Abstract
Nontypeable Haemophilus influenzae (NTHi) is a leading causative agent of otitis media. Much of the inflammation occurring during NTHi disease is initiated by lipooligosaccharides (LOS) on the bacterial surface. Phosphorylcholine (PCho) is added to some LOS forms in a phase-variable manner, and these PCho+ variants predominate in vivo. Thus, we asked whether this modification confers some advantage during infection. Virulence of an otitis media isolate (NTHi strain 86-028NP) was compared with that of an isogenic PCho transferase (licD) mutant using a chinchilla (Chinchilla lanigera) model of otitis media. Animals infected with NTHi 86-028NP licD demonstrated increased early inflammation and a delayed increase in bacterial counts compared to animals infected with NTHi 86-028NP. LOS purified from chinchilla-passed NTHi 86-028NP had increased PCho content compared to LOS purified from the inoculum. Both strains were recovered from middle ear fluids as long as 14 days postinfection. Biofilms were macroscopically visible in the middle ears of euthanized animals infected with NTHi 86-028NP 7 days and 14 days postchallenge. Conversely, less dense biofilms were observed in animals infected with NTHi 86-028NP licD 7 days postinfection, and none of the animals infected with NTHi 86-028NP licD had a visible biofilm by 14 days. Fluorescent antibody staining revealed PCho+ variants within biofilms, similar to our prior results with tissue culture cells in vitro (S. L. West-Barnette, A. Rockel, and W. E. Swords, Infect. Immun. 74:1828-1836, 2006). Animals coinfected with equal proportions of both strains had equal persistence of each strain and somewhat greater severity of disease. We thus conclude that PCho promotes NTHi infection and persistence by reducing the host inflammatory response and by promoting formation of stable biofilm communities.
Haemophilus influenzae is a gram-negative pleiomorphic bacterium that is a common commensal/mutualist within the human airways (30). Encapsulated H. influenzae strains are overt pathogens causing invasive disease (3) and have largely been contained by a vaccine effective against the predominant capsular serotype b strains (32). In contrast, the so-called nontypeable H. influenzae (NTHi) strains lacking capsular polysaccharides remain predominant in asymptomatic carriage and localized airway infections (14, 29). These infections are mostly opportunistic in nature and include bronchiopneumonia, sinusitis, and otitis media (OM). OM is among the most common pediatric infections, causing an estimated ∼$5 billion in costs of treatment and parents' missed work days per year (20). OM infections include chronic OM that is difficult to resolve with antibiotic therapy, and it has long been postulated that chronic OM involves the formation of bacterial biofilm communities (5, 35). In support of that hypothesis, biofilms have been visualized in tympanostomy drain tubes removed from patients with OM and on middle ear tissue from experimentally infected chinchillas (7, 18, 33). More recent evidence shows that NTHi and other bacterial agents are present within biofilms on tissue specimens obtained from patients with chronic and recurrent OM (13).
The H. influenzae surface is covered with lipooligosaccharide (LOS) endotoxins that lack a repeating O side chain. Instead, the H. influenzae LOS features a diverse collection of LOS glycoforms that differ in the length, content, and nature of the chemical linkages found in the oligosaccharide portion. These LOS oligosaccharides include structures that are antigenically similar to host cell-surface glycolipids and may also contain the host membrane constituents sialic acid (NeuAc) and phosphorylcholine (PCho) (41). LOS confers resistance to host killing (8, 9, 37) and is also the primary target of the Toll-like receptor 4 pathway that mediates protection against H. influenzae in the airways (47). It has been established that NTHi strains that express NeuAc-LOS forms comprise a greater proportion of biofilm communities than of planktonic cultures, and that mutations eliminating these forms decrease biofilm formation and bacterial persistence in animal models of OM (4, 12, 18, 43). More recently, we showed that LOS purified from biofilms has decreased potency as an inflammatory agonist, which correlated with an increase in PCho+ LOS forms that were present within biofilms (55). In this study, we compared the virulence of a well-defined otitis media isolate (NTHi 86-028NP) with an isogenic PCho transferase (licD) mutant in a chinchilla middle ear infection model. The data showed that the PCho− mutant evoked increased host inflammatory responses early in the course of infection and correspondingly induced a delayed progression of disease. NTHi 86-028NP recovered from middle ear fluids of challenged animals revealed an increase in PCho+ variants during the course of the infection. At later times postinfection, while bacteria were recovered from all animals infected with either strain, the majority of animals infected with the PCho− mutant had no discernible biofilm. Coinfection with parental and PCho− strains resulted in increased severity of disease and increased counts of both bacterial strains. Based on these data, we conclude that PCho confers a fitness advantage on bacteria in the middle ear which may be related to a difference in the magnitude of the induced host inflammatory response and/or to the formation of stable biofilm communities.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All NTHi strains were cultivated on supplemented brain heart infusion media, which consists of brain heart infusion medium (Difco) with added NAD (Sigma) and hemin (ICN). NTHi 86-028NP is a pediatric OM isolate (2) for which virulence in the chinchilla has been extensively documented (1, 19, 25, 26) and for which a complete genomic sequence is available (16, 28). NTHi 86-028NP licD is a PCho− mutant derived from NTHi 86-028NP that has been described previously (55).
