Abstract
The gene encoding the Treponema denticola factor H-like protein 1 (FHL-1) binding protein, FhbB, was recovered and characterized. Sequence conservation, expression, and properties of FhbB were analyzed. The identification of FhbB represents an important step in understanding the contribution of FHL-1 binding in T. denticola pathogenesis and in development of periodontal disease.
Treponema denticola is an important contributor to the development of acute and chronic periodontal disease in humans (44, 48). Periodontal disease has been linked to systemic disease, including heart disease (21), low birth weight (34), and esophageal cancers (33). Periodontal disease affects nearly all individuals at some point in their lives. This disease results from the synergistic action of a polymicrobial population of endogenous bacteria in association with several host-determined factors. The association of spirochetes with periodontal disease has been firmly established (45). High numbers of T. denticola cells have been found in periodontal lesions and at the leading front of periodontitis-associated subgingival plaque (41).
T. denticola, a member of the “red microbial complex” (45), binds the complement regulatory protein, factor H-like protein 1 (FHL-1) (25). The molecular mass of the FHL-1 binding protein produced by T. denticola is ∼12 kDa, and this protein has been tentatively designated FhbB (FHL-1 binding protein B). This protein is unique in that it binds FHL-1 but not the closely related factor H protein (FH) (25). FHL-1, which is derived from the FH mRNA via alternative splicing, consists of the N-terminal domain of FH (11, 50). Both FH and FHL-1, as well as other members of the FH protein family, have similar structural organizations in that they are comprised of a series 50- to 60-amino-acid repeat units called short consensus repeats (SCRs). FHL-1 is comprised of the first seven SCRs of FH, but in addition it has four unique C-terminal residues as a result of alternate splicing of the FH mRNA. In mammals, FH and FHL-1 contribute to regulation of the alternative complement pathway by serving as cofactors for factor I-mediated cleavage of C3b (39, 40). They also regulate complement by inhibiting the initial formation and accelerating the dissociation of the alternative pathway C3 convertase. While the importance of FH and FHL-1 binding by microbial pathogens as an immune evasion mechanism has been clearly demonstrated (for a review, see reference 20), some pathogens may also exploit the interaction as a way to facilitate adherence and intracellular localization (35). The different functional activities associated with these otherwise very similar proteins most likely result from the different ways that they fold and present individual SCR domains on their surfaces. Previously, we demonstrated that T. denticola cleaves C3b through a predominantly FHL-1-independent mechanism (25). This observation suggests that FHL-1 binding may contribute to other aspects of T. denticola pathogenesis, such as adherence to the extracellular matrix (ECM) or to anchorage-dependent cell types that present FHL-1. The interaction of T. denticola with cell- or ECM-anchored FHL-1 could promote biofilm formation, plaque development, and the progression of periodontal disease.
To allow future analysis of FhbB and the contribution of FHL-1 binding to T. denticola pathogenesis, the first goal of this study was to identify the gene that encodes FhbB. To do this, a proteomics-based approach was used. Since most spirochetal FH/FHL-1 binding proteins are lipoproteins (2, 15, 18), we focused on lipoprotein-encoding genes, of which there are more than 160 in strain 35405 (42, 43). In view of the fact that FH/FHL-1 binding proteins lack conserved primary sequence elements or an identifiable functional domain, we focused on the genes annotated as having unknown functions (n = 63). Of these 63 genes, 9 were predicted to encode proteins having molecular masses in a broad range (8 to 17 kDa) similar to the molecular mass of FhbB (∼12 kDa). These nine open reading frames (ORFs) were then scanned for the presence of possible coiled-coil domains using the COILS program (22). Coiled coils have been demonstrated to be critical structural elements involved in FH/FHL-1 binding by several spirochetal proteins (16, 23, 27) and by the M protein of the group A streptococci (3). The predictive probability of coiled-coil formation was highest for tde0108 and tde1135 (Table 1). For one ORF (tde0851) no coiled-coil probability was predicted, and this ORF was not considered further. The remaining eight ORFs were the focus of additional screening analyses.
