Abstract
Bacterial pathogens produce a variety of exotoxins, which often become associated with the bacterial outer membrane component lipopolysaccharide (LPS) during their secretion. LPS is a potent proinflammatory mediator; however, it is not known whether LPS contributes to cell signaling induced by those microbial components to which it is attached. This is partly due to the common view that LPS present in bacterial component preparations is an experimental artifact. The Escherichia coli exotoxin hemolysin (Hly) is a known inducer of proinflammatory signaling in epithelial cells, and the signal transduction pathway involves fluctuation of the intracellular-Ca2+ concentration. Since LPS is known to interact with Hly, we investigated whether it is required as a cofactor for the activity of Hly. We found that the LPS/Hly complex exploits the CD14/LPS-binding protein recognition system to bring Hly to the cell membrane, where intracellular-Ca2+ signaling is initiated via specific activation of the small GTPase RhoA. Hly-induced Ca2+ signaling was found to occur independently of the LPS receptor TLR4, suggesting that the role of LPS/CD14 is to deliver Hly to the cell membrane. In contrast, the cytolytic effect triggered by exposure of cells to high Hly concentrations occurs independently of LPS/CD14. Collectively, our data reveal a novel molecular mechanism for toxin delivery in bacterial pathogenesis, where LPS-associated microbial compounds are targeted to the host cell membrane as a consequence of their association with LPS.
The exotoxin hemolysin (Hly) produced by uropathogenic Escherichia coli (UPEC) is an important virulence factor in extraintestinal infections, such as urinary tract infections, newborn meningitis, bacteremia, and septicemia (33). Hly exerts a cytolytic effect when present in high concentrations, while low, sublytic concentrations have been demonstrated to induce intracellular-Ca2+ signaling in epithelial cells, leading to proinflammatory responses, such as production of interleukin-6 (IL-6) and IL-8 (47). Hly is secreted from bacteria via the type I secretion pathway (26) and is found associated with bacterial outer membranes, in supernatants, and in outer membrane vesicles (5, 34). Hly forms high-molecular-weight complexes with lipopolysaccharide (LPS), and this interaction has been suggested to prevent aggregation and degradation of Hly as well as to promote an active conformation of the toxin (6, 12, 15). Whether there is also a molecular function of LPS in the Ca2+ signaling and cytolytic activities of Hly remains unknown.
Detailed information regarding the signal transduction pathway elicited by LPS does exist, however. LPS is a large amphipathic molecule composed of the acylated, hydrophobic lipid A component, the LPS core, and the O-antigen polysaccharide. Host recognition of LPS is a multistep process initiated by the binding of LPS by the LPS-binding protein (LBP). This interaction enhances the binding of LPS to the glycosylphosphatidylinositol-linked receptor CD14, whose protein structure contains an NH2 terminal hydrophobic pocket where lipid A binds (25). CD14 subsequently presents LPS to the TLR4/MD-2 receptor complex, which initiates the intracellular signaling cascade. The number and nature of lipid A's acyl chains are major determinants of the potency of LPS in eliciting TLR4-dependent host responses, a dynamic feature which is important for inflammation (3, 20).
In the current study, the biological relevance of LPS to the dual activities of Hly was studied. We found that LPS is essential for Hly-induced Ca2+ oscillations and that this signal is dependent on CD14 and LBP, both known for their involvement in the recruitment of LPS to the eukaryotic membrane. Interestingly, the Hly-induced signaling occurs independently of the LPS receptor TLR4. To increase our understanding of the mechanism by which the Hly-induced Ca2+ signaling occurs, the involvement of small GTPases was investigated. Small GTPases transmit signals from cell surface receptors via several signaling pathways. We found that activation of the small GTPase RhoA, but not Rac and Cdc42, was required for Ca2+ signaling. In addition, our data revealed that the cytolytic activities of Hly occur independently of the LPS/LBP/CD14 complex, suggesting that cytolysis is induced via a mechanism different from that used for induction of Ca2+ oscillations.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains are listed in Table 1. The Hly operon (hlyCABD) from the fully sequenced human and rat pyelonephritogenic E. coli strain CFT073 (American Type Culture Collection [ATCC] no. 700928) was amplified together with an approximately 1.3-kbp-long 5′ flanking region, using oligonucleotides Hlyforw-XbaI (TTATCTAGAGGGTACTGGGAAGACCAGGGTTA) and Hlyrev-KpnI (ATAGGTACCTTAACGCTCATGTAAACTTTCTGTT). The resulting 8.6-kbp-long PCR product was digested by XbaI/KpnI and ligated into pUC18, generating pGNH404 (ampicillin resistant [Ampr]), or into the chloramphenicol-resistant (Chlr) pUC18 derivate pPCR-Script Cam SK(+) (Stratagene, Sweden), generating pLT404. In addition, Hly expression plasmids pANN202-812 (the wild-type [wt] hly operon from pHly152, Ampr) (47), pANN202-812B (with an insertion of TT at position 421 in hlyC of pANN202-812, producing Hly lacking acyl chains; Ampr) (31, 47), and pSF4000 (the hly operon from strain J96, Chlr) (49) were used. Bacteria were grown in Luria-Bertani medium (37°C) and supplemented with 20 μg/ml chloramphenicol or 100 μg/ml ampicillin as indicated.
TABLE 1.
E. coli strains used in this study
| Strain | Relevant characteristics for this study | Source or reference |
|---|---|---|
| CFT073 | O6:K2:H1, smooth LPS | 48 |
| W3110 | K-12, rough LPS | 24 |
| ARD20 | W3110 pANN202-812, Hly+, Ampr | 47 |
| ARD21 | W3110 pANN202-812B, Hly+, HlyC−, Ampr | 47 |
| ARD31 | W3110 pLT404, Hly+, Chlr | This study |
| ARD32 | W3110 ΔmsbB (MLK1067) pGNH404, penta-acylated LPS, Hly+, Chlr, Ampr | 24 and this study |
| WAM 1824 | CL633 pSF4000, K-12, rough LPS, Hly+, Chlr | 13, 49 |
Toxin preparations.