Chinchilla NTHi challenge model.
Healthy adult chinchillas (Chinchilla lanigera; three animals/group) were purchased from Rauscher's Chinchilla Ranch (LaRue, OH). All animals were allowed to acclimate to the vivarium for 1 week prior to challenge, and none had any visible signs of middle ear infection as detected by otoscopy or tympanometry. NTHi strains were harvested from overnight plate cultures, bacterial counts were estimated by optical density, and samples were suspended in pyrogen-free phosphate-buffered saline (PBS) solution. The estimated bacterial density was confirmed by plate count. Approximately 2,500 CFU of each bacterial strain was used to challenge chinchillas (three animals/group) via transbullar injection. The chinchillas were anesthetized and challenged according to previously described methods (36), and the bacterial loads in the inocula were confirmed by plate count. At times indicated in the text, the progression of OM was monitored by daily visual otoscopy inspection and tympanometry measurement. At appropriate times after challenge, and as indicated in the text, middle ear fluid was aspirated from anesthetized animals. Bacterial counts were obtained from tapped middle ear fluids by serial dilution and plate count, and all remaining middle ear fluids were stored at −80°C until further study. Examination of biofilms within the middle ear was performed essentially as described previously (18). Briefly, after euthanasia, the bullae were aseptically opened to expose the middle ear cavity. Effusions were collected, and the presence of biofilms within the middle ear was confirmed by visual inspection. Where applicable, the biofilms were fixed for 15 min in 2% paraformaldehyde-PBS and cryosectioned and then subjected to immunofluorescence microscopy analysis as indicated in the text. The challenge protocols were approved by the relevant animal care and use committees of the Columbus Children's Research Institute/Ohio State University and Wake Forest University Health Sciences.
Measurement of inflammation.
The degree of inflammation was assessed during otoscopic examination using a scoring system, and a qualitative score of 1 to 4 was recorded for each of the following criteria: erythema, tympanic membrane discoloration and opacity, vessel dilation, and fluid accumulation. Changes in middle ear pressure, tympanic width, and tympanic membrane compliance were measured by tympanometry (EarScan, Daytona, FL) as previously described (27). As indicated in the text, the presence of inflammatory mediators in middle ear fluids was compared using RayBiotek protein arrays. Quantitative comparisons were made on fluids obtained from animals with comparable bacterial loads as defined from plate-count experiments.
Purification and analysis of LOS.
Samples of inocula and chinchilla-passed bacterial strains were stored at −80°C with minimal passage. LOS was purified from these strains according to established methods (17) and analyzed by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot using the monoclonal antibody TEPC-15 (Sigma) or HAS (Statens Serum Institut), as previously described (39).
Cryosections.
At various time points postchallenge, chinchillas were euthanized and the bullae were excised. The superior bulla was removed, and the inferior bulla was rinsed with 1 ml of sterile pyrogen-free saline and then filled with Tissue-Tek OCT (Fisher Scientific, Pittsburgh, PA) and snap frozen over liquid nitrogen. Bullae were stored at −80°C until further processing. The frozen bullae were placed on a bed of dry ice, and the external bone of the inferior bulla was chipped away. The resulting block was split in a plane perpendicular to the tympanic membrane and re-embedded in OCT. Initially, 4-μm serial sections were cut using a cryotome and placed on StarFrost adhesive slides (Mercedes Medical, Sarasota, FL). For immunofluorescence staining, the sections were fixed in 2% paraformaldehyde-PBS prior to antibody staining as indicated in the text.
Microscopy.
Tissue samples were fixed for 15 min in 2% paraformaldehyde-PBS, cryosectioned, and then analyzed by immunofluorescence microscopy as indicated in the text. For immunofluorescent microscopy, biofilms were stained with rabbit anti-NTHi outer membrane protein preparation (17) and goat anti-rabbit-fluorescein isothiocyanate antibody conjugate as well as anti-PCho monoclonal antibody and donkey anti-mouse-Texas Red antibody conjugate. All secondary antibodies were purchased from Jackson Laboratories (Bar Harbor, ME). The samples were visualized using a Nikon Eclipse TE300 fluorescent microscope or a Zeiss LSM510 confocal laser-scanning microscope, as indicated in the text. For scanning electron microscopy, the biofilm samples were fixed for 60 min with 2.5% glutaraldehyde-PBS and then rinsed twice (10 min/wash) in PBS prior to dehydration in a graded ethanol series. The samples were then subjected to critical point drying, mounted onto stubs, and sputter coated with palladium prior to viewing with a Phillips SEM-515 scanning electron microscope.
Statistical analysis.
Data were analyzed using unpaired, nonparametric t test with Welch's correction for unequal variance. Data sets with P ≤ 0.05 were deemed to have significant differences.
RESULTS
A mutant lacking PCho induced a slower progression of OM disease in the chinchilla middle ear.
