean percentage of total streptococci 13 days after infection, shown as bars and standard errors. Geometric mean recoveries of S. sobrinus ranged from 1.8 × 105 to 6.0 × 105 per swab. *, differences when all groups are compared by SNK, statistically significant; P < 0.05. (C) Rat cervical lymph node cells (at termination) were cultured in triplicate in the presence and absence of MAP constructs or native GTF. [3H]thymidine (0.5 μCi/well) was added for the last 16 h of culture. Radioactivity is expressed as counts/minute (cpm). Group means are indicated by bars and standard errors. *, P < 0.05; **, P < 0.01 (differences between groups statistically significant) when analyzed by SNK. (D) Bars show the mean total caries scores, including smooth and sulcal surfaces, and standard errors for 8 to 11 rats per group. **, differences among groups compared by SNK, statistically significant; P < 0.01.
T-cell responses to the individual MAPs and to GTF were assessed with rat lymph node T cells by in vitro stimulation with GTF and the MAPs. Significant proliferative reactivity to GTF was observed only after immunization with peptide 11. Similarly, after immunization with peptide 11, T cells responded to peptide 11 but not to peptide 16, affirming that MAP 11 also gave rise to a T-cell response to GTF (Fig. 4C). Immunization with MAP 11 plus MAP 16 also gave rise to highly significant proliferative activity specific to MAP 11 and reactivity to MAP 16 and GTF (Fig. 4C).
Scores of dental caries were obtained at the termination of the experiment. Animals immunized with MAP 11 exhibited significantly reduced dental caries score levels (Fig. 4D). Rats immunized with MAP 16 alone did not demonstrate caries levels different from those of sham-immunized/infected rats, although significant protection was also observed after immunization with the combination of MAPs 11 and 16. However, addition of MAP 16 to MAP 11 for immunization did not improve the level of protection observed.
Experiment 2.
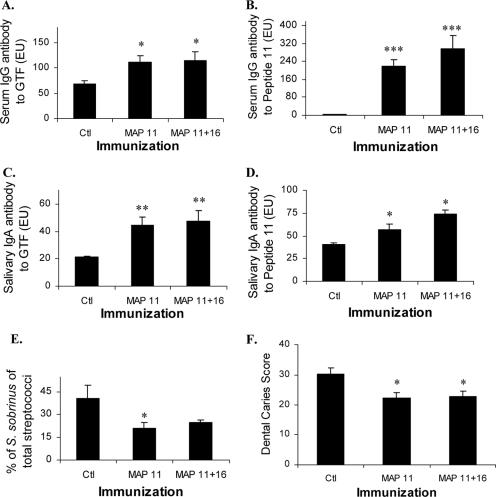
In order to verify and extend our observations, a similar experimental protocol of immunization and infection was followed in the second experiment using groups sham-immunized or immunized with MAP 11 or MAP 11 plus MAP 16. Sera and salivas harvested before infection confirmed that inclusion of MAP 11 resulted in significant elevations of serum IgG and salivary IgA antibody to the inciting peptide and to S. sobrinus GTF (Fig. 5A to D). The means of bacterial recoveries of S. sobrinus as a percentage of total streptococci 1 and 13 days after the last infection and at the end of the experiment were significantly lower for the MAP 11-immunized group compared to those for the sham immunized control (Fig. 5E). Also corroborating the experiment 1 observations, inclusion of MAP 11 in the immunogen resulted in significantly lower mean dental caries scores (Fig. 5F).
FIG. 5.