To ensure that natural interactions between Hly and other bacterial components remained, Hly-containing supernatants from stationary overnight cultures were used. Hly was prepared fresh for each experiment, and lytic activity was measured in sheep blood assays (9), where the Hly concentration resulting in 60% hemolysis of a 2.5% erythrocyte suspension was defined as 1,000 hemolytic units (HU).
A highly purified preparation of Hly was prepared from E. coli strain WAM 1824 (CL633 pSF4000). Hly was concentrated by acid-ethanol precipitation from mid-log-phase culture supernatants, denatured by boiling with 1% sodium dodecyl sulfate (SDS), and separated by SDS gel electrophoresis. The Hly polypeptide was eluted from excised gel fragments and dialyzed, before being dissolved in 0.9% NaCl containing 1 mM CaCl2. As an internal control for noxious SDS levels in the end product, proHlyA (the inactive, nonacylated, extracellular proform of Hly that is produced in the absence of HlyC) was purified in parallel. The LPS-free proHlyA preparation showed no lytic or cytotoxic activity even at high concentrations. The details of this purification method will be described elsewhere (S. Pellett and R. A. Welch, unpublished data). The Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD) was used to measure endotoxic units (EU), showing that the purified Hly preparation contained at least 105-fold less LPS (<10−2 EU/ml) than culture supernatants (6.4 × 103 EU/ml). The concentration of toxin was monitored by comparison of the results for Coomassie blue staining of the HlyA band in SDS-polyacrylamide gel electrophoresis gels to known amounts of beta-galactosidase as previously described (6).
Cell lines and primary cell preparation.
The human bladder carcinoma cell line T24 (ATCC HTB-4) was grown on glass coverslips to 40 to 60% confluence as previously described (4), while primary renal epithelial cells (proximal tubule cells [PTC]) were prepared from 20-day-old Sprague-Dawley rats (47). All cells were serum starved (1%) in the absence of antibiotics 12 to 18 h prior to experiments (47). Animal studies were approved by Stockholms Norra Djurförsöksetiska Nämnd.
Single-cell Ca2+ recording.
Cells were incubated with 2 μM Fura-2/AM (acetylmethyl ester; Molecular Probes) in P buffer (100 mM NaCl, 4 mM KCl, 20 mM HEPES, 25 mM NaHCO3, 1 mM CaCl2, 1.2 mM MgCl2, 1 mM NaH2PO4-H2O, and 10 mM d-glucose) for 1 h before ratiometric imaging was performed. Ratiometric imaging was performed at 37°C using a heated chamber (Warner Instruments), mounted on a Nikon TS-100 microscope using a 40×/0.75-numerical-aperture Plan Fluor objective. Emission fluorescence was collected via a PTI image intensifier connected to a PTI Photometrics Coolsnap-cooled charge-coupled-device camera and analyzed using acquisition software from PTI (ImageMaster 3). Clusters containing 15 to 35 cells were chosen in each experiment. Cells were excited at 340 nm and 380 nm every 60 s, and emission was collected at 510 nm. Data shown represent single-cell traces where the ratio, presented in arbitrary units, reflects the change in intracellular-Ca2+ concentration. Sublytic (130- to 200-HU) and lytic (1,500- to 2,000-HU) amounts of Hly were used as specified. Where indicated, cells were pretreated with monoclonal antibodies (1 h, 37°C) directed toward CD14 (BIG-7 [Biometec, Germany] and MEM18 [EXBIO, Czech Republic]), LBP (BIG-412; Biometec, Germany), or isogenic immunoglobulin G control antibody (Biometec, Germany) or with 5 mM methyl-β-cyclodextrin (45 min, 37°C; Sigma, Sweden). Where stated, Hly was preincubated on ice with 5 U/ml polymyxin B (PmB) (30 min; Sigma, Sweden), 10 μg/ml penta-acylated LPSW3110ΔmsbB (4) (15 min), 6 μg/ml rhCD14 from a cell culture supernatant of p-POL-DHFR-hCD14-transfected CHO-cells (15 min; Biometec, Germany), or a corresponding control supernatant (15 min; Biometec, Germany) (mock treatment).
Dominant-negative RhoA.
The enhanced green fluorescent protein (EGFP)-tagged dominant-negative RhoAN19 (pEGFP-RhoAN19) expression vector was constructed by amplification of the RhoAN19 sequence from the pEXVRhoAN19 plasmid (36), using primers RhoA_BamHI_F (ATTCGGATCCATGGCTGCCATCCGGAAGAAAC) and RhoA_XbaI_R (CGATCTAGATCACAAGACAAGGCAACCAGA). The PCR fragment was cloned into the BamHI and XbaI restriction sites of the pEGFP-C1 expression vector (BD Biosciences). pEGFP-RhoAN19 was transfected into PTC using Lipofectamine 2000 (Invitrogen).
Affinity precipitation of GTP-Rho, GTP-Rac, and GTP-Cdc42.
