Abstract
Vaccination with live attenuated Yersinia pestis confers protection against pneumonic plague but is not considered safe for general use. Subunit plague vaccines containing the Y. pestis F1 and LcrV proteins prime robust antibody responses but may not provide sufficient protection. To aid the development of a safe and effective plague vaccine, we are investigating roles for T cells during defense against Y. pestis infection. Here we demonstrate that vaccination of mice with live Y. pestis primes specific CD4 and CD8 T cells that, upon purification and direct transfer to naïve mice, synergistically protect against lethal intranasal Y. pestis challenge. While not preventing extrapulmonary dissemination, the coadministered T cells promote bacterial clearance and reduce bacteremia. These observations strongly suggest that development of pneumonic plague vaccines should strive to prime both CD4 and CD8 T cells. Finally, we demonstrate that vaccination with live Y. pestis primes CD4 and CD8 T cells that respond to Y. pestis strains lacking the capacity to express F1, LcrV, and all pCD1/pPCP-encoded proteins, suggesting that protective T cells likely recognize antigens distinct from those previously defined as targets for humoral immunity.
Plague is caused by Yersinia pestis, a gram-negative facultative intracellular bacterium. Humans naturally acquire Y. pestis infections from fleas, which acquire the bacteria from rodent reservoirs. Pandemics of flea-borne plague have killed hundreds of millions of people during recorded history. While improved sanitation, coupled with the development of antibiotics, has dramatically reduced the incidence of plague today, the documented existence of virulent antibiotic-resistant Y. pestis strains raises concern that plague may be exploited as a biological weapon (13, 16).
Pneumonic plague is the most feared and deadly disease caused by Y. pestis. This rapidly progressing disease results from inhalation of aerosolized Y. pestis and can be spread from person to person. Pneumonic plague is nearly always fatal unless antibiotic treatment is initiated soon after infection (19). While natural outbreaks of pneumonic plague are uncommon, they have been reported (31), and Cold War scientists developed means to purposefully aerosolize Y. pestis (3, 44). Effectively aerosolized, antibiotic-resistant Y. pestis would constitute a formidable biological weapon.
Vaccination against plague would thwart the use of antibiotic-resistant Y. pestis as a biological weapon, but safe and effective pneumonic plague vaccines are not currently available (36). A live attenuated “EV76” vaccine effectively protects nonhuman primates against pneumonic plague (9, 23) and induces high-titer antibody in humans (2), but it also causes chronic infections in nonhuman primates and severe adverse reactions in humans (22, 25, 36-38). As such, the EV76 vaccine is not suitable for wide-scale use. An alternative vaccine (Plague Vaccine, USP) was widely available in the United States until 1999. This killed, whole-cell vaccine protects against flea-borne bubonic plague and produces fewer side effects than the EV76 vaccine but is not effective against pneumonic plague (10, 22, 36).
Recent efforts to create a safe and effective pneumonic plague vaccine have focused on the development of recombinant subunit vaccines that elicit antibodies against two well-characterized Y. pestis antigens: F1, a component of the capsule, and LcrV, a component of the type III secretion system. However, Y. pestis strains deficient in the F1 antigen retain virulence and have been recovered from patients (39). In addition, atypical virulent Y. pestis strains producing variants of LcrV exist (41, 42), and serological studies suggest that vaccines based on a single LcrV protein may fail to protect against LcrV variants (6, 32). Perhaps most disconcerting, the U.S Army reported that the current formulation of their F1-LcrV fusion protein vaccine does not protect all nonhuman primate species against pneumonic plague (M. L. Pitt, Animal Models and Correlates of Protection for Plague Vaccines Workshop, Gaithersburg, MD, 13 to 14 October 2004, http://www.fda.gov/cber/minutes/workshop-min.htm). Thus, there is concern that the F1-LcrV vaccines under development may not protect humans against pneumonic plague, particularly that caused by weaponized Y. pestis strains that lack expression of F1 and produce variant forms of LcrV.
As noted, recent efforts have focused primarily on generating subunit vaccines that elicit protective antibodies. While Y. pestis-specific antibodies certainly confer significant protection in animal models, it is not clear that antibodies alone will suffice in conferring robust protection. For example, we recently demonstrated that gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and nitric oxide synthase 2, host proteins classically associated with cellular immunity, play important roles in antibody-mediated protection against pulmonary Y. pestis infection in mice (29). Given that all pathogenic Yersinia species are facultative intracellular pathogens, cellular immunity may well play important roles in clearing intracellular Yersinia reservoirs. In the cases of Y. enterocolitica and Y. pseudotuberculosis, cellular immunity orchestrated by T cells has long been appreciated to contribute to defense against infection (7, 27). While prior literature also suggested roles for cellular immunity in defense against plague (18, 40), only recently have model systems been described in which roles for T cells can be documented and studied. Specifically, we established that vaccination with live attenuated Y. pestis can protect B-cell-deficient μMT mice against pulmonary Y. pestis infection and that this protection was abrogated upon depletion of T cells, IFN-γ, or TNF-α (28, 29). Moreover, after expansion in vitro by stimulation with Y. pestis-treated antigen-presenting cells (APC), T cells from vaccinated μMT mice could adoptively transfer protection to naïve mice (28). These observations strongly suggest that cellular immunity can contribute to defense against pneumonic plague and, thus, that development of pneumonic plague vaccines should strive to prime both cellular and humoral immune responses.