TABLE 1.
T. denticola ORFs analyzed in this study
| ORF | Predicted protein molecular mass (kDa) | Highest coiled-coil formation probabilitya |
|---|---|---|
| tde0108 | 11.4 | 0.702 |
| tde0429 | 8.5 | 0.235 |
| tde0940 | 16.0 | 0.039 |
| tde1135 | 13.5 | 0.295b |
| tde1190 | 16.6 | 0.142c |
| tde1191 | 16.3 | 0.101 |
| tde1361 | 12.7 | 0.200d |
| tde2448 | 16.7 | 0.040e |
Unless indicated otherwise, probabilities were determined using a 14-amino-acid window without weighting of the a and d positions of the coiled-coil heptad repeat sequence (positions a to g).
For a 28-amino-acid window, the probability was 0.852.
For a 21-amino-acid window, the probability was 0.269.
Probability with a and d positions weighted.
For a 21-amino-acid window (a and d positions weighted), the probability was 0.085.
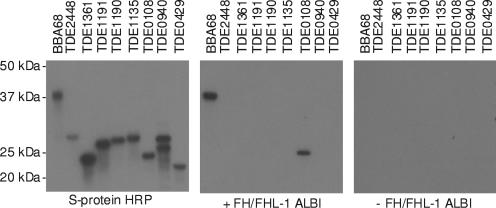
r-Protein was generated for each of the eight ORFs listed in Table 1 using the pET32 Ek-LIC cloning vector and methods that have been described previously (8). The primers used in all PCRs, including those used for cloning, are shown in Table 2. Protein expression in isopropyl-β-d-thiogalactopyranoside (IPTG)-induced Escherichia coli was demonstrated by screening an immunoblot with S-protein (Fig. 1). All immunoblot assays were conducted exactly as described previously (24). All expressed r-proteins were the predicted size and exhibited little or no degradation. The ability of the proteins to bind FHL-1 was assessed using the affinity ligand binding immunoblot (ALBI) assay (32). The r-protein derived from ORF tde0108 displayed strong FHL-1 binding, while no binding was detected to other r-proteins (Fig. 1). As a control, an identical blot was screened using the ALBI assay except that no FH/FHL-1 was added; as expected, no signal was observed. From these analyses we concluded that tde0108 encodes the FhbB protein previously described by McDowell et al. (25).
TABLE 2.
Oligonucleotide primers
| Primer | Oligonucleotide sequencea |
|---|---|
| TDE0108FLIC | GACGACGACAAGATTACTTTCAAAATGAATACTGCAC |
| TDE0108RLIC | GAGGAGAAGCCCGGTTTACTTTATCTTTTTGGGTAT |
| TDE1135FLIC | GACGACGACAAGATCTGCACAAGAAGCGGAATA |
| TDE1135RLIC | GAGGAGAAGCCCGGTTCAATCTTCTTTTGCTTTTCC |
| TDE2448FLIC | GACGACGACAAGATTAAGAGCCGCCGAATCGCCGAAC |
| TDE2448RLIC | GAGGAGAAGCCCGGTTTATTTCTTTTCGATTTTGCG |
| TDE0429FLIC | GACGACGACAAGATTAAAACAACCGATACAAGTAAAA |
| TDE0429RLIC | GAGGAGAAGCCCGGTTTAGATAGGCTTCAATATAAGC |
| TDE1191FLIC | GACGACGACAAGATTTCTAAGACAGCGATAAAGGC |
| TDE1191RLIC | GAGGAGAAGCCCGGTTTAGTACTCTCCACTATTGAGC |
| TDE1190FLIC | GACGACGACAAGATTAAAACAAATGAGAAAAAAAATGCTC |
| TDE1191RLIC | GAGGAGAAGCCCGGTCTAATATTCCGTATGCTTAAAATC |
| TDE1361FLIC | GACGACGACAAGATTAAACAATTTATTGCCGATATTG |
| TDE1361RLIC | GAGGAGAAGCCCGGTTTAAGCTCGTAGTCGGTACCATTG |
| TDE0940FLIC | GACGACGACAAGATCAAGACAAAGCAAATTCAGCC |
| TDE0940RLIC | GAGGAGAAGCCCGGTCTATAATTCGATATTAAAAACATTC |
| FhbB RT F | ACGCGCTTGAGAATGAATTA |
| FhbB RT R | AATCTAATGCAAGGGCTTCAG |
| FlaA RT F | GCTCAGGTTGATGATCAGG |
| FlaA RT R | GCAATTGATTTGATAACGCCG |
| TDE0109R1 | GCTCATCAGCTTGCAAAGGC |
| TDE0109R2 | CGATATTCATGACGTTTACTAC |
| FhbB Up | CTCTTGACAGTACGTATAGTG |
| FhbB78R | GGGTTTTTTATCCACAATTTG |
Underlining indicates the tail sequences added to allow annealing into the pET32 Ek/LIC vector.