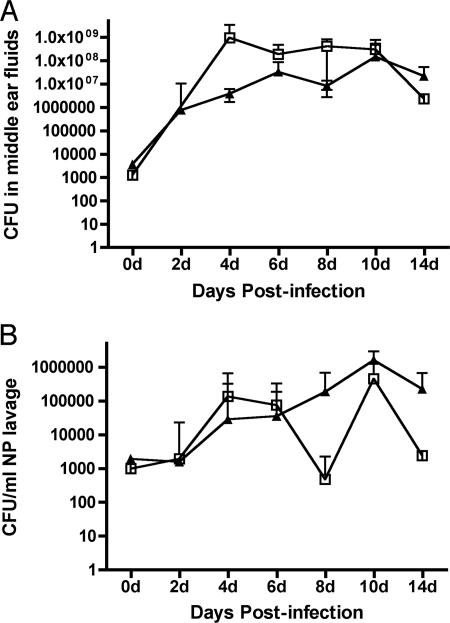
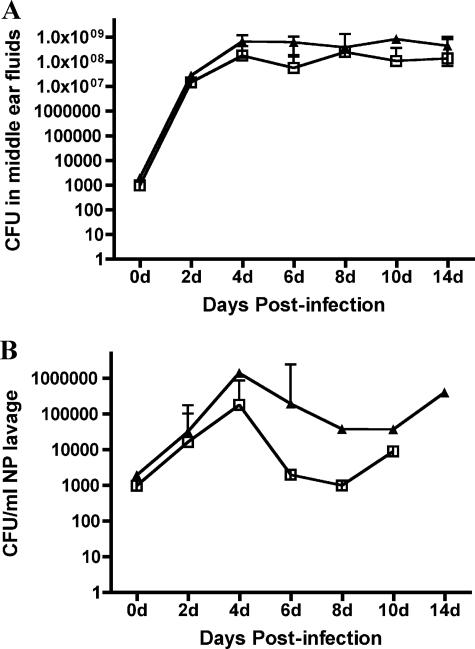
Comparable infectious doses (2,100 and 3,200 CFU/ear) of NTHi 86-028NP and NTHi 86-028NP licD were inoculated into the middle ears of healthy adult chinchillas. The progression of disease was monitored by video otoscopy and tympanometry every 2 days postinfection. When the results of these measures indicated the presence of a recoverable effusion within the middle ear cavity (i.e., an inflammatory score of >2.0), fluids were collected from anesthetized animals by epitympanic tap. Bacterial counts obtained from middle ear fluids recovered from infected chinchillas by epitympanic puncture are shown in Fig. 1. Although both strains were able to survive within the chinchilla ear, the licD mutant strain had a slower increase in bacterial counts within the middle ears of all of the infected animals. Statistical analysis showed that the progression of disease as measured by bacterial load was significantly different (P = 0.02 across the time course of the infection). In contrast, plate counts obtained from nasopharyngeal lavages (Fig. 1B) did not show a significant difference (P = 0.19). Comparison of the numbers of animals exhibiting clinical symptoms of OM during the first 5 days postchallenge indicated that NTHi 86-028NP licD elicited greater inflammatory symptoms than the parental strain. From these data, we conclude that loss of PCho results in an increased inflammatory response that would likely subsequently slow or prevent bacterial replication, thus slowing the progression of OM.
FIG. 1.
Bacterial counts obtained from middle ear fluids (A) or nasopharyngeal (NP) lavages (B) from chinchillas infected with NTHi 86-028NP (open squares) or NTHi 86-028NP licD (closed triangles). Dotted lines indicate the limit of detection.
A mutant lacking PCho elicits greater inflammatory responses early after challenge.
Otoscopy images were captured by digital imaging and assigned a score for measures of inflammation and OM between 1 and 4, with 4 representing the most severe disease. These measures of inflammation included vessel dilation, fluid accumulation, and opacity of the tympanic membrane. For all of the animals infected with the licD mutant strain, the signs of inflammation were initiated earlier and had greater magnitude than in animals infected with NTHi 86-028NP (Fig. 2). For example, on day 1 postchallenge, 5/6 ears infected with NTHi 86-028NP licD were scored positive (>2) for erythema, whereas only 1/6 ears infected with NTHi 86-028NP had positive scores. Likewise, greater numbers of ears infected with NTHi 86-028NP licD were scored positive for opacity, fluid accumulation, and membrane discoloration at day 1 postchallenge than ears infected with NTHi 86-028NP. On day 3 postinfection, 5/6 ears infected with NTHi 86-028NP licD were scored positive for blood vessel dilation, compared to 0/6 ears infected with NTHi 86-028NP. A full summary of the otoscopy scores is provided in Table S1 in the supplemental material.
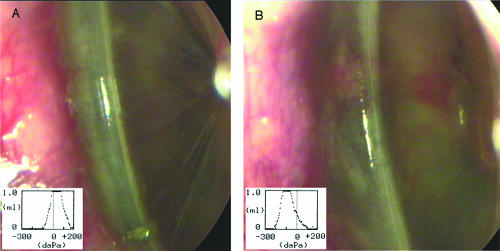
FIG. 2.
Video otoscopy images and tympanometry plots demonstrating changes in the middle ear during infections. Panels are representative images obtained from animals infected with NTHi 86-028NP (A) or NTHi 86-028NP licD (B). Tympanometry readings are shown in the inset of each panel and were obtained at the same time as the otoscopy images.
