Abstract
Yersinia pestis is the causative agent of plague, a disease that can manifest as either bubonic or pneumonic plague. An interesting feature of plague is that it is a rapidly progressive disease, suggesting that Y. pestis either evades and/or suppresses the innate immune response to infection. Therefore, the early host response during the course of primary pneumonic plague was investigated in two mouse strains, the outbred strain CD1 and the inbred strain C57BL/6. A comparative analysis of the course of disease in these two strains of mice indicated that they are susceptible to intranasal Y. pestis CO92 infection and have similar 50% lethal doses and kinetics of infection with respect to colonization of the lung, liver, and spleen. Significantly, in both strains of mice, robust neutrophil recruitment to the lungs was not observed until 48 h after infection, suggesting that there was a delay in inflammatory cell recruitment to the site of infection. In addition, proinflammatory cytokines (interleukin-6 [IL-6], tumor necrosis factor alpha, gamma interferon, IL-12p70, monocyte chemoattractant protein 1) and chemokines (KC, MIP-2) in the bronchoalveolar lavage fluids were not readily detected until 48 h after infection, which coincided with the increase in polymorphonuclear leukocyte (PMN) recruitment to the lungs. In comparison, CD1 mice with gram-negative pneumonia caused by Klebsiella pneumoniae exhibited strong inflammatory responses early in infection, with PMNs comprising the majority of the cells in the bronchoalveolar lavage fluid 24 h postinfection, indicating that PMN recruitment to the lungs could occur earlier in this infection than in Y. pestis infection. Together, our results indicate that there is a delay in the recruitment of neutrophils to the lungs in the mouse model of primary plague pneumonia that correlates with delayed expression of proinflammatory cytokines and chemokines in both outbred and inbred mice.
The genus Yersinia contains three species that are human pathogens. Yersinia enterocolitica and Yersinia pseudotuberculosis are enteropathogens that cause gastrointestinal disease, whereas Yersinia pestis is the causative agent of plague. Plague generally manifests as either the bubonic or pneumonic form of the disease. Bubonic plague arises from inoculation of Y. pestis into the host due to the bite of an infected flea and is the most common presentation of the disease. In contrast, inhalation of Y. pestis results in development of primary pneumonic plague, a contagious disease that can be spread via aerosols. Primary pneumonic plague usually develops 2 to 3 days after exposure and is a rapidly progressive and aggressive pneumonia with mortality rates approaching 100% if treatment is delayed (9, 38). Little is known about the host response or the pathogenesis of primary pneumonic plague.
The rapid onset of symptoms and then death in plague pneumonia suggests that the host's innate immune defenses are not effective for fighting the disease. This could be due to suppression of immune responses by bacterially encoded virulence factors or evasion of immune recognition. In order to better understand the pathogenesis of plague pneumonia, insight into the early host response to infection is required.
Progress has been made in understanding the pathogenesis of bubonic plague, while insights into the pathogenesis of pneumonic plague have lagged due to our poor understanding of the early immune response to pneumonic Y. pestis infection. In previous studies workers used a number of different hosts to experimentally study Y. pestis infection, including nonhuman primates, cats, and rodents (mice and rats), each of which has advantages and disadvantages (4, 5, 13, 14, 19, 31, 37). The utility of rodent models, particularly mice, make them attractive models, as rodents are natural hosts of Y. pestis and have been used extensively in studies of both pathogenesis and the efficacy of vaccines against Y. pestis infection using both bubonic (22, 26) and pneumonic (36, 39) models. In previous studies workers have used a variety of mouse strains, including outbred strains (1, 18, 39) and inbred strains, such as BALB/c (11, 16) and C57BL/6 (11), as well as other strains (12). When a mouse model is used, the host genotype can have a strong impact on the pathogenesis of the disease caused by the enteropathogenic yersiniae, as well as a variety of other organisms (2, 7, 8, 17, 24). Even though mouse models of pulmonary Y. pestis infection have been described (19, 31, 32), currently there is no consensus concerning or data on whether inbred or outbred mice are more appropriate host models for studying the pathogenesis of plague pneumonia.
In this study the pathogenesis of primary pneumonic plague caused by infection with both high and low doses of the wild-type Y. pestis CO92 strain (9) was evaluated in outbred (CD1) and inbred (C57BL/6) mice. We observed that within the parameters of this study, there were only subtle differences in the innate immune responses of the CD1 and C57BL/6 strains of mice during acute pneumonic plague infection. However, our investigations revealed that there was a distinct delay in the recruitment of inflammatory cells to the site of infection in both strains of mice. A delay in inflammatory cell recruitment may be a key step in the pathogenesis of primary plague pneumonia.
MATERIALS AND METHODS
Bacteria.
Y. pestis CO92, a biovar Orientalis strain recently isolated from a case of pneumonic plague, was obtained from the Select Agent Distribution Activity, Centers for Disease Control and Prevention, Fort Collins, CO. This strain was confirmed to contain the pigmentation (pgm) locus phenotypically by the production of red colonies on Congo red plates and by PCR. The presence of the low-calcium-response virulence plasmid (Lcr) was confirmed by PCR of the lcrV, yopH, and yopJ genes. Virulence in mice was confirmed as described below. Klebsiella pneumoniae ATCC 43816 was obtained from the American Type Culture Collection (Manassas, VA) and was grown on LB at 37°C. The estimated 50% lethal dose (LD50) of K. pneumoniae ATCC 43816 has been reported to be 3 × 103 CFU (21).
Animal infection.