Here, we report that wild-type mice vaccinated with live Y. pestis generate Y. pestis-specific T cells that confer protection against pulmonary Y. pestis infection upon their direct transfer to naïve mice without any in vitro culture. Moreover, we demonstrate that CD4 and CD8 T cells confer synergistic protection in this model and that they each respond well in vitro to a Y. pestis strain that lacks expression of both the F1 and LcrV antigens. These findings strongly support efforts to incorporate cellular immunity into pneumonic plague vaccines and provide a model system for identifying Y. pestis antigens recognized by protective T cells.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice (6 to 8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME) or were bred at the Trudeau Institute. Animals were cared for according to Trudeau Institute Animal Care and Use Committee guidelines.
Bacteria.
Pigmentation-negative Y. pestis strains KIM5 (pCD1+, pMT+, pPCP+) and KIM6 (pCD1−, pMT+, pPCP+) were obtained from Robert R. Brubaker (Michigan State University) (these strains are denoted D27 and D28, respectively, in his nomenclature). Strain KIM5/caf1−, an F1-negative KIM5 mutant, was provided by Susan C. Straley (University of Kentucky). Strain KIM10+/caf1− (pCD1−, pMT+, pPCP−), a pigmentation-positive, F1-negative mutant, was provided by James B. Bliska (State University of New York at Stony Brook).
Y. pestis strains were grown overnight at 26°C in heart infusion broth (Difco Laboratories, Detroit, MI) supplemented with 2.5 mM CaCl2. For in vitro and in vivo infections, bacteria were collected in logarithmic growth, washed once with phosphate-buffered saline (PBS) (135 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 1.8 mM KH2PO4, pH 7.4), and diluted in room temperature RPMI 1640 medium (Invitrogen Corporation, Grand Island, NY). Bacteria were quantified by measuring the optical density at 620 nm (1 optical density unit = 5.8 × 108 CFU). Heat-killed bacteria were prepared as described above, washed twice with PBS, and then inactivated by heating at 60°C for 1 h.
Infections.
In all instances, mice were infected intranasally by applying bacteria (50 μl) to the nares of lightly anesthetized animals (isofluorane; Webster Veterinary Supply, Inc., Sterling, MA). For primary vaccinations with live Y. pestis, mice were infected intranasally with 5 × 104 CFU KIM5 or 1 × 106 CFU KIM6. On average, 60% of mice receiving KIM5 failed to survive this primary vaccination, whereas all mice survived vaccination with KIM6. For booster vaccinations, mice were intranasally delivered the same dose of the same Y. pestis strain 14 days after the primary vaccination. Routinely, all mice survived the booster vaccinations. For challenge experiments, mice were infected intranasally with 2 × 105 CFU KIM5.
T-cell purifications.
Spleens were harvested from ≥5 mice/vaccination group and pooled. T cells were purified by magnetic sorting using CD4 and CD8 microbeads, as directed by the manufacture (Miltenyi Biotec, Inc., Auburn, CA). In brief, total splenocytes were isolated, red blood cells were lysed (Red Blood Cell Lysing Buffer; Sigma Aldrich, St. Louis, MO), and the remaining cells were washed twice with cold PBS containing 0.5% bovine serum albumin and 2 mM EDTA. Fc receptors were then blocked with 30 μg/ml monoclonal antibody 24G2 (BioExpress, West Lebanon, NH) prior to adding the recommended quantity of CD4 and/or CD8 microbeads. Cells were simultaneously incubated with CD4 and CD8 beads for experiments using “copurified” T cells. After incubation on ice, the bead-labeled cells were loaded onto magnetized LS columns. Where indicated, the flowthrough was collected and used as a control. After washing, columns were removed from magnets and CD4 and/or CD8 T cells collected. Purity was routinely greater than 90%, as assessed by flow cytometry using monoclonal antibodies specific for mouse CD4, CD8, and CD3.
Measurement of T-cell responses in vitro.
For all in vitro assays, total splenocytes from naïve C57BL/6 mice were used as APC. The APC were pretreated with mitomycin C (50 μg/ml; Sigma) for 33 min at 37°C in antibiotic-free complete medium (Dulbecco modified Eagle medium supplemented with 1% l-glutamine, 1% sodium pyruvate, 0.1% β-mercaptoethanol, and 10% fetal bovine serum). After washing, the mitomycin C-treated APC were infected with Y. pestis (multiplicity of infection of 10:1) for 2 h at 37°C in antibiotic-free complete medium. To prevent further growth of Y. pestis organisms, penicillin and streptomycin (Invitrogen) were then added to achieve final concentrations of 100 units/ml and 100 μg/ml, respectively. The infected APC cells were then combined with purified T cells in 96-well flat-bottom plates. Each well contained 1 × 106 APC and 1 × 105 purified T cells in a total volume of 200 μl antibiotic-containing complete medium. After incubation at 37°C in 5% CO2 for 48 h, 100 μl culture supernatant was removed for IFN-γ enzyme-linked immunosorbent assay (BD Biosciences, San Diego, CA). Each T-cell culture was routinely performed in triplicate.
T-cell adoptive transfers.
