Abstract
Transgenic Leishmania parasites that encode the murine chemokine monocyte chemoattractant protein 1 (MCP-1) were generated. These parasites transcribed MCP-1 mRNA and secreted MCP-1 protein. Infection of BALB/c, C57BL/6, or MCP-1 knockout (KO) mice with these parasites resulted in minimal lesion development with fewer parasites in the infected foot, lymph node, and spleen compared to wild-type-infected mice. In contrast, transgenic parasites caused substantial lesions with relatively high numbers of parasites in CC chemokine receptor 2 (CCR2) KO mice, indicating that the parasites are viable and healthy and that the lack of lesion development is CCR2 dependent. Prior infection of mice with transgenic parasites offered no protection to subsequent wild-type L. major challenge, suggesting that the transgenic parasites are controlled by an early innate immune response. Consistent with innate immunity, flow cytometry of cells from the ears of mice infected with transgenic parasites revealed an increase in the number of CCR2-positive macrophages by day 7 postinfection. The enumeration of transgenic parasites in ear lesions demonstrated a significant reduction in parasite numbers, which coincided with the increased CCR2-positive macrophage migration. CCR2-positive macrophages isolated from ears of mice infected with transgenic parasites contained virtually no parasites. In vitro studies revealed that optimal parasite killing required the recruitment of CCR2-positive macrophages, followed by stimulation with a combination of both MCP-1 and gamma interferon (IFN-γ). This work suggests that the parasite-derived MCP-1 can recruit a restrictive population of CCR2-positive macrophages into lesions that can be optimally stimulated by MCP-1 and IFN-γ to efficiently kill Leishmania parasites.
Leishmania organisms are obligate intracellular parasites that reside primarily within host tissue macrophages. They can cause a wide spectrum of diseases ranging from a self-healing cutaneous form to a potentially fatal visceral state (4). The disease outcome often depends on the particular Leishmania sp. and strain causing the disease and the host's immune response. It has been suggested that the host's early innate immune response is critical for parasite containment and for the resolution of disease (18, 19, 40).
Chemokines are small chemotactic proteins that are divided into four families based on the number and positioning of the N-terminal cysteines (29, 37). Chemokines regulate both innate and adaptive immune responses by coordinating leukocyte trafficking with immune cell differentiation and effector functions (20). As a result, chemokines and their receptors play a critical role in the development of immunity against a wide variety of pathogens (28).
Monocyte chemoattractant protein 1 (MCP-1) is a CC chemokine known to attract monocytes, dendritic cells (DCs), natural killer cells, and memory T lymphocytes (8, 29). CC chemokine receptor 2 (CCR2) the receptor that binds MCP-1 (28, 29), has been shown to be vital for host defense to a number of pathogens (11, 12, 17, 30, 36, 38, 41, 42). Likewise, it has been suggested that MCP-1/CCR2 may play a variety of roles in host defense against Leishmania (38). It has been reported that high doses of MCP-1 activate anti-Leishmania macrophage killing mechanisms either directly (23), by inducing reactive oxygen intermediates (33), or via nitric oxide production (5-7). In addition, MCP-1 has been reported to activate NK cells (47). Likewise, mice normally resistant to L. major infection become susceptible when lacking the CCR2 receptor (38). In human leishmaniasis, there is evidence of elevated MCP-1 levels in localized self-healing cutaneous lesions. In contrast, there is no detectable MCP-1 in diffuse, nonhealing cutaneous lesions (25, 32, 34).
Recently, a subset of CCR2-positive monocytes has been identified and shown to migrate into inflammatory sites. It was suggested that this population of cells may play a role in pathogen clearance (9, 10, 45). In addition, CCR2-positive tumor necrosis factor/inducible nitric oxide synthase-producing DCs (42) and CCR2-positive Gr-1-positive activated macrophages (26, 36) have also been shown to play an active role in pathogen clearance in particular infections.
