Abstract
Pro- and anti-inflammatory cytokines and their signaling pathways play key roles in protection from and pathogenesis of mycobacterial infection, and their balance and dynamic changes may control or predict clinical outcome. Peripheral blood cells' capacity to produce proinflammatory (tumor necrosis factor alpha [TNF-α], interleukin-12/23p40 [IL-12/23p40], and gamma interferon [IFN-γ]) and anti-inflammatory (IL-10) cytokines in response to Mycobacterium tuberculosis or unrelated stimuli (lipopolysaccharide, phytohemagglutinin) was studied in 93 pulmonary tuberculosis (TB) patients and 127 healthy controls from Indonesia. Their cells' ability to respond to IFN-γ was examined to investigate whether M. tuberculosis infection can also inhibit IFN-γ receptor (IFN-γR) signaling. Although there was interindividual variability in the observed responses, the overall results revealed that M. tuberculosis-induced TNF-α and IFN-γ levels showed opposite trends. Whereas TNF-α production was higher in active-TB patients than in controls, IFN-γ production was strongly depressed during active TB, correlated inversely with TB disease severity, and increased during therapy. By contrast, mitogen-induced IFN-γ production, although lower in patients than in controls, did not change during treatment, suggesting an M. tuberculosis-specific and reversible component in the depression of IFN-γ. Depressed IFN-γ production was not due to decreased IL-12/IL-23 production. Importantly, IFN-γ-inducible responses were also significantly depressed during active TB and normalized during treatment, revealing disease activity-related and reversible impairment in IFN-γR signaling in TB. Finally, IFN-γ/IL-10 ratios significantly correlated with TB cure. Taken together, these results show that M. tuberculosis-specific stimulation of IFN-γ (but not TNF-α) production and IFN-γR signaling are significantly depressed in active TB, correlate with TB disease severity and activity, and normalize during microbiological TB cure. The depression of both IFN-γ production and IFN-γR signaling may synergize in contributing to defective host control in active TB.
Although one-third of the world's population is thought to be latently infected with Mycobacterium tuberculosis (15), it remains largely unclear why in only 5 to 10% of individuals' infections will progress to active tuberculosis (TB) during their lifetimes (53). Given the impact of mycobacterial exposure and its immunoregulatory consequences for host immunity, it is important to study the integrity and regulation of immune responses and their downstream signaling pathways in areas where TB is endemic since most individuals will be exposed to tuberculous and environmental mycobacteria.
The control of mycobacterial infection is dependent on cell-mediated immunity (CMI), involving activated macrophages, T cells, and type 1 cytokines (23, 40). Upon triggering of innate microbial pattern recognition receptors, such as Toll-like receptors (TLRs) (22), mannose receptors (19, 30), type C lectins like DC-SIGN (25, 48), and NOD/NACHT receptors (21), phagocytes are activated to produce proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-12, and particularly in the case of DC-SIGN, also anti-inflammatory cytokines, including IL-10 and transforming growth factor beta (TGF-β) (22). TNF-α is a monocyte-activating cytokine which stimulates antimycobacterial activity and helps to maintain the integrity of the tuberculous granulomas in which M. tuberculosis is contained (52). IL-12 (p40/p35) links innate and adaptive immunity; drives T cells and NK cells to produce Th1 proinflammatory cytokines, including IFN-γ and TNF-α; and regulates IL-17 production (28, 40, 43). In synergy with TNF-α, IFN-γ activates infected macrophages to eliminate intracellular pathogens as a major effector mechanism of CMI. IL-10 is generally considered to be an anti-inflammatory cytokine and is produced by alternatively activated macrophages (57), DC subsets, and Th2, Th3, and subsets of T-regulatory cells (8, 37). IL-10 down-regulates IL-12 production, decreases IFN-γ production, and regulates antigen presentation (5, 32). Genetic deficiencies in the type 1 cytokine network (IL-12/IL-23/IL-12R/IL-23R/IFN-γ/IFN-γR/STAT1) have been found in patients suffering from severe infections due to otherwise poorly pathogenic mycobacteria and salmonellae (reviewed in references 41 and 55). These unfortunate experiments of nature lead to a failure to produce or to respond to IFN-γ and underline the crucial role of this network in optimal host defense against mycobacterial pathogens.
There is ample evidence suggesting strong immunoregulation and temporary immune suppression in active TB. It is well known that the capacity of active-TB patients to produce IFN-γ as determined in blood stimulation assays is depressed (18, 27, 29, 39, 47, 51). Although there is evidence suggesting that sequestration of reactive cells into active lesions may contribute to the lower responses in the peripheral compartment, it is thought that active suppressive mechanisms contribute to these depressed responses. Indeed, TB patients have enhanced levels of IL-10 and TGF-β (17, 26, 50). However, it is unknown whether this impairment in IFN-γ production during active TB is antigen specific or rather a generalized phenomenon. Moreover, besides the depressed IFN-γ production, in vitro experiments have suggested that M. tuberculosis or components thereof are able to down-regulate IFN-γ receptor (IFN-γR) signaling. This could further undermine the impact of the already diminished levels of IFN-γ on immune activation. As an example, M. tuberculosis 19-kDa lipoprotein can inhibit IFN-γ-induced regulation of various genes via TLR/MyD88-dependent and TLR/MyD88-independent mechanisms, impairing binding of Stat1 to downstream transcription factors (24, 42, 49). Most of these studies, however, have been carried out in in vitro model systems, and it is has not been well studied whether this effect also impacts on the human host defense against M. tuberculosis in infected individuals, including active-TB patients.
We therefore decided to study possible defects—acquired or inherent—in the type 1 cytokine network in TB in a comprehensive fashion in a setting where TB is highly endemic. Indonesia currently ranks third among the world's countries in TB prevalence (59). We evaluated the capacity of TB patients' peripheral blood cells before, during, and after treatment to produce pro- versus anti-inflammatory cytokines (TNF-α, IL-12/23p40, IFN-γ, IL-10) in response to M. tuberculosis or unrelated stimuli (lipopolysaccharide [LPS], phytohemagglutinin [PHA]). Simultaneously, we studied their ability to respond to IFN-γ in view of the cited evidence that M. tuberculosis infection inhibits IFN-γR signaling in vitro. Cytokine responses and profiles were analyzed cross-sectionally in relation to clinical severity and longitudinally in relation to treatment outcome.
MATERIALS AND METHODS
Study subjects.