Immune response to MAP constructs and pathogenesis of dental caries (pathogenesis experiment 2). Rats (8 to 10/group) injected with buffer (control [ctl]) or MAP construct 11 or 11 plus 16 were infected with S. sobrinus for 33 days. (A) Serum IgG antibody to GTF (ELISA units [′EU]) 7 days after booster immunization and before infection is shown as bars depicting group means and standard errors. *, different from control animal group by SNK multiple-comparisons test; statistically significant, P < 0.05. (B) Serum IgG antibody to MAP 11 (EU) after immunization and before infection is shown as bars depicting group means and standard errors. ***, different from control group by SNK multiple-comparisons test; statistically significant, P < 0.05. (C) Saliva harvested after immunization and before infection contained levels of IgA (EU) to GTF, shown as bars depicting group means and standard errors. **, Different from control group by SNK; statistically significant, P < 0.01. (D) Salivary IgA antibody (EU) to MAP 11 as in panel C; *, P < 0.05. (E) Recovery of S. sobrinus expressed as percentage of total streptococcus mean of recoveries on 18 and 30 days after infection, shown as bars and standard errors. *, differences when MAP 11- and 11-plus-16-immunized groups are compared to infected control group by SNK paired one-tailed t test; statistically significant, P < 0.05. (F) Dental caries scores of animals immunized and infected with S. sobrinus for 33 days. Bars show the mean total caries scores, including smooth and sulcal surfaces, and standard errors for 8 to 10 rats/group. *, differences among groups compared by SNK test; statistically significant, P < 0.05.
DISCUSSION
Human MHC may be directly deduced from pooled sequence data of MHC alleles; thus, algorithms can then be used to estimate binding probability. Two independent systems were employed, a proprietary system, Epimatrix (Epivax; (15), and a public access system, ProPred (19). Epivax uses a set of as many as 17 MHC alleles to recognize known binding motifs in peptides in question, whereas the latter uses a matrix tested on the 51 alleles with 97 to 99% accuracy. We have identified peptide sequences of GTF-I of S. sobrinus on the basis of MHC binding probabilities using a consensus of the two different computer-generated algorithms. Twenty candidate linear peptides were synthesized and subjected to in vitro and in vivo investigation with rodents. We initially identified three immunologically promising linear peptides, which were then synthesized as MAP constructs (see Table 1). All three constructs, 7, 11, and 16, are of themselves immunogenic (Fig. 2). However, only one such MAP, MAP 11 (amino acids [aa] 847 to 866 [Table 1]) generated antibody (IgG and IgA) that bound to GTF (Fig. 3 A and C) and inhibited function of the GTF enzyme (Fig. 3D) in rodent studies. Such antibody was also found to inhibit increases in accumulation of S. sobrinus relative to the total streptococcal population (Fig. 4B and 5E) and most importantly to inhibit dental caries (Fig. 4D and 5F). These investigations demonstrated an effective approach to peptide selection and assessment for vaccines. These rodent studies with the candidate MAPs as antigens demonstrated that such functionally significant antibody is most meaningful in the inhibition of dental caries.
The question arises as to the relationship between MHC binding potential, immunogenicity, and protective response. All three peptides, 7, 11, and 16, tested herein were of themselves immunogenic (Fig. 2). However, only immunization with MAP 11 resulted in antibody to GTF (Fig. 3) and protection (Fig. 4D). This is not a function of immunogenicity but rather of the nature of the epitope, which shares structure with the GTF molecule. It would be reasonable to assume that the protective aspects of immunization with MAP 11 were attributable to this cross-reaction and thereby interference with a main function of GTF in the molecular pathogenesis of dental caries. As indicated, protection and immunogenicity are not necessarily related, since all peptides tested were immunogenic but only MAP 11 elicited protection. Also, for example, observation of peptide 5 (Table 1) indicates that while this peptide was mildly immunogenic (had no T-cell epitope and low binding scores), MAP 5 elicited modest levels of antibody (27, 30) that was protective (30). Since this peptide is found in the major catalytic site of GTF, the elicited protective antibody interferes with function because of its proximity to a vital site of enzyme function in disease pathogenesis.
Previously we have used the matrix-based algorithm (EpiMatrix) to estimate the MHC class II binding characteristics of glucan-binding protein B (GbpB) from Streptococcus mutans (22). Such an approach was valuable because there is little or no indication of the functionally associated regions of that protein. Therefore, we sought an immunogenic portion of the molecule to initiate significant immunity. Of two peptides initially selected and four others ultimately selected (18, 20), one (called “SYI”) when synthesized as the MAP construct induced a more consistent and somewhat higher response in rats to the GbpB protein and was used successfully for immunization to afford protection against dental caries with the rat model (22).