Six- to 8-week-old female outbred CD1 mice (Charles River Laboratories, Wilmington, MA) or inbred C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were used. Fully virulent Y. pestis strain CO92 was grown for 20 h at 37°C in heart infusion broth (US Biological, Swampscott, MA) supplemented with 0.2% xylose. Cultures then were harvested, washed once with sterile phosphate-buffered saline (PBS), and diluted in endotoxin-free PBS to obtain a concentration of either 106 bacteria/ml for the high-dose experiments or 104 bacteria/ml for the low-dose experiments. Bacterial counts were verified by determining the number of CFU on Congo red plates. K. pneumoniae was grown for 16 h at 37°C in LB broth (US Biological, Swampscott, MA). Cultures were then harvested, washed once with sterile PBS, and diluted in endotoxin-free PBS to obtain a concentration of 107 bacteria/ml. Depending on the experimental protocol, mice were anesthetized either by intramuscular injection of 0.1 ml of mouse cocktail (2 mg/ml xylazine and 15 mg/ml ketamine in PBS) or by intraperitoneal injection of 0.5 ml of Avertin (2,2,2-tribromoethanol; 20 mg/ml in PBS; Sigma, St. Louis, MO). Mice were then intranasally infected with 20 μl of inoculum; each mouse received approximately 0.8 × 104 to 2.9 × 104 CFU for the high-dose Y. pestis group, 250 to 1,170 CFU for the low-dose Y. pestis group, or 1 × 105 CFU for the K. pneumoniae group. Mice were euthanized with an overdose of isoflurane (Iso-Thesia; Vetus Animal Health, Burns Veterinary Supply, Inc., Westbury, NY), which was followed by cervical dislocation, at 24 and 48 h after infection for the high-dose Y. pestis and K. pneumoniae groups or at 24, 48, and 72 h after infection for the low-dose Y. pestis group. All experiments with Y. pestis were performed at biosafety level 3 in accordance with Institutional Biosafety Committee and Institutional Animal Use and Care Committee protocols.
Bacterial burden.
The bacterial burdens in target organs of the high-dose Y. pestis groups were determined as previously described (17). Briefly, lungs, livers, and spleens were harvested at different times and weighed prior to homogenization. Tissue homogenates were then diluted in PBS, and dilutions were plated on Congo red plates. Bacterial growth was evaluated after 72 h of incubation at 37°C. The results are expressed below in CFU/gram of tissue, and the values represent the data for at least two independent experiments performed with three to five animals per genotype per time.
LD50.
The LD50 for Y. pestis was estimated by the method of Reed and Muench (29). Briefly, mice were inoculated intranasally with 10-fold serial dilutions containing 105 to 1 CFU of CO92. The survival of the mice was then monitored every 12 h. Each LD50 reported below is the average of the data from at least two independent experiments performed with five mice per strain per dose of bacteria.
Bronchoalveolar lavage.
Mice were euthanized, and a 1-cm longitudinal incision was made to expose the trachea. Bronchoalveolar lavage was performed by catheterizing the trachea using 18-gauge catheters (Becton-Dickinson Infusion Therapy Systems, Inc., Sandy, UT). Each mouse was lavaged with three 1-ml aliquots of PBS with protease inhibitors (pepstatin, phenylmethylsulfonyl fluoride, aprotinin, and leupeptin [Sigma, St. Louis, MO]). Bronchoalveolar lavage fluids (BALF) were placed immediately on ice. Cellular BALF infiltrates were analyzed using BALF smears placed on poly-l-lysine-coated microscope slides (Electron Microscopy Sciences, Hatfield, PA) and were subsequently stained with modified Wright's stain (Sigma, St. Louis, MO). The total number of cells per milliliter of BALF was determined by visual counting using a hemocytometer. The percentage of polymorphonuclear cells (PMNs) in the total cell infiltrate was determined by visual examination after staining with modified Wright stain (Sigma, St. Louis, MO). At least 300 infiltrating cells from each sample were counted, and the results reported below are the percentages of PMNs in BALF infiltrates. The BALF was then filtered with a 0.2-μm syringe filter (SFCA; Fisher Scientific, Pittsburg, PA). BALF samples were then stored at −80°C for future analysis. The results reported below are representative results of two independent experiments performed with four or five animals per genotype and time.
Cytokine analysis. (i) Cytometric bead array.
BALF samples from the high-dose infection group were assayed to determine the presence of interleukin-10 (IL-10), IL-6, IL-12p70, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and monocyte chemoattractant protein 1 (MCP-1) using a mouse inflammation cytometric bead array kit (BD Biosciences, Franklin Lakes, NJ) as recommended by the manufacturer. Cytokine levels were determined using the cytometric BEAD ARRAY ANALYSIS software, version 1.1. Seven to 10 samples per genotype per time were used in the assay, and each sample was assayed in a single tube.
(ii) ELISA.
BALF samples from the high-dose Y. pestis group were diluted as appropriate and used in enzyme-linked immunosorbent assays (ELISA) for mMIP-2/CXCL-2 and mKC/CXCL-1 (R&D Systems, Minneapolis, MN). Seven to 10 samples per genotype per time were used in the assay, and each sample was assayed in triplicate.
Histopathology.
The histopathology of infection was also investigated. Following intranasal infection, the lungs, liver, and spleen were harvested at different times. The gross appearance of each organ was evaluated at necropsy. At least 10 animals per genotype per dose per time were examined. Tissues were fixed in 10 ml of 10% neutral buffered formalin. Formalin-fixed tissues were then embedded in paraffin, and 3- to 4-μm sections were cut and placed on slides. The tissue sections were then stained with hematoxylin and eosin. The tissues were examined and evaluated in a blind fashion to determine inflammatory cell infiltration, bacterial colonization, the presence of inflammatory exudates in airways, edema, necrosis, hemorrhage, and the presence of fibrin. Images were captured digitally with a Zeiss Axioscope 2 microscope equipped with a digital camera. Images were processed using the Axiovision V.4 software suite (Carl Zeiss, Inc., Thornwood, NY).
Statistical analysis.
All results were expressed as the mean ± standard error of the mean. Statistical differences were determined using a two-tailed Student t test or a Mann-Whitney U test with GraphPad In-Stat3 (GraphPad Software). A P value of <0.05 was considered significant.