We wanted to take advantage of the strong correlation between CCR2 expression and host defense; thus, we genetically engineered transgenic MCP-1 secreting Leishmania parasites to specifically recruit CCR2-positive cells during leishmaniasis. To our knowledge, this is the first example of Leishmania parasites that have been engineered to recruit specific immune cells to the site of infection. We hypothesized that the parasites that we developed would recruit particular cell populations and that this approach would allow us to correlate the lack of lesion progression with specific immune cell recruitment.
MATERIALS AND METHODS
Animal studies.
These studies were reviewed and approved by the University of Maryland Institutional Animal Care and Use Committee. BALB/c and C57BL/6 mice were purchased from Taconic (Rockville, MD). CCR2 knockout (KO) mice on a C57BL/6 background and C5-deficient mice were purchased from Jackson Laboratories (Bar Harbor, Maine). MCP-1 KO mice (21) on a BALB/c background were obtained from Barrett Rollins (Harvard Medical School, Boston, MA).
Cell and parasite culture.
D10 media, which consists of Dulbecco's modification Eagle's medium (Fisher Scientific, Pittsburgh, PA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT), 100 U of penicillin/ml (Fisher Scientific), 100 μg of streptomycin/ml (Fisher Scientific), and 2 mM glutamine (Fisher Scientific), was used to culture peritoneal and bone marrow-derived macrophages (BMDM). Both wild-type and transgenic parasites were grown in 50:50 media (50% Schneider's insect medium [Sigma-Aldrich, St. Louis, MO] supplemented with 20% FBS, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM glutamine, and 50% M199 media [Invitrogen, Rockville, MD]). Transgenic parasites were grown in the presence of 100 μg of nourseothricin (SAT; WERNER BioAgents, Germany)/ml. Leishmania amazonensis axenic amastigotes were cultured as previously described (46).
Blood agar plates were prepared as previously described (16) containing SAT. A concentration of 100 μg of SAT/ml was used to select for transgenic L. major parasites, whereas 50 μg of SAT/ml was used to select for transgenic L. amazonensis parasites.
Parasites, infection, and parasite quantitation.
Lesion-derived wild-type and transgenic L. major (WHO MHOM/IL/80/Friedlin) and L. amazonensis (WHOM/BR/75/Josefa) were isolated from infected mice as previously described (27).
Mice were injected in the right hind footpad with 1 × 105 or 5 × 106 wild-type or transgenic stationary L. major promastigotes, depending on the experiment. Lesion size was determined by using a caliper to measure the thickness of the infected footpad and subtracting the thickness of the contralateral uninfected footpad as described previously (1). For ear infections, mice were injected in the right and left ears with 5 × 104 wild-type or transgenic L. major parasites. Ear lesion progression was monitored by measuring the diameter of the lesion using an Absolute Digimatic Caliper (Mitutoyo, Ontario, Canada) as previously described (3). For protection experiments, C57BL/6 mice were infected with 5 × 104 wild-type L. major or transgenic L. major MCP-1 parasites in the right footpad. After 5 weeks, these mice, along with uninfected (unprotected) mice, were infected in both ears with 105 wild-type L. major parasites and monitored for lesion development over the next 5 weeks. For all of the in vivo experiments, error bars represent the standard error of the mean of three separate experiments done with a minimum of five mice per group.
Parasite burdens were determined by serial dilution of single cell suspensions made from excised footpads, ears, lymph nodes, or spleens as previously described (1).
SOE-PCR.