FIG. 1.
Identification of the T. denticola strain 35405 ORF that encodes the FHL-1 binding protein, FhbB. S-tagged fusion proteins were generated for potential FHL-1 binding proteins. Expression was verified using horseradish peroxidase (HRP)-conjugated S protein (left panel), and FHL-1 binding was assessed using the ALBI assay (center panel) (32). As a negative control one blot was screened with primary and secondary antibodies without FH/FHL-1 added (right panel). The positions of molecular mass markers are indicated on the left, and the ORF designations are indicated above the lanes. r-BBA68 protein, a Borrelia burgdorferi FH binding protein, served as the positive control.
Analysis of the fhbB gene sequence revealed that it is 309 bp long and encodes a putative lipoprotein with a predicted molecular mass of 11.4 kDa and a pI of 10.6. The gene has a strong ribosomal binding site (AAGGA) and is followed 43 bp downstream by a rho-independent transcriptional terminator with the sequence 5′-CCATCGGAAGATTCCGTCCTCCGATGG-3′. At the protein level, FhbB lacks potential transmembrane-spanning helices and is predicted to be presented on the surface of the cell, anchored by a lipid moiety (lipidation signal peptide motif, MKNKKIFTVLFLLAVSALLFTSC) (42). FHL-1 binding to the surface of T. denticola cultivated in vitro has been demonstrated previously (25).
FhbB differs from other FH/FHL-1 binding proteins in terms of its binding specificity (it binds only FHL-1) and predicted structure. It has a single coiled coil, whereas other FH/FHL-1 binding proteins of spirochetes contain two or more coiled coils (16, 23, 27). Multiple coiled coils within a protein could mediate intramolecular interactions that define or present the FH/FHL-1 binding pocket. The occurrence of only a single coiled coil in FhbB raises the possibility that this domain is involved in an intermolecular interaction that is necessary for FH/FHL-1 binding. The interaction could be a direct interaction with FHL-1 or could facilitate FhbB dimer formation which allows for presentation of the FHL-1 binding pocket. It is important to note that coiled-coil interactions are very stable and are resistant to heat and sodium dodecyl sulfate (17, 46). This could explain why the FhbB protein retains FHL-1 binding activity even after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
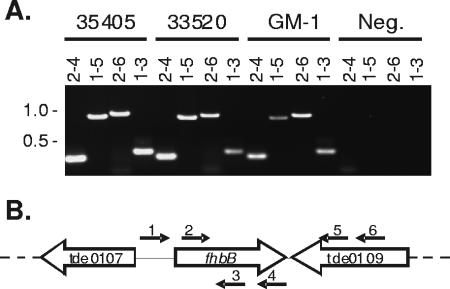
To determine if fhbB is present in other T. denticola strains, PCR analyses were conducted. The fhbB gene was successfully amplified from T. denticola strains 35405, 33520, and GM-1 (Fig. 2A). Sequence analyses of the amplicons revealed that the gene is highly conserved, suggesting that it has an important functional role in T. denticola biology. The fhbB genes from the 35405 and 33520 strains had identical sequences, while GM-1 fhbB differed at one nucleotide position (G-to-A transition), which results in a His-to-Arg change at position 95. To determine if fhbB has the same orientation relative to its flanking sequence in other strains, PCR analyses were performed. The binding site for each primer tested is shown in Fig. 2B. Amplicons that were the same size were obtained from all three strains tested, indicating that the gene orientation is conserved. fhbB is located between tde0107, which encodes an alpha-amylase family protein, and tde0109, which encodes the alpha subunit of phenylalanyl tRNA synthetase. The localization of fhbB between genes that encode housekeeping functions suggests that fhbB is a gene that has a bacterial origin and is not a gene that was recently acquired by, or subject to, lateral transfer. This is in contrast to the FH binding OspE proteins of the Lyme disease spirochetes, which are carried by prophage (9, 49).