Assessment of the compliance and thickness of the tympanic membrane and pressure within the middle ear by tympanometry also gave evidence of greater early inflammatory signs in animals infected with NTHi 86-028NP licD (Fig. 2, inset).
Inflammatory responses were further compared by semiquantitative immunoblot analysis of archived middle ear fluids using a RayBiotech protein array (Table 1). The results showed a two- to eightfold difference in levels of interleukin-1α (IL-1α), IL-1β, IL-6, monocyte chemoattractant protein 1, MIP-1α, and tumor necrosis factor alpha. The observed differences in these mediators were confined to the earliest time point (4 days). At later time points, comparable results were obtained for both experimental groups (data not shown). Notably, these differences occurred at time points when fewer NTHi 86-028NP licD bacteria were present in the middle ear fluids than were bacteria of the parental strain; thus, fewer viable bacteria of this strain caused greater inflammation. One possible explanation of these results that we considered was a greater early killing of the mutant bacteria immediately postchallenge compared to that of the parental strain, thus resulting in increased amounts of free LOS and other inflammatory agonists. Similar increased early inflammation has been noted for defensin-sensitive mutants of NTHi (25). Thus, we compared the two strains for their relative ability to resist complement-mediated killing and the chinchilla beta-defensin cBD-1 (15). There was no detectable increase in in vitro killing by these two mechanisms as a result of the mutation (data not shown). Because mutations that alter sialylation can also render NTHi bacteria susceptible to clearance, we also compared sialylation of the two strains by colony immunoblot using Limax flavus or Maackia amurensis lectins conjugated to alkaline phosphatase (EY Laboratories, San Mateo, CA). No difference in sialylation of the two strains was observed (data not shown). Therefore, we conclude that loss of PCho slows the infection of the middle ear by NTHi by a mechanism that is independent of bacterial sialylation or sensitization to host antimicrobials found within the middle ear.
TABLE 1.
Differences in inflammatory mediators in middle ear fluids as assessed by RayBiotech protein arraysa
| Cytokine | Fold induction |
|---|---|
| IL-1α | 2.3 ± 0.9 |
| IL-1β | 1.8 ± 0.6 |
| IL-6 | 3.4 ± 1.4 |
| MCP-1 | 2.2 ± 0.7 |
| MIP-1α | 4.3 ± 2.7 |
| TNF-α | 6.2 ± 3.2 |
Analyses on middle ear fluids were made using the C1000 array according to the manufacturer's instructions. Fold induction values were calculated as ratios of data obtained from middle ear fluids from animals infected with NTHi 86-028NP licD relative to values obtained from NTHi 86-028NP. MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha.
Bacterial survival within the middle ear enriches for PCho+ variants.
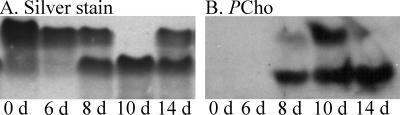
Previous work has shown that PCho+ variants of NTHi predominate in vivo (45, 52, 53). Thus, we sought to define how the proportion of these variants changed during the course of the infection studies. LOS was purified from the inoculum and from NTHi recovered from the chinchilla middle ear fluids as the infection progressed, and these were compared by Tricine-SDS-PAGE and ammoniacal silver stain (46) and Western blot using anti-PCho monoclonal antibody. We observed visible shifts in electrophoretic mobility of the major LOS glycoform populations in samples taken from later in the infection (Fig. 3A), which correlated with increased proportions of PCho+ LOS in all animals infected with NTHi 86-028NP (Fig. 3B). It is noteworthy that prior studies have shown a net decrease in LOS oligosaccharide chain length in accordance with PCho substitutions, possibly because for many strains PCho is a terminal oligosaccharide structure (23).
FIG. 3.
Analysis of lipooligosaccharides purified from NTHi 86-028NP inoculum and isolates from chinchilla middle ear fluids. LOS were separated by Tricine-SDS-PAGE and visualized by ammonium-silver stain (A) or immunoblot using anti-PCho monoclonal antibody (B). Lanes: 1, inoculum; 2, 6 days postinfection; 3, 8 days postinfection; 4, 10 days postinfection; 5, 14 days postinfection.
PCho promotes formation of stable biofilm communities in vivo.
