Abstract
The facultative intracellular bacterial pathogen Listeria monocytogenes induces severe fetal infection during pregnancy. Little is known about the molecular mechanisms allowing the maternofetal transmission of bacteria. In this work, we studied fetoplacental invasion by infecting mice with various mutants lacking virulence factors involved in the intracellular life cycle of L. monocytogenes. We found that the placenta was highly susceptible to bacteria, including avirulent bacteria, such as an L. monocytogenes mutant with an hly deletion (ΔLLO) and a nonpathogenic species, Listeria innocua, suggesting that permissive trophoblastic cells, trapping bacteria, provide a protective niche for bacterial survival. The ΔLLO mutant, which is unable to escape the phagosomal compartment of infected cells, failed to grow in the trophoblast tissue and to invade the fetus. Mutant bacteria with inlA and inlB deletion (ΔInlAB) grew in the placenta and fetus as well as did the wild-type virulent stain (EGDwt), indicating that in the murine model, internalins A and B are not involved in fetoplacental invasion by L. monocytogenes. Pregnant mice were then infected with an actA deletion (ΔActA) strain, a virulence-attenuated mutant that is unable to polymerize actin and to spread from cell to cell. With the ΔActA mutant, fetal infection occurs, but with a significant delay and restriction, and it requires a placental bacterial load 2 log units higher than that for the wild-type virulent strain. Definitive evidence for the role of ActA was provided by showing that a actA-complemented ΔActA mutant was restored in its capacity to invade fetuses. ActA-mediated cell-to-cell spreading plays a major role in the vertical transmission of L. monocytogenes to the fetus in the murine model.
Listeria monocytogenes is a facultative intracellular fast-growing gram-positive bacterium widely spread in the environment. It is a food-borne pathogen responsible for severe and life-threatening infections in both humans and a large variety of animal species (13). Immunocompromised patients, including the elderly and pregnant women, represent high-risk groups for listeriosis (9). During pregnancy, listeriosis can be asymptomatic or can give rise to subclinical symptoms like a nonspecific fever despite the insidious development of fetoplacental infection resulting in abortion, stillbirth, or severe and disseminated neonatal infections markedly described as granulomatosis infantiseptica (9, 24). Little is known about molecular mechanisms implicated in the placental infection by L. monocytogenes and the subsequent vertical transmission to the fetus.
Most virulence factors involved in the intracellular growth and survival of L. monocytogenes have been identified and extensively studied (8, 10, 30). Adhesion and invasion of nonprofessional phagocytes are mainly dependent upon the expression of internalin A (InlA), which interacts with E-cadherin expressed on eukaryotic cells. After phagocytosis, bacteria produce listeriolysin O (LLO), a pore-forming cytolysin (1), allowing bacterial escape from the phagosomal compartment. Once in the cytoplasm, bacteria divide, move, and spread from cell to cell. This is due to ActA, a bacterial surface-exposed protein, which induces actin cytoskeleton rearrangements and polymerization. Thus, bacteria protrude into and infect neighboring cells favoring the persistence of the intracellular life cycle of L. monocytogenes (28).
The placenta is a dynamic organ constituted of intricate maternal and fetal tissues, whose structure and function change throughout the pregnancy. The physiological barrier separating fetal and maternal blood in the placenta is mainly formed by fetally derived trophoblastic cells. Very few pathogens are capable of crossing the placental biological barrier. This includes some viruses (17); parasites such as Toxoplasma gondii (26) and Plasmodium falciparum (27); and very rare bacteria, including Chlamydia psittaci, Coxiella burnetti, and L. monocytogenes (6, 23, 25).
There is compelling evidence that the trophoblast plays a central role in vertical transmission of pathogens from mothers to the fetus (21). First of all, the trophoblast acts as a pregnancy-specific component of the innate immune system (14). During pregnancy, the trophoblast is responsive to CSF-1 which acts to organize the maternal immune response to bacterial infection at the uteroplacental interface through recruitment of polymorphonuclear neutrophils. These inflammatory cells are the main effector cells mobilized in the placenta to destroy L. monocytogenes, as opposed to macrophages that are mostly excluded from the murine placenta (14, 21).
Little is known about the pathophysiological process of placental invasion during listeriosis. Using a murine model, we recently demonstrated that placental invasion by L. monocytogenes is associated with bacterial growth within trophoblastic cells, thus allowing outward spreading from the initial foci to the adjacent structures (21). In human fetoplacental listeriosis, the involvement of InlA through its interaction with E-cadherin has been recently reported, using an in vitro model (19). Mouse E-cadherin compared with human E-cadherin has a single-amino-acid mutation which results in a decrease of its affinity for InlA (18, 20). However, although the InlA-E-cadherin interaction occurs with the same affinity in guinea pigs as in humans, it has been very recently published that an InlA mutant behaved as wild-type virulent strain in a pregnant guinea pig model (4). These authors provided evidence for a role of ActA in the vertical transmission of L. monocytogenes in this model (4).