For adoptive transfer studies, CD4 and/or CD8 T cells were purified from spleens of naïve and vaccinated mice, as described above, and 5 × 106 cells (in 100 μl RPMI) were transferred intravenously to naïve mice. Challenge infections were performed the following day.
Statistics.
Student t tests were used for CFU and enzyme-linked immunosorbent assay data. Log rank tests were used for survival data.
RESULTS
Vaccination with live Y. pestis primes specific T cells.
Vaccination with live, attenuated, pigmentation-negative Y. pestis strain EV76 confers protection against pneumonic plague in murine and primate models but is not widely used in humans due to safety concerns (15, 24, 36). Pigmentation-negative Y. pestis strains are only modestly attenuated when administered aerogenically or intranasally, but vaccination via these routes engenders robust immunity against pneumonic plague (2, 9, 28, 37). To better understand the mechanisms by which vaccination with pigmentation-negative strains confer protection, we investigated immune responses induced by intranasal vaccination with KIM5, a pigmentation-negative derivative of the well-characterized, fully sequenced Y. pestis KIM strain (11).
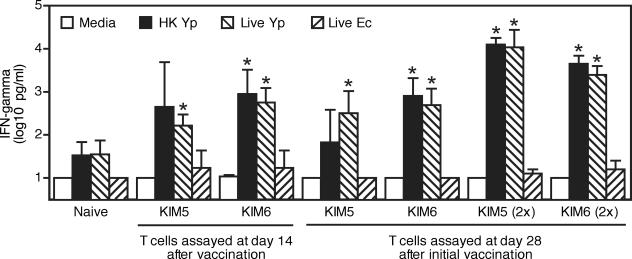
We intranasally vaccinated wild-type C57BL/6 mice with 5 × 104 CFU KIM5. At days 14 and 28 thereafter, we copurified splenic CD4 and CD8 T cells and measured their capacity to produce IFN-γ upon reexposure to KIM5 in vitro. To assess specificity, we also measured responses of unprimed T cells purified from naïve mice. As a further specificity control, we measured responses to Escherichia coli. As shown in Fig. 1, naïve T cells produced barely detectable levels of IFN-γ upon exposure to KIM5 in vitro, and the magnitude of this IFN-γ production was not significantly greater than that elicited by exposure to E. coli. In contrast, T cells purified from mice vaccinated with KIM5 produced significantly increased levels of IFN-γ upon exposure to KIM5 in vitro, compared with exposure to E. coli. We also vaccinated mice intranasally with 1 × 106 CFU KIM6, a highly attenuated derivative of KIM5 that lacks the LcrV-encoding pCD1 plasmid. T cells purified from mice vaccinated with KIM6 responded similarly to those purified from mice vaccinated with KIM5 (Fig. 1), suggesting that pCD1 does not encode the dominant antigens recognized by T cells primed by vaccination with live Y. pestis. Notably, the Y. pestis-primed T cells also responded to KIM5 that had been heat inactivated after growth at 26°C, suggesting that the F1 protein, which is upregulated at 37°C, also is not a dominant T-cell antigen in this model.
FIG. 1.
Vaccination with live Y. pestis generates T cells that produce IFN-γ upon reexposure to Y. pestis. Mice were vaccinated intranasally with live Y. pestis (Yp) KIM5 (5 × 104 CFU) or KIM6 (1 × 106 CFU). Where indicated by “(2×),” mice were boosted by revaccinating intranasally with the same strains 14 days later. At 14 and 28 days after the initial vaccination, splenic CD4 and CD8 T cells were copurified from the vaccinated mice and from naïve control mice. In parallel with the T-cell purifications, mitomycin C-treated splenocyte APC from naïve mice were exposed to antibiotic-free medium alone, medium containing live KIM5, medium containing heat-inactivated (HK) KIM5, or medium containing live E. coli (Ec) for 2 h. The purified T cells and antigen-exposed APC were then combined in the presence of antibiotics, and supernatants were assayed for levels of IFN-γ protein after 48 h of culture. Data depict averages and standard deviations from triplicate cultures (*, P < 0.05 compared with responses to E. coli). Values below the detection limit of our assay were assigned that level (10 pg/ml). Similar results were observed in a second experiment of similar design.
To assess the protective capacities of vaccine-primed T cells, we copurified CD4 and CD8 T cells from mice vaccinated 14 days prior, adoptively transferred 5 × 106 of these cells to naïve mice, and then challenged the mice with a lethal intranasal dose of KIM5 (2 × 105 CFU). The transferred T cells did not confer significant protection (not shown). We conclude that a single vaccination with live Y. pestis KIM5 or KIM6 primes T cells that specifically produce IFN-γ upon exposure to Y. pestis but that these T cells do not suffice in conferring robust protection against lethal intranasal Y. pestis challenge.
Prime-boost vaccination with live Y. pestis generates protective T cells.
Prime-boost vaccination with EV76 generates greater resistance to Y. pestis infection than does a single vaccination (9). To investigate whether prime-boost vaccination with live Y. pestis increases the protective capacity of vaccine-primed T cells, we intranasally vaccinated C57BL/6 mice with KIM5 or KIM6 and then revaccinated the same mice with the same strains 14 days later. At 28 days after the initial vaccination, we copurified splenic CD4 and CD8 T cells and assayed responses in vitro. In comparison with T cells purified from mice that received a single vaccination with KIM5, T cells purified after prime-boost vaccination with KIM5 produced 30- to 150-fold more IFN-γ upon exposure to Y. pestis in vitro (Fig. 1). By comparison, T cells purified after prime-boost vaccination with KIM6 produced only 5- to 20-fold more IFN-γ than T cells purified after a single vaccination with KIM6. Again, exposure to either live or heat-inactivated KIM5 produced responses of similar magnitude.