RESULTS
Kinetics of infection and LD50.
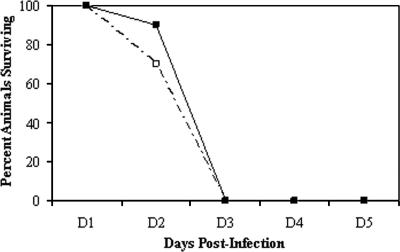
The pathogenesis of Y. pestis infection in both outbred CD1 mice and inbred C57BL/6 mice was assessed in order to evaluate the impact of host genotype in a primary pneumonic plague model. Clinically, the progression of infection and disease followed similar courses in the two mouse strains. The animals infected with a high dose did not show clinical signs of disease until 48 h after infection, when their fur began to ruffle and they began to hunch over, crowd together, and became inactive. All of the animals died by 72 h postinfection (Fig. 1).
FIG. 1.
Survival curve. Mice were infected intranasally with ∼104 CFU Y. pestis CO92, and survival was monitored for 3 days postinfection (all animals had succumbed to the infection after 3 days). The numbers of days to death and the survival percentages were similar for the two strains of mice when they were infected with the same dose. ▪, CD1; □, C57BL/6. A total of 10 mice per genotype were used in the experiments.
(i) Kinetics of infection.
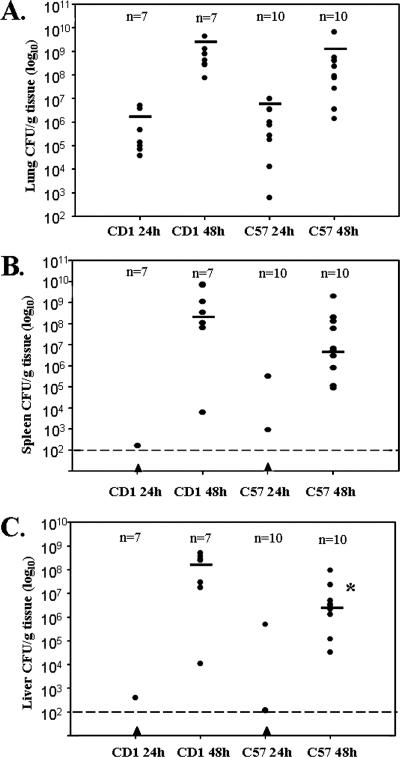
The bacterial burdens in the lungs, livers, and spleens of outbred CD1 mice were quantitatively compared to the bacterial burdens in the tissues of inbred C57BL/6 mice. When mice were infected with 0.8 × 104 to 2.9 × 104 CFU of CO92, we initially observed low levels of bacterial growth (102- to 103-fold changes) in the lungs 24 h postinfection; the average bacterial burdens in the lungs were 1.4 × 106 CFU/g of tissue for the CD1 mice and 2.0 × 106 CFU/g of tissue for the C57BL/6 mice, and few or no bacteria were detected in the liver and spleen (Fig. 2). However, between 24 and 48 h postinfection, there was tremendous outgrowth of bacteria (106- to 107-fold changes) as the average bacterial burdens in the lungs increased to 1.1 × 109 CFU/g of tissue for the CD1 mice and 8.4 × 108 CFU/g of tissue for the C57BL/6 mice. Likewise, 24 to 48 h postinfection was the period when heavy colonization of the spleen and liver was detected, with bacterial burdens reaching 108 and 109 CFU/g of tissue in the liver and spleen, respectively. These data indicate that both dissemination and outgrowth of the bacteria occurred in these tissues (Fig. 2). The course of dissemination and outgrowth in the C57BL/6 inbred mice was similar to the course recently reported by Lathem et al. (19).
FIG. 2.
Kinetics of infection. Mice were infected intranasally with ∼104 CFU of CO92. At different times, lungs (A), spleens (B), and livers (C) were harvested. Seven CD1 mice and 10 C57BL/6 mice were used in this experiment. A solid triangle indicates that the number of CFU/g tissue was below the limit of detection, which occurred only with organs harvested and plated at 24 h. The asterisk indicates that the P value is 0.0431, as determined by a Mann-Whitney U test, for a comparison of CD1 liver data and C57BL/6 liver data at 48 h. The bars indicate median values.
(ii) Determination of LD50.
An excellent way to determine if the host genotype has a strong impact on the pathogenesis of infection is to compare the 50% lethal doses of two strains of mice. The intranasal LD50 of CO92 for each of the strains was estimated using the method of Reed and Muench (29), and the LD50s were found to be similar for the two mouse strains. The LD50s of CO92 were estimated to be 1.2 × 102 CFU for the CD1 mice and 0.4 × 102 CFU for the C57BL/6 mice (Table 1), a finding consistent with the results of other studies in which the intranasal LD50 of CO92 for C57BL/6 mice was evaluated (19). The threefold difference in LD50 between the two strains is not statistically significant, as the method of Reed and Muench provides an estimate of the LD50. Together, these data indicate that the progression of disease in the CD1 mice and the progression of disease in the C57BL/6 mice were very similar and that gross indicators of pathogenesis (bacterial burden in tissues and LD50) revealed no differences between these strains of mice.
TABLE 1.
Intranasal LD50 of Y. pestis CO92 for two strains of mouse
| Mouse strain | LD50 (CFU)
|
||
|---|---|---|---|
| Expt 1 | Expt 2 | Avg ± SEM | |
| CD1 | 156 | 88 | 122 ± 34 |
| C57BL/6 | 58 | 21 | 40 ± 18 |
Cellular infiltrates in BALF during pneumonic Y. pestis infection.