Interestingly, in those experiments, immunization with the SYI-MAP construct produced levels of protection (22) similar to those with previous immunization with the intact GbpB protein (25). In the experiments performed herein, reductions observed in caries levels were of the order of 40 to 27%, as found in other immunization experiments with GTF and peptides (21, 22, 30). The finding of similar protection potency when rats are immunized with GbpB or with the SYI-MAP construct is remarkable in the context of the GbpB experiments (22, 25) and also in the current GTF immune peptide experiments. In this regard, we have demonstrated that combinations of peptides from more than one functional region (i.e., diepitopic constructs) may enhance immunogenicity and the caries-protective response (29). Thus, a combination of a highly immunogenic peptide, e.g., peptide 11, combined with another peptide derived from a functional region and possibly containing complementary immunological properties (e.g., T- and B-cell epitopes), could result in a very potent diepitopic construct (29). This was recently reiterated when a diepitopic putative subunit dental caries vaccine containing SYI from GbpB (S. mutans) and the catalytic domain peptide (CAT) from GTF (S. sobrinus) was evaluated (23). This combination diepitopic construct enhanced the immunological response to CAT and GTF epitopes and extended protection against dental caries to include S. mutans and S. sobrinus. Coupled with this information, the current discovery of peptide 11 adds an extra immunogenic peptide for consideration to peptides from functional regions of S. sobrinus GTF and immunogenic regions of S. mutans GbpB. Combinations of these peptides as constructs bearing four peptide components might further enhance the effectiveness of such diepitopic constructs substantially and potentially produce a more universal dental caries vaccine.
The reactive peptide sequences are summarized in Table 1, along with the rationale for selection of the peptide. In human in vitro testing, peptides 5, 7, and 16 demonstrated potent immunoreactivity, whereas peptide 11 exhibited only modest activity (2). Peptides 5, 6, and 9 were chosen previously on the basis of identity to peptides CAT, EAW, and HDS (Table 1), which had been identified as functionally significant, immunogenic peptides (21, 27, 29) but were predicted to have low probable binding to MHC. Of the remaining reactive peptides, 7, 11, and 16, all had been predicted to be highly likely to bind class II MHC (Table 1). However, peptide 11 exhibited exceptional reactivity with IgG antibody and with lymphocytes from GTF-immunized rats (Fig. 1A and B).
That peptides reacted differently with humans and rats is not surprising, since the binding probabilities are based on human MHC algorithms. While these findings highlight some difficulties of using rodent models to mirror human systems, in this case sufficient similarity in reactivity does exist. For example, peptide 11 was somewhat immunogenic for humans and was particularly potent in generating protective antibody in rats. This similarity enabled the effects of peptide immunization to be assessed with Sprague-Dawley rats, a system routinely used as an animal model of dental caries. The results obtained from rodent studies demonstrated that select MAP constructs, namely, from aa 502 to 521, aa 847 to 866, and aa 1376 to 1395 (peptides 7, 11, and 16), showed strong reactivity with rat antibody to the homologous MAP. For example, peptide 11 was predicted to bind multiple class II alleles in humans and was highly immunogenic for the rat.
Interestingly, peptides 7, 11, and 16, all with predicted class II binding, are also associated with or close to functional domains. Peptide 7 lies within the catalytic domain with glycosylhydrolase homology (8, 27, 30, 33). Peptide 11 also lies at the boundary of a region in an area with particular homology to α-amylase (12, 21). Peptide 16 lies within the glucan-binding domain (analyzed by NCBI conserved domain search [13, 26, 30]). Peptide 11 demonstrates in vivo immunogenicity and elicits antibody that inhibits the function of the GTF enzyme. This epitope lies on the boundary of a functional domain and therefore may have previously escaped selection based exclusively on enzymatic function. This demonstrates that peptide antigen identification may be enhanced by considering MHC binding possibilities. Therefore, this strategy may represent a significant improvement in identifying such antigens compared to selection only on the basis of functionally active peptides.