In this work, using a murine model of pregnant mice (21). We studied the role of virulence factors (InlA, InlB, LLO, and ActA) involved in the intracellular life cycle of L. monocytogenes. To decipher the role of virulence factors for the crossing of the fetoplacental barrier, we systematically monitored for each mutant of L. monocytogenes the correlation between the infection of the placenta and its corresponding fetus. We show that ActA-dependent cell-to-cell spreading promotes fetal invasion. Final evidence for the crucial role of ActA was obtained by restoring fetal invasion in an actA-complemented ActA mutant.
MATERIALS AND METHODS
Bacterial strains and cultures.
We used the wild-type virulent strain of L. monocytogenes EGDe (EGDwt) (12) various isogenic mutants (Table 1) and L. innocua. Bacteria were grown overnight in brain heart infusion broth (Difco Laboratories, Detroit, MI) at 37°C without antibiotics, reexpanded the next day, and collected at the end of the exponential phase and then were centrifuged at 5,000 × g for 30 min at 4°C, washed twice with lipopolysaccharide-free Hanks' balanced salt solution (Gibco, Long Island, NY), and resuspended in RPMI 1640 medium (Difco) before being stored at −80°C in 1-ml aliquots. Bacteria were titrated by serial dilution and plated on brain heart infusion agar. Before each experiment, an aliquot was thawed and diluted as convenient for the experiment.
TABLE 1.
Bacterial strains used in this work
| Bacterial strain | Characteristic(s) | Source or reference |
|---|---|---|
| Listeria monocytogenes | ||
| Wild type (EGDwt) | Virulent EGDe strain | 12 |
| EGD mutants | ||
| ΔInlAB | InlAB deleted | P. Cossart (Institut Pasteur, Paris) |
| ΔLLO | 1,080-bp deletion in hly gene | 15 |
| ΔActA | 1,752-bp deletion in actA gene | 7 |
| ΔActA+actA | Insertion of actA gene in ΔActA mutant | This work |
| Listeria innocua | ||
| L. innocua CIP 11254 | Wild type | Institut Pasteur Collection |
DNA techniques.
Obtaining of chromosomal DNA, plasmid isolation, restriction enzyme analyses, and PCR amplifications were performed as previously described by Autret et al. (3). Oligonucleotides were synthesized by Eurogentec (Paris, France). We used the AmpliTaq Gold DNA polymerase of Thermus aquaticus from Roche (Branchburg, NJ) and the pAT113/pAT145 system (29) as previously described (3).
Cloning of actA and complementation of the ΔActA mutant strain.
The entire actA sequence including its promoter was amplified by PCR from EGDwt chromosomal DNA using primers actA-prom (5′-TGAAGCTTGGGAAGCAGTTGGGGT-3′), which contains a HindIII site (underlined), and actA-term (5′-TTGAATTCTGAATTTCATATCATTCACCTCACT-3′), which contains an EcoRI site (underlined). The fragment was subcloned into the pCR II plasmid (Invitrogen, Carlsbad, CA), and transferred to Escherichia coli TOP10 (Invitrogen, Cergy Pontoise, France). Recombinant bacteria were selected onto ampicillin-containing agar, and clones were checked by PCR. The plasmid of one clone was prepared and submitted to restriction by HindIII and EcoRI (New England Biolabs). The HindIII/EcoRI actA fragment was cloned into the HindIII/EcoRI-digested pAT113 plasmid, leading to the pAT113-actA plasmid.
Chromosomal integration of the pAT113 Tn1545 transposon requires the presence of its integrase, provided in trans by pAT145 plasmid. Competent EGD-ΔActA bacteria were thus prepared as described previously (3), and 2 μg of QIAGEN-purified plasmid pAT145 was used for electroporation. The resulting Kanr transformants were named ΔActa-145. Then L. monocytogenes ΔActA-145 cells were transformed by electroporation with 2 μg of purified pAT113-actA plasmid. Plasmid integration into chromosomal DNA was checked by PCR. The DNA sequences flanking the transposon carrying the wild-type actA allele were determined using ligation-mediated PCR, as described previously (3), and the site of transposon insertion into the genome was identified by sequence analysis. The transposon was inserted at position 75350 in open reading frame lmo0068, encoding a putative 107-amino-acid protein of unknown function. Protein secretion was checked by Western blot analysis.