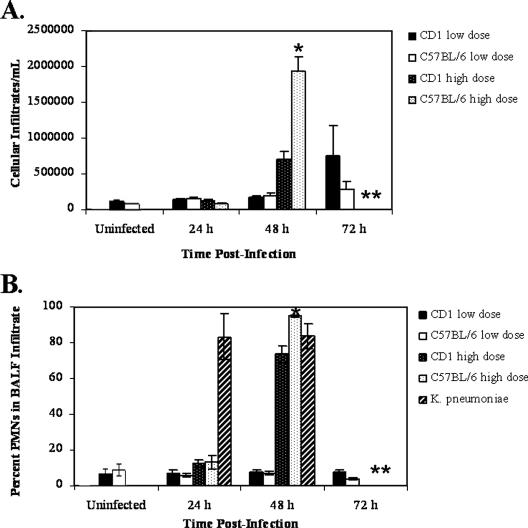
While gross indicators of the host response to infection suggested that CD1 and C57BL/6 mice responded similarly to pulmonary infection with Y. pestis, we checked for specific differences between the immune responses of the mouse strains. Because the BALF is a rich source of information about the host response to pulmonary infection, we examined the BALF from both strains of mice. The total number of cells per ml infiltrating the BALF was determined for both the high- and low-dose groups. For the mice infected with a high dose, the total number of cells/ml BALF did not change significantly for either strain until 48 h postinfection (P value for a comparison of uninfected CD1 mice and CD1 mice infected for 48 h with CO92, 0.001; P value for a comparison of uninfected C57BL/6 mice and mice infected for 48 h with CO92, <0.0001) (Fig. 3A). Interestingly, for mice infected with a low dose of Y. pestis, the total number of cells/ml BALF did not increase significantly over the course of infection for either mouse strain (Fig. 3A), indicating that the bacteria did not induce strong inflammatory cell recruitment to the site of infection. Differential counts of BALF cellular infiltrates revealed that PMNs accounted for a very small percentage (∼6 to 12%) of the infiltrating cells at 24 h postinfection for both the high- and low-dose groups. The levels were approximately equal to the levels found in uninfected animals (∼8%). The percentages increased drastically (74% and 95% for CD1 and C57BL/6 mice, respectively) 48 h postinfection for the high-dose groups (Fig. 3B). However, the PMNs accounted for a low percentage of the total infiltrating cells (∼7%) in the low-dose group throughout the course of infection (Fig. 3B). For comparison, the presence of PMNs in cellular infiltrates of the BALF of CD1 mice infected intranasally with K. pneumoniae was evaluated. At 24 h postinfection, the infiltrating cells were largely PMNs (∼83%), and the level remained high at 48 h postinfection (∼83%) (Fig. 3B and 4E). These data suggest that there was not a general defect in the ability of the mice to respond to bacterial infection. Together, the data further suggest that there was a delay in the initial recruitment of PMNs to the site of Y. pestis infection in the lungs that may have represented an important component of Yersinia pathogenesis. These data indicate that despite infection with a lethal dose of Y. pestis, there is little or no inflammatory cell response early in infection in the BALF, a striking contrast to the expected host response to a lethal dose of bacteria, particularly a gram-negative organism that causes pneumonia.
FIG. 3.
Cellular infiltrates in BALF. Mice were infected intranasally with ∼104 CFU (high dose) or ∼102 CFU (low dose) of Y. pestis CO92 or ∼105 CFU of K. pneumoniae. At different times, mice were euthanized, and bronchoalveolar lavage was performed. (A) Infiltrating cells were counted using a hemocytometer, and the results are expressed as the numbers of infiltrating cells/ml BALF. (B) Infiltrating cells were pelleted, placed on poly-l-lysine-coated slides, stained with Wright's stain (modified), and examined. Visual counts were obtained, and PMNs were differentiated based on morphology. The results are expressed as the percentages of PMNs in BALF infiltrates. One asterisk indicates that the CD1 and C57BL/6 data are significantly different (P < 0.05). Two asterisks indicate that there were no data for the high-dose Y. pestis group at this time because the mice had succumbed to infection and no data for the K. pneumoniae mice at this time because the experiment was not carried out past day 2. Eight to 10 mice were used to obtain each data point for the Y. pestis experiments, and four mice were used to obtain each data point for the K. pneumoniae experiments.
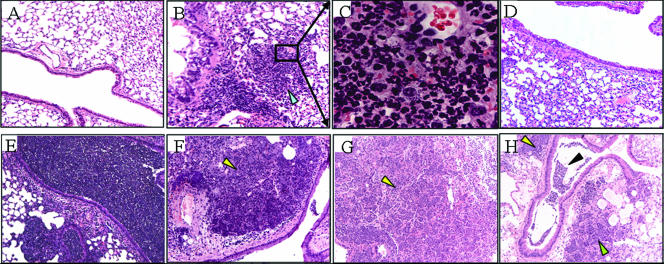
FIG. 4.
Histopathology of lungs during Y. pestis CO92 intranasal infection. Mice were infected intranasally with ∼104 CFU of CO92. At different times, mice were euthanized, and lungs were removed, placed in 10% neutral buffered formalin, embedded in paraffin, cut into 3- to 4 μm-sections, and stained with hematoxylin and eosin. (A) Normal lung tissue with representative conducting airway. (B) CD1 lung tissue 24 h postinfection, showing a small focal infiltration of inflammatory cells (arrowhead). (C) Higher magnification (×630) of the lung tissue indicated by the box in panel B. (D) C57BL/6 lung tissue 24 h postinfection. (E) CD1 lung tissue 24 h after infection with K. pneumoniae, showing dense PMN infiltrates in the lungs. (F and G) Lung tissue of CD1 and C57BL/6 mice, respectively, 48 h postinfection, showing dense bacterial sheets with many infiltrating immune cells, largely PMNs (arrowheads). (H) CD1 lung tissue 48 h postinfection, showing dense exudates of PMNs, bacteria, and red blood cells in the large airway (black arrowhead) with the smaller airspaces obliterated (green arrowhead). Magnification, ×100 unless indicated otherwise.
Cytokine secretion into the BALF during high-dose pneumonic Y. pestis infection.