To assess the capacity of prime-boost vaccination to generate protective T cells, we again copurified CD4 and CD8 T cells and adoptively transferred 5 × 106 of these cells to naïve mice. For comparison, additional groups of mice received either unprimed T cells purified from naïve mice or the residual non-T cells (90% CD19+) derived from each of the T-cell purifications. We then intranasally challenged all groups with a lethal dose of KIM5. As shown in Fig. 2A, prime-boost vaccination with KIM5 generated T cells that provided significant protection against lethal intranasal challenge (P < 0.002). Prime-boost vaccination with KIM6 also generated T cells that conferred some degree of protection, although this did not achieve statistical significance (60% survival) (Fig. 2B). T cells purified from naïve mice failed to provide any measurable protection (Fig. 2C), as did the non-T cells derived from either naïve or vaccinated mice (Fig. 2A to C). We conclude that prime-boost vaccination of wild-type mice with live Y. pestis generates T cells that protect against lethal intranasal challenge.
FIG. 2.
Prime-boost vaccination with live Y. pestis generates protective T cells. CD4 and CD8 T cells were copurified from prime-boost-vaccinated mice, as well as naïve control mice, as described for Fig. 1. The residual non-T cells were also collected from each purification. Both the T-cell and non-T-cell populations (5 × 106) were individually transferred intravenously to naïve mice. On the following day, all recipient mice were challenged intranasally with a lethal dose of KIM5 (2 × 105 CFU) and then monitored for 20 days. (A) T cells isolated from mice that were prime-boost vaccinated with KIM5 provided significant protection compared with non-T cells isolated from the same mice (P < 0.002; n = 5 mice/group; ratio of CD4/CD8 = 1.7). (B) T cells isolated from mice that were prime-boost vaccinated with KIM6 provided protection that did not achieve statistical significance in this experiment (P = 0.1; n = 5 mice/group; ratio of CD4/CD8 = 1.6). (C) T cells isolated from naïve mice did not provide any measurable protection (n = 5 mice/group; ratio of CD4/CD8 = 1.6).
Prime-boost vaccination with live Y. pestis generates T cells that promote bacterial clearance and reduce bacteremia.
We next investigated how T cells primed by vaccination with live Y. pestis confer protection. To assess whether vaccine-primed T cells reduce bacterial growth and/or dissemination, we prime-boost vaccinated mice with KIM5, transferred T cells to naïve animals, challenged these mice with a lethal intranasal dose of KIM5, and measured bacterial CFU on subsequent days. Control animals received T cells from unvaccinated mice. At day 1 after challenge, we observed similar numbers of CFU in the lung tissues of control animals and mice that received KIM5-primed T cells (Fig. 3A). We conclude that primed T cells do not prevent bacteria from colonizing the lung. However, by day 3 after challenge, the number of pulmonary CFU declined significantly in mice that received KIM5-primed T cells but not in the control mice. Moreover, mice that received KIM5-primed T cells displayed nearly 1,000-fold fewer pulmonary CFU than control mice by day 5 after challenge. Thus, prime-boost vaccination with live Y. pestis primes T cells that promote the clearance of bacteria from pulmonary tissue.
FIG. 3.
Prime-boost vaccination with live Y. pestis generates T cells that limit bacterial growth in vivo. CD4 and CD8 T cells (5 × 106) were copurified from prime-boost-vaccinated mice, as well as naïve control mice, and transferred intravenously to naïve mice as described for Fig. 2. On the following day, all recipient mice were challenged intranasally with a lethal dose of KIM5 (2 × 105 CFU). At 1, 3, and 5 days later, cohorts of mice were euthanatized and bacterial CFU in the lung (A), blood (B), spleen (C), and liver (D) were measured. Where indicated (*), significantly reduced bacterial CFU were observed in mice that received primed T cells compared with those that received naïve T cells (P < 0.0001; n = 5 mice/condition/time point except for the naïve T cells on day 5, where bacterial CFU were measured in the three mice that survived to that time). Error bars indicate standard deviations.
To investigate the impact of primed T cells on Y. pestis dissemination and extrapulmonary growth, we also measured bacterial CFU in the blood, spleen, and liver. In the mice that received control T cells from unvaccinated mice, we observed evidence of bacteremia at day 1 after challenge, and the number of CFU recovered from blood samples then steadily increased as time progressed (Fig. 3B). In striking contrast, bacteremia never reached detectable levels in mice that received KIM5-primed T cells. However, the primed T cells did not fully prevent bacterial dissemination, as seeding of bacteria in the spleen and liver was evident by day 1 after challenge and increased by day 3 after challenge (Fig. 3C and D). Nevertheless, by day 5 after challenge, we observed significantly fewer CFU in the spleens and livers of mice that received KIM5-primed T cells than in those of mice that received control T cells. We conclude that vaccine-primed T cells reduce bacteremia and promote clearance of bacteria from the spleen and liver.
CD4 and CD8 cells synergistically protect against Y. pestis infection.