GTP-Rac and GTP-Cdc42 bind the p21-binding domain of p21-activated kinase, and GTP-Rho binds the Rho-binding domain (RBD) of the effector protein rhotekin. The glutathione S-transferase (GST)-RBD and GST-p21-binding domain (PDB) proteins were purified as previously described (17). Cytosolic lysates of PTC exposed to sublytic concentrations of Hly for the indicated times or untreated were prepared in ice-cold buffer and cleared by centrifugation (13,000 rpm, 4°C). They were further incubated with the GST-RBD or GST-PDB protein coupled to glutathione Sepharose (4°C, 45 min), washed, and resuspended in SDS-polyacrylamide gel electrophoresis sample buffer (28) for Western blot analysis using antibodies anti-RhoA (sc179), anti-Cdc42 (sc87) (Santa Cruz Biotechnology), and anti-Rac (clone 102) (Transduction Laboratories, Lexington, KY). The total GTPase content of the cytosolic lysate was similarly analyzed.
RESULTS
Role of LPS in the dual, concentration-dependent activities of Hly.
When analyzing the concentration-dependent effects of the UPEC virulence factor Hly, epithelial cells from the urinary tract are commonly used (27, 29, 47). When these cells are exposed to a high dose of the toxin, the cytolytic response can be observed as a rapid and sustained increase of Ca2+, while a low dose induces an oscillating intracellular-Ca2+ signal, leading to proinflammatory responses. Both scenarios can be monitored at a single-cell level, using ratiometric imaging based on the Ca2+-binding properties of the fluorophore Fura-2/AM. A typical oscillatory fluctuation of intracellular-Ca2+ concentration is shown by the pseudocoloration of a cell in Fig. 1A. Alternatively, semicontinuous Ca2+ recording can be represented in a graph format. Since this communicates a more detailed representation of Ca2+ fluctuations and enables comparison of Ca2+ recordings from individual cells, the graph format was chosen for presentation of data throughout this report.
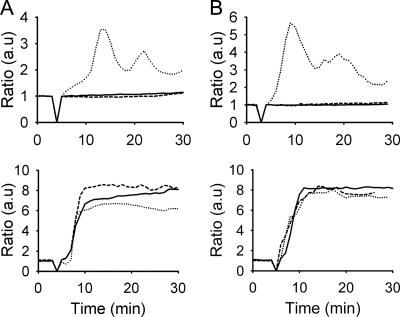
FIG. 1.
LPS is required for Hly-induced Ca2+ signaling but not for cytolysis. (A) Intracellular-Ca2+ fluctuation visualized by ratiometric imaging in a single cell upon Hly stimulation. The pseudocolored cell shows the basal level of intracellular-Ca2+ concentration as blue, while increases in intracellular Ca2+ are depicted as yellow, red, and black. (B) Comparison of the hemolytic activity on sheep erythrocytes by LPS-depleted Hly (dotted line) and Hly-containing supernatant from the source strain (WAM 1824) (continuous line). (C and D) Single-cell recordings of intracellular Ca2+ in PTC stimulated with a sublytic (130-HU) (C) or lytic (1,500-HU) (D) concentration of Hly-containing supernatant from ARD31 in the absence (continuous line) or presence (dashed line) of PmB. The dotted line represents cells stimulated with LPS-depleted Hly, while supernatant from strain W3110, which lacks Hly, serves as a negative control (dashed-dotted line). (E) Hemolysis of erythrocytes measured in HU upon stimulation with Hly-containing supernatant from ARD31 in the absence (continuous line) or presence (dashed line) of PmB or with non-Hly-containing control supernatant (W3110) with (dashed-dotted line) or without (dotted) PmB. A minimum of three independent experiments was performed, with 15 to 20 cells recorded in each experiment. The basal level of Ca2+ in cells during the first 3 to 5 min of the recordings is shown; the addition of stimuli is indicated by closing the shutter for one time point (ratio = 0). a.u., arbitrary units; Conc., concentration; Sup., supernatant.
When cells are exposed to Hly, the toxin is naturally complexed with bacterial membrane components. To investigate whether these are of importance for the activity of Hly, a highly purified Hly preparation, depleted of LPS and other membrane components, was compared to supernatants from Hly-expressing bacterial cultures. The activities of Hly originating from different sources were possible to compare because HU, determined by contact-dependent hemolysis assays, were used to define the amount of stimuli to be used in each experiment, rather than the specific protein concentrations. Thus, the slight decrease in the hemolytic activity of the purified Hly preparation compared to that of the supernatant from which it was purified (Fig. 1B) is compensated for by the fact that the amounts used for stimulation of epithelial cells in the experiments described below were based on HU. The sublytic concentrations used in the experiments described throughout this paper correspond to 130 to 200 HU, while the lytic concentrations of Hly correspond to 1,500 to 2,000 HU.
The effect of sublytic concentrations of purified Hly on primary rat PTC was compared to that of supernatant originating from strain ARD31. In addition to containing Hly, this supernatant contains LPS and other shedded membrane components. Interestingly, the purified Hly preparation was unable to induce any Ca2+ response (Fig. 1C), while the supernatant originating from strain ARD31 rapidly induced an oscillating Ca2+ signal (Fig. 1C). W3110, an isogenic strain which lacks the Hly-encoding plasmid, did not affect Ca2+ homeostasis (Fig. 1C). This observation is in accordance with previously published data (47) and confirms that Hly is essential for the induction of Ca2+ oscillations. A different scenario was revealed when lytic concentrations of the two Hly stimuli were used. Rapid, sustained increases in the intracellular-Ca2+ concentration of PTC were observed when either the purified Hly preparation or the Hly-containing supernatant from ARD31 was used (Fig. 1D). A high, sustained concentration of intracellular Ca2+ represents a prestage to cytolysis (47) and is eventually lethal for cells (7). Supernatant from W3110, lacking the Hly-encoding plasmid, did not induce any increase in Ca2+ concentration (Fig. 1D). Collectively, this suggests that a cofactor required for induction of Ca2+ signaling but not for cell lysis has been removed from the purified Hly preparation. One obvious candidate is LPS, which is reduced by a factor of 105 in the purified Hly preparation, as analyzed by Limulus assays.