Because of the delay in PMN recruitment to the lungs during Y. pestis infection, we investigated the cytokine and chemokine concentrations in the BALF of mice infected with 0.8 × 104 to 2.9 × 104 CFU of CO92. We examined a variety of cytokines and chemokines previously implicated in either the pathogenesis of Yersinia infection or the recruitment of neutrophils, including IFN-γ, TNF-α, MCP-1, KC, MIP-2, IL-6, IL-10, and IL-12 (2, 3, 10, 17). Overall, there were no overt differences in cytokine and chemokine production between the two mouse strains with the exception of the KC levels at 48 h postinfection (Table 2). The levels of KC in the BALF of CD1 mice were significantly higher (P = 0.0098) than the levels detected in the BALF of C57BL/6 mice 48 h postinfection; however, this did not seem to affect the outcome of infection. Appreciable levels of proinflammatory cytokines and chemokines (TNF-α, IL-6, MCP-1, KC, MIP-2) produced in response to Y. pestis infection in both strains of mice were not detected until 48 h postinfection (Table 2). We were particularly interested in the production of KC and MIP-2 as these chemokines predominately recruit PMNs to the site of an infection (15, 30, 35). The time course of KC and MIP-2 protein expression in the lungs coincided with the delayed kinetics of PMN infiltration into the BALF. The subtle differences in cytokine production between the strains included detection of small amounts of IFN-γ and IL-12p70 in the BALF of CD1 mice but not in the BALF of C57BL/6 mice (Table 2) at 48 h postinfection. There was also a subtle increase in the level of IL-10 at 24 h postinfection in the BALF, but the IL-10 concentration returned to basal levels by 48 h postinfection.
TABLE 2.
Mean cytokine and chemokine levels in BALF
| Cytokine or chemokine | Mouse strain | Concn(pg/ml) (mean ± SEM) at the following times postinfectiona:
|
||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| IL-12p70b | CD1 | NDc | ND | 23 ± 9 |
| C57BL/6 | ND | ND | ND | |
| TNF-αb | CD1 | ND | 5 ± 5 | 533 ± 133 |
| C57BL/6 | ND | ND | 356 ± 75 | |
| IFN-γb | CD1 | ND | ND | 53 ± 35 |
| C57BL/6 | ND | ND | ND | |
| MCP-1b | CD1 | ND | ND | 134 ± 63 |
| C57BL/6 | ND | ND | 110 ± 40 | |
| IL-10b | CD1 | 10 ± 10 | 36 ± 2 | 19 ± 7 |
| C57BL/6 | 12 ± 12 | 39 ± 1 | 18 ± 2 | |
| IL-6b | CD1 | ND | 16 ± 9 | 2,187 ± 563 |
| C57BL/6 | ND | 5 ± 5 | 2,820 ± 358 | |
| MIP-2b | CD1 | ND | 24 ± 9 | 2,198 ± 569 |
| C57BL/6 | ND | 7 ± 5 | 1,821 ± 287 | |
| KCd | CD1 | ND | 32 ± 14 | 3,830 ± 679 |
| C57BL/6 | ND | ND | 1,548 ± 352e | |
The data were obtained using 8 to 10 mice.
Values were determined using a cytometric bead assay (BD Bioscience).
ND, not detected.
Values were determined using an ELISA (R&D Systems).
The value is significantly different (P < 0.05) from the value obtained for KC in CD1 mice at 48 h.
Histopathology.
Data on the histopathology of primary pneumonic plague during disease development are limited, and data comparing the progression of disease in CD1 mice and the progression of disease in C57BL/6 mice are nonexistent. Therefore, we compared the histopathological findings for the lungs, livers, and spleens of CD1 and C57BL/6 mice infected intranasally with 0.8 × 104 to 2.9 × 104 CFU of CO92 (24 h and 48 h postinfection). Tissue sections were examined in a blind manner for cellular infiltration, microscopic evidence of bacterial colonization, edema, the presence of inflammatory exudates in airways, necrosis, hemorrhaging, and the presence of fibrin. A minimum of 10 organs per time were examined.
No gross differences between the two strains of mice were observed. At 24 h mild erythema was observed, but otherwise the lungs appeared normal. We observed a remarkable lack of PMNs in the lungs of both strains of mice, with pulmonary congestion observed in 75% of the lungs 24 h after infection compared to the lungs of uninfected animals. The remaining 25% of the lungs appeared normal. There were a few small focal infiltrations of inflammatory cells in the lungs at 24 h in 20% of the CD1 mice (Fig. 4B and C), but focal infiltrations were not observed with the C57BL/6 mice (Fig. 4D).
At 48 h erythema, pulmonary effusion, gross hemorrhaging, petechia, and pulmonary consolidation were evident. These findings are consistent with previous descriptions of the gross pathology of plague pneumonia (19, 32). PMN infiltration into the airspaces and interstitium was observed in all of the mice, along with lobular consolidation, areas of necrosis, fibrin deposition, and hemorrhaging. Pulmonary effusion, often bilateral, was evident at 48 h postinfection in both strains of mice, especially in the lower lobes. Other workers have reported the presence of pleural effusions (13, 32), and we have observed plural effusions in nonhuman primates (S. Bubeck, J. Elliot, and P. Dube, unpublished data), but such effusions were not observed in this study. In both the CD1 and C57BL/6 animals there were large areas of bacterial colonization (visualized by dark purple staining with hematoxylin and eosin stain) which were embedded within inflammatory cells and debris found readily throughout the lungs (Fig. 4F and G). Dense exudates of PMNs, bacteria, and red blood cells were present in conducting airways (Fig. 4H), whereas many of the smaller airways were obliterated by inflammatory cell infiltration.