In a case-control study, 120 newly diagnosed active pulmonary TB patients (ages, 15 to 60 years) were recruited from January 2002 to December 2004 at Perkumpulan Pemberantasan Tuberkulosis Indonesia, an outpatient TB clinic in central Jakarta. TB diagnosis was performed according to World Health Organization criteria, on the basis of the clinical presentation and a chest X-ray radiograph (CXR), and was confirmed by microscopic detection of acid-fast bacilli in Ziehl-Nielsen-stained sputum smears and positive culture of M. tuberculosis. Human immunodeficiency virus (HIV)-seropositive patients (3.3%), diabetes mellitus (DM)-affected patients (10.7%), patients with heart diseases (1.7%), and patients with incomplete data records (5%) were excluded. TB patients entering the study (n = 93) were classified as having mild-to-moderate TB (n = 41) or advanced TB (n = 52) on the basis of the extent of lesions on CXR as described elsewhere (20). Free anti-TB drug treatment was provided to all patients and consisted of a standard regimen of isoniazid, rifampin, pyrazinamide, and ethambutol (2HRZE/4H3R3) according to the national TB program. Treatment was supervised once weekly by a directly observed treatment program. A subgroup of patients (n = 53) was followed longitudinally. This group was selected according to exactly the same criteria as above.
In the same period, 144 healthy individuals matched for sex and age (±10%) and living within the same rukun tetangga, the smallest residential unit in Jakarta (consisting of 15 to 30 households), were included as control subjects. Controls were interviewed by using the same standardized questionnaire and subjected to the same physical examination, blood testing, and CXR scheme as the TB patients. Controls with signs, symptoms, and CXR results suggestive of active TB (2.1%), a history of prior anti-TB treatment (1.4%), DM (1.4%), or incomplete data entry (6.9%) were excluded. HIV status was not tested in the control group. Indonesia is classified as a country with a low HIV prevalence of ≤0.1% in 2003 (33, 59). Included controls (n = 127) had either a normal CXR (n = 110) or a CXR with minimal pulmonary calcifications (n = 17) suggestive of postprimary TB infection. The latter were not excluded because they had never taken anti-TB drugs.
This study was approved by the Ethical Committee of the Faculty of Medicine, University of Indonesia, Jakarta, and by the Eijkman Institute Research Ethics Committee, Jakarta, and written informed consent was voluntarily signed by all patients and control subjects.
Ex vivo stimulation of whole blood and isolated mononuclear cells. Ex vivo cytokine production was examined in patients before initiation of TB treatment. In addition, a group of patients was examined again after 2 and 6 months of TB treatment, respectively. Healthy controls were examined only at the moment of enrollment in the study. To assess cytokine responses, 200 μl of heparinized venous blood was diluted (1:10) in Iscove's modified Dulbecco's medium (Gibco Invitrogen) and incubated in 96-well round-bottom plates in triplicate in the presence of 10 μg/ml M. tuberculosis H37Rv sonicate (heat killed and ultrasonicated; gift from D. van Soolingen, Rijksinstituut voor Volksgezondheid en Milieu, Bilthoven, The Netherlands) or 100 ng/ml LPS (Escherichia coli serotype O55:B5; Sigma-Aldrich Chemie BV) in the presence or absence of 50 or 500 U/ml IFN-γ (Immukine; Boehringer Ingelheim). Cultures were incubated at 37°C in a fully humidified incubator (5% CO2), and supernatants were harvested after 4 h to measure TNF-α production and after 24 h to measure IL-12/23p40 and IL-10 production.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Ficoll-Hypaque (Pharmacia Biotech) density gradient centrifugation. Freshly isolated PBMCs (1.5 × 105 per well) were incubated in 96-well round-bottom plates in triplicate for 6 days in the presence of 2 or 10 μg/ml M. tuberculosis sonicate or 4 μg/ml PHA (Murex Biotech Ltd.). Cultures were incubated at 37°C in a fully humidified incubator (5% CO2). Supernatants were harvested after 6 days and stored at −20°C until IFN-γ enzyme-linked immunosorbent assay measurement.
Cytokine measurement.
TNF-α, IL-12/23p40, IL-10 (Biosource), and IFN-γ (U-CyTech) were measured with a standard enzyme-linked immunosorbent assay according to the manufacturer's protocol. To determine precise concentrations, serial dilutions of the test samples' supernatants were always tested (in duplicate). The detection limits of the assays were 8 pg/ml for TNF-α, IL-12/23p40, and IL-10 and 15 pg/ml for IFN-γ. The cutoff value used was 30 pg/ml, corresponding to three standard deviations above the mean values found in the standard negative control cultures. Detectable values in unstimulated cultures from an individual were subtracted from the values of the stimulated cultures from the same individual.
Statistical analyses.
The t test (independent or paired) was used to compare means of clinical laboratory parameters (Table 1). The Kruskal-Wallis nonparametric test was used to test differences between responses among three or more groups, whereas the Mann-Whitney U test was used to test two groups. In the follow-up study, Wilcoxon signed-rank tests were used to compare median values obtained before, during, and at the end of TB therapy. All statistical analyses were two sided, and a P value of ≤0.05 was considered statistically significant.
TABLE 1.
Clinical characteristics of pulmonary TB patients before and at the end of TB therapy compared to those of control subjectsa
| Parameter | Pulmonary TB patients
|
Controls (n = 127) | ||
|---|---|---|---|---|
| Before TB therapy
|
End of TB therapy (n = 86) | |||
| Mild-to-moderate TB (n = 41) | Advanced TB (n = 52) | |||
| Median age (range), yr | 29 (17-52) | 30 (18-55) | 30 (17-58) | |
| No. (%) of males | 23 (56.1) | 30 (57.7) | 70 (55.1) | |
| No. (%) of individuals with BCG scar | 20 (48.8) | 14 (26.9)b | 58 (45.7) | |
| Mean symptom duration ± SE (wk) | 17.8 ± 3.2 | 23.7 ± 3.0 | ||
| Mean BMI ± SE (kg/m2) | 18.6 ± 0.4 | 16.9 ± 0.3b | 19.8 ± 0.3c,e | 22.4 ± 0.3d |
| Mean ESR ± SE (mm/h) | 71.8 ± 5.0 | 93.7 ± 4.2b | 22.4 ± 3.2c,f | 17.2 ± 1.3d |
| Mean CRP level ± SE (mg/liter) | 45.5 ± 5.5 | 83.0 ± 5.7b | 8.0 ± 1.1c,e | 6.0 ± 0.7d |
| Mean Hb level ± SE (g/dl) | 12.2 ± 0.2 | 11.4 ± 0.2b | 13.7 ± 0.3c,g | 14.5 ± 0.1d |
| Mean WBC ± SE (103/μl) | 10.9 ± 0.6 | 13.2 ± 0.6b | 7.8 ± 0.5c,g | 8.3 ± 0.2d |
| Mean lymphocyte count ± SE (103/μl) | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.6 ± 0.1c,g | 2.9 ± 0.1d |
| Mean granulocyte count ± SE (103/μl) | 7.8 ± 0.5 | 10.0 ± 0.6b | 4.7 ± 0.4c,g | 4.9 ± 0.1d |
Clinical severity of pulmonary TB in patients was determined on the basis of the extent of lesions on CXR and classified into mild-to-moderate TB and advanced TB. Eighty-six patients were cured on the basis of conversion to negative sputum microscopy examinations, improved clinical appearance, and a reduction of the lesion area in the CXR. All blood parameters in treated TB patients approached normal values.
Statistically significant difference (P < 0.05) between mild-to-moderate and advanced pulmonary TB before therapy (t test).