Components of the LPS recognition system are required for Hly-induced Ca2+ oscillations.
LPS is a major component of the bacterial supernatant, and it is known to form a complex together with Hly (6, 12; Pellett and Welch, unpublished observations). To address whether there is a specific role for LPS in the activity of Hly, the Hly samples were preincubated with the LPS-binding polycationic peptide PmB. Treatment with PmB alters LPS interactions and neutralizes its immune-stimulatory effect (50). We found that preincubation of sublytic concentrations of Hly-containing supernatant from ARD31 with PmB abolished Hly-induced Ca2+ signals in PTC (Fig. 1C). In contrast, PmB did not affect the lytic effect on these cells, as illustrated by the rapid and sustained elevation of Ca2+ (Fig. 1D). Similarly, we found that PmB did not affect the lytic activity in a contact-dependent hemolysis assay on red blood cells (Fig. 1E). To exclude the possibility that PmB exerted any direct effect on erythrocyte viability, control experiments in which red blood cells were treated with supernatant originating from the isogenic strain W3110, thus lacking Hly, were performed. This supernatant showed no lytic effect on the red blood cells, either in the presence or in the absence of PmB (Fig. 1E). Collectively, these results strongly indicate that LPS is required as a cofactor for Hly-induced Ca2+ oscillations, while the cytolytic activities of Hly occur independently of LPS.
This finding prompted us to analyze whether the Hly-induced Ca2+ response requires intersecting signaling pathways initiated by the interaction between LPS and its signaling receptor TLR4. Molecular characterization of TLR4 signaling in epithelial cells originates mainly from studies using different uroepithelial cell lines (2, 21, 40). In T24 cells, it was shown that wt LPS, expressing hexa-acylated lipid A, rapidly induces TLR4-dependent proinflammatory responses, while underacylated LPS, exemplified by penta-acylated LPS from the E. coli strain W3110 ΔmsbB, was found to be an insufficient activator (4, 24). This effect was recently ascribed to an altered interaction between penta-acylated LPS and TLR4 (44). Knowing that penta-acylated LPS is unable to induce TLR4 signaling, we introduced a plasmid encoding Hly into strain W3110 ΔmsbB (ARD32). Supernatant from this strain (containing Hly, penta-acylated LPS, and other membrane components) was used to investigate whether activation of TLR4 is required for the onset of Hly-induced Ca2+ oscillations. We found that sublytic concentrations of Hly induced Ca2+ oscillations in T24 cells (Fig. 2A, upper panel). This response was similar to those seen in T24 cells and PTC when the cells were stimulated with Hly-containing supernatant from the isogenic strain ARD31, expressing hexa-acylated LPS (Fig. 1B and data not shown). This finding suggests that TLR4 signaling is dispensable for the activity of Hly. This is also supported by previously published data showing that Hly induces Ca2+ oscillations in the TLR4-negative epithelial cell line A498 (4, 47).
FIG. 2.
Hly-induced Ca2+ signaling requires LBP and CD14 but not TLR4. Ratiometric single-cell recording of intracellular Ca2+ in TLR4-expressing T24 cells stimulated with sublytic (170- to 200-HU) (A and B, upper panels) or lytic (1,600- to 2,000-HU) (A and B, lower panels) concentrations of Hly-containing supernatant from ARD32. For panel A, cells were preincubated for 60 min with the anti-CD14 antibody BIG-7 (continuous line), the anti-LBP antibody BIG-412 (dashed line), or the isotypic immunoglobulin G control antibody (dotted line). For panel B, the Hly stimuli was preincubated with LPSW3110ΔmsbB (continuous line), rhCD14 (dashed line) or a mock treatment supernatant (dotted line) for 30 min prior to addition to the cells. A minimum of three independent experiments was performed, and 15 to 35 cells were recorded in each experiment. a.u., arbitrary units.
Although hexa-acylated and penta-acylated LPS interact differently with TLR4, they do not differ in their interactions with the elements acting upstream of the receptor. Both LPS molecules form complexes with components involved in the recruitment of LPS to the eukaryotic cell membrane, e.g., LBP and CD14 (44). There is a possibility that the role of LPS in the induction of Ca2+ oscillations by Hly may be related to an analogous mechanism for the recruitment of the Hly-LPS complexes to the target cells. To test this hypothesis, T24 cells were stimulated in the presence of two antibodies inhibiting the interactions between LPS and CD14: BIG-7, which binds to amino acids 9 to 13 and 39 to 44, and MEM18, which binds to amino acids 39 to 44 or amino acids 57 to 64, in the LPS-binding pocket of CD14 (25). Both antibodies completely abrogated the oscillatory Ca2+ response to sublytic concentrations of Hly (in supernatant from ARD32) (Fig. 2A, upper panel [for BIG-7], and data not shown [for MEM-18]), while the isotype-matched control antibody did not affect the Ca2+ response (Fig. 2A, upper panel). Hly stimulation of cells in the presence of antibody BIG-412, which blocks LBP-mediated presentation of LPS to CD14, also prevented the induction of Ca2+ oscillations (Fig. 2A, upper panel). Thus, Hly utilizes the well-established LPS/CD14 interaction to target the cell membrane. To further test the hypothesis that LPS acts as a bridging molecule between Hly and CD14, we preincubated a sublytic concentration of Hly supernatant with excess LPS (10 μg/ml) prior to stimulation of the T24 cells, assuming that this would out-compete the interaction between Hly and CD14. We found that this treatment completely abrogated Ca2+ oscillations (Fig. 2B, upper panel). As predicted, addition of excess recombinant human CD14 (6 μg/ml) also prevented Ca2+ signaling (Fig. 2B, upper panel), while mock-treated cells (see Materials and Methods for details) responded with oscillations (Fig. 2B, upper panel). However, none of these treatments influenced the cytolytic activity of the toxin on epithelial cells (Fig. 2A and B, lower panels), verifying that the cytolytic activity of Hly occurs via a separate mechanism that differs from the LPS/LBP/CD14-dependent, Hly-induced Ca2+ oscillations.