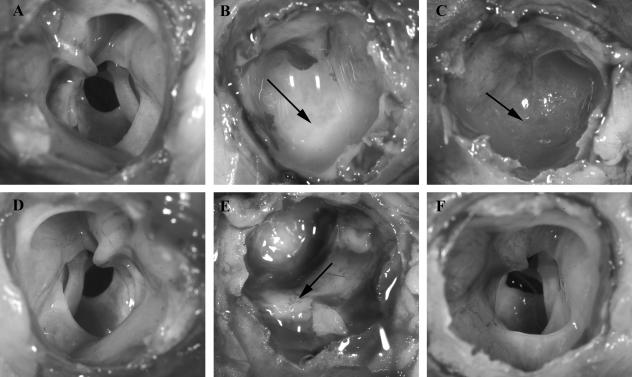
The existing data showed that PCho promoted NTHi residence within the chinchilla middle ear. However, because all of the colony count data were obtained from middle ear fluids it was possible that these represented planktonic populations rather than biofilm communities. Thus, we performed an additional challenge study with NTHi 86-028NP and NTHi 86-028NP licD, and the presence of biofilms within the middle ear was assessed at 7 days and 14 days postinfection (Fig. 4). Compared to uninfected control animals (Fig. 4A and D), animals infected with NTHi 86-028NP had a dense, opaque biofilm that was macroscopically visible within the middle ear cavity 7 days postchallenge (see the arrow in Fig. 4B). In contrast, a thin translucent biofilm was observed in animals infected with NTHi 86-028NP licD at 7 days postchallenge (see the arrow in Fig. 4C). At 14 days postinfection, there was a marked difference in the biofilms observed within the chinchilla ears (Fig. 4). For the animals infected with NTHi 86-028NP, a thick biofilm was observed within the middle ears of 2/4 infected ears (Fig. 4E; see also the figure in the supplemental material). However, in the animals infected with NTHi 86-028NP licD, 0/6 ears had visible biofilms at 14 days postchallenge. Despite the apparent difference in biofilm, bacteria were recovered from middle ear fluids or lavages from all animals infected with NTHi 86-028NP and from 2/6 animals infected with NTHi 86-028NP licD. In those animals from which bacterial counts were obtained, there were no significant differences in the numbers of bacteria of either strain present within the middle ears. Effusions were observed in 6/8 middle ears in the NTHi 86-028NP group and 4/6 of the middle ears in the NTHi 86-028NP licD group at 7 days postchallenge. On day 14 postchallenge, there were visible effusions present in 2/4 middle ears in the NTHi 86-028NP group and 0/6 middle ears in the NTHi 86-028NP licD group. A full summary of the infection results is provided in the figure in the supplemental material. Our interpretation of these results is consistent with the prior study, in that loss of PCho decreases persistent colonization of the middle ear by NTHi. There was also a notable difference in the presence and apparent density of NTHi biofilms within the middle ears of animals challenged with NTHi 86-028NP licD.
FIG. 4.
Visualization of biofilms within the middle ear of infected chinchillas 7 days (B and C) and 14 days (E and F) after challenge. Uninfected control animals are shown in panels A and D. Animals in panels B and E were challenged with NTHi 86-028NP, while animals in panels C and F were challenged with NTHi 86-028NP licD.
Biofilm communities recovered from the chinchilla middle ear contain PCho+ variants.
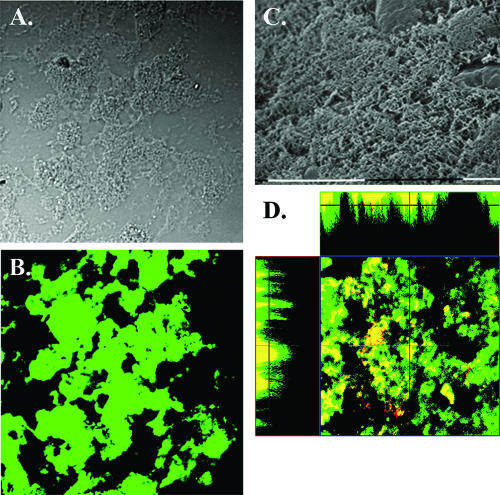
We next performed microscopic analyses on biofilms recovered from the chinchilla middle ear. Biofilm material was excised from the middle ears of infected animals and was stained with anti-NTHi serum and fluorescent antibody conjugate to confirm that the biofilm was composed of NTHi bacteria (Fig. 5A and B). More detailed analysis by scanning electron microscopy showed dense multilayered bacterial communities (Fig. 5C) that are reminiscent of those observed by our group and others in vitro (12, 38, 43, 48).
FIG. 5.
Microscopy analysis of NTHi biofilms recovered from chinchilla middle ears. A) Nomarski image of biofilm. B) Same field of view stained with rabbit anti-NTHi serum and fluorescent antibody conjugate. C) Scanning electron micrograph of biofilm removed from chinchilla middle ear. Scale bars, 10 μm. D) Fluorescent confocal laser-scanning microscopy analysis of middle ear mucosa from a chinchilla infected with NTHi 86-028NP. Middle ear tissue was cryosectioned and stained for all NTHi (green) or PCho+ variants (red). Colocalization of both signals appears yellow. A horizontal Z-slice image (center) and vertical stacked Z images (margins) show PCho+ forms within NTHi biofilms.
We also examined infected chinchilla middle ear tissue for the presence of biofilms. Tissue cryosections were examined by immunohistochemical staining with polyclonal anti-NTHi serum, monoclonal antibody against PCho, and appropriate secondary antibody conjugates, followed by confocal laser-scanning microscopy (Fig. 5D). The results clearly show that the NTHi biofilms formed within the chinchilla middle ear contain PCho+ variants that are within the biofilm structure. These results are consistent with our recently published work in which NTHi biofilms formed on immortalized HMEEC-1 middle ear epithelial cells were shown to contain PCho+ variants (55).
Competitive infection studies.