Two different PCR products were created in separate PCRs and used to generate a hybrid “gene splicing by overlapping extension” (SOE)-PCR product (13, 14) that begins with a 5′-SmaI restriction site followed by the L. major gp63 signal sequence and the entire murine MCP-1/JE gene and ends with a 3′-SmaI site (Fig. 1A). The first PCR generated a 148-bp PCR product that contained a 5′-SmaI restriction site followed by the L. major gp63 signal sequence and a short sequence corresponding to the murine MCP-1/JE gene. This fragment was created by using the following primers: sense, 5′-TATCCCGGGATGTCCGTCGACAGCAG-3′, and antisense, 5′-GCATGACAGGGACCTGAGCGGCGTGTGCCCACGC-3′. These primers were used, along with a plasmid template that contained the entire L. major gp63 gene (accession no. Y00647). The second PCR generated a 450-bp PCR product that contained the murine MCP-1/JE gene, followed by a 3′-SmaI restriction site, using the following primers: sense, 5′-GCTCAGGTCCCTGTCATGCTTCTGGGC-3′, and antisense, 5′-TACCCGGGGTTCACTGTCACACTGGTCACTCCTAC-3′. These primers were used along with the template pORF mMCP-1 (Invivogen, San Diego, CA) to create MCP-1 PCR#2.
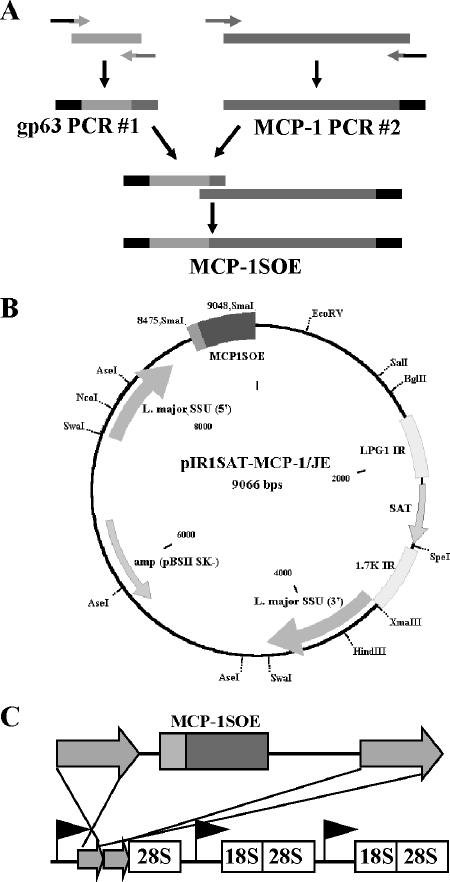
FIG. 1.
Generation of transgenic MCP-1 Leishmania parasites. (A) gp63 PCR#1 and MCP-1 PCR#2 were amplified in separate PCRs and then spliced together to generate the hybrid PCR product MCP-1SOE. (B) MCP-1SOE was ligated into the Leishmania expression plasmid, pIR1SAT. (C) pIR1SAT-MCP-1/JE was linearized and transfected into Leishmania parasites to promote stable integration into the Leishmania genome via homologous recombination.
The hybrid PCR product MCP-1SOE was created by using gp63 PCR#1 and MCP-1 PCR#2 as templates (Fig. 1A). The PCR product was purified by using a PCR purification kit (QIAGEN) and ligated into the TA cloning vector, pCRII (Invitrogen).
The hybrid PCR product was excised from the TA cloning vector with SmaI, gel purified, and ligated into the multiple cloning site of the Leishmania expression plasmid, pIR1SAT (Fig. 1B), which was generously provided by Steven Beverley (Washington University, St. Louis, MO). The ligated expression plasmid, pIR1SAT-MCP-1/JE was transformed into Max Efficiency DH10B competent cells (Invitrogen) and isolated by using a plasmid maxi kit (QIAGEN). The plasmid was digested with SwaI and transfected into Leishmania parasites to permit integration into the parasite genome (Fig. 1C).
Electroporation.
L. major or L. amazonensis parasites (108 parasites) were resuspended in 400 μl of electroporation buffer (21 mM HEPES [pH 7.5], 137 mM NaCl, 5 mM KCl, 0.7 mM Na2PO4, and 6 mM glucose). This suspension was mixed with 5 μg of linearized pIR1SAT-MCP-1/JE, added to a 0.4-cm Gene Pulser cuvette (Bio-Rad, Hercules, CA), and electroporated (0.5 kV, 0.5 μF) using Bio-Rad's Gene Pulser II. The cuvette was put on ice for 10 min. Electroporated parasites were added to blood agar plates containing SAT.