Infection of mice.
Inbred BALB/c pregnant mice purchased from Elevage Janvier (Le Genest-St-Isle, France) were used for bacterial growth studies and histological staining. Couplings were carried out with 8- to 10-week-old BALB/c female mice. Mating was assessed by the appearance of a vaginal plug, denoting the first embryonic day of pregnancy. The gestation was checked at the 12th day and nonpregnant females were used as control mice. Mice were housed in wire-bottom cages, with free access to food and water, and held under these conditions for at least 24 h before infection. Animal experiments were approved by the Animal Welfare Committee of the University Paris-Descartes.
BALB/c female mice were inoculated intravenously (i.v.) at the 14th day of gestation via the lateral tail vein with 0.5 ml of a calibrated suspension of bacteria, extemporarily obtained by appropriate dilution into saline isotonic solution from a frozen stock. All mice were daily examined. At intervals after infection (1, 6, 24, 48, and 72 h), groups of mice were anesthetized by intramuscular injection of 200 μl of a mixture of ketamine at 200 mg kg−1 (Imalgène 100; Merial, Lyon-France) and xylazine hydrochloride at 10 mg kg−1 (Rampun, Bayer, Puteaux, France) and were sacrificed. The abdominal cavity was then aseptically opened, and each mouse was bled by intracardiac puncture with heparinized syringe (Heparin sodic; Sanofi-Wintrop, France). Organs (livers, spleens, and brains) and each fetoplacental unit were aseptically removed and homogenized for bacterial counts and histological studies. Each placenta and its respective fetus were independently dissected and analyzed. Bacterial counts were determined by plating serial 10-fold dilutions of each organ homogenate on BHI agar plates incubated at 37°C during 24 to 48 h. For each mouse, 100 μl of each placenta or fetus homogenate was separately pooled to determine the mean bacterial load. The results were expressed as a mean ± standard error expressed as log10 CFU per organ (bacteria/organ). The 50% lethal doses (LD50) estimated by the probit method on groups of five mice were assessed in this work at 104.3 and 104.6 bacteria per mouse for EGDwt and actA-complemented ΔActA strains, respectively.
Histology.
Histological studies were performed on placentas removed from mice at day 17 of gestation, 72 h after i.v. infection with 2 × 105 bacteria. Fetoplacental units were removed from the uterus corns by dissociation between implantation sites. One half of each placenta was used to quantify bacterial load, and the second half was used for histological analysis. For light microscopy studies (Nikon Eclipse E600; digital camera DXM1200), placentas were fixed overnight in 10% formalin, dehydrated with an alcohol gradient, and embedded in paraffin blocks. Sequential 5- to 7-μm placental sections were stained by Gram-Weigert techniques.
Statistical analysis.
All values are given as the mean ± standard error of the mean. We used several statistical methods to determine the relationship between placental infection and the corresponding fetal infection. To compare the mean values at the indicated times between mutants, we used multifactorial analysis of variance in which the two analyzed factors were “time” (6 h and 1, 2, and 3 days postinfection) and “mutants” (EGD, ΔInlAB, ΔActA, and ΔActA+actA). The differences were considered significant for P ≤ 0.05. Placentas and fetuses were considered infected when bacterial counts were superior or equal to 1 bacterium/placenta or 50 bacteria/fetus. Receiver-operator characteristics analysis was used to identify the cutoff values for placental bacterial load associated with the maximal probability of fetal infection, according to a method previously described (21).
RESULTS
Crossing of fetoplacental barrier requires the expression of virulence factors.
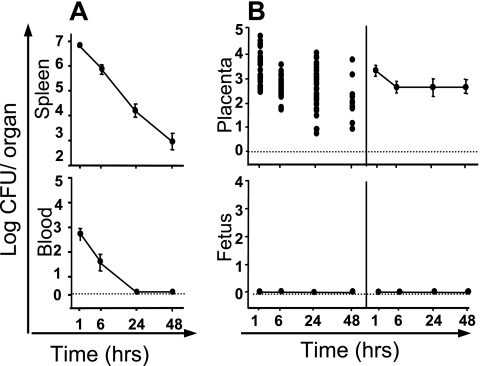
We recently reported that L. monocytogenes inoculated i.v. into pregnant mice can invade the placenta and cross the placental barrier to subsequently proliferate in the fetus (21). With wild-type virulent bacteria, fetal infection occurred when the placenta was infected with doses as low as 1 × 103 bacteria. To study the role of virulence factors in the vertical transmission of L. monocytogenes from mother to fetus, we first i.v. infected pregnant mice (14 days) with a high dose (5 × 107 bacteria) of L. innocua, a nonvirulent, nonhemolytic species that does not display the prfA-dependent virulent genes. Bacterial growth was monitored in the blood, spleen, placenta, and fetuses from 1 h to 48 h after infection (Fig. 1). As expected, bacteria were rapidly eliminated from the blood and the organs (data in the liver are not shown). In contrast, we found that all placentas were infected by L. innocua as early as 1 h after inoculation. Bacteria survived in placental tissues for at least 2 days, at a low titer (∼1 × 103 bacteria), without any infection of the respective fetus. These data suggest that although the placentas become infected with L. innocua, the crossing of the fetoplacental barrier requires bacterial growth and the expression of virulence factors.