Statistically significant difference (P < 0.05) between pulmonary TB patients before and at the end of therapy (paired t test).
Statistically significant difference (P < 0.05) between all pulmonary TB patients before therapy and control subjects (t test).
Value measured in 81 patients.
Value measured in 39 patients.
Value measured in 21 patients.
RESULTS
Clinical severity of TB in patients.
The clinical severity of pulmonary TB in patients was determined on the basis of the extent of lesions on CXR and classified into mild-to-moderate TB (n = 41) and advanced TB (n = 52) and is compared to that of healthy control subjects (n = 127) in Table 1. Tuberculin skin tests were not performed in our study. BCG status was determined by the presence of a BCG scar. Interestingly, the mild-to-moderate TB group had significantly more BCG scars (48.8%) than the advanced-TB group (26.9%, P < 0.05). There was no such difference between the mild-to-moderate TB patient group and the healthy control group (45.7%). More than 95% of the active-TB patients had persistent coughing as the main complaint. The duration of this symptom was shorter in mild-to-moderate TB (17.8 ± 3.2 weeks) than in advanced TB (23.7 ± 3.0 weeks), although this difference was not statistically significant. The body mass index (BMI) in advanced-TB patients was significantly lower than that of patients with mild-to-moderate TB (16.9 ± 0.3 versus 18.6 ± 0.4, P < 0.05) and control subjects (22.4 ± 0.3, P < 0.05). Anemia (corrected by gender, the hemoglobin [Hb] level for males was ≤13 g/dl and that for females was ≤12 g/dl) was more prominent in advanced TB (86.0%; mean Hb, 11.4 g/dl) than in mild-to-moderate TB (57.5%; mean Hb, 12.2 g/dl). Laboratory parameters, such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, white blood cell indices (WBC), and granulocyte numbers were all significantly different between TB patients and controls. They were also significantly higher in advanced-TB than in mild-to-moderate TB patients, in agreement with the CXR results.
After the completion of 6 months of anti-TB therapy, 86 patients were cured on the basis of conversion to negative sputum microscopy results. They also showed improved clinical appearance and reduction of the lesion area in the CXR. Treated TB patients had significant weight gain (19.8 kg/m2; P < 0.01) and a decreased ESR (21.5 ± 3.1 mm/h; P < 0.01), while CRP levels decreased to normal (≤10 mg/liter; P < 0.01). Also, all other blood parameters approached normal values in treated TB patients (Table 1). Seven TB patients showed persistently positive sputum microscopy results. None of them were infected with multidrug-resistant M. tuberculosis strains. Only one of these seven patients still showed a high CRP level (31 mg/liter) but had a normalized BMI (20.8 kg/m2) at month 6. Two patients had normalized CRP levels with normalized BMIs, while four other patients had normalized CRP levels with BMIs still under 18.5 kg/m2. Those four patients, however, had gained significant weight compared to when they were in the active-TB phase.
Clinical TB severity is often classified on the basis of chest radiography results, ranging from infiltration in a limited upper lung segment to extensive bilateral lung involvement with tissue destruction and cavitation (56). Since CXR readings are subject to intra- and interobserver variations, we compared our CXR-based classification of mild-to-moderate TB versus advanced TB with other clinical presentations and laboratory parameters. CXR severity classifications matched well with clinical symptoms, including wasting, a prominent feature of TB (56), with the extent of lymphopenia associated with anemia (38), and with increases in white blood cell numbers with high numbers of granulocytes.
Proinflammatory (TNF-α, IL-12/23p40) and anti-inflammatory (IL-10) cytokine production in response to M. tuberculosis or LPS.
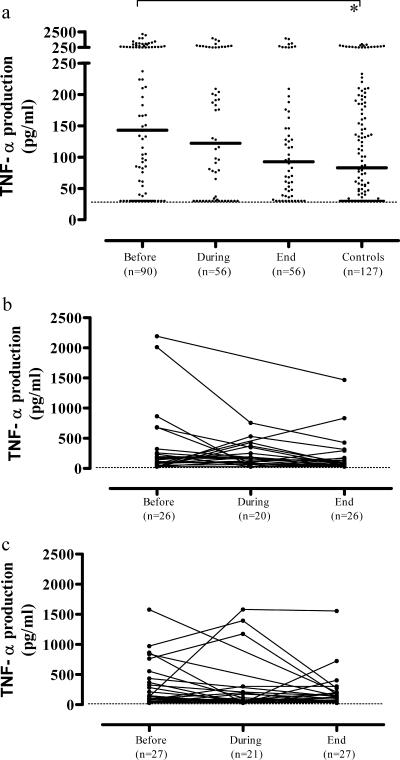
Cytokine responses were analyzed cross-sectionally as well as longitudinally. In the cross-sectional analysis, M. tuberculosis-induced (Fig. 1a) or LPS-induced (data not shown) TNF-α production was highest in untreated TB patients, intermediate during therapy, and lowest at the end of TB therapy (medians, 143, 122, and 93 pg/ml, respectively), closely approaching the values measured in control subjects (median, 83 pg/ml). TNF-α production levels in untreated TB patients were significantly increased compared to those of controls (P = 0.007), correlating with TB disease-related inflammation. Induced TNF-α levels were significantly higher following stimulation with LPS compared to M. tuberculosis sonicate (P < 0.001), but the same decrease in production during treatment was noticeable (data not shown).
FIG. 1.
(a) M. tuberculosis-induced TNF-α production in the cross-sectional analysis was highest in untreated TB patients, decreased during therapy, and was lowest at the end of TB therapy, closely approaching the values measured in control subjects, correlating with the TB disease-related inflammatory process. A smaller set of patients was followed up in a longitudinal-observation study. No significant differences in TNF-α production were observed between the mild-to-moderate TB (b) and advanced-TB (c) groups before, during, and at the end of the therapy. Each dot in the cross-sectional study represents one individual. A horizontal bar indicates the median of each group. A dashed line indicates the lower detection limit of the assay. A statistically significant difference (P < 0.05) is indicated by the asterisk (Mann-Whitney U test).
A smaller set of patients (mild-to-moderate TB, n = 26; advanced TB, n = 27) could be followed-up in a longitudinal-observation study. No significant differences in TNF-α production were observed between the mild-to-moderate TB (Fig. 1b) and advanced-TB (Fig. 1c) groups before, during, and at the end of therapy, although group sizes were limited. Taken together, these data suggest that TNF-α production is enhanced in active TB, normalizes during curative treatment, and correlates with TB disease activity rather than disease severity.
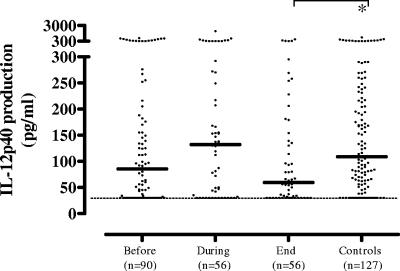
In contrast to TNF-α, IL-12/23p40 production tended to be slightly increased after 2 months of TB therapy but was decreased at the end of anti-TB therapy in the cross-sectional analysis. This trend was most evident in the longitudinal study in the mild-to-moderate TB patients, compared to the advanced-TB patients, who showed a gradual decline in IL-12/23p40 production during TB therapy (Fig. 2). However, these trends failed to reach statistical significance (not shown). Like TNF-α, IL-12/23p40 production levels were significantly higher upon stimulation with LPS compared to M. tuberculosis (P < 0.01), although the observed trends were the same (data not shown).