One potential interpretation of the preceding infection studies was that PCho+ variants had a selective survival advantage within the chinchilla middle ear. Therefore, we tested this hypothesis by coinfecting animals with equal proportions of NTHi 86-028NP and NTHi 86-028NP licD. The animals were infected via transbullar injection, the progression of OM was monitored, and middle ear fluids were collected at varying time points as before. The results showed no significant differences in density of the two bacterial populations within the chinchilla middle ear during the course of the infection (Fig. 6A). Of note, NTHi 86-028NP licD persisted for a longer period of time within middle ear fluids of the coinfected animals than in the preceding study for NTHi 86-028NP licD alone, suggesting that the presence of PCho+ variants was indirectly protective. As in the previous study, there was no significant difference in relative bacterial loads of either strain within nasopharyngeal lavages (Fig. 6B). Moreover, the severity of induced experimental OM was markedly greater in coinfected animals than in the animals infected with NTHi 86-028NP alone and were comparable with the scores of animals infected with NTHi 86-028NP licD. For example, greater numbers of coinfected ears had positive scores for erythema, opacity, vessel dilation, fluid accumulation, and membrane discoloration than ears infected with NTHi 86-028NP alone. A summary of the otoscopy scores is provided in Table S1 in the supplemental material. Based on these data, we conclude that the presence of PCho+ variants within a community was sufficient to confer protection from clearance to PCho− variants.
FIG. 6.
Bacterial counts obtained from middle ear fluids (A) or nasopharyngeal lavages (NP) (B) from chinchillas coinfected with NTHi 86-028 (open squares) and NTHi 86-028 licD (closed triangles). Dotted lines indicate the limit of detection.
DISCUSSION
The addition of PCho to bacterial surfaces is a recurring theme among bacteria that inhabit mucosal surfaces (11, 21, 44, 51, 54). For many of these bacteria, carriage in vivo enriches for bacterial populations that express higher levels of PCho (6, 45, 50, 52, 53). Contributions of PCho to the persistence of NTHi in vivo include increased adherence to and invasion of airway epithelial cells (39, 40, 42), resistance to some host antimicrobials (23), and diminished potency of LOS as an inflammatory agonist (55). The results of this study show that PCho promotes the establishment and persistence of NTHi communities within the chinchilla middle ear.
Biofilms have long been thought to promote bacterial persistence due to their increased resistance to clearance by host defenses or antimicrobial therapy (10, 22, 34). Our recent work shows that NTHi biofilms contain increased proportions of PCho+ variants, and that LOS purified from these biofilms evokes less robust host inflammatory responses (55). Thus, in addition to biofilm resistance phenotypes, NTHi also has the capacity to avoid triggering the host's innate responses during the biofilm mode of growth. The focus of this study was to ask how PCho impacts the course of OM disease elicited by NTHi. Our results demonstrate that PCho moderates the early host inflammatory response after initial NTHi infection and that variants lacking these LOS forms ultimately fail to form persistent biofilm communities (see Fig. 4; see also the figure in the supplemental material). However, it is noteworthy that we were consistently able to recover viable bacteria from middle ear effusions of those animals infected with NTHi 86-028NP licD that had recoverable middle ear effusion fluids. This finding is reminiscent of prior work showing that some variants can persist in the planktonic phase within the middle ear despite failing to form biofilms (18).
A large body of work shows that populations of NTHi in vivo contain variants that are sialylated (18, 24) and PCho+ (45, 52, 53). Moreover, prior work has clearly shown that NeuAc promotes biofilm formation and bacterial persistence in animal models of NTHi disease (4, 18, 43). Our previous studies did not show any biofilm phenotype associated with loss of PCho, at least in terms of initial attachment or numbers of viable bacteria found within biofilms (55). Conversely, the present data show that loss of PCho compromises the ability of NTHi to form biofilms during long-term (14 days) infections (Fig. 4; also see the figure in the supplemental material). Possible explanations for these differences include more discrete effects of PCho on biofilm structure and/or differences in susceptibility of the bacteria to host clearance. While we considered the possibility that loss of PCho sensitized NTHi to killing via complement or other factors within the middle ear, this hypothesis was not supported by the results of experiments comparing in vitro killing of NTHi 86-028NP and NTHi 86-028NP licD by chinchilla or human serum or by cBD-1, a homolog of one of several known antimicrobial peptides involved in innate immunity in the airways (data not shown). Remaining possibilities include differences in biofilm maturation and resistance phenotypes, which could explain why the PCho− variants had increased persistence in the presence of the parental strain. These are questions that we are presently addressing.
Understanding how stable biofilm communities form and persist in vivo is an essential stage in the eventual design of new diagnostic, preventive, and/or therapeutic strategies for controlling NTHi infections. It has long been recognized that NTHi populations vary considerably both within and between strains (31), and there is apparent conservation of bacterial adaptations that promote this diversity (49). This study clearly shows that the addition of PCho to the bacterial surface was important in the establishment of NTHi biofilms and persistence of NTHi within the chinchilla middle ear. Of note, the results of the coinfection studies also showed that the presence of PCho+ variants within a population was sufficient to permit persistence of PCho− bacteria within the middle ear. These results suggest that the NTHi biofilm is a differentiated community in which PCho+ variants play a key role. Our ongoing work will define the contributions of various bacterial subpopulations in infection and persistence of NTHi within the airways.