Hly-induced Ca2+ signaling requires activation of the small GTPase RhoA.
Numerous signaling pathways in eukaryotic cells originate from receptors located in distinct cholesterol-rich membrane compartments called lipid rafts. These compartments recruit and concentrate receptors and signaling molecules, such as CD14. Lipid rafts were recently shown to be crucial for LPS signaling in monocytes and CHO cells (45). Signal components, such as small GTPases of the Rho subfamily (RhoA, Rac, and Cdc42), which may be recruited to lipid rafts, are well-known regulators of the actin cytoskeleton (11). In addition, recent data suggest that RhoA also transduces signals upon cell stimulation, involving PIP2, PLCγ2, and Ca2+ mobilization (39). To address whether lipid rafts and associated signaling components are required for Hly-triggered Ca2+ oscillations, experiments were performed with the isogenic strains ARD20 (wt Hly) and ARD21 (HlyC−). The culture supernatants from these strains have very similar compositions, though ARD20 expresses active Hly, while ARD21 expresses a nonhemolytic proform (31, 47). In addition, the HlyC− mutant is unable to induce intracellular-Ca2+ oscillations in PTC (47). To investigate whether lipid raft structures are involved in mediating Hly-induced Ca2+ oscillation, PTC were treated with the cholesterol-depleting agent methyl-β-cyclodextrin (5 mM) prior to stimulation with sublytic concentrations of Hly from ARD20. We found that this treatment completely abrogated Ca2+ oscillations (Fig. 3A), which suggests that intact lipid rafts are a prerequisite for Hly-induced signaling.
FIG. 3.
Hly-induced cell signaling occurs in lipid rafts and involves RhoA activation. (A) Ratiometric single-cell recording of intracellular Ca2+ in PTC reveals an abrogated Ca2+ response in cells treated with methyl-β-cyclodextrin prior to stimulation with sublytic concentrations of Hly-containing supernatant from ARD20 (130 HU). Three independent experiments were performed, and 15 to 20 cells were recorded in each experiment. (B and C) Affinity precipitation of GTP-RhoA, GTP-Cdc42, and GTP-Rac from untreated cells (lane 1), cells exposed to sublytic concentrations of Hly-containing supernatants from ARD20 (wt Hly) (lane 2), or cells exposed to Hly-containing supernatant from ARD21 (inactive proform of Hly) (lane 3). Panel B shows a statistical overview of the means ± standard deviations for three independent experiments, while panel C shows the details of one representative experiment. Cytotoxic necrotizing factor (CNF)-treated cells were used as a positive control (41). Values were normalized to the total amount of RhoA, Cdc42, and Rac present in the cytosolic extract (indicated as Rho*, Cdc42*, and Rac*) and reported as “Fold increase,” which represents the ratio between the optical density of the GTP-bound GTPase and that of the GTPase present in the cytosolic extract. a.u., arbitrary units.
Affinity-binding assays were performed to investigate whether activation of any of the small GTPases RhoA, Rac, and Cdc42 is required to mediate Hly-induced Ca2+ signaling. Thus, an assay based on the ability of activated GTP-bound forms of the small GTPases to interact with the specific effector domains rhotekin (RhoA) and p21-activated kinase (Rac, Cdc42) was used. GTPase activation levels were analyzed in PTC stimulated with sublytic concentrations of Hly, which in parallel experiments were shown to elicit Ca2+ oscillations. As these were initiated a few minutes after Hly was added, activation of small GTPases was analyzed 15 and 60 min after the addition of Hly-containing culture supernatant. Cells stimulated for 15 min with ARD20 showed a two- to threefold increase in activated RhoA compared to untreated cells (Fig. 3B, lanes 1 and 2, and C, top panel). Activation was specific for RhoA since no increase in GTP-bound Cdc42 or Rac could be detected (Fig. 3B and C, middle and lower panels). In control experiments, we found that none of the small GTPases were significantly activated in cells stimulated with supernatant from ARD21, containing the proform of Hly (Fig. 3B, lane 3, and C). This confirms that activation of RhoA occurs as a consequence of Hly stimulation and is not caused by unspecific stimulation by other bacterial-membrane-associated compounds.
To analyze whether activated RhoA also is functionally involved in mediating Hly-induced Ca2+ signaling, ratiometric Ca2+ imaging experiments in which PTC had been genetically engineered to express an EGFP-tagged dominant-negative mutant form of RhoA (pEGFP-RhoN19) were performed. These cells were identified in the microscope based on their expression of EGFP, while neighboring, nontransfected cells served as internal controls. When cells were exposed to sublytic doses of Hly-containing supernatant from ARD20, the dominant-negative form of RhoA was found to completely abrogate the Ca2+ response. One such cell is depicted as circled in Fig. 4A. In addition, the semicontinuous recording from the same experiment is represented by a continuous line in the graph presented in Fig. 4B. In contrast, the same stimulus was found to induce a prompt Ca2+ response in neighboring, nontransfected cells within the same cell cluster (Fig. 4). Similarly, Ca2+ oscillations were also induced in cells transfected with the pEGFP-C1 vector control (data not shown). In summary, our data suggest that activation of RhoA is a prerequisite for mediating Hly-induced Ca2+ signaling.