FIG. 2.
M. tuberculosis-induced IL-12/23p40 production tended to be slightly increased after 2 months of TB therapy but decreased at the end of anti-TB therapy in the cross-sectional analysis. This trend was most evident in the longitudinal study (see text; not shown). Each dot in the cross-sectional study represents one individual. A horizontal bar indicates the median of each group. A dashed line indicates the lower detection limit of the assay. A statistically significant difference (P < 0.05) is indicated by the asterisk (Mann-Whitney U test).
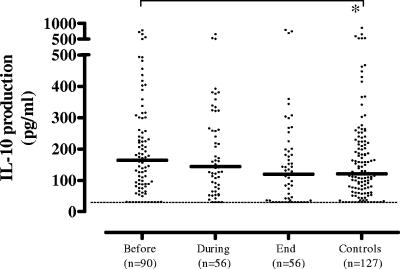
IL-10 production in response to M. tuberculosis sonicate was below the detection limit of our assay in most patients and controls. This was presumably due to the limited number of cells present in the assay. In response to LPS, however, IL-10 production was slightly but significantly increased in active TB (median, 164 pg/ml) and normalized at the end of therapy (median, 120 pg/ml) compared to that of the control group (median, 121 pg/ml; P < 0.03) (Fig. 3). In the longitudinal study, no significant differences were detectable between the mild-to-moderate TB and advanced-TB subgroups (not shown).
FIG. 3.
LPS-induced IL-10 production of pulmonary TB patients was slightly but significantly increased in active TB and normalized at the end of therapy compared to that of the control group. In the longitudinal study, no significant differences were detectable between the mild-to-moderate TB and advanced-TB subgroups (see text; not shown). Each dot in the cross-sectional study represents one individual. A horizontal bar indicates the median of each group. A dashed line indicates the lower detection limit of the assay. A statistically significant difference (P < 0.05) is indicated by the asterisk (Mann-Whitney U test).
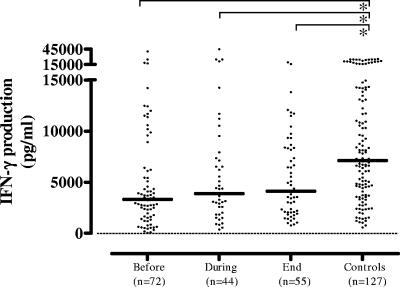
IFN-γ production in response to M. tuberculosis compared to PHA.
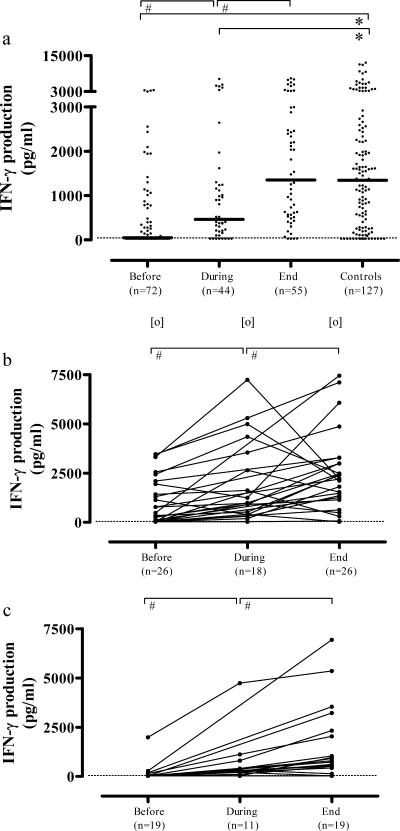
PBMC IFN-γ production was strongly depressed in response to M. tuberculosis in untreated TB but increased during the course of therapy, approaching values measured in control subjects at the end of therapy (medians, 49, 463, and 1,353 pg/ml compared to 1,343 pg/ml in controls). Thus, IFN-γ production correlated inversely with TB disease activity (Fig. 4a). There was a clear dose effect, since levels of IFN-γ production were lower in response to 2 μg/ml M. tuberculosis sonicate than in response to 10 μg/ml M. tuberculosis sonicate, but the same trend was observed (data not shown). Exclusion of the 17 controls with CXR suggestive of postprimary M. tuberculosis infection (n = 17; median, 1,604 pg/ml) did not change the IFN-γ production results in the control group (n = 110; median, 1,283 pg/ml) compared to TB patients. P values in the case of the total control group (n = 127) or the group from which the 17 subjects had been excluded (n = 110), compared to the TB group, were as follows: prior to treatment, P < 0.001 in both cases; during treatment, P = 0.01 and 0.03; following completion of therapy, not different, as expected (P = 0.5 and 0.3, respectively).
FIG. 4.
(a) M. tuberculosis-induced IFN-γ production of PBMCs was strongly suppressed in untreated TB but increased during the course of therapy, approaching values measured in control subjects at the end of therapy, correlating inversely with TB disease activity. Overall, IFN-γ production was higher in mild-to-moderate TB patients (b) than in advanced-TB patients (c) at all of the time points studied, showing that, in contrast to TNF-α, IL-10, and IL-12/23p40, the depression in IFN-γ production correlates not only with TB disease activity but also with TB disease severity. Each dot in the cross-sectional study represents one individual. A horizontal bar indicates the median in each group. A dashed line indicates the lower detection limit of the assay. Statistically significant differences (P < 0.05) are indicated by the symbol # between median values before, during, and at the end of the therapy (Wilcoxon signed-rank test); by the symbol * compared to control subjects (Mann-Whitney U test); and by the symbol [o] between mild-to-moderate TB and advanced-TB patients (Mann-Whitney U test).
A smaller set of patients (mild-to-moderate TB, n = 19; advanced TB, n = 29) could be studied longitudinally. Overall, IFN-γ production in response to M. tuberculosis sonicate was higher in mild-to-moderate TB than in advanced TB at all of the time points studied (medians: before treatment, 370 versus <30 pg/ml [P < 0.01]; during treatment, 963 versus 235 pg/ml [P = 0.02]; end of therapy, 2,154 versus 795 pg/ml [P = 0.05], respectively) (Fig. 4b and c). These results show that, in contrast to the observed changes in TNF-α, IL-10, and IL-12/23p40, the decreased IFN-γ production correlates not only with TB disease activity but also with TB disease severity.