Supplementary Material
Acknowledgments
We acknowledge excellent technical assistance by Gayle Foster and Vadim Ciobanu and assistance from the WFUHS Medical Center Graphics department in photography of in vivo biofilms. The staff of the WFUHS MicroMed microscopy core facility provided assistance with microscopy studies.
This work was supported by NIH grants AI054425, DC007444 (W.E.S.), DC003915, and DC005847 (L.O.B.). Kevin Mason was supported by an individual NIH F32 fellowship (DC006320).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 27 November 2006.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bakaletz, L. O., E. R. Leake, J. M. Billy, and P. T. Kaumaya. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15:955-961. [DOI] [PubMed] [Google Scholar]
- 2.Bakaletz, L. O., B. M. Tallan, T. Hoepf, T. F. DeMaria, H. G. Birck, and D. J. Lim. 1988. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect. Immun. 56:331-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bol, P., L. Spanjaard, L. van Alphen, and H. C. Zanen. 1987. Epidemiology of Haemophilus influenzae meningitis in patients more than 6 years of age. J. Infect. 15:81-94. [DOI] [PubMed] [Google Scholar]
- 4.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Cundell, D. R., J. N. Weiser, J. Shen, A. Young, and E. I. Tuomanen. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich, G. D., R. Veeh, X. Wang, J. W. Costerton, J. D. Hayes, F. Z. Hu, B. J. Daigle, M. D. Ehrlich, and J. C. Post. 2002. Mucosal biofilm formation on middle ear mucosa in the chinchilla model of otitis media. JAMA 287:1710-1715. [DOI] [PubMed] [Google Scholar]
- 8.Erwin, A. L., S. Allen, D. K. Ho, P. J. Bonthius, J. Jarisch, K. L. Nelson, D. L. Tsao, W. C. Unrath, M. E. Watson, Jr., B. W. Gibson, M. A. Apicella, and A. L. Smith. 2006. Role of lgtC in resistance to human serum of nontypeable Haemophilus influenzae strain R2866. Infect. Immun. 74:6226-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erwin, A. L., P. J. Bonthuis, J. L. Geelhood, K. L. Nelson, K. W. McCrea, J. R. Gilsdorf, and A. L. Smith. 2006. Heterogeneity in tandem octanucleotides within Haemophilus influenzae lipopolysaccharide biosynthetic gene losA affects serum resistance. Infect. Immun. 74:3408-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fux, C. A., P. Stoodley, L. Hall-Stoodley, and J. W. Costerton. 2003. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert. Rev. Anti-Infect. Ther. 1:667-683. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie, S. H., S. Ainscough, A. Dickens, and J. Lewin. 1996. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J. Med. Microbiol. 44:35-40. [DOI] [PubMed] [Google Scholar]
- 12.Greiner, L. L., H. Watanabe, N. J. Phillips, J. Shao, A. Morgan, A. Zaleski, B. W. Gibson, and M. A. Apicella. 2004. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect. Immun. 72:4249-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall-Stoodley, L., F. Z. Hu, A. Gieseke, L. Nistico, D. Nguyen, J. Hayes, M. Forbes, D. P. Greenberg, B. Dice, A. Burrows, P. A. Wackym, P. Stoodley, J. C. Post, G. D. Ehrlich, and J. E. Kerschner. 2006. Direct detection of bacterial biofilms on the middle ear mucosa of children with chronic otitis media. JAMA 296:202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy, G. G., S. M. Tudor, and J. W. St Geme, 3rd. 2003. The pathogenesis of disease due to nontypeable Haemophilus influenzae. Methods Mol. Med. 71:1-28. [DOI] [PubMed] [Google Scholar]
- 15.Harris, R. H., D. Wilk, C. L. Bevins, R. S. Munson, Jr., and L. O. Bakaletz. 2004. Identification and characterization of a mucosal antimicrobial peptide expressed by the chinchilla (Chinchilla lanigera) airway. J. Biol. Chem. 279:20250-20256. [DOI] [PubMed] [Google Scholar]
- 16.Harrison, A., D. W. Dyer, A. Gillaspy, W. C. Ray, R. Mungur, M. B. Carson, H. Zhong, J. Gipson, M. Gipson, L. S. Johnson, L. Lewis, L. O. Bakaletz, and R. S. Munson, Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187:4627-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, P. A., N. A. Samuels, N. J. Phillips, R. S. Munson, J. A. Bozue, J. A. Arseneau, W. A. Nichols, A. Zaleski, B. W. Gibson, and M. A. Apicella. 2002. Haemophilus influenzae type B strain A2 has multiple sialyltransferases involved in lipooligosaccharide sialylation. J. Biol. Chem. 277:14598-14611. [DOI] [PubMed] [Google Scholar]
- 18.Jurcisek, J., L. Greiner, H. Watanabe, A. Zaleski, M. A. Apicella, and L. O. Bakaletz. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy, B. J., L. A. Novotny, J. A. Jurcisek, Y. Lobet, and L. O. Bakaletz. 2000. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect. Immun. 68:2756-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein, J. O. 2000. The burden of otitis media. Vaccine 19:S2-8. [DOI] [PubMed] [Google Scholar]
- 21.Kolberg, J., E. A. Hoiby, and E. Jantzen. 1997. Detection of the phosphorylcholine epitope in streptococci, Haemophilus and pathogenic Neisseriae by immunoblotting. Microb. Pathog. 22:321-329. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lysenko, E. S., J. Gould, R. Bals, J. M. Wilson, and J. N. Weiser. 2000. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 68:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandrell, R. E., R. McLaughlin, Y. A. Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S. A. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialyated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2005. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect. Immun. 73:599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton, D. J., L. O. Bakaletz, J. A. Jurcisek, T. M. VanWagoner, T. W. Seale, P. W. Whitby, and T. L. Stull. 2004. Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin-haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media. Microb. Pathog. 36:25-33. [DOI] [PubMed] [Google Scholar]