Additionally, none of the immunoreactive peptide sequences showed any significant homology to human proteins (analyzed by NCBI BLAST [16, 17]), further emphasizing the likely safety of a subunit vaccine formulated with such select peptides. These data provide a rationale for the use of MHC binding prediction in association with protein function considerations when selecting peptides as vaccine candidates. The study has identified novel sequences that are immunogenic and merit further consideration for investigation in preclinical trials (in particular, peptide 11) of subunit dental caries vaccines. This study suggests a new approach for identifying potential novel vaccine candidates against dental caries.
Acknowledgments
This work was supported by NIH grant DE-04733 from the National Institute of Dental and Craniofacial Research.
We acknowledge the kind assistance of Rick Huntress in obtaining animals devoid of mutans streptococci and of James Fox in devising the curative protocols.
Editor: D. L. Burns
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Chia, J. S., R. H. Lin, S. W. Lin, J. Y. Chen, and C. S. Yang. 1993. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect. Immun. 61:4689-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Culshaw, S. E., K. B. LaRosa, J. W. Eastcott, D. J. Smith, and M. A. Taubman. 2005. Novel immunogenic peptides selected for a caries vaccine by MHC-binding. J. Dent. Res. 83:168. [Google Scholar]
- 3.Department of Health and Human Services. 2000. Oral health in America: a report of the surgeon general—executive summary. Department of Health and Human Services, NIH, NIDCR, Rockville, MD.
- 4.Germain, R. N., and D. H. Margulies. 1993. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 11:403-450. [DOI] [PubMed] [Google Scholar]
- 5.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaste, L. M., R. H. Selwitz, R. J. Oldakowski, J. A. Brunelle, D. M. Winn, and L. J. Brown. 1996. Coronal caries in the primary and permanent dentition of children and adolescents 1-17 years of age: United States, 1988-1991. J. Dent. Res. 75:631-641. [DOI] [PubMed] [Google Scholar]
- 7.Kubo, R. T., A. Sette, H. M. Grey, E. Appella, K. Sakaguchi, N. Z. Zhu, D. Arnott, N. Sherman, J. Shabanowitz, H. Michel, et al. 1994. Definition of specific peptide motifs for four major HLA-A alleles. J. Immunol. 152:3913-3924. [PubMed] [Google Scholar]
- 8.Laloi, P., C. L. Munro, K. R. Jones, and F. L. Macrina. 1996. Immunologic characteristics of a Streptococcus mutans glucosyltransferase B sucrose-binding site peptide-cholera toxin B-subunit chimeric protein. Infect. Immun. 64:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehner, T., S. J. Challacombe, J. M. Wilton, and J. Caldwell. 1976. Cellular and humoral immune responses in vaccination against dental caries in monkeys. Nature 264:69-72. [DOI] [PubMed] [Google Scholar]
- 10.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGhee, J. R., S. M. Michalek, J. Webb, J. M. Navia, A. F. Rahman, and D. W. Legler. 1975. Effective immunity to dental caries: protection of gnotobiotic rats by local immunization with Streptococcus mutans. J. Immunol. 16:300-305. [PubMed] [Google Scholar]
- 12.Monchois, V., J. H. Lakey, and R. R. Russell. 1999. Secondary structure of Streptococcus downei GTF-1 glucansucrase. FEMS Microbiol. Lett. 177:243-248. [DOI] [PubMed] [Google Scholar]
- 13.Mooser, G., and C. Wong. 1988. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect. Immun. 56:880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitts, N. B., I. G. Chestnutt, D. Evans, D. White, B. Chadwick, and J. G. Steele. 2006. The dentinal caries experience of children in the United Kingdom, 2003. Br. Dent. J. 200:313-320. [DOI] [PubMed] [Google Scholar]
- 15.Roberts, C. G., G. E. Meister, B. M. Jesdale, J. Lieberman, J. A. Berzofsky, and A. S. De Groot. 1996. Prediction of HIV peptide epitopes by a novel algorithm. AIDS Res. Hum. Retrovir. 12:593-610. [DOI] [PubMed] [Google Scholar]