Nonspecific stimulation with the T-cell mitogen PHA, which assesses the overall capacity of T cells to produce cytokines, also revealed significantly depressed IFN-γ production in TB patients compared to controls (P < 0.0001). In contrast to the results from M. tuberculosis-specific stimulation, however, there was no increase in IFN-γ production during anti-TB therapy (Fig. 5). There was also no significant difference between mild-to-moderate TB and advanced TB at the first and third time points studied, although there were slightly enhanced levels in mild-to-moderate TB compared to advanced-TB cases after two months of therapy (not shown). Taken together, these results show suppression of IFN-γ immune responses during active TB disease, with a prominent antigen-specific component which seems reversible and an antigen nonspecific component which seems more permanent.
FIG. 5.
PHA-induced IFN-γ production of PBMCs was strongly suppressed in patients with untreated TB compared to that in controls. In contrast to the results from M. tuberculosis-specific stimulation, no increased IFN-γ production during anti-TB therapy was observed. There was overall no significant difference between mild-to-moderate TB patients and advanced-TB patients (see text; not shown). Each dot in the cross-sectional study represents one individual. A horizontal bar indicates the median in each group. A dashed line indicates the lower detection limit of the assay. Statistically significant differences (P < 0.05) are indicated by asterisks (Mann-Whitney U test).
IFN-γ production and clinical severity.
The presence of a BCG scar correlated with protection against advanced TB (Table 1). However, IFN-γ production levels within each of the patient groups did not correlate with the presence of a BCG scar. IFN-γ production also did not correlate with any other laboratory parameters such as Hb, WBC, or granulocyte numbers. Low IFN-γ production induced by M. tuberculosis (≤100 pg/ml) during the entire treatment period was found in 4 out of 31 patients. Low responses to M. tuberculosis were also observed in 18 (14%) out of 127 control subjects. Of these, only one showed calcification in CXR suggestive of postprimary TB. However, all of these individuals responded well to PHA. Two advanced-TB patients with a low response to PHA before treatment showed increased production at the end of therapy.
We also separately analyzed IFN-γ production in TB patients in relation to their DM status, since 13 patients had concomitant DM and DM has a strong relationship with TB in Indonesia (1). However, no differences were found between the two groups. In a more recent study that we have carried out in the same area, also no differences in ex vivo cytokine production were found in TB patients with or without DM (J. Stalenhoef et al., unpublished data).
Integrity of the IFN-γR signaling pathway in TB.
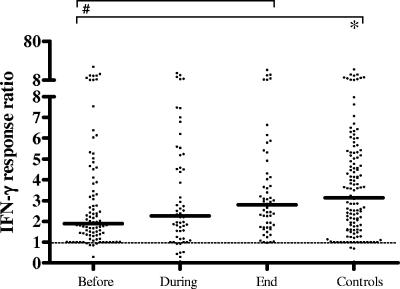
In order to assess the integrity of the IFN-γR signaling pathway in TB, we analyzed the ability of patients' and controls' cells to respond to exogenous IFN-γ by enhancing M. tuberculosis or LPS induction of TNF-α. IFN-γ significantly enhanced M. tuberculosis- and LPS-driven production of TNF-α (and IL-12/23p40 [not shown]). In Fig. 6, IFN-γ responsiveness is expressed as the ratio of M. tuberculosis plus IFN-γ to M. tuberculosis only. During active TB, the ratio of M. tuberculosis plus IFN-γ- to M. tuberculosis-induced production of TNF-α was decreased significantly compared to that of controls but normalized during the course of therapy (P < 0.05) to levels comparable to those of the control group. These data thus may indicate that IFN-γR signaling is compromised during active TB. An alternative explanation might be that active-TB patients already have higher starting levels of TNF-α which may be more difficult to upregulate further. However, we also found impairment of IFN-γ-dependent TNF-α upregulation at the lower stimulatory M. tuberculosis concentrations used, which give rise to lower primary levels of TNF-α, as well as in the case of LPS stimulation, which gives rise to higher TNF-α levels, such that we favor the first interpretation.
FIG. 6.
IFN-γ responsiveness as measured by synergy in TNF-α induction upon stimulation with M. tuberculosis sonicate (ratio of M. tuberculosis plus IFN-γ to M. tuberculosis only). IFN-γ significantly enhanced M. tuberculosis-driven production of TNF-α. IFN-γ responsiveness was decreased significantly in active-TB patients compared to that of controls and normalized during therapy to levels comparable to those of the control group. Each dot represents the IFN-γ response ratio of one individual. A dashed line indicates a ratio of 1. A horizontal bar indicates the median level of each group. Statistically significant differences (P < 0.05) are indicated by the symbol # between median values before, during, and at the end of the therapy (Wilcoxon signed-rank test) and by the symbol * compared to control subjects (Mann-Whitney U test).
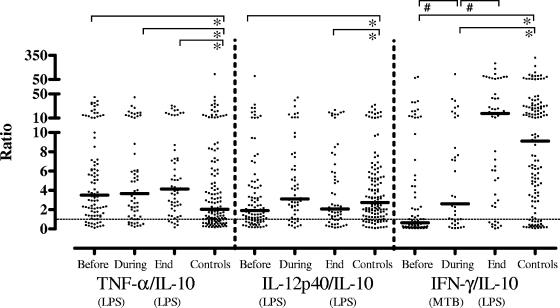
Proinflammatory and anti-inflammatory cytokine ratios.
Since the balance between proinflammatory and anti-inflammatory cytokines is important in clinical outcome in several human diseases, we calculated ratios of TNF-α to IL-10 and IL-12/23p40 to IL-10. TNF-α/IL-10 ratios were significantly increased in TB patients at all time points before, during, and following completion of therapy compared to those of the control subjects (P < 0.05). By contrast, IL-12/23p40/IL-10 ratios were only temporarily increased during anti-TB therapy and decreased at the end of therapy. The ratio of M. tuberculosis-specific induced IFN-γ over IL-10 production showed a strong increase during the course of therapy and was slightly increased at the end of therapy compared to that of control subjects (Fig. 7). Differences were statistically significant at all times points. A similar result was found for IFN-γ over TNF-α ratios (not shown). These results strongly suggest a shift toward a proinflammatory host immune phenotype during control of infection and also suggest that IFN-γ/IL-10 ratios may be a useful biomarker signature to assess this (Fig. 7). The IFN-γ/IL-10 ratio, in particular, was slightly more sensitive than IFN-γ measurements alone; although before therapy both were significantly differed from those in the control group at a level of P < 0.0001, during therapy the IFN-γ/IL-10 ratio showed a slightly more pronounced difference compared to IFN-γ alone (P = 0.004 versus 0.01). As expected, after therapy neither was different from the control group (P = 0.2 and 0.5 for IFN-γ/IL-10 and IFN-γ alone, respectively). The slightly higher sensitivity of the IFN-γ/IL-10 ratio is due to the fact that IL-10 levels decrease (Fig. 3) while IFN-γ levels increase (Fig. 4) during treatment.
FIG. 7.