- 28.Munson, R. S., Jr., A. Harrison, A. Gillaspy, W. C. Ray, M. Carson, D. Armbruster, J. Gipson, M. Gipson, L. Johnson, L. Lewis, D. W. Dyer, and L. O. Bakaletz. 2004. Partial analysis of the genomes of two nontypeable Haemophilus influenzae otitis media isolates. Infect. Immun. 72:3002-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, T. F. 2003. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr. Opin. Infect. Dis. 16:129-134. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, T. F., and M. A. Apicella. 1987. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens and the human response to infection. Rev. Infect. Dis. 9:1-15. [DOI] [PubMed] [Google Scholar]
- 31.Musser, J. M., S. J. Barenkamp, D. M. Granoff, and R. K. Selander. 1986. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect. Immun. 52:183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post, J. C. 2001. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083-2094. [DOI] [PubMed] [Google Scholar]
- 34.Post, J. C., P. Stoodley, L. Hall-Stoodley, and G. D. Ehrlich. 2004. The role of biofilms in otolaryngologic infections. Curr. Opin. Otolaryngol. Head Neck Surg. 12:185-190. [DOI] [PubMed] [Google Scholar]
- 35.Rayner, M. G., Y. Zhang, M. C. Gorry, Y. Chen, J. C. Post, and G. D. Ehrlich. 1998. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA 279:296-299. [DOI] [PubMed] [Google Scholar]
- 36.Sirakova, T., P. E. Kolattukudy, D. Murwin, J. Billy, E. Leake, D. Lim, T. DeMaria, and L. Bakaletz. 1994. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect. Immun. 62:2002-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starner, T. D., W. E. Swords, M. A. Apicella, and P. B. McCray, Jr. 2002. Lipid A acylation is a determinant of resistance of nontypeable Haemophilus influenzae to killing by beta-defensins. Infect. Immun. 70:5287-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starner, T. D., N. Zhang, G. Kim, M. A. Apicella, and P. B. McCray, Jr. 2006. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am. J. Respir. Crit. Care Med. 174:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swords, W. E., B. Buscher, K. Ver Steeg, W. Nichols, A. Preston, J. N. Weiser, B. Gibson, and M. A. Apicella. 2000. Nontypeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells by an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 40.Swords, W. E., D. L. Chance, L. A. Cohn, J. Shao, M. A. Apicella, and A. L. Smith. 2002. Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infect. Immun. 70:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swords, W. E., P. A. Jones, and M. A. Apicella. 2003. The lipooligosaccharides of Haemophilus influenzae: an interesting assortment of characters. J. Endotoxin Res. 9:131-144. [DOI] [PubMed] [Google Scholar]
- 42.Swords, W. E., M. R. Ketterer, J. Shao, C. A. Campbell, J. N. Weiser, and M. A. Apicella. 2001. Binding of the nontypeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signaling. Cell Microbiol. 3:525-536. [DOI] [PubMed] [Google Scholar]
- 43.Swords, W. E., M. L. Moore, L. Godzicki, G. Bukofzer, M. J. Mitten, and J. VonCannon. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect. Immun. 72:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasz, A. 1967. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in pneumococcus. Science 157:694-697. [DOI] [PubMed] [Google Scholar]
- 45.Tong, H. H., L. E. Blue, M. A. James, Y. P. Chen, and T. F. DeMaria. 2000. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:4593-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 47.Wang, X., C. Moser, J. P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 168:810-815. [DOI] [PubMed] [Google Scholar]
- 48.Webster, P., S. Wu, S. Webster, K. A. Rich, and K. McDonald. 2004. Ultrastructural preservation of biofilms formed by non-typeable Haemophilus influenzae. Biofilms 1:165-182. [Google Scholar]
- 49.Weiser, J. N. 2000. The generation of diversity by Haemophilus influenzae. Trends Microbiol. 8:433-435. [DOI] [PubMed] [Google Scholar]
- 50.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiser, J. N., J. B. Goldberg, N. Pan, L. Wilson, and M. Virji. 1998. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect. Immun. 66:4263-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiser, J. N., and N. Pan. 1998. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol. Microbiol. 30:767-775. [DOI] [PubMed] [Google Scholar]
- 53.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiser, J. N., M. Shchepetov, and S. T. Chong. 1997. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect. Immun. 65:943-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West-Barnette, S. L., A. Rockel, and W. E. Swords. 2006. Biofilm growth increases phosphorylcholine content and decreases potency of nontypeable Haemophilus influenzae endotoxins. Infect. Immun. 74:1828-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.