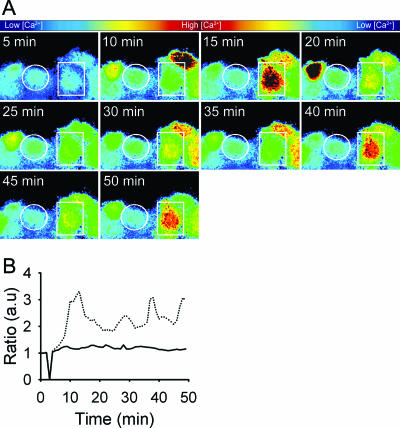
FIG. 4.
Hly-induced cell signaling requires RhoA. Ratiometric single-cell recording of intracellular-Ca2+ concentration in PTC stimulated with sublytic concentrations (130 HU) of Hly containing supernatant from ARD20. (A) Time series of images depicting the intracellular-Ca2+ concentrations in pseudocolor according to the bar. The white circle indicates one cell transfected with dominant-negative RhoA (pEGFP-RhoN19). The boxed cell, as well as surrounding cells, is nontransfected. (B) Single-cell tracings of the circled cell (transfected with dominant-negative RhoA [pEGFP-RhoN19]) (continuous line) and the boxed nontransfected cell (dotted line). A minimum of three independent experiments was performed, and 15 to 20 cells were recorded in each experiment. a.u., arbitrary units.
DISCUSSION
UPEC is the most common causative agent of urinary tract infections (16). During the course of infection, bacteria ascend into the urinary tract. Depending on the virulence factors expressed, bacteria will preferentially colonize the bladder, causing cystitis, or the kidney, resulting in pyelonephritis. In addition to the attachment organelle P pili, the exotoxin Hly is one of the virulence factors that are most frequently associated with pyelonephritogenic isolates of UPEC (16, 32). Functional studies of Hly activity have until recently almost exclusively been focused on the toxin's cytolytic activity. In these experiments, red blood cells are often used as targets, which may not reflect the full biological relevance of Hly. According to the natural route of infection, bacteria first interact with epithelial cells lining the urogenital tract before breakage of this barrier allows bacteria to reach into deeper tissues. Once they reach the renal interstitium, a massive inflammatory response which commonly results in the clearance of bacteria at the cost of irreversible scar formation is initiated (23, 32). On some occasions during the infection process, bacteria may come into contact with the vasculature, where both nucleated cells and erythrocytes may become targets for the cytolytic activity of Hly. However, at early stages of infection, when bacteria ascend through and colonize the urinary tract, Hly may act on the epithelial cells of the tubular linings, thereby directly or indirectly participating in the cross talk between the mucosal lining and inflammatory cells. An important role for Hly in the kinetics of inflammation-related tissue responses during the first 8 h of infection was recently shown using multiphoton-microscopy-based intravital imaging of infected rat cortical proximal tubules (32).
Evidence supporting this hypothesis was reported some years ago when Hly was shown to exert dual, concentration-dependent activities on primary epithelial cells originating from the renal proximal tubules (29). While high toxin concentrations result in cell lysis, sublytic concentrations were shown to elicit oscillatory fluctuations of intracellular-Ca2+ concentration, occurring with a periodicity of 12 min (47). The Ca2+-signaling system is known to operate over a wide temporal range (8), and the slow, Hly-induced Ca2+ oscillation, in which the intracellular-Ca2+ concentration differed within the physiological range of 0.1 μM to 1.5 μM, was found to contribute to the pathophysiology of infection by triggering the production of the proinflammatory cytokines IL-6 and IL-8 (47). Induction of similar oscillating Ca2+ responses has also been reported to be triggered by other pore-forming toxins. Listeriolysin O produced by Listeria monocytogenes elicits Ca2+ oscillations in HEK293 cells (37), while those oscillations induced by pneumolysin from Streptococcus pneumoniae activate the p38 mitogen-activated protein kinase in neuroblastoma cells (43). Thus, it seems that the induction of signal transduction cascades involving oscillating Ca2+ signals is a more widespread activity of pore-forming toxins than previously appreciated.
Interestingly, proinflammatory effects of sublytic doses of Hly have also been reported in neutrophils, where the production of large quantities of lipoxygenase and superoxide is induced (10, 19). Although the details of the signals leading to these responses are unknown, it is interesting to note that the concept of sublytic doses of Hly evoking cellular reactions independently from transmembrane pore formation and cytolysis was hypothesized more than 15 years ago (10).
Hly has long been known to physically interact with LPS; however, the biological role of LPS in toxin activity is unclear (12, 15). Our experiments and previous results reveal that LPS at best plays a marginal or indirect role in the cytolytic activity of Hly, both in erythrocytes and in epithelial cells (6). In contrast, the ability of Hly to induce Ca2+ oscillations strictly requires LPS as a cofactor. Based on data presented in this report, we propose the following model: LPS acts as a carrier molecule that delivers Hly to CD14, which aids in directing this multimolecular complex to the cell membrane, where signaling is initiated. Alternatively, Hly may also directly interact with CD14, which has been shown to interact with other diacylated lipopeptides (42). It is unlikely, however, that CD14 functions as a signaling receptor, since this is a glycosylphosphatidylinositol-linked protein lacking an intracellular signaling domain (46). Although the nature of the signaling receptor is currently unknown, several candidate proteins have been suggested to act as receptors for both sublytic and lytic concentrations of Hly on nucleated cells and erythrocytes (14, 30). Others have suggested that the activity of Hly on target cells depends on the toxins' pore-forming capacities rather than receptor interactions (27). However, these results are difficult to comprehend in light of the fact that the same group has previously published contradictory findings relating to pore-independent signaling of low concentrations of the Hly toxin in nucleated cells (10).