TNF-α/IL-10 ratios were significantly increased in TB patients before, during, and following completion of therapy compared to those of control subjects. By contrast, IL-12/23p40/IL-10 ratios were only temporarily increased during anti-TB therapy and decreased at the end of therapy. The ratio of M. tuberculosis-specific IFN-γ to IL-10 production showed a strong increase during the course of therapy and was slightly increased at the end of therapy compared to that of control subjects, suggesting a shift toward a proinflammatory host immune phenotype during control of infection. Each dot represents one individual. A horizontal bar indicates the median level of each group. A dashed line indicates a ratio of 1. A statistically significant difference (P < 0.05) is indicated by the symbol # (Wilcoxon signed-rank test) between median values before, during, and at the end of therapy or by the symbol * (Mann-Whitney U test) compared to control subjects.
DISCUSSION
M. tuberculosis has evolved efficient ways to evade host defense by down-regulating various key elements of the cell-mediated immune system. Since we and others have reported that immunity to mycobacteria is critically dependent on type 1 immunity, involving the IL-12/23/IFN-γ/IFN-γR, NFκB, and TNF-α/TNF-αR axes (14, 31, 44), we hypothesized that impairment of these pathways could be related to TB disease. We therefore designed a study to analyze the integrity of these pathways in active and cured TB in an area where TB is highly endemic in order to take into account the impact of environmental as well as tuberculous mycobacterial exposure on the population's immunity (9). We report that in this population where TB is endemic—with high exposure to tuberculous and nontuberculous mycobacteria—M. tuberculosis-induced IFN-γ production, as well as IFN-γR signaling, was significantly down-regulated during active TB. Decreased IFN-γ production had an M. tuberculosis-specific component which was reversible and a nonspecific component which did not seem to normalize during treatment. Longer follow-up studies are needed to substantiate the possible permanence of the decreased IFN-γ production in TB-susceptible individuals. Furthermore, M. tuberculosis-specific (but not nonspecific) IFN-γ production levels correlated inversely with TB disease severity. IFN-γ-inducible responses, as measured by synergy with M. tuberculosis or LPS stimulation, were also significantly depressed during active disease and normalized during treatment, suggesting a disease activity-related, reversible impairment in IFN-γR signaling in TB. TNF-α and IFN-γ levels showed opposite trends, since TNF-α production was higher in active-TB patients than in controls. IL-12/23p40 production kinetics did not correlate with disease activity, showing that the depressed IFN-γ production found is not due to decreased IL-12/IL-23 production. IFN-γ/IL-10 and IFN-γ/TNF-α ratios were found to be particularly significantly regulated biomarker signatures that correlated strongly with TB cure, showing a shift toward a proinflammatory cytokine profile during successful treatment. Taken together, M. tuberculosis appears to be capable of interfering in multiple key steps in innate and adaptive IFN-γ-dependent immunity. The depression of both IFN-γ production and IFN-γR signaling may synergize in contributing to defective host control of M. tuberculosis infection in active TB.
From in vitro models, it is well known that M. tuberculosis infection in macrophages can inhibit the induction of a subset of IFN-γ-responsive genes, including Fcγ receptor type I and major histocompatibility complex class II transactivator (12, 36, 46). Our results clearly suggest that during active TB disease, not only M. tuberculosis-specific induced IFN-γ production but also IFN-γR signaling is impaired. Consequently, there is suppression of both innate and adaptive immunity in active TB. Human IFN-γ production depends on IL-12/23, and IL-12/23 is critical for the control of tuberculous and nontuberculous mycobacterial infections in mice and humans (2, 3, 13). M. tuberculosis inhibits in vitro IL-12/23p40 mRNA and protein production in human (34) and mouse (16) macrophages, indicating active repression of IL-12/23p40 induction by live M. tuberculosis. One study has reported depressed IL-12 production in response to the M. tuberculosis 30/32-kDa antigen in active pulmonary TB (45). In our study, however, there was no suppression of IL-12/23p40 in active pulmonary TB patients compared to controls. We cannot exclude the possibility that in untreated patients M. tuberculosis infection masks increased inflammatory IL-12/23p40 responses, compatible with our observation that there was a temporary increase in IL-12/23p40 production after 2 months of treatment. The latter could have resulted from treatment-induced killing of M. tuberculosis, which would both inhibit IL-12/23p40 suppression and drive stimulation of inflammatory responses, including IL-12/23p40 production, by the concomitant release of M. tuberculosis products. Regardless of the peak in IL-12/23p40 production at 2 months, however, IFN-γ responses had not normalized. The high IL-12/23p40 levels at 0 and 2 months in the absence of high IFN-γ production suggest that there is active suppression of IFN-γ production, which is not dependent on IL-12/23p40 regulation. In previous studies, we showed that human macrophages, when stimulated by M. tuberculosis, release IL-23 (p40/p19) but no IL-12p70 (p40/p35). Only in the presence of IFN-γ as an accessory factor were IL-12p35 gene transcription and IL-12p70 (p40/p35) protein production induced (57). Thus, both IL-12 and IL-23 can be released by human macrophages in response to M. tuberculosis but this depends on the context of stimulation. In the present study, we attempted to measure the production of IL-23 but were unable to detect IL-23 protein over the assay's background.
Furthermore, the suppression of IFN-γ production weakly correlated with increased levels of IL-10 (Fig. 3). IL-10 production has been observed in active TB (11, 50), and a recent study suggested that the combined production of IL-10 and TGF-β might act to down-modulate pulmonary immunity to M. tuberculosis, allowing M. tuberculosis to evade type 1 immunity (10). In our study, the increase in IFN-γ production during TB therapy also coincided with slightly reduced IL-10 production, in agreement with the possibility that IL-10 and IFN-γ cross-regulate. Furthermore, IL-10 down-regulates the expression of the IL-12 receptor chain in TB. Since TB patients also have reduced levels of IL-12R expression (60), their cells may be less responsive to IL-12, which may partly account for the lower level of IFN-γ production in active TB. The latter study also reported that anti-IL-10 and anti-TGF-β enhanced IL-12Rβ1 and IL-12Rβ2 expression and IFN-γ production.
In our study, active-TB patients had a striking depression of IFN-γ production in response to specific M. tuberculosis stimulation. While this has been reported before (18, 27, 29, 39, 47, 51), our comparative analysis of M. tuberculosis- versus mitogen-induced IFN-γ production suggests that there may be a more general—acquired or inherent—impairment in maximum IFN-γ production capacity in TB patients—at least during the first 6 months following the start of treatment—next to a prominent defect which is antigen specific. It is not clear whether IFN-γ production in these individuals will normalize at later time points, and additional studies are required to resolve this. The observation that the depression of IFN-γ production following stimulation with a specific antigen (M. tuberculosis) normalized following treatment is compatible both with the disappearance of specific suppression and with the recruitment of antigen-specific T cells from sequestered lesions into the peripheral compartment (6, 7). However, the fact that mitogen-induced responses remain significantly lower in active-TB patients than in controls is less easily compatible with these possibilities and might argue in favor of a more permanent defect in IFN-γ production capacity. The observation that this latter difference between patients and controls failed to become apparent following M. tuberculosis stimulation may be due to the recruitment of increased numbers of antigen-specific cells from active-TB sites into the peripheral compartment following microbiological cure. However, these parameters were not measured in this study, and future work will need to determine frequencies of such responsive cells by using quantitative assays.