FIG. 1.
Infection of pregnant mice with L. innocua. Pregnant BALB/c mice were injected i.v. with 5 × 107 bacteria by day 14 of gestation. Animals were monitored by quantifying bacterial growth at intervals. (A) Bacterial growth in spleen and blood of pregnant mice. The results shown are means ± standard errors from groups of five mice (each experiment was repeated twice) and are expressed as the log10 bacteria (CFU) per organ or log10 bacteria per ml of blood. (B) The kinetics of bacterial growth are represented in the left panels by dot plots corresponding to individual bacterial counts for each placenta (top panel) and each fetus (lower panel) for the indicated times. In the right panels, means ± standard errors for each time are given.
LLO is required for bacterial growth in the placenta and fetal invasion.
We recently showed in pregnant mice that the virulent strain of L. monocytogenes (EGDwt) first targets the trophoblastic cells before crossing the fetoplacental barrier (21). Thus, fetal infection requires bacterial growth which is clearly associated with the intracellular life cycle of L. monocytogenes within trophoblast cells acting as phagocytes. To decipher the role of virulence factors in the placental invasion and the subsequent fetal infection, we studied L. monocytogenes mutants inactivated for the expression of various virulent factors.
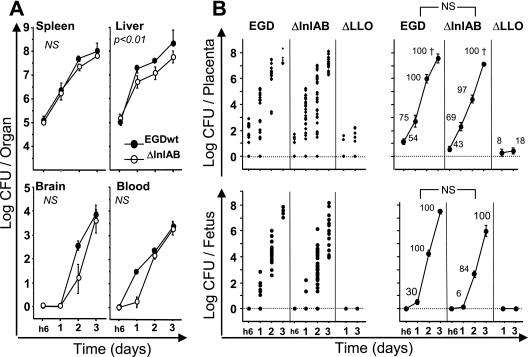
We first tested the involvement of internalins A and B in placental infection. Pregnant mice were infected i.v. with 5 × 105 cells of the ΔInlAB mutant. The kinetics of bacterial growth was then observed for 3 days in the blood and organs (liver, spleen, and brain) and in all fetoplacental units. As compared to the EGDwt strain used as control, bacterial growth of the ΔInlAB mutant was similar in blood and organs except for the liver, in which the growth of the ΔInlAB mutant was moderately lower (Fig. 2A). Surprisingly, this held true for bacterial growth of ΔInlAB bacteria in placenta and fetus (Fig. 2B). As wild-type bacteria, the ΔInlAB mutant rapidly multiplied in these tissues, with a daily increase of about 2 log units. By day 3, all placentas were infected and the comparison between the EGDwt and ΔInlAB mutant strains was not statistically different. We then determined statistically which bacterial load in the placenta was associated with a higher probability of fetal infection. Thus, the cutoff values were assessed at 1.7 × 103 and 1.0 × 103 CFU/placenta for the EGDwt and ΔInlAB strains, respectively. The comparison of the means for fetal infection between wild-type EGDwt and ΔInlAB mutant was not statistically different. These results suggest that in the murine model, InlA and InlB are not required for the invasion of placenta and the subsequent fetal infection.
FIG. 2.
Infection of pregnant mice with ΔInlAB and ΔLLO mutants. Pregnant BALB/c mice were injected i.v. at day 14 of gestation with 5 × 105 of wild-type L. monocytogenes (EGD) and its isogenic hly or inlAB deletion mutants. Animals were monitored by quantifying bacterial growth at intervals. (A) Bacterial growth in organs (spleen, liver and brain) and in blood of pregnant mice for EGDwt and ΔInlAB strains. Results shown are means ± standard errors of the means from groups of three to five mice and are expressed as the log10 bacteria (CFU) per organ or log10 bacteria per ml of blood. (B) The kinetics of bacterial growth are represented on the left panels by dot plots corresponding to individual bacterial counts for each placenta (top panel) and each fetus (lower panel) for the indicated times and strains. In the right panels, means ± standard errors for each time are given for wild-type, ΔInlAB, and ΔLLO strains. The numbers reported near each value correspond to the percentage of infected placenta among all analyzed placentas. When observed, fetal losses are symbolized by a cross. P values reported in each graph correspond to the comparison between EGDwt and the indicated mutant. NS, not statistically different.