FIG. 2.
PCR analyses of FhbB and its flanking regions in diverse T. denticola strains. Regions internal to or flanking fhbB were PCR amplified from T. denticola strains 35405, 33520, and GM-1. Control reactions were performed with no DNA template added (Neg.) (A). The primers used are indicated above the lanes. The primer numbers correspond to the following primers: primer 1, FhbB Up; primer 2, TDE0108FLIC; primer 3, FhbB78R; primer 4, TDE0108RLIC; primer 5, TDE0109R1; and primer 6, TDE0109R2. All primer sequences are shown in Table 2. The target sites for the primers are indicated in panel B.
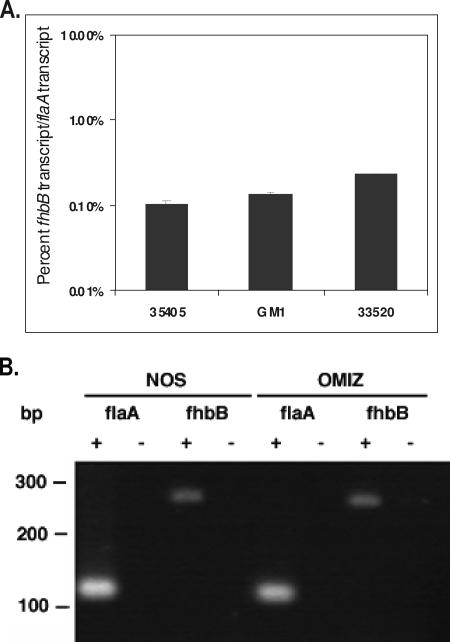
To verify that fhbB is transcribed by strains 35405, 33520, and GM-1 during anaerobic cultivation in NOS medium (ATCC medium 1357), spirochetes were cultivated at 37°C in an anaerobe jar for ∼8 days, RNA was extracted, and real-time reverse transcription (RT)-PCR was performed. All methods used in these analyses have been described previously (49). Standard curves generated using cloned PCR amplicons allowed calculation of transcript numbers. fhbB was determined to be highly expressed, and the transcript levels ranged from 0.1% to 0.5% of the transcript levels of flaA (Fig. 3A). There was no significant difference in the level of fhbB expression between strains 35405, GM-1, and 33520. In a previous study it was demonstrated that the composition of the growth medium can influence protein expression profiles of T. denticola (38). To assess fhbB expression in the two most commonly used T. denticola growth media, RNA was extracted from strain 35405 grown in NOS or OMIZ medium (5), and RT-PCR was performed. Detection of the constitutively produced flaA transcript served as a positive control, and reactions in which RT was omitted served as a negative control. Expression of fhbB was observed in spirochetes cultivated in both media (Fig. 3B). The constitutive expression of fhbB suggests that FhbB has an important role in T. denticola biology.
FIG. 3.