- 16.Robinson, J., M. J. Waller, P. Parham, J. G. Bodmer, and S. G. Marsh. 2001. IMGT/HLA database—a sequence database for the human major histocompatibility complex. Nucleic Acids Res. 29:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson, J., M. J. Waller, P. Parham, N. de Groot, R. Bontrop, L. J. Kennedy, P. Stoehr, and S. G. Marsh. 2003. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 31:311-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen, B., W. F. King, M. A. Taubman, and D. J. Smith. 2004. Immune potential of glutamine-rich peptides from S. mutans GbpB. J. Dent. Res. 83:188. [Google Scholar]
- 19.Singh, H., and G. P. Raghava. 2001. ProPred: prediction of HLA-DR binding sites. Bioinformatics 17:1236-1237. [DOI] [PubMed] [Google Scholar]
- 20.Smith, C., W. F. King, M. A. Taubman, and D. J. Smith. 2005. Epitope immunogenicity in putative functional domains of S. mutans GbpB. J. Dent. Res. 84:145. [Google Scholar]
- 21.Smith, D. J., R. L. Heschel, W. F. King, and M. A. Taubman. 1999. Antibody to glucosyltransferase induced by synthetic peptides associated with catalytic regions of alpha-amylases. Infect. Immun. 67:2638-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, D. J., W. F. King, L. A. Barnes, Z. Peacock, and M. A. Taubman. 2003. Immunogenicity and protective immunity induced by synthetic peptides associated with putative immunodominant regions of Streptococcus mutans glucan-binding protein B. Infect. Immun. 71:1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, D. J., W. F. King, J. Rivero, and M. A. Taubman. 2005. Immunological and protective effects of diepitopic subunit dental caries vaccines. Infect. Immun. 73:2797-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, D. J., B. Shoushtari, R. L. Heschel, W. F. King, and M. A. Taubman. 1997. Immunogenicity and protective immunity induced by synthetic peptides associated with a catalytic subdomain of mutans group streptococcal glucosyltransferase. Infect. Immun. 65:4424-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, D. J., and M. A. Taubman. 1996. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect. Immun. 64:3069-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, D. J., M. A. Taubman, C. F. Holmberg, J. W. Eastcott, W. F. King, and P. Ali-Salaam. 1993. Antigenicity and immunogenicity of a synthetic peptide derived from a glucan-binding domain of mutans streptococcal glucosyltransferase. Infect. Immun. 61:2899-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, D. J., M. A. Taubman, W. F. King, S. Eida, J. R. Powell, and J. W. Eastcott. 1994. Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect. Immun. 62:5470-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stack, W. E., M. A. Taubman, T. Tsukuda, D. J. Smith, J. L. Ebersole, and R. Kent. 1990. Dental caries in congenitally athymic rats. Oral Microbiol. Immunol. 5:309-314. [DOI] [PubMed] [Google Scholar]
- 29.Taubman, M. A., C. J. Holmberg, and D. J. Smith. 2001. Diepitopic construct of functionally and epitopically complementary peptides enhances immunogenicity, reactivity with glucosyltransferase, and protection from dental caries. Infect. Immun. 69:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taubman, M. A., C. J. Holmberg, and D. J. Smith. 1995. Immunization of rats with synthetic peptide constructs from the glucan-binding or catalytic region of mutans streptococcal glucosyltransferase protects against dental caries. Infect. Immun. 63:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taubman, M. A., and D. J. Smith. 1974. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody experimental dental caries in rats. Infect. Immun. 9:1079-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taubman, M. A., D. J. Smith, C. J. Holmberg, and J. W. Eastcott. 2000. Coimmunization with complementary glucosyltransferase peptides results in enhanced immunogenicity and protection against dental caries. Infect. Immun. 68:2698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsumori, H., T. Minami, and H. K. Kuramitsu. 1997. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. J. Bacteriol. 179:3391-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]