We then tested the role of LLO, the major virulence factor of L. monocytogenes needed to escape phagosomes. Pregnant mice were i.v. infected with 5 × 105 bacteria of a ΔLLO mutant. As previously described, bacteria were rapidly eliminated from the blood and organs (spleen and liver), without brain infection (data not shown) (11). By day 1 and day 3 postinfection, about 10% of placentas (3/30 at day 1 and 4/36 at day 3) were infected at low levels (10 to 100 bacteria/placenta). Under these conditions, fetuses were never infected (Fig. 2). Thus, nonvirulent ΔLLO bacteria can infect some placentas for at least 3 days, revealing that once infected, the placenta cannot easily eliminate bacteria, in contrast to the spleen and the liver. As for L. innocua, ΔLLO bacteria were unable to grow in the placenta and to subsequently invade fetuses, because they are probably retained within the phagosomes of trophoblastic cells.
ActA promotes the crossing of the fetoplacental barrier.
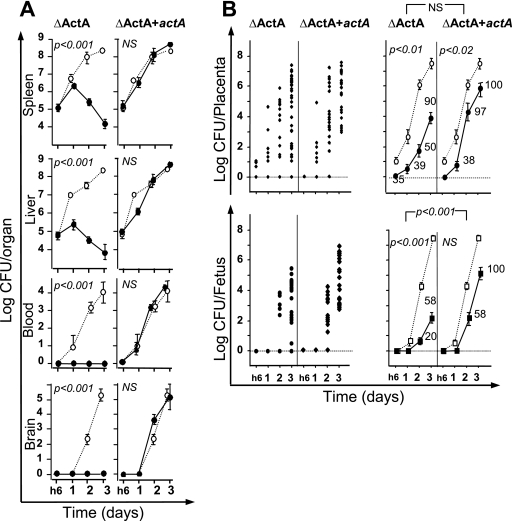
The murine fetoplacental barrier consists of two layers of trophoblastic cells and one of endothelial cells. We then studied the role of ActA, a virulence factor promoting cell-to-cell spreading. We first analyzed the infectious process in pregnant mice i.v. inoculated with 5 × 105 bacteria of a ΔActA mutant. After infection, bacterial growth was monitored in the blood and organs (spleen, liver, and brain) for 3 days. No difference was observed for the bacterial growth in organs between pregnant and nonpregnant mice (data not shown). The ΔActA bacteria were completely eliminated from the blood within 6 h postinfection, which was correlated with the absence of brain infection, as previously described. After initial growth in the liver and the spleen by day 1 of infection, mutant bacteria then declined rapidly, indicating that the infectious process was well controlled in these organs. With this inoculum (5 × 105 bacteria), about 35% of placentas were infected by ΔActA bacteria as early as 6 h postinfection, compared to 54% of placentas being infected with EGDwt. The percentage of placentas infected by EGDwt reached 100% by day 2, whereas mutant bacteria infected only 50% and 90% of placentas by days 2 and 3 postinfection, respectively. Although the means for placental infection between EGDwt and the ΔActA mutant were statistically different, the rate of mutant growth in the placenta was similar to that of EGDwt, which might reflect rapid multiplication inside permissive trophobastic cells (see below). As illustrated in Fig. 3, fetal infection was significantly delayed for 2 to 3 days in mice infected by ΔActA bacteria, as compared to EGDwt bacteria. The rate of mutant growth was lower in fetuses during the 3 first days postinfection (Fig. 3B). This was also quantified by calculating the cutoff values corresponding to the placental bacterial load associated with the higher probability to induce fetal infection. The cutoff value was estimated at 1.2 × 105 ΔActA bacteria per placenta, compared to 1.7 × 103 for EGDwt bacteria (Table 2).
FIG. 3.