Several bacterial toxins exert their cellular effects by initial interaction with lipid rafts, as exemplified by cholera toxin, anthrax, and listeriolysin (1, 18, 38). Our data suggest that this may also be true for Hly-induced cell signaling, although this must be addressed in further detail. CD14 has been shown to direct LPS to lipid rafts for induction of cell signaling (45), suggesting that the same may occur in CD14-mediated cell targeting of Hly. Due to their dynamic features, lipid rafts can modulate intracellular signal transduction cascades via spatial and temporal recruitment and compartmentalization of relevant signaling molecules. Indeed, we found that disruption of lipid raft integrity abolished Ca2+ signaling. RhoA has been shown to concentrate in lipid rafts, and its interaction with downstream effector molecules leads to intracellular signaling (35). Interestingly, we found that Ca2+ signaling required the recruitment of activated RhoA to the plasma membrane and that the introduction of dominant-negative RhoA completely abolished the Ca2+ oscillations. Further experiments will reveal whether RhoA mediates a direct or indirect link to L-type voltage-operated Ca2+ channels and the IP3 receptor, both known to be required for Hly-induced Ca2+ oscillations (47).
The novel function of the LPS recognition system presented in this study may represent a general mechanism for activation of alternate signal transduction events by other LPS-associated microbial molecules. Whether such molecules are expressed from bacteria expressing smooth or rough LPS is irrelevant, since lipid A is reported as the major CD14-interacting part of LPS (25), and accordingly, CD14 is unable to discriminate between these different chemotypes (22). Similarly, Ca2+ oscillations are induced by Hly originating from strains expressing smooth, rough, and deep-rough LPS (47; data not shown). Thus, we suggest that the common view of LPS as a contaminant in bacterial protein preparations ought to be reevaluated, and LPS should be considered a potential virulence mechanism in targeting exotoxins to the correct membrane compartment.
Acknowledgments
We thank M. Kuehn and C. Oxhamre for materials and technical assistance.
This work was supported by the Swedish Foundation for Strategic Research (A.R.D.), The Royal Swedish Academy of Sciences (A.R.D.), the Swedish Research Council (A.R.D. and T.F.), The Swedish Cancer Society (T.F.), and U.S. Public Health Service grant DK63250 (R.A.W.).
Editor: F. C. Fang
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed, F., L. Meijer, S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell. Microbiol. 4:493-501. [DOI] [PubMed] [Google Scholar]
- 3.Backhed, F., S. Normark, E. K. Schweda, S. Oscarson, and A. Richter-Dahlfors. 2003. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 5:1057-1063. [DOI] [PubMed] [Google Scholar]
- 4.Backhed, F., M. Soderhall, P. Ekman, S. Normark, and A. Richter-Dahlfors. 2001. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell. Microbiol. 3:153-158. [DOI] [PubMed] [Google Scholar]
- 5.Balsalobre, C., J. M. Silvan, S. Berglund, Y. Mizunoe, B. E. Uhlin, and S. N. Wai. 2006. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 59:99-112. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, M. E., and R. A. Welch. 1997. Pleiotropic effects of a mutation in rfaC on Escherichia coli hemolysin. Infect. Immun. 65:2218-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge, M. J., M. D. Bootman, and P. Lipp. 1998. Calcium—a life and death signal. Nature 395:645-648. [DOI] [PubMed] [Google Scholar]
- 8.Berridge, M. J., M. D. Bootman, and H. L. Roderick. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517-529. [DOI] [PubMed] [Google Scholar]
- 9.Bhakdi, S., N. Mackman, J. M. Nicaud, and I. B. Holland. 1986. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect. Immun. 52:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhakdi, S., and E. Martin. 1991. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect. Immun. 59:2955-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop, A. L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 12.Bohach, G. A., and I. S. Snyder. 1985. Chemical and immunological analysis of the complex structure of Escherichia coli alpha-hemolysin. J. Bacteriol. 164:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, L., and W. G. Coleman, Jr. 1993. Cloning and characterization of the Escherichia coli K-12 rfa-2 (rfaC) gene, a gene required for lipopolysaccharide inner core synthesis. J. Bacteriol. 175:2534-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortajarena, A. L., F. M. Goni, and H. Ostolaza. 2001. Glycophorin as a receptor for Escherichia coli alpha-hemolysin in erythrocytes. J. Biol. Chem. 276:12513-12519. [DOI] [PubMed] [Google Scholar]
- 15.Czuprynski, C. J., and R. A. Welch. 1995. Biological effects of RTX toxins: the possible role of lipopolysaccharide. Trends Microbiol. 3:480-483. [DOI] [PubMed] [Google Scholar]
- 16.Emody, L., M. Kerenyi, and G. Nagy. 2003. Virulence factors of uropathogenic Escherichia coli. Int. J. Antimicrob. Agents 22(Suppl. 2):29-33. [DOI] [PubMed] [Google Scholar]
- 17.Frisan, T., X. Cortes-Bratti, E. Chaves-Olarte, B. Stenerlow, and M. Thelestam. 2003. The Haemophilus ducreyi cytolethal distending toxin induces DNA double-strand breaks and promotes ATM-dependent activation of RhoA. Cell. Microbiol. 5:695-707. [DOI] [PubMed] [Google Scholar]
- 18.Gekara, N. O., T. Jacobs, T. Chakraborty, and S. Weiss. 2005. The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell. Microbiol. 7:1345-1356. [DOI] [PubMed] [Google Scholar]