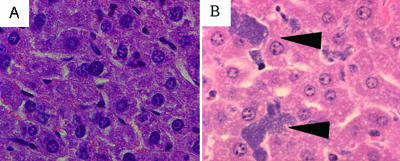
Our findings for the spleen and liver were consistent with previous findings reported for pneumonic (32), bubonic, and systemic plague models (28, 31). We observed breakdown of the spleen architecture at 48 h postinfection, with foci of bacteria present throughout the tissue (data not shown). In the liver, the results were similar, and small foci of bacteria were dispersed throughout the tissue without appreciable inflammatory cell infiltration (Fig. 5B). Interestingly, because the bacterial burdens approached 109 CFU/g at this time, these data suggest that the inflammatory response observed in the lung may have been due to tissue damage rather than bacterial products.
FIG. 5.
Y. pestis microcolonies in the liver do not elicit an inflammatory response. Mice were infected intranasally with ∼104 CFU of CO92. Livers were harvested at 48 h postinfection for histopathological analysis. (A) Normal liver. Magnification, ×630. (B) Liver from an infected CD1 mouse. Magnification, ×630. Note the multiple bacterial microcolonies indicated by arrowheads. Also note the striking lack of inflammatory cell infiltration at the site of bacterial colonization.
DISCUSSION
In studies of the pathogenesis of Y. pestis infection workers have used a number of different animal models (4, 5, 13, 14, 19, 31, 37). Currently, there is no consensus concerning which model system is best. However, similar results are often obtained when terminal stages of infection are observed using a variety of animal models. A reevaluation of the mouse models of primary pneumonic plague was warranted because many of the older studies (32-34) predate our modern understanding of both the host immune response and the ability of Y. pestis to modulate this response. In this study we examined the early pathogenesis (0 to 72 h) of pulmonary infection with Y. pestis using two different infectious doses with both inbred and outbred strains of mice to determine if genotype-based differences could be observed.
Our studies revealed an early quiescent phase with respect to bacterial growth at the site of infection, followed by strong outgrowth of bacteria after 24 h postinfection. These findings agree with the findings of previous studies in which the workers looked at the kinetics of Y. pestis infection in both mouse and rat models (19, 31). We also observed that the kinetics of infection were essentially the same for both an outbred mouse strain (CD1) and an inbred strain (C57BL/6). The estimated intranasal LD50 in our studies was consistent with that reported by Lathem et al., and the LD50s were determined to be similar for the two strains of mice. These data indicate that there were no major differences in the outcome and course of bacterial spread in pulmonary Y. pestis infection for these two strains of mice.
Members of the genus Yersinia which are able to cause disease in humans have a common 70-kb virulence plasmid. It is well established that the 70-kb virulence plasmid (pCD1) contains genes that code for proteins (Yops and LcrV) that downmodulate the host immune response to infection (6). It has been hypothesized that these virulence factors enable the bacteria to avoid setting off major innate immune warning signals early during infection that would trigger a strong host response. Certainly, it is possible that the virulence factors encoded on the 70-kb plasmid contribute to the phenotypes which we observed in the first 24 h of infection.
One of the initial steps in mounting an effective immune response to gram-negative bacterial pathogens like Y. pestis is the recruitment of polymorphonuclear cells to the area of infection. These cells are part of the innate immune system and are very efficient at phagocytosing bacteria and releasing proinflammatory signals to help orchestrate the immune response. Indeed, with bacterial burdens ranging from 106 to 109 CFU/g tissue in the lungs one would expect a robust proinflammatory response. However, our studies revealed a distinct delay in recruitment of PMNs to the alveolar spaces and lung tissue compared to both our observations of pulmonary infection with other gram-negative pathogens, such as K. pneumoniae, and the observations of other workers (20, 21). Importantly, mice from the same shipment as the mice used in the Y. pestis infections that were infected with a lower number of LD50s of K. pneumoniae had a strong inflammatory response as early as 24 h postinfection. While the initial dose of K. pneumoniae was larger than that of Y. pestis, when the LD50s were compared, the K. pneumoniae group was inoculated with ∼30 times the LD50 (1 × 103 CFU [21]) rather than the nearly 100 times the LD50 (40 to 250 CFU [19]) of Y. pestis that was given to the high-dose Y. pestis group. Despite the higher dose given to the K. pneumoniae mice, we believe that given the difference in the LD50s of the two bacteria, this is a valid comparison. Furthermore, the results of a recent study by Lawlor et al. describing pulmonary K. pneumoniae infection suggested that there was a relative increase in lung bacterial burdens similar to the increase which we observed with Y. pestis (21). Together, these data suggest that the mice were capable of generating an inflammatory response to infection and link the lack of a response to Y. pestis.
The delayed arrival of PMNs to the site of infection in a pneumonic plague model is consistent with recent observations reported for C57BL/6 mice (19) and the observations reported for a bubonic plague model using rats (31). The fact that this delayed arrival was evident in both outbred and inbred mice suggests that it was linked to the pathogen and not the host. A recent study revealed evidence that neutrophils are important in the phagocytosis and control of Y. pestis infection in the spleens of mice infected subcutaneously with wild-type Y. pestis strain GB (23). Together, these data suggest that controlling PMN responses during the initial phases of pulmonary Y. pestis infection is an important aspect of the pathogenesis of disease and may partially explain the extraordinary virulence of Y. pestis pneumonia.
The bronchoalveolar lavage fluid from the animals that received a high dose of Y. pestis was analyzed for the presence of multiple cytokines and chemokines. The overall theme of what we found was that there was a delay in the detection of proinflammatory cytokines and chemokines in the BALF. A small increase in the level of IL-10 was observed at 24 h postinfection, and the level of IL-10 decreased by 48 h postinfection. However, the IL-10 levels were close to the level of detection for the assay, and it remains to be determined if this finding is biologically relevant; nevertheless, IL-10 has potent anti-inflammatory effects, and it is possible that it plays a role in modulating inflammation early in infection. Often proinflammatory cytokines and chemokines were undetectable until 48 h after infection, which is further evidence for a delay in the inflammatory response to infection. The delay in cytokine and chemokine expression is consistent with the delay in PMN recruitment to the lung, which requires both a chemokine gradient and inflammatory signals (cytokines) to activate the endothelium and promote efficient transmigration from the circulation to the tissue.