Role of ActA in the crossing of the fetoplacental barrier. Pregnant BALB/c mice were injected intravenously at day 14 of gestation with 5 × 105 L. monocytogenes actA deletion mutant cells (ΔActA) or the ΔActA mutant complemented with actA (ΔActA+actA). As compared to the isogenic mutants, bacterial growth for the EGDwt strain is indicated by the dotted lines. Animals were monitored by quantifying bacterial growth at the indicated times. (A) Bacterial growth in organs (spleen, liver, and brain) and in blood of pregnant mice infected with ΔActA (left panels) and actA-complemented ΔActA+actA (right panels) strains. Results shown are means ± standard errors from groups of three to five mice and are expressed as the log10 bacteria (CFU) per organ or log10 bacteria per ml of blood. (B) The kinetics of bacterial growth are represented on the left panels by dot plots corresponding to individual bacterial counts for each placenta (top panel) and each fetus (lower panel) for the indicated times and strains. In the right panels, the means ± standard errors for each time and strain are given for ΔActA and the actA-complemented ΔAct mutant and P values for the comparison between the two strains are also reported. The numbers reported near each value correspond to the percentage of infected placenta among all analyzed placentas. All data are representative of three independent experiments. P values reported in each graph correspond to the comparison between EGDwt and the indicated mutant. NS, not statistically different.
TABLE 2.
Cutoff values for bacterial placental load associated with the highest probability of fetal infection
| Bacterial strain | No. of fetoplacental units analyzed | Cutoff value (103 CFU)a | Sensitivity (%) | Specificity (%) | PPV (%)b | NPV (%)c |
|---|---|---|---|---|---|---|
| EGDwt | 185 | 1.7 | 91 | 99 | 99 | 91 |
| ΔInlAB | 48 | 1.0 | 60 | 92 | 85 | 73 |
| ΔActA | 72 | 120.0 | 93 | 82 | 82 | 93 |
| ΔActA+ actA | 36 | 2.0 | 89 | 100 | 100 | 87 |
Cutoff values correspond to level of bacterial load of the placenta giving the highest probability of fetal infection.
PPV, positive predictive value.
NPV, negative predictive value.
Final evidence for the role of the virulence factor ActA in the crossing of fetoplacental barrier was obtained by inserting a new actA gene in the chromosome of the ΔActA mutant strain (see Materials and Methods). After actA insertion, the expression of this surface-exposed protein was restored. As illustrated in Fig. 3, the virulence of the actA-complemented ΔActA strain was almost completely restored. Complemented bacteria produced bacteremia and grew rapidly in organs (liver, spleen, and brain), as well as did EGDwt bacteria. This was also observed in placentas and fetuses, where complemented bacteria behaved similarly to EDGwt bacteria (Fig. 3B), with cutoff values in the placenta estimated at 1.7 × 103 bacteria for EDGwt and 2.0 × 103 for the actA-complemented ΔActA strain (Table 2).
Bacterial cell-to-cell spreading is a key step for crossing the fetoplacental barrier.
Histologic examination of the placental labyrinthine zone was performed 48 h after infection with 5 × 104 bacteria of the EGDwt, ΔInlAB, or ΔActA strains (Fig. 4). After infection with EGDwt and ΔInlAB, the lesions observed in placental villosities were similar, consisting of a centrifugal dissemination all over the syncytiotrophoblastic cells following the villous axis. In contrast, ΔActA bacteria induced infectious foci in the placenta, where bacteria mostly visible inside trophoblastic cells were unable to spread in an outward manner, as did EGDwt bacteria. These results clearly demonstrate that spreading within the labyrinthine zone is a crucial step for the crossing the fetoplacental barrier in the murine model.
FIG. 4.
Histological examination of placental infectious foci obtained with wild-type L. monocytognes and its ΔInlAB and ΔActA isogenic mutants. Gram-Weigert staining at different magnifications (top and bottom panels) were performed on placental sections 72 h after intravenous infection with 2 × 105 wild-type L. monocytogenes cells or with mutants with the inlAB locus (ΔInlAB) or actA (ΔActA) deleted. Arrows indicate the important spreading to adjacent and distant cells. Arrowheads symbolized the well-delimited peripheral zones from foci induced by infection due to the ΔActA mutant.
DISCUSSION
Our results first show that the placenta is an advantageous and protective niche for any bacteria, even the nonvirulent ΔLLO L. monocytogenes mutant and L. innocua, which could survive at a stable and low level for several days in the placenta. Trophoblastic cells appear to play an immunological role against pathogens, protecting the fetuses from bacterial aggression, but are also the first placental target cells for intracellular pathogens as L. monocytogenes (14, 21). Our data are in agreement with previous reports demonstrating that trophoblastic cells express an important phagocytic capacity, mainly during the two first trimesters of pregnancy in humans, a function still conserved in the late stage of pregnancy (2). The bacterial survival of nonvirulent bacteria in the placenta might be due to several causes, including the presence of latent bacteria confined in the phagosomal compartment; low intracellular growth due to the escape of few ΔLLO bacteria by production of phospholipases (22); and extracellular replication in the placenta, which constitutes a protective environment for bacterial growth. Indeed, the immunological status of the placenta is characterized by a predominant Th2 anti-inflammatory response to prevent the rejection of the semiallogeneic fetus, thus restraining the recruitment of inflammatory cells at the early onset of the infection.