Analysis of fhbB expression using RT-PCR. Real-time RT-PCR analyses were performed as described in the text. (A) Data for strains 35405, GM-1, and 33520. (B) RT-PCR performed to assess fhbB expression by strain 35405 grown in either NOS or OMIZ medium. The amplicons were analyzed by agarose gel electrophoresis in 2% MetaPhor agarose gels.
Identification of the gene encoding FhbB is an important step that will facilitate future analyses of the role of FHL-1 binding in T. denticola pathogenesis. The importance of FH and/or FHL-1 binding as a microbial virulence mechanism is becoming increasingly apparent. Numerous viruses, parasites, and bacteria, including several spirochetes, exploit FH and/or FHL-1 binding as a means of facilitating C3b cleavage and hence immune evasion (1, 6, 7, 10, 12-14, 16, 19, 25, 26, 28-32, 35-37). However, we previously demonstrated that while T. denticola cleaves C3b, this activity is not dependent on FHL-1 binding (25). C3b cleavage by T. denticola appears to be due to dentilisin, a chymotrypsin-like protease which is one of several identified proteases produced by T. denticola (47). FHL-1 binding may instead be more important in adherence and tissue invasion, as has been demonstrated for some streptococci (35) and Actinobacillus (4). We previously demonstrated that T. denticola binds to SCR7 of FHL-1. Our hypothesis is that T. denticola binds primarily to cell- or ECM-anchored FHL-1, an interaction mediated by the RGD motif contained in SCR4, via FhbB. The outcome of this interaction may facilitate biofilm and plaque formation and thus development and progression of periodontal disease. Future analyses will seek to test this hypothesis.
The GenBank accession numbers for sequences determined for this study are EF032155 and EF032156.
Acknowledgments
This work was supported in part by grants from the NIAID, NIH, to R.T. Marconi. J. Frederick was supported in part by an NIAID training grant in molecular pathogenesis to the Department of Microbiology and Immunology at VCU.
We thank D. Haake for assistance with T. denticola genome analysis.
Editor: D. L. Burns
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. T. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alitalo, A., M. T., H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 3.Andre, I., J. Persson, A. M. Blom, H. Nilsson, T. Drakenberg, G. Lindahl, and S. Linse. 2006. Streptococcal M protein: structural studies of the hypervariable region, free and bound to human C4BP. Biochemistry 45:4559-4568. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa, R., H. Komatsuzawa, T. Kawai, S. Yamada, R. B. Goncalves, S. Izumi, T. Fujiwara, Y. Nakano, H. Shiba, M. A. Taubman, H. Kurihara, and M. Sugai. 2003. Outer membrane protein 100, a versatile virulence factor of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 50:1125-1139. [DOI] [PubMed] [Google Scholar]
- 5.Brissette, C. A., L. G. Simonson, and S. A. Lukehart. 2004. Resistance to human beta-defensins is common among oral treponemes. Oral Microbiol. Immunol. 19:403-407. [DOI] [PubMed] [Google Scholar]
- 6.Dave, S., S. Carmicle, S. Hammerschmidt, M. K. Pangburn, and L. S. McDaniel. 2004. Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J. Immunol. 173:471-477. [DOI] [PubMed] [Google Scholar]
- 7.Duthy, T. G., R. J. Ormsby, E. Giannakis, A. D. Ogunniyi, U. H. Stroeher, J. C. Paton, and D. L. Gordon. 2002. The human complement regulator factor H binds pneumococcal surface protein PspC via short consensus repeats 13 to 15. Infect. Immun. 70:5604-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earnhart, C. G., E. L. Buckles, J. S. Dumler, and R. T. Marconi. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC antibody response. Infect. Immun. 73:7869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggers, C. H., S. Casjens, S. F. Hayes, C. F. Garon, C. J. Damman, D. B. Oliver, and D. S. Samuels. 2000. Bacteriophages of spirochetes. J Mol. Microbiol. Biotechnol. 4:365-373. [PubMed] [Google Scholar]