It appears that the bacteria take advantage of the time when they seem to remain largely undetected by the host immune system, replicating and producing large numbers of cells in host tissues. Although the host does mount a response to the bacteria in the lungs between 24 and 48 h postinfection through increased production of proinflammatory cytokines and chemokines and increased neutrophil recruitment to the site of infection, it appears that this response is effectively too late to prevent a morbid outcome of the infection.
Currently, it is unclear whether the initiation of an inflammatory response is a result of bacterial products or a result of damage to host tissues. One finding addressing this issue is the observation that at 48 h postinfection, when bacterial burdens commonly reached 109 CFU/g of liver tissue, multiple microcolonies were present in the liver without an appreciable inflammatory response. This suggests that the inflammatory response in the lung, observed late in infection, may be a response to host tissue damage rather than a response to bacterial products.
It has been shown previously that administration of proinflammatory cytokines, namely TNF-α and IFN-γ, prior to bacterial challenge can provide protection for the animals in an intravenous mouse model of Y. pestis infection (27). More recently, Montminy and coworkers demonstrated that Y. pestis expressing lipopolysaccharide lipid A at 37°C that more closely resembled the more immunogenic lipopolysaccharide lipid A expressed at 26°C enhanced inflammatory responses to Y. pestis and eliminated the lethality of this modified strain (25). Based on the information from these studies and our study, it is intriguing to speculate that if the bacteria are unable to deter and delay important proinflammatory proteins, such as TNF-α, MIP-2, and KC, early in the infection, the host may be able to effectively control the infection and recover.
Consistent with the delay in PMN recruitment to the lungs and the delay in cytokine and chemokine expression, substantial changes in histopathology are not evident until late in infection. The pronounced pathology reported for terminal infections of humans and primates is not evident in mice until 36 to 48 h postinfection, when the mice are moribund. In this study we focused on the histopathology that develops during the first 48 h of primary pneumonic plague in both outbred and inbred mice using an infectious dose of 0.8 × 104 to 2.9 × 104 CFU. In contrast to previous studies, we did not observe eosinophilia in either mouse strain at any time, nor did we observe pronounced neutrophil infiltration until after 24 h, which is in contrast to what was reported by Smith (32). This difference was probably due to differences in mouse strains (it is unclear what strain Smith used in his study) or to the fact that we used specific-pathogen-free animals maintained in barrier facilities that were uncommon in 1959. However, most of our other observations were similar to what Smith reported and to what was recently reported by Latham et al. Liver and spleen lesions were similar to what was described by Brubaker and coworkers, but less severe. This difference may have been due to differences in strains, to the severity of spleen and liver lesions in pneumonic plague models compared to spleen and liver lesions in systemic plague models, or to differences in the duration of disease in the two models. Together, the histopathological findings for the outbred CD1 mice and the inbred C57BL/6 mice were very similar.
Previous studies of a variety of pathogens illustrated that in many cases, the host genotype had a large effect on the outcome of infection (2, 8, 17, 24). In order to evaluate the influence of host genotype on the outcome of infection, we compared Y. pestis infection in an outbred strain, CD1, and Y. pestis infection in an inbred strain, C57BL/6. Our results indicate that in these two host strains the progression and outcome of disease were very similar, suggesting that, at least for CD1 and C57BL/6 animals, the host genotype has little effect on the outcome of Y. pestis infection. We speculate that a possible reason for this lies in the fact that pneumonic plague is an acute infection, killing the host within a few days, which leaves clearance of the bacteria exclusively to innate immune mechanisms. Many of the phenotypes dependent on the host genotype appear later in infection, when the adaptive response begins. For pneumonic plague, the host does not live long enough to develop an adaptive immune response. Because of this, the general trends in Th1 T-cell responses versus Th2 T-cell responses in different mouse strains would be predicted to have less effect on the outcome of pneumonic plague, which is supported by the results of our study.
Overall, we concluded that there are some subtle differences in pneumonic plague infection between the inbred mouse strain C57BL/6 and the outbred mouse strain CD1. For example, we observed low levels of IFN-γ and IL-12p70 in the BALF of CD1 mice at 48 h after infection, while the levels in C57BL/6 mice were undetectable. However, these subtle differences did not significantly change the outcome or progression of disease in this model, as the LD50, kinetics of infection, and general delays in the proinflammatory response to infection all followed similar courses. More significantly, in both strains of mice, a prolonged delay in detection of proinflammatory cytokines and chemokines, as well as the appearance of inflammatory cells, was evident. We hypothesize that the delayed inflammatory response to infection is beneficial to Y. pestis growth and may be a key virulence strategy of this pathogen.