We observed that the survival of ΔLLO mutants and L. innocua in the placenta was associated with neither bacterial growth nor fetal infection, as opposed to virulent wild-type L. monocytogenes as previously observed in the guinea pig model (5). This indicates that the virulence factor LLO is absolutely required in vivo for bacterial growth in trophoblastic cells and the subsequent fetal invasion. The vertical transmission of L. monocytogenes to the fetus is therefore dependent upon the expression of virulence factors promoting intracellular multiplication of this pathogen. Consequently, we first tested in pregnant mice a ΔInlAB mutant of L. monocytogenes, which might be affected in its invasive capacity for trophoblastic cells. It has been published that internalization of L. monocytogenes in a human trophoblastic cell line (BeWo) infected in vitro requires the expression of InlA (4), a surface-exposed protein interacting with high affinity with human and guinea pig E-cadherin. The affinity of mouse E-cadherin for InlA is lower than that of guinea pig E-cadherin (18). However, this single in vitro interaction does not account for the mechanism of in vivo infection of trophoblastic cells by L. monocytogenes, since the rate of placental infection with an InlA mutant was not reduced in the guinea pig model (4). Similarly, we found in the mouse model that a strain with InlA and InlB deletion invaded placentas and fetuses as well as did wild-type bacteria. Therefore, InlA and InlB are not implicated in the crossing of the murine fetoplacental barrier as for guinea pigs (4).
We then studied the role of ActA in placental infection by using a ΔActA mutant in pregnant mice. Growth of this virulence-attenuated mutant was restricted in vivo, as indicated by the absence of bacteremia and the rapid elimination of bacteria in organs, as previously described (16). However, we observed that the ΔActA strain retained the ability to grow in the placenta, although at a low rate compared to wild-type bacteria. As expected, ΔActA bacteria were strongly impaired in cell-to-cell spreading through placental tissues, bacteria often being seen packed inside trophoblastic cells, contrasting with the outward spreading and the rapid dissemination of wild-type bacteria to neighboring cells. This growth defect of ΔActA bacteria was correlated to a delayed and decreased rate of fetal infection. The placental bacterial load required for fetal infection with the ΔActA mutant was 2 log units higher than that seen with EGDwt. However, impairment of placental growth of the ΔActA mutant might also be partly explained by the lack of bacteremia, which was observed for wild-type bacteria in pregnant mice. Our results in the murine model strongly support the major role of cell-to-cell spreading in the vertical transmission of L. monocytogenes.
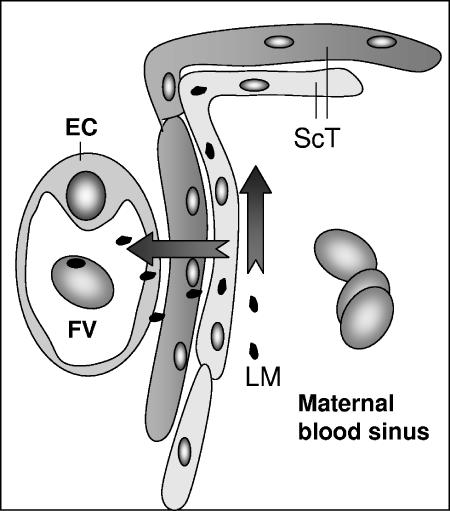
In conclusion, together with those of Barkadjiev et al. from guinea pigs (4, 5), our data demonstrate in the murine model (i) that the surface-exposed proteins InlA and InlB are not necessary for placental invasion and (ii) the crucial role of ActA-dependent cell-to-cell spreading in the vertical transmission of L. monocytogenes to the fetus, including direct evidence obtained by fully restoring fetal invasion in an actA-complemented ΔActA mutant. Therefore, the crossing of the murine fetoplacental barrier requiring ActA-dependent cell-to-cell spreading allows bacteria to cross the two layers of trophoblastic cells and endothelial cells from the fetal vessel, as illustrated in Fig. 5.
FIG. 5.
Model of crossing of the fetoplacental barrier by L. monocytogenes. Two layers of syncytiotrophoblastic cells (ScT) and one layer of endothelial cells (EC) surrounding a fetal vessel (FV) constitute the murine fetoplacental barrier. L. monocytogenes (LM) infects the syncytiotrophoblastic layer and crosses the fetoplacental barrier by ActA-dependent cell-to-cell spreading.
Acknowledgments
We thank P. Cossart (Institue Pasteur, Paris) for the gift of various Listeria mutants and G. Pivert for the technical assistance.