- 10.Fischetti, V. A., R. D. Horstmann, and V. Pancholi. 1995. Location of the complement factor H binding site on streptococcal M6 protein. Infect. Immun. 63:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friese, M. A., J. Hellwage, T. S. Jokiranta, S. Meri, H. H. Peter, H. Eibel, and P. F. Zipfel. 1999. FHL-1/reconection and factor H: two human complement regulators which are encoded by the same gene are differentially expressed and regulated. Mol. Immunol. 36:809-818. [DOI] [PubMed] [Google Scholar]
- 12.Giannakis, E., T. S. Jokiranta, D. A. Male, S. Ranganathan, R. J. Ormsby, V. A. Fischetti, C. Mold, and D. L. Gordon. 2003. A common site within factor H SCR7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur. J. Immunol. 33:962-969. [DOI] [PubMed] [Google Scholar]
- 13.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 14.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic acitivity of streptoccocal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 85:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovis, K., J. V. McDowell, L. Griffin, and R. T. Marconi. 2004. Identification and characterization of a linear plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete, Borrelia hermsii. J. Bacteriol. 186:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovis, K. M., J. P. Jones, T. Sadlon, G. Raval, D. L. Gordon, and R. T. Marconi. 2006. Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 74:2007-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi, S. B., R. E. Dutch, and R. A. Lamb. 1998. A core trimer of the Paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology 248:20-34. [DOI] [PubMed] [Google Scholar]
- 18.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. S. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279S:2421-2429. [DOI] [PubMed] [Google Scholar]
- 19.Kraiczy, P., C. Skerka, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraiczy, P., and R. Würzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 21.Li, X., K. M. Kolltveit, L. Tronstad, and I. Olsen. 2000. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 13:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 23.McDowell, J. V., M. E. Harlin, E. Rogers, and R. T. Marconi. 2005. Putative coiled-coil structural elements of the BBA68 protein of the Lyme disease spirochetes are required for formation of its factor H binding site. J. Bacteriol. 187:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDowell, J. V., K. M. Hovis, H. Zhang, E. Tran, J. Lankford, and R. T. Marconi. 2006. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 74:3030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDowell, J. V., J. Lankford, L. Stamm, T. Sadlon, D. L. Gordon, and R. T. Marconi. 2005. Demonstration of factor H-like protein 1 binding to Treponema denticola, a pathogen associated with periodontal disease in humans. Infect. Immun. 73:7126-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell, J. V., E. Tran, D. Hamilton, J. Wolfgang, K. Miller, and R. T. Marconi. 2003. Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave C3b. J. Clin. Microbiol. 41:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDowell, J. V., J. Wolfgang, L. Senty, C. M. Sundy, M. J. Noto, and R. T. Marconi. 2004. Demonstration of the involvement of outer surface protein E coiled-coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H. J. Immunol. 173:7471-7480. [DOI] [PubMed] [Google Scholar]
- 28.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meri, T., A. Hartmann, D. Lenk, R. Eck, R. Wurzner, J. Hellwage, S. Meri, and P. F. Zipfel. 2002. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect. Immun. 70:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meri, T., T. S. Jokiranta, J. Hellwage, A. Bialonski, P. F. Zipfel, and S. Meri. 2002. Onchocerca volvulus microfilariae avoid complement attach by direct binding of factor H. J. Infect. Dis. 185:1786-1793. [DOI] [PubMed] [Google Scholar]
- 31.Meri, T., R. Murgia, P. Stefanel, S. Meri, and M. Cinco. 2005. Regulation of complement activation at the C3-level by serum resistant leptospires. Microb. Pathog. 39:139-147. [DOI] [PubMed] [Google Scholar]