- 19.Grimminger, F., C. Scholz, S. Bhakdi, and W. Seeger. 1991. Subhemolytic doses of Escherichia coli hemolysin evoke large quantities of lipoxygenase products in human neutrophils. J. Biol. Chem. 266:14262-14269. [PubMed] [Google Scholar]
- 20.Hajjar, A. M., R. K. Ernst, J. H. Tsai, C. B. Wilson, and S. I. Miller. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3:354-359. [DOI] [PubMed] [Google Scholar]
- 21.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39:542-552. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, Z., P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, M. Huber, C. Kalis, S. Keck, C. Galanos, M. Freudenberg, and B. Beutler. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6:565-570. [DOI] [PubMed] [Google Scholar]
- 23.Kabore, A. F., M. Simard, and M. G. Bergeron. 1999. Local production of inflammatory mediators in an experimental model of acute obstructive pyelonephritis. J. Infect. Dis. 179:1162-1172. [DOI] [PubMed] [Google Scholar]
- 24.Karow, M., and C. Georgopoulos. 1992. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J. Bacteriol. 174:702-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, J. I., C. J. Lee, M. S. Jin, C. H. Lee, S. G. Paik, H. Lee, and J. O. Lee. 2005. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J. Biol. Chem. 280:11347-11351. [DOI] [PubMed] [Google Scholar]
- 26.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 27.Koschinski, A., H. Repp, B. Unver, F. Dreyer, D. Brockmeier, A. Valeva, S. Bhakdi, and I. Walev. 2006. Why Escherichia coli alpha-hemolysin induces calcium oscillations in mammalian cells—the pore is on its own. FASEB J. 20:973-975. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Laestadius, A., A. Richter-Dahlfors, and A. Aperia. 2002. Dual effects of Escherichia coli alpha-hemolysin on rat renal proximal tubule cells. Kidney Int. 62:2035-2042. [DOI] [PubMed] [Google Scholar]
- 30.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig, A., M. Vogel, and W. Goebel. 1987. Mutations affecting activity and transport of haemolysin in Escherichia coli. Mol. Gen. Genet. 206:238-245. [DOI] [PubMed] [Google Scholar]
- 32.Mansson, L. E., K. Melican, J. Boekel, R. M. Sandoval, I. Hautefort, G. A. Tanner, B. A. Molitoris, and A. Richter-Dahlfors. 2006. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell. Microbiol. [Epub ahead of print.] [DOI] [PubMed]
- 33.Menestrina, G., C. Moser, S. Pellet, and R. Welch. 1994. Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology 87:249-267. [DOI] [PubMed] [Google Scholar]
- 34.Oropeza-Wekerle, R. L., E. Muller, P. Kern, R. Meyermann, and W. Goebel. 1989. Synthesis, inactivation, and localization of extracellular and intracellular Escherichia coli hemolysins. J. Bacteriol. 171:2783-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palazzo, A. F., C. H. Eng, D. D. Schlaepfer, E. E. Marcantonio, and G. G. Gundersen. 2004. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303:836-839. [DOI] [PubMed] [Google Scholar]
- 36.Qiu, R. G., J. Chen, F. McCormick, and M. Symons. 1995. A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. USA 92:11781-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Repp, H., Z. Pamukci, A. Koschinski, E. Domann, A. Darji, J. Birringer, D. Brockmeier, T. Chakraborty, and F. Dreyer. 2002. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell. Microbiol. 4:483-491. [DOI] [PubMed] [Google Scholar]
- 38.Rouquette-Jazdanian, A. K., A. Foussat, L. Lamy, C. Pelassy, P. Lagadec, J. P. Breittmayer, and C. Aussel. 2005. Cholera toxin B-subunit prevents activation and proliferation of human CD4+ T cells by activation of a neutral sphingomyelinase in lipid rafts. J. Immunol. 175:5637-5648. [DOI] [PubMed] [Google Scholar]
- 39.Saci, A., and C. L. Carpenter. 2005. RhoA GTPase regulates B cell receptor signaling. Mol. Cell 17:205-214. [DOI] [PubMed] [Google Scholar]
- 40.Schilling, J. D., M. A. Mulvey, C. D. Vincent, R. G. Lorenz, and S. J. Hultgren. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148-1155. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729.9192900 [Google Scholar]
- 42.Schroder, N. W., H. Heine, C. Alexander, M. Manukyan, J. Eckert, L. Hamann, U. B. Gobel, and R. R. Schumann. 2004. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J. Immunol. 173:2683-2691. [DOI] [PubMed] [Google Scholar]
- 43.Stringaris, A. K., J. Geisenhainer, F. Bergmann, C. Balshusemann, U. Lee, G. Zysk, T. J. Mitchell, B. U. Keller, U. Kuhnt, J. Gerber, A. Spreer, M. Bahr, U. Michel, and R. Nau. 2002. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol. Dis. 11:355-368. [DOI] [PubMed] [Google Scholar]
- 44.Teghanemt, A., D. Zhang, E. N. Levis, J. P. Weiss, and T. L. Gioannini. 2005. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 175:4669-4676. [DOI] [PubMed] [Google Scholar]
- 45.Triantafilou, M., K. Miyake, D. T. Golenbock, and K. Triantafilou. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603-2611. [DOI] [PubMed] [Google Scholar]
- 46.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 47.Uhlen, P., A. Laestadius, T. Jahnukainen, T. Soderblom, F. Backhed, G. Celsi, H. Brismar, S. Normark, A. Aperia, and A. Richter-Dahlfors. 2000. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405:694-697. [DOI] [PubMed] [Google Scholar]
- 48.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welch, R. A., R. Hull, and S. Falkow. 1983. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect. Immun. 42:178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiese, A., M. Munstermann, T. Gutsmann, B. Lindner, K. Kawahara, U. Zahringer, and U. Seydel. 1998. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J. Membr. Biol. 162:127-138. [DOI] [PubMed] [Google Scholar]