This work was supported by University Paris-Descates, Inserm (ANR-IRAP.2005), and Assistance Publique—Hopitaux de Paris.
Editor: D. L. Burns
Footnotes
Published ahead of print on 21 November 2006.
REFERENCES
- 1.Alouf, J. E. 2000. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 290:351-356. [DOI] [PubMed] [Google Scholar]
- 2.Amarante-Paffaro, A., G. S. Queiroz, S. T. Correa, B. Spira, and E. Bevilacqua. 2004. Phagocytosis as a potential mechanism for microbial defense of mouse placental trophoblast cells. Reproduction 128:207-218. [DOI] [PubMed] [Google Scholar]
- 3.Autret, N., I. Dubail, P. Trieu-Cuot, P. Berche, and A. Charbit. 2001. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69:2054-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakardjiev, A. I., B. A. Stacy, S. J. Fisher, and D. A. Portnoy. 2004. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect. Immun. 72:489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakardjiev, A. I., B. A. Stacy, and D. A. Portnoy. 2005. Growth of Listeria monocytogenes in the Guinea pig placenta and role of cell-to-cell spread in fetal infection. J. Infect. Dis. 191:1889-1897. [DOI] [PubMed] [Google Scholar]
- 6.Buendia, A. J., R. M. De Oca, J. A. Navarro, J. Sánchez, F. Cuello, and J. Salinas. 1999. Role of polymorphonuclear neutrophils in a murine model of Chlamydia psittaci-induced abortion. Infect. Immun. 67:2110-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty, T., F. Ebel, E. Domann, K. Niebuhr, B. Gerstel, S. Pistor, C. J. Temm-Grove, B. M. Jockusch, M. Reinhard, U. Walter, et al. 1995. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 14:1314-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossart, P. 2002. Molecular and cellular basis of the infection by Listeria monocytogenes: an overview. Int. J. Med. Microbiol. 291:401-409. [DOI] [PubMed] [Google Scholar]
- 9.Doganay, M. 2003. Listeriosis: clinical presentation. FEMS Immunol. Med. Microbiol. 35:173-175. [DOI] [PubMed] [Google Scholar]
- 10.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58:587-610. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard, J. L., P. Berche, and P. Sansonetti. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun. 52:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 13.Gray, M. L., and A. H. Killinger. 1966. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 30:309-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guleria, I., and J. W. Pollard. 2000. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 6:589-593. [DOI] [PubMed] [Google Scholar]
- 15.Guzman, C. A., M. Rohde, T. Chakraborty, E. Domann, M. Hudel, J. Wehland, and K. N. Timmis. 1995. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect. Immun. 63:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 17.Koi, H., J. Zhang, and S. Parry. 2001. The mechanisms of placental viral infection. Ann. N. Y. Acad. Sci. 943:148-156. [DOI] [PubMed] [Google Scholar]
- 18.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecuit, M., D. M. Nelson, S. D. Smith, H. Khun, M. Huerre, M. C. Vacher-Lavenu, J. I. Gordon, and P. Cossart. 2004. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proc. Natl. Acad. Sci. USA 101:6152-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 21.Le Monnier, A., O. F. Join-Lambert, F. Jaubert, P. Berche, and S. Kayal. 2006. Invasion of the placenta during murine listeriosis. Infect. Immun. 74:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquis, H., V. Doshi, and D. A. Portnoy. 1995. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63:4531-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mylonakis, E., M. Paliou, E. L. Hohmann, S. B. Calderwood, and E. J. Wing. 2002. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 81:260-269. [DOI] [PubMed] [Google Scholar]
- 25.Pappas, G., N. Akritidis, M. Bosilkovski, and E. Tsianos. 2005. Brucellosis. N. Engl. J. Med. 352:2325-2336. [DOI] [PubMed] [Google Scholar]
- 26.Pfaff, A. W., S. Georges, A. Abou-Bacar, V. Letscher-Bru, J. P. Klein, M. Mousli, and E. Candolfi. 2005. Toxoplasma gondii regulates ICAM-1 mediated monocyte adhesion to trophoblasts. Immunol. Cell Biol. 83:483-489. [DOI] [PubMed] [Google Scholar]
- 27.Scherf, A., B. Pouvelle, P. A. Buffet, and J. Gysin. 2001. Molecular mechanisms of Plasmodium falciparum placental adhesion. Cell Microbiol. 3:125-131. [DOI] [PubMed] [Google Scholar]
- 28.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1991. An integrative vector exploiting the transposition properties of Tn1545 for insertional mutagenesis and cloning of genes from gram-positive bacteria. Gene 106:21-27. [DOI] [PubMed] [Google Scholar]
- 30.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]