The studies described thus far establish that CD4 and CD8 T cells copurified from mice vaccinated with live Y. pestis confer significant protection against lethal Y. pestis challenge. To examine the relative protective capacities of CD4 and CD8 T cells, we intranasally prime-boost vaccinated mice with KIM5 and individually purified splenic CD4 cells and CD8 cells. We then adoptively transferred 5 × 106 CD4 or CD8 cells to naïve mice. For comparison, additional groups of mice received a mixture of equal parts CD4 and CD8 cells from prime-boost-vaccinated mice or from unvaccinated naïve mice. Subsequently, all groups were challenged intranasally with a lethal dose of KIM5. Consistent with our earlier studies of copurified CD4 and CD8 T cells, transfer of a mixture of CD4 and CD8 T cells from prime-boost-vaccinated mice enabled a significantly greater number of mice to survive the challenge infection, compared with the transfer of CD4 and CD8 T cells from naïve mice (Fig. 4) (P < 0.0001). Interestingly, 23% of mice survived the infection if they received only CD8 T cells from vaccinated mice, while no mice survived if they received only CD4 T cells (Fig. 4). The data presented in Fig. 4 are pooled from three independent experiments, each of which yielded similar results. We conclude that CD4 and CD8 T cells synergize in conferring protection against lethal Y. pestis challenge.
FIG. 4.
Prime-boost vaccination with live Y. pestis generates CD4 and CD8 T cells that synergistically protect against lethal Y. pestis challenge. CD4 and CD8 T cells were individually purified from mice that were prime-boost vaccinated with KIM5. Control CD4 and CD8 T cells were individually purified from naïve control mice. The CD4 and CD8 T cells were transferred intravenously individually to naïve mice (5 × 106 T cells) or were pooled and then transferred together to naïve mice (2.5 × 106 of each type of T cell). On the following day, all recipient animals were challenged intranasally with a lethal dose of KIM5 (2 × 105 CFU) and then monitored for 20 days. Pooled CD4 and CD8 T cells from mice that were prime-boost vaccinated with KIM5 provided significant protection (P < 0.0001; n = 13; ratio of CD4/CD8 = 1.0) compared with pooled CD4 and CD8 T cells from naïve mice (n = 5). CD8 T cells from mice that were prime-boost vaccinated with KIM5 provided modest but significant protection (P < 0.002; n = 13) compared with pooled CD4 and CD8 T cells from naïve mice. CD8 T cells also conferred significantly protection in comparison with CD4 T cells (P < 0.0001; n = 13 each), which did not provide any measurable protection on their own (n = 13). Data depicting KIM5-primed T cells are combined from three independent experiments.
Vaccination with live Y. pestis generates T cells that recognize antigens other than F1, LcrV, and all pCD1/pPCP-encoded proteins.
Given that CD4 and CD8 T cells confer protection synergistically, our findings suggest that the development of subunit vaccines should aim to prime both CD4 and CD8 T cells. Accomplishing that goal requires knowledge of Y. pestis antigens that prime both CD4 T cells and CD8 T cells. The subunit vaccines currently under development contain the Y. pestis pMT-encoded F1 protein and the pCD1-encoded LcrV protein. Vaccination with several other pCD1-encoded antigens also reportedly confers protection in mice (5, 35). In all these cases, it is believed that protection is conferred primarily by antibody-mediated humoral immunity. While the antigens that prime protective humoral responses may also prime protective cellular responses, there is no a priori reason to assume that the same antigens will most effectively prime both antibody-mediated and T-cell-mediated immunity.
To begin investigating which antigens are recognized by T cells primed by vaccination with live Y. pestis, we prime-boost vaccinated mice with KIM5, isolated CD4 and CD8 T cells, incubated these cells with APC infected with various Y. pestis mutant strains, and measured T-cell activation by assaying levels of IFN-γ in culture supernatants. We individually measured responses of CD4 and CD8 T cells to KIM5 (pigmentation negative), KIM5/caf1− (pigmentation negative, F1 negative), KIM6 (pigmentation negative, pCD1 negative), KIM10+/caf1− (pigmentation positive, F1 negative, pCD1 negative, pPCP negative), and E. coli, which served as a negative control. As depicted in Fig. 5, both CD4 and CD8 T cells responded well to each of the tested Y. pestis strains. In several independent experiments, T cells responded equally well to KIM5 and KIM5/caf1−, indicating that F1 is not a dominant T-cell antigen in this vaccine model. By comparison, responses to KIM6 and KIM10+/caf1− were slightly reduced but always much higher than responses to E. coli. As KIM10+/caf1− lacks the capacity to produce F1, LcrV, and all pCD1-encoded antigens, these observations suggest that the dominant antigens recognized by T cells primed by vaccination with live Y. pestis have yet to be discovered. This model system provides an assay for those antigens, which may constitute useful components of subunit plague vaccines.
FIG. 5.