There was no difference in M. tuberculosis-induced IFN-γ production in patients or controls with or without BCG scars, even though the presence of a scar was associated with protection against advanced TB. However, the absence of a BCG scar cannot rule out possible past BCG vaccination.
In contrast to IL-12 and IFN-γ production, TNF-α production was increased during active TB, suggesting that proinflammatory cytokine production was not generally impaired in active TB (4, 58). TNF-α production normalized during curative treatment and correlated with TB disease activity rather than disease severity. The balance of pro- versus anti-inflammatory cytokines is important in clinical outcome in several human diseases. TNF-α/IL-10 ratios were significantly increased in TB patients at all time points before, during, and following completion of therapy compared to those of the control subjects (P < 0.05). By contrast, IL-12p40/IL-10 ratios were only temporarily increased during anti-TB therapy and decreased at the end of therapy.
The ratios of M. tuberculosis-specific IFN-γ production over IL-10 and TNF-α production both showed a strong increase during the course of therapy and were slightly increased at the end of therapy compared to those of control subjects. These results strongly suggest a shift toward a proinflammatory host immune phenotype during control of infection.
We did not measure IL-17 in this study. IL-17 is an important inflammatory cytokine whose production is differentially regulated by IL-23 and IL-12; IL-23 promotes while IL-12 inhibits IL-17 production, even though both IL-12 and IL-23 enhance IFN-γ production (28). Through this anti-inflammatory property, IL-12 may limit inflammation while enhancing specific adaptive immunity by increasing T-cell IFN-γ production. In mice, IL-12 can modulate pathology by reducing the numbers of immune cells involved in inflammatory tuberculous lesions (35). Moreover, IL-12 is also able to enhance IL-10 production, a property often not recognized (32, 54). Thus, IL-12 likely has several different roles in host responses to infection, including a previously unknown anti-inflammatory role.
Collectively, our results show that M. tuberculosis-specific stimulation of IFN-γ production and IFN-γR signaling are both significantly depressed in clinically active TB. Both correlated with TB disease severity and activity and normalized during microbiological TB cure. We propose that the depression of both IFN-γ production and IFN-γR signaling synergizes in contributing to defective host control of M. tuberculosis infection in active TB.
Acknowledgments
This study was supported mainly by a grant from the Royal Netherlands Academy of Arts and Sciences (KNAW99MED01) and received supplementary support from NWO-PRIOR and LUMC.
We thank M. R. Klein for reviewing the manuscript.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Alisjahbana, B., R. van Crevel, E. Sahiratmadja, M. den Heijer, A. Maya, E. Istriana, H. Danusantoso, T. H. Ottenhoff, R. H. Nelwan, and J. W. van der Meer. 2006. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int. J. Tuberc. Lung Dis. 10:696-700. [PubMed] [Google Scholar]
- 2.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 3.Altare, F., D. Lammas, P. Revy, E. Jouanguy, R. Doffinger, S. Lamhamedi, P. Drysdale, D. Scheel-Toellner, J. Girdlestone, P. Darbyshire, M. Wadhwa, H. Dockrell, M. Salmon, A. Fischer, A. Durandy, J. L. Casanova, and D. S. Kumararatne. 1998. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 102:2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antas, P. R., F. L. Cardoso, K. C. Pereira, K. L. Franken, K. S. Cunha, P. Klatser, E. N. Sarno, T. H. Ottenhoff, and E. P. Sampaio. 2005. T cell immune responses to mycobacterial antigens in Brazilian tuberculosis patients and controls. Trans. R. Soc. Trop. Med. Hyg. 99:699-707. [DOI] [PubMed] [Google Scholar]
- 5.Aste-Amezaga, M., X. Ma, A. Sartori, and G. Trinchieri. 1998. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 160:5936-5944. [PubMed] [Google Scholar]
- 6.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, P. F., S. D. Mistry, C. L. Cooper, C. Pirmez, T. H. Rea, and R. L. Modlin. 1989. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J. Immunol. 142:1114-1119. [PubMed] [Google Scholar]
- 8.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 9.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 10.Bonecini-Almeida, M. G., J. L. Ho, N. Boechat, R. C. Huard, S. Chitale, H. Doo, J. Geng, L. Rego, L. C. Lazzarini, A. L. Kritski, W. D. Johnson, Jr., T. A. McCaffrey, and J. R. Silva. 2004. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-β) and analysis of TGF-β receptors I and II in active tuberculosis. Infect. Immun. 72:2628-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boussiotis, V. A., E. Y. Tsai, E. J. Yunis, S. Thim, J. C. Delgado, C. C. Dascher, A. Berezovskaya, D. Rousset, J. M. Reynes, and A. E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan, J., X. D. Fan, S. W. Hunter, P. J. Brennan, and B. R. Bloom. 1991. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 59:1755-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 14.Doffinger, R., S. Y. Patel, and D. S. Kumararatne. 2006. Host genetic factors and mycobacterial infections: lessons from single gene disorders affecting innate and adaptive immunity. Microbes. Infect. 8:1141-1150. [DOI] [PubMed] [Google Scholar]
- 15.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 16.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellner, J. J. 1996. Immunosuppression in tuberculosis. Infect. Agents Dis. 5:62-72. [PubMed] [Google Scholar]
- 18.Ellner, J. J., C. S. Hirsch, and C. C. Whalen. 2000. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin. Infect. Dis. 30(Suppl. 3):S279-S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst, J. D. 1998. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falk, A., J. B. O'Connor, and P. C. Pratt. 1969. Classification of pulmonary tuberculosis, p. 68-76. In Diagnosis standards and classification of tuberculosis, 12th ed. National Tuberculosis and Respiratory Disease Association, New York, N.Y.