Acknowledgments
We thank Murry Stein and Rebecca A. Cox for helpful discussions and evaluation of the manuscript.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Anderson, G. W., Jr., S. E. Leary, E. D. Williamson, R. W. Titball, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., M. Beer, E. Bohn, S. H. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohn, E., E. Schmitt, C. Bielfeldt, A. Noll, R. Schulte, and I. B. Autenrieth. 1998. Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect. Immun. 66:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, T. H., L. E. Foster, and K. F. Meyer. 1974. Comparison of the immune response to three different Yersinia pestis vaccines in guinea pigs and langurs. J. Infect. Dis. 129:(Suppl.)S53-S61. [DOI] [PubMed] [Google Scholar]
- 5.Chen, T. H., and K. F. Meyer. 1965. Susceptibility of the langur monkey (Semnopithecus entellus) to experimental plague: pathology and immunity. J. Infect. Dis. 115:456-464. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darville, T., C. W. Andrews, Jr., K. K. Laffoon, W. Shymasani, L. R. Kishen, and R. G. Rank. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, L. Braswell, and R. G. Rank. 2001. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect. Immun. 69:7419-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doll, J. M., P. S. Zeitz, P. Ettestad, A. L. Bucholtz, T. Davis, and K. Gage. 1994. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51:109-114. [DOI] [PubMed] [Google Scholar]
- 10.Dube, P. H., S. A. Handley, J. Lewis, and V. L. Miller. 2004. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect. Immun. 72:3561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elvin, S. J., and E. D. Williamson. 2004. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb. Pathog. 37:177-184. [DOI] [PubMed] [Google Scholar]
- 12.Eyles, J. E., G. J. Sharp, E. D. Williamson, I. D. Spiers, and H. O. Alpar. 1998. Intranasal administration of poly-lactic acid microsphere co-encapsulated Yersinia pestis subunits confers protection from pneumonic plague in the mouse. Vaccine 16:698-707. [DOI] [PubMed] [Google Scholar]
- 13.Finegold, M. J. 1969. Pneumonic plague in monkeys. An electron microscopic study. Am. J. Pathol. 54:167-185. [PMC free article] [PubMed] [Google Scholar]
- 14.Finegold, M. J., J. J. Petery, R. F. Berendt, and H. R. Adams. 1968. Studies on the pathogenesis of plague. Blood coagulation and tissue responses of Macaca mulatta following exposure to aerosols of Pasteurella pestis. Am. J. Pathol. 53:99-114. [PMC free article] [PubMed] [Google Scholar]
- 15.Frevert, C. W., S. Huang, H. Danaee, J. D. Paulauskis, and L. Kobzik. 1995. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J. Immunol. 154:335-344. [PubMed] [Google Scholar]
- 16.Garmory, H. S., K. F. Griffin, K. A. Brown, and R. W. Titball. 2003. Oral immunisation with live aroA attenuated Salmonella enterica serovar Typhimurium expressing the Yersinia pestis V antigen protects mice against plague. Vaccine 21:3051-3057. [DOI] [PubMed] [Google Scholar]
- 17.Handley, S. A., P. H. Dube, P. A. Revell, and V. L. Miller. 2004. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect. Immun. 72:1645-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarrett, C. O., F. Sebbane, J. J. Adamovicz, G. P. Andrews, and B. J. Hinnebusch. 2004. Flea-borne transmission model to evaluate vaccine efficacy against naturally acquired bubonic plague. Infect. Immun. 72:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 102:17786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawlor, M. S., S. A. Handley, and V. L. Miller. 2006. Comparison of the host responses to wild-type and cpsB mutant Klebsiella pneumoniae infections. Infect. Immun. 74:5402-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor, M. S., J. Hsu, P. D. Rick, and V. L. Miller. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 58:1054-1073. [DOI] [PubMed] [Google Scholar]
- 22.Leary, S. E., K. F. Griffin, E. E. Galyov, J. Hewer, E. D. Williamson, A. Holmstrom, A. Forsberg, and R. W. Titball. 1999. Yersinia outer proteins (YOPS) E, K and N are antigenic but non-protective compared to V antigen, in a murine model of bubonic plague. Microb. Pathog. 26:159-169. [DOI] [PubMed] [Google Scholar]
- 23.Lukaszewski, R. A., D. J. Kenny, R. Taylor, D. G. Rees, M. G. Hartley, and P. C. Oyston. 2005. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 73:7142-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magee, D. M., and R. A. Cox. 1996. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect. Immun. 64:3609-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7:1066-1073. [DOI] [PubMed] [Google Scholar]
- 26.Morton, M., H. S. Garmory, S. D. Perkins, A. M. O'Dowd, K. F. Griffin, A. K. Turner, A. M. Bennett, and R. W. Titball. 2004. A Salmonella enterica serovar Typhi vaccine expressing Yersinia pestis F1 antigen on its surface provides protection against plague in mice. Vaccine 22:2524-2532. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 30.Schmal, H., T. P. Shanley, M. L. Jones, H. P. Friedl, and P. A. Ward. 1996. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J. Immunol. 156:1963-1972. [PubMed] [Google Scholar]
- 31.Sebbane, F., D. Gardner, D. Long, B. B. Gowen, and B. J. Hinnebusch. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 166:1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, P. N. 1959. Pneumonic plague in mice: gross and histopathology in untreated and passively immunized animals. J. Infect. Dis. 104:78-84. [DOI] [PubMed] [Google Scholar]
- 33.Smith, P. N. 1959. Pneumonic plague in mice: modification of the infection by antibody against specific components of Pasteurella pestis. J. Infect. Dis. 104:85-91. [DOI] [PubMed] [Google Scholar]
- 34.Smith, P. N. 1959. Pneumonic plague in mice; the effect of polyvinylpyrrolidone. Am. J. Hyg. 69:21-24. [DOI] [PubMed] [Google Scholar]
- 35.Standiford, T. J., S. L. Kunkel, M. J. Greenberger, L. L. Laichalk, and R. M. Strieter. 1996. Expression and regulation of chemokines in bacterial pneumonia. J. Leukoc. Biol. 59:24-28. [DOI] [PubMed] [Google Scholar]
- 36.Wang, S., D. Heilman, F. Liu, T. Giehl, S. Joshi, X. Huang, T. H. Chou, J. Goguen, and S. Lu. 2004. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 22:3348-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson, R. P., T. W. Blanchard, M. G. Mense, and P. W. Gasper. 2001. Histopathology of experimental plague in cats. Vet. Pathol. 38:165-172. [DOI] [PubMed] [Google Scholar]
- 38.Werner, S. B., C. E. Weidmer, B. C. Nelson, G. S. Nygaard, R. M. Goethals, and J. D. Poland. 1984. Primary plague pneumonia contracted from a domestic cat at South Lake Tahoe, Calif. JAMA 251:929-931. [PubMed] [Google Scholar]
- 39.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 2000. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine 19:566-571. [DOI] [PubMed] [Google Scholar]