Prime-boost vaccination with live Y. pestis generates T cells that respond well to Y. pestis strains lacking the capacity to produce F1 and LcrV. CD4 and CD8 T cells were individually purified from naïve mice and mice that were prime-boost vaccinated with KIM5. The T cells were then incubated with APC preexposed to live bacteria as described for Fig. 1. The bacterial strains included KIM5 (pigmentation negative), KIM5/caf1− (pigmentation negative, F1-negative), KIM6 (pigmentation negative, pCD1 negative), KIM10+/caf1− (pigmentation positive, F1 negative, pCD1 negative, pPCP negative), and E. coli, which served as a negative control. The figure depicts levels of IFN-γ protein in culture supernatants, presented as the averages and standard deviations from triplicate cultures (*, P < 0.001 compared with E. coli). Values below the level of detection were assigned the level of detection (10 pg/ml).
DISCUSSION
To date, development of Y. pestis subunit vaccines has aimed primarily to elicit humoral immunity. An alternative approach is to incorporate antigens that stimulate both humoral and cellular immunity. This strategy has yet to attract much attention, in part because the importance of cellular immunity during defense against plague remains controversial. Several reports have documented that Y. pestis can survive and multiply within the phagolysosomes of naïve macrophages (20, 30, 34), suggesting that intracellular bacteria contribute to pathogenesis. However, the relevance of intracellular bacteria has been questioned, in part because extracellular Y. pestis organisms dominate the infection in vivo (21, 33). Indeed, when grown at 37°C, Y. pestis produces a capsule and type III secretion system that permit the bacteria to downregulate inflammation, resist phagocytosis, and suppress lymphocyte activation (4, 8, 12, 14, 43). Together, these observations suggest that Y. pestis virulence is mediated, at least in part, by its capacity to powerfully combat cellular immunity. Thus, cellular immunity may not have an opportunity to combat Y. pestis infections.
An alternative viewpoint is that the evolution of Y. pestis to encode virulence factors that disrupt cellular immunity implies that cellular immunity must be detrimental to Y. pestis. Thus, vaccines that preestablish a capacity to rapidly activate Y. pestis-specific cellular immune mechanisms should aid defense against this deadly pathogen. Consistent with this hypothesis, Nakajima and Brubaker demonstrated that pretreatment of naïve mice with IFN-γ and TNF-α, products of cellular immunity, improved the animals' capacity to resist Y. pestis infection (26). In a vaccine model, we recently demonstrated that T cells, IFN-γ, and TNF-α can each play critical roles in protecting antibody-deficient μMT mice against lethal Y. pestis infection (28). Subsequently, we demonstrated that these same cytokines also participate in antibody-mediated defense against Y. pestis infection (29). Together, these observations strongly suggest that plague vaccines should include antigens that prime both humoral and cellular defenses against Y. pestis infection. Humoral immunity will presumably aid the clearance of extracellular bacterial reservoirs while inactivating mechanisms used by Y. pestis to disable cellular immunity, in turn enabling cellular immunity to efficiently eradicate intracellular bacterial reservoirs.
With these concepts in mind, the work undertaken in this study aimed to augment our understanding of roles for T cells during vaccine-primed defense against Y. pestis infection and to investigate the types of antigens recognized by T cells that protect against plague. We employed live Y. pestis as the vaccine because live replicating agents are appreciated to prime robust T-cell responses. We focused on Y. pestis strain KIM5 because this strain has been widely used by plague researchers in the United States and because a large number of well-characterized mutant KIM strains are available for study.
We observed that a single KIM5 vaccination primed T cells that responded specifically to Y. pestis in vitro. However, transferring these T cells to naïve mice did not suffice in conferring protection against lethal Y. pestis infection. In contrast, prime-boost vaccination with KIM5 generated T cells that adoptively transferred significant protection. Importantly, this T-cell-mediated protection did not require any ex vivo expansion of antigen-specific T cells, indicating that the frequency and/or potency of the T cells generated by KIM5 prime-boost vaccination is quite high. In vitro, the T cells from prime-boost-vaccinated mice generated greater levels of IFN-γ than did an equal number of T cells from singly vaccinated mice. We speculate that prime-boost vaccination generates a greater number of plague-specific T cells and/or generates T cells that produce greater quantities of cytokines. Identification of the specific antigens recognized by plague-specific T cells will facilitate the development of sensitive enzyme-linked immunospot and flow cytometry-based major histocompatibility complex multimer assays that, in turn, will permit quantitative dissection of plague-specific T-cell responses.
The live attenuated EV76 Y. pestis vaccine often elicits severe side effects (22, 25). During the course of our studies, we observed that vaccination with KIM5 likewise induced significant morbidity: on average, 60% of mice succumbed to the initial vaccine administration. Those animals that survived were well protected against subsequent booster vaccinations and, as demonstrated here, generated T cells that transferred protection to naïve animals. In an attempt to suppress vaccine-induced morbidity, we first reduced the initial vaccination dose. Unfortunately, doing so suppressed the generation of protective T cells (not shown). In further attempts to reduce vaccine-associated mortality, we administered either antibiotics or antiplague serum the day after KIM5 vaccination. Prior studies found that antibiotic treatment beginning 24 h after infection with virulent Y. pestis significantly reduced the development of immunity to reinfection, whereas treatment with antiplague serum did not (1). However, we found that treatment with either antibiotics or antiplague serum suppressed the generation of protective T cells (not shown). Finally, as demonstrated in Fig. 2, we also investigated whether vaccination with more attenuated Y. pestis strains, such as KIM6, would prime protective T cells without causing morbidity. However, thus far we have successfully generated T cells that transfer significant protection to naïve animals only by vaccinating with near-lethal doses of KIM5. We speculate that the robust generation of protective T cells, unlike the generation of protective antibodies, requires persistence of the antigen in an inflammatory environment. Ongoing studies are aimed at more precisely defining the specific requirements for priming T cells that protect against pneumonic plague.