- 21.Ferwerda, G., S. E. Girardin, B. J. Kullberg, L. Le Bourhis, D. J. de Jong, D. M. Langenberg, R. van Crevel, G. J. Adema, T. H. Ottenhoff, J. W. van der Meer, and M. G. Netea. 2005. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathogens 1:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 23.Flynn, J. L., and J. D. Ernst. 2000. Immune responses in tuberculosis. Curr. Opin. Immunol. 12:432-436. [DOI] [PubMed] [Google Scholar]
- 24.Fortune, S. M., A. Solache, A. Jaeger, P. J. Hill, J. T. Belisle, B. R. Bloom, E. J. Rubin, and J. D. Ernst. 2004. Mycobacterium tuberculosis inhibits macrophage responses to IFN-γ through myeloid differentiation factor 88-dependent and -independent mechanisms. J. Immunol. 172:6272-6280. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch, C. S., R. Hussain, Z. Toossi, G. Dawood, F. Shahid, and J. J. Ellner. 1996. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc. Natl. Acad. Sci. USA 93:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch, C. S., Z. Toossi, C. Othieno, J. L. Johnson, S. K. Schwander, S. Robertson, R. S. Wallis, K. Edmonds, A. Okwera, R. Mugerwa, P. Peters, and J. J. Ellner. 1999. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J. Infect. Dis. 180:2069-2073. [DOI] [PubMed] [Google Scholar]
- 28.Hoeve, M. A., N. D. Savage, T. de Boer, D. M. Langenberg, M. R. de Waal, T. H. Ottenhoff, and F. A. Verreck. 2006. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur. J. Immunol. 36:661-670. [DOI] [PubMed] [Google Scholar]
- 29.Jo, E. K., J. K. Park, and H. M. Dockrell. 2003. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr. Opin. Infect. Dis. 16:205-210. [DOI] [PubMed] [Google Scholar]
- 30.Kang, P. B., A. K. Azad, J. B. Torrelles, T. M. Kaufman, A. Beharka, E. Tibesar, L. E. Desjardin, and L. S. Schlesinger. 2005. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202:987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald, D. R., R. Janssen, and R. Geha. 2006. Lessons learned from molecular defects in nuclear factor κB dependent signaling. Microbes Infect. 8:1151-1156. [DOI] [PubMed] [Google Scholar]
- 32.Moore, K. W., M. R. de Waal, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 33.National AIDS Commission of the Republic of Indonesia. 2005. Country report on follow-up to the Declaration of Commitment on HIV/AIDS (UNGASS) reporting period 2003-2004. National AIDS Commission of the Republic of Indonesia, Jakarta, Indonesia.
- 34.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolt, D., and J. L. Flynn. 2004. Interleukin-12 therapy reduces the number of immune cells and pathology in lungs of mice infected with Mycobacterium tuberculosis. Infect. Immun. 72:2976-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noss, E. H., C. V. Harding, and W. H. Boom. 2000. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell. Immunol. 201:63-74. [DOI] [PubMed] [Google Scholar]
- 37.O'Garra, A., and P. Vieira. 2004. Regulatory T cells and mechanisms of immune system control. Nat. Med. 10:801-805. [DOI] [PubMed] [Google Scholar]
- 38.Onwubalili, J. K. 1990. Untreated tuberculosis may be associated with lymphopoenia, not lymphocytosis. Afr. J. Med. Med. Sci. 19:181-183. [PubMed] [Google Scholar]
- 39.Onwubalili, J. K., G. M. Scott, and J. A. Robinson. 1985. Deficient immune interferon production in tuberculosis. Clin. Exp. Immunol. 59:405-413. [PMC free article] [PubMed] [Google Scholar]
- 40.Ottenhoff, T. H., F. A. Verreck, M. A. Hoeve, and E. van de Vosse. 2005. Control of human host immunity to mycobacteria. Tuberculosis 85:53-64. [DOI] [PubMed] [Google Scholar]
- 41.Ottenhoff, T. H., F. A. Verreck, E. G. Lichtenauer-Kaligis, M. A. Hoeve, O. Sanal, and J. T. van Dissel. 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat. Genet. 32:97-105. [DOI] [PubMed] [Google Scholar]
- 42.Pai, R. K., M. E. Pennini, A. A. Tobian, D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Prolonged Toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogge, L., L. Barberis-Maino, M. Biffi, N. Passini, D. H. Presky, U. Gubler, and F. Sinigaglia. 1997. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanal, O., T. Turul, T. de Boer, E. V. Vosse, I. Yalcin, I. Tezcan, C. Sun, L. Memis, T. H. Ottenhoff, and F. Ersoy. 2006. Presentation of interleukin-12/-23 receptor β1 deficiency with various clinical symptoms of Salmonella infections. J. Clin. Immunol. 26:1-6. [DOI] [PubMed] [Google Scholar]
- 45.Song, C. H., H. J. Kim, J. K. Park, J. H. Lim, U. O. Kim, J. S. Kim, T. H. Paik, K. J. Kim, J. W. Suhr, and E. K. Jo. 2000. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect. Immun. 68:4477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steimle, V., C. A. Siegrist, A. Mottet, B. Lisowska-Grospierre, and B. Mach. 1994. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265:106-109. [DOI] [PubMed] [Google Scholar]
- 47.Subronto, Y. W., K. E. van Meijgaarden, A. Geluk, S. M. Arend, T. Sunardi, K. L. Franken, B. Hisyam, R. R. de Vries, and T. H. Ottenhoff. 2003. Interferon-gamma production in response to M. tuberculosis antigens in TB patients in Indonesia. Adv. Exp. Med. Biol. 531:249-260. [DOI] [PubMed] [Google Scholar]
- 48.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ting, L. M., A. C. Kim, A. Cattamanchi, and J. D. Ernst. 1999. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898-3906. [PubMed] [Google Scholar]
- 50.Toossi, Z., and J. J. Ellner. 1998. The role of TGFβ in the pathogenesis of human tuberculosis. Clin. Immunol. Immunopathol. 87:107-114. [DOI] [PubMed] [Google Scholar]
- 51.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tufariello, J. M., J. Chan, and J. L. Flynn. 2003. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 3:578-590. [DOI] [PubMed] [Google Scholar]
- 53.van Crevel, R., T. H. Ottenhoff, and J. W. van der Meer. 2002. Innate immunity to Mycobacterium tuberculosis. Clin. Microbiol. Rev. 15:294-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van de Vosse, E., R. A. de Paus, J. T. van Dissel, and T. H. Ottenhoff. 2005. Molecular complementation of IL-12Rβ1 deficiency reveals functional differences between IL-12Rβ1 alleles including partial IL-12Rβ1 deficiency. Hum. Mol. Genet. 14:3847-3855. [DOI] [PubMed] [Google Scholar]
- 55.van de Vosse, E., M. A. Hoeve, and T. H. Ottenhoff. 2004. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect. Dis. 4:739-749. [DOI] [PubMed] [Google Scholar]
- 56.van Lettow, M., J. J. Kumwenda, A. D. Harries, C. C. Whalen, T. E. Taha, N. Kumwenda, C. Kang'ombe, and R. D. Semba. 2004. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int. J. Tuberc. Lung Dis. 8:211-217. [PubMed] [Google Scholar]
- 57.Verreck, F. A., T. de Boer, D. M. Langenberg, M. A. Hoeve, M. Kramer, E. Vaisberg, R. Kastelein, A. Kolk, R. Waal-Malefyt, and T. H. Ottenhoff. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 101:4560-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson, R. J., H. M. Vordermeier, K. A. Wilkinson, A. Sjolund, C. Moreno, G. Pasvol, and J. Ivanyi. 1998. Peptide-specific T cell response to Mycobacterium tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J. Infect. Dis. 178:760-768. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. 2005. WHO report. WHO/HTM/TB/2005.349. Global tuberculosis control: surveillance, planning, financing. World Health Organization, Geneva, Switzerland.
- 60.Zhang, M., J. Gong, D. H. Presky, W. Xue, and P. F. Barnes. 1999. Expression of the IL-12 receptor β1 and β2 subunits in human tuberculosis. J. Immunol. 162:2441-2447. [PubMed] [Google Scholar]