- 32.Metts, S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in the binding of factor H and OspE targeting antibodies elicited during infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narikiyo, M., C. Tanabe, Y. Yamada, H. Igaki, Y. Tachimori, H. Kato, M. Muto, Montensano, H. Sakamoto, Y. Nakajima, and H. Sasaki. 2004. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 95:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Offenbacher, S., H. L. Jared, P. G. O'Reilly, S. R. Wells, G. E. Salvi, H. P. Lawrence, S. S. Socransky, and J. D. Beck. 1998. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann. Periodontol. 3:233-250. [DOI] [PubMed] [Google Scholar]
- 35.Pandiripally, V., L. Wei, C. Skerka, P. F. Zipfel, and D. Cue. 2003. Recruitment of complement factor H-like protein 1 promotes intracellular invasion by group A streptococci. Infect. Immun. 71:7119-7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialyated Neisseria gonorrhoeae. J. Exp. Med. 188:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ram, S., A. K. Sharma, and S. D. Simpson. 1998. A novel sialic acid binding site on factor H mediates serum resistance of non-sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riviere, G. R., K. S. Smith, S. G. Willis, and K. H. Riviere. 1999. Phenotypic and genotypic heterogeneity among cultivable pathogen-related oral spirochetes and Treponema vincentii. J. Clin. Microbiol. 37:3676-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruddy, S., and K. F. Austen. 1969. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J. Immunol. 102:533-543. [PubMed] [Google Scholar]
- 40.Ruddy, S., and K. F. Austen. 1971. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J. Immunol. 107:742-750. [PubMed] [Google Scholar]
- 41.Saglie, R., M. G. Newman, F. A. Carranza, and G. L. Pattison. 1982. Bacterial invasion of the gingiva in advanced periodontitis in humans. J. Periodontol. 53:217-222. [DOI] [PubMed] [Google Scholar]
- 42.Seshadri, R., G. S. A. Myers, H. Tettelin, J. A. Eisen, J. F. Heidelberg, R. J. Dodson, T. M. Davidsen, R. T. DeBoy, D. E. Fouts, D. H. Haft, J. Selengut, Q. Ren, L. M. Brinkac, R. Madupu, J. Kolonay, S. A. Durkin, S. C. Daugherty, J. Shetty, A. Shvartsbeyn, E. Gebregeorgis, K. Geer, G. Tsegaye, J. Malek, B. Ayodeji, S. Shatsman, M. P. McLeod, D. Smajs, J. K. Howell, S. Pal, A. Amin, P. Vashisth, T. Z. McNeill, Q. Xiang, E. Sodergren, E. Baca, G. M. Weinstock, S. J. Norris, C. M. Fraser, and I. T. Paulsen. 2004. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc. Natl. Acad. Sci. USA 101:5646-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setubal, J. C., M. Reis, J. Matsunaga, and D. A. Haake. 2006. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonson, L. G., C. H. Goodman, J. J. Bial, and H. E. Morton. 1988. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect. Immun. 56:726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socransky, S., A. Haffajee, M. Cugini, C. Smith, and R. J. Kent. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 46.Wosten, H. A. B. 2001. Hydrophobins: multipurpose proteins. Annu. Rev. Microbiol. 55:625-646. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki, T., M. Miyamoto, S. Yamada, K. Okuda, and K. Ishihara. 2006. Surface protease of Treponema denticola hydrolyzes C3 and influences function of polymorphonuclear leukocytes. Microbes Infect. 8:1758-1763. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida, A., M. Kawada, N. Suzuki, Y. Nakano, T. Oho, T. Saito, and Y. Yamashita. 2004. TaqMan real-time polymerase chain reaction assay for the correlation of Treponema denticola numbers with the severity of periodontal disease. Oral Microbiol. Immunol. 19:196-200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, H., and R. T. Marconi. 2005. Demonstration of cotranscription and 1-methyl-3-nitroso-nitroguanidine induction of a 30-gene operon of Borrelia burgdorferi: evidence that the 32-kb circular plasmids are prophage. J. Bacteriol. 187:7985-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zipfel, P. F., and C. Skerka. 1999. FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol. Today 20:135-141. [DOI] [PubMed] [Google Scholar]