Another goal of this study was to determine which T-cell subpopulations confer protection. Prior literature indicates that both CD4 and CD8 T cells can protect against intracellular bacteria (17). Our prior studies suggested that both CD4 and CD8 T cells can confer significant protection against Y. pestis infection, as treatment with monoclonal antibodies that deplete both CD4 and CD8 T cells significantly abrogated protection in KIM5-vaccinated μMT mice, whereas depleting only CD4 T cells or only CD8 T cells failed to significantly suppress protection (28). However, given that antibody-based in vivo depletion protocols never remove 100% of the targeted cells, we reasoned that the adoptive transfer model used in the present study would assess roles for CD4 and CD8 T cells more precisely. We observed that the transfer of purified CD8 T cells provided modest but significant protection. In contrast, the transfer of CD4 T cells never provided measurable protection. However, cotransfer studies clearly demonstrated synergistic protection by CD4 and CD8 T cells (Fig. 5). While the mechanism accounting for this synergy remains to be determined, this striking observation strongly suggests that pneumonic plague vaccines should strive to prime both CD4 and CD8 T cells.
Given our demonstration of synergistic protection by CD4 and CD8 T cells, we now believe it imperative to identify the protective antigens recognized by each of these subsets. Our most notable finding thus far is that vaccination with KIM5 primes CD4 and CD8 T cells that respond well to KIM10+/caf1− in vitro. As KIM10+/caf1− lacks the capacity to produce F1, LcrV, and all pCD1/pPCP-encoded proteins, we conclude that protective T cells likely recognize antigens distinct from those previously defined as targets for humoral immunity. We anticipate that further application of the vaccination protocols and T-cell assays described here will help to identify these new protective antigens. We further anticipate that modified versions of the in vitro assays will find utility as surrogate assays for plague vaccine efficacy in humans.
Acknowledgments
This work was supported by Public Health Service grant AI061577 (to S.T.S.) and by funds from the Trudeau Institute.
We thank Robert Brubaker, James Bliska, and Susan Straley for generously providing bacterial strains and the employees of the Trudeau Institute animal facilities for dedicated care of the mice used in these studies.
Editor: D. L. Burns
Footnotes
Published ahead of print on 21 November 2006.
REFERENCES
- 1.Abramova, G. F., A. L. Kartashova, and E. L. Semenova. 1956. Degree of immunity in convalescence following experimental therapy with streptomycin and sera. Zh. Mikrobiol. Epidemiol. Immunobiol. 27:54-57. [PubMed] [Google Scholar]
- 2.Aleksandrov, N. I., N. E. Gefen, K. G. Gapochko, N. S. Garin, S. S. Daniliuk, L. L. Egorova, R. F. Kuzina, G. G. Koridze, A. P. Labinskii, V. A. Lebedinskii, A. I. Maslov, N. P. Osipov, V. A. Silich, M. S. Smirnov, and N. I. Tsyganova. 1963. Study of a method of aerosol immunization with powdered plague vaccine in extensive population groups. Zh. Mikrobiol. Epidemiol. Immunobiol 40:22-28. [PubMed] [Google Scholar]
- 3.Alibek, K. 1999. Biohazard. Random House, Inc., New York, NY.
- 4.Alonso, A., N. Bottini, S. Bruckner, S. Rahmouni, S. Williams, S. P. Schoenberger, and T. Mustelin. 2004. Lck dephosphorylation at Tyr-394 and inhibition of T cell antigen receptor signaling by Yersinia phosphatase YopH. J. Biol. Chem. 279:4922-4928. [DOI] [PubMed] [Google Scholar]
- 5.Andrews, G. P., S. T. Strachan, G. E. Benner, A. K. Sample, G. W. Anderson, Jr., J. J. Adamovicz, S. L. Welkos, J. K. Pullen, and A. M. Friedlander. 1999. Protective efficacy of recombinant Yersinia outer proteins against bubonic plague caused by encapsulated and nonencapsulated Yersinia pestis. Infect. Immun. 67:1533-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anisimov, A. P., L. E. Lindler, and G. B. Pier. 2004. Intraspecific diversity of Yersinia pestis. Clin. Microbiol. Rev. 17:434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byvalov, A. A., V. N. Pautov, V. Chicherin Iu, V. A. Lebedinskii, and V. I. Evtigneev. 1984. Effectiveness of revaccinating hamadryas baboons with NISS live dried plague vaccine and fraction I of the plague microbe. Zh. Mikrobiol. Epidemiol. Immunobiol. 4:74-76. [PubMed] [Google Scholar]
- 10.Cohen, R. J., and J. L. Stockard. 1967. Pneumonic plague in an untreated plague-vaccinated individual. JAMA 202:365-366. [PubMed] [Google Scholar]
- 11.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galimand, M., A. Guiyoule, G. Gerbaud, B. Rasoamanana, S. Chanteau, E. Carniel, and P. Courvalin. 1997. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 337:677-680. [DOI] [PubMed] [Google Scholar]
- 14.Gerke, C., S. Falkow, and Y. H. Chien. 2005. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T cell activation. J. Exp. Med. 201:361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard, G. 1963. Immunity in plague. Acquisitions supplied by 30 years of work on the “Pasteurella pestis Ev” (Girard and Robic) strain. Biol. Med. (Paris) 52:631-731. [PubMed] [Google Scholar]
- 16.Guiyoule, A., G. Gerbaud, C. Buchrieser, M. Galimand, L. Rahalison, S. Chanteau, P. Courvalin, and E. Carniel. 2001. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 7:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harty, J. T., L. L. Lenz, and M. J. Bevan. 1996. Primary and secondary immune responses to Listeria monocytogenes. Curr. Opin. Immunol. 8:526-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtrel, B., J. M. Alonso, P. H. Lagrange, and M. Hurtrel. 1981. Delayed-type hypersensitivity and acquired resistance to plague in mice immunized with killed Yersinia pestis and immunoregulators. Immunology 44:297-304. [PMC free article] [PubMed] [Google Scholar]
- 19.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, K. Tonat, et al. 2000. Plague as a biological weapon: medical and public health management. JAMA 283:2281-2290. [DOI] [PubMed] [Google Scholar]
- 20.Janssen, W. A., and M. J. Surgalla. 1969. Plague bacillus: survival within host phagocytes. Science 163:950-952. [DOI] [PubMed] [Google Scholar]
- 21.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 102:17786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer, K. F. 1970. Effectiveness of live or killed plague vaccines in man. Bull. W. H. O. 42:653-666. [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer, K. F. 1961. Pneumonic plague. Bacteriol. Rev. 25:249-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer, K. F., D. C. Cavanaugh, P. J. Bartelloni, and J. D. Marshall, Jr. 1974. Plague immunization. I. Past and present trends. J. Infect. Dis. 129(Suppl.):S13-S18. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, K. F., G. Smith, L. Foster, M. Brookman, and M. Sung. 1974. Live, attenuated Yersinia pestis vaccine: virulent in nonhuman primates, harmless to guinea pigs. J. Infect. Dis. 129(Suppl.):S85-S112. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noll, A., A. Roggenkamp, J. Heesemann, and I. B. Autenrieth. 1994. Protective role for heat shock protein-reactive alpha beta T cells in murine yersiniosis. Infect. Immun. 62:2784-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parent, M. A., K. N. Berggren, L. W. Kummer, L. B. Wilhelm, F. M. Szaba, I. K. Mullarky, and S. T. Smiley. 2005. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect. Immun. 73:7304-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parent, M. A., L. B. Wilhelm, L. W. Kummer, F. M. Szaba, I. K. Mullarky, and S. T. Smiley. 2006. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun. 74:3381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujol, C., J. P. Grabenstein, R. D. Perry, and J. B. Bliska. 2005. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc. Natl. Acad. Sci. USA 102:12909-12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratsitorahina, M., S. Chanteau, L. Rahalison, L. Ratsifasoamanana, and P. Boisier. 2000. Epidemiological and diagnostic aspects of the outbreak of pneumonic plague in Madagascar. Lancet 355:111-113. [DOI] [PubMed] [Google Scholar]
- 32.Roggenkamp, A., A. M. Geiger, L. Leitritz, A. Kessler, and J. Heesemann. 1997. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect. Immun. 65:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebbane, F., D. Gardner, D. Long, B. B. Gowen, and B. J. Hinnebusch. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 166:1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straley, S. C., and P. A. Harmon. 1984. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect. Immun. 45:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swietnicki, W., B. S. Powell, and J. Goodin. 2005. Yersinia pestis Yop secretion protein F: purification, characterization, and protective efficacy against bubonic plague. Protein Expr. Purif. 42:166-172. [DOI] [PubMed] [Google Scholar]
- 36.Titball, R. W., and E. D. Williamson. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4:965-973. [DOI] [PubMed] [Google Scholar]
- 37.Welkos, S., M. L. Pitt, M. Martinez, A. Friedlander, P. Vogel, and R. Tammariello. 2002. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in non-human primate and mouse models of pneumonic plague. Vaccine 20:2206-2214. [DOI] [PubMed] [Google Scholar]
- 38.Williamson, E. D. 2001. Plague vaccine research and development. J. Appl. Microbiol. 91:606-608. [DOI] [PubMed] [Google Scholar]
- 39.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull. W. H. O. 23:408-409. [PMC free article] [PubMed] [Google Scholar]
- 40.Wong, J. F., and S. S. Elberg. 1977. Cellular immune response to Yersinia pestis modulated by product(s) from thymus-derived lymphocytes. J. Infect. Dis. 135:67-78. [DOI] [PubMed] [Google Scholar]
- 41.Worsham, P., and M. Hunter. 1998. Characterization of pestoides F, an atypical strain of Y. pestis. Medische Microbiologie 6:S34-S35. [Google Scholar]
- 42.Worsham, P. L., and C. Roy. 2003. Pestoides F, a Yersinia pestis strain lacking plasminogen activator, is virulent by the aerosol route. Adv. Exp. Med. Biol. 529:129-131. [DOI] [PubMed] [Google Scholar]
- 43.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zilinskas, R. A. 2006. The anti-plague system and the Soviet biological warfare program. Crit. Rev. Microbiol. 32:47-64. [DOI] [PubMed] [Google Scholar]