RNA isolation.
RNA was isolated from 106 wild-type or transgenic L. major or L. amazonensis parasites during either promastigote or axenic development using TRIzol (Invitrogen). The RNA was converted to cDNA using the manufacturer's protocol. Murine MCP-1/JE was amplified from the cDNA samples using the following primers: sense, 5′-GCTCAGGTCCCTGTCATGCTTCTGGG-3′, and antisense, 5′-GTTCACTGTCACACTGGTCACTCCTAC. gp63 was amplified by using the following primers: sense, 5′-ATCCTCACCGACGAGAAGCGCGAC-3′, and antisense, 5′-ACGGAGGCGACGTACAACACGAAG-3′.
Transgenic parasite MCP-1 production.
Costar high-binding enzyme-linked immunosorbent assay (ELISA) plates (Fisher Scientific) were coated with monoclonal goat anti-mouse MCP-1/JE antibody (capture antibody) from the DuoSet MCP-1/JE ELISA kit (R&D Systems, Minneapolis, MN). Wild-type or transgenic parasites (5 × 106) were added to the ELISA plate wells for 24 h. The following day, the parasites were washed away, and the MCP-1 ELISA was completed according to the manufacturer's protocol using biotinylated anti-mouse MCP-1/JE detection antibody, streptavidin-horseradish peroxidase, and horseradish peroxidase substrate.
Leishmania survival assay and staining.
Peritoneal macrophages were isolated from peritoneal cavities using cold phosphate-buffered saline (PBS) as previously described (27). Macrophages (105) were added to coverslips in 100-μl bubbles for 2 h to allow macrophage attachment. D10 medium was added, and the cells were rested for 2 h. Prior to infection, L. major promastigotes were opsonized with 10% serum from C5-deficient mice for 20 min at room temperature. The peritoneal macrophages were then infected at a multiplicity of infection (MOI) of 10:1 using opsonized wild-type or transgenic promastigotes for 2 h. Cells were washed and fixed with methanol for 10 min immediately after infection or after further 72 h of incubation. The experiment was also performed using lesion-derived amastigotes from either wild-type or transgenic infected CCR2 KO mice. The amastigotes were used at an MOI of 10:1 and added for 1 h before washing. Cells were fixed immediately after 1 h or after a further 72 h of incubation. For immunofluorescence visualization, glass coverslips were blocked with PBS containing 5% FBS. Mouse anti-Leishmania serum (1:250) was used to stain Leishmania amastigotes for 1 h at 4°C. The coverslips were then washed twice and a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG; H+L; Jackson Immunoresearch) at 1:100 was added for 30 min. After the coverslips were washed again, they were treated with a 1:1,000 dilution of 1 mg of propidium iodide/ml for 2 min to stain the macrophage nucleus. Coverslips were then mounted onto slides using MOWIOL (EMD Biosciences, San Diego, CA).
Parasite-derived MCP-1 intracellular staining.
BMDM were prepared from MCP-1 KO mice as previously described (43). Macrophages were plated, infected for 48 h with C5-deficient serum opsonized wild-type or transgenic MCP-1 parasites, and fixed as described above. Coverslips were blocked with 6% bovine serum albumin for 1 h prior to staining. Parasite-derived MCP-1 was stained with 100 ng of goat anti-mouse MCP-1 antibody (R&D Systems)/ml for 45 min at 4°C, followed by rhodamine (i.e., TRITC [tetramethyl rhodamine isothiocyanate])-conjugated donkey anti-goat IgG (Jackson Immunoresearch) at 1:100 for 30 min at 4°C. The parasites were then stained as described above using mouse anti-Leishmania serum for 45 min, along with FITC-conjugated rabbit anti-mouse IgG (Jackson Immunoresearch) at 1:100 for 30 min at 4°C. The macrophage nucleus was stained with Hoechst stain (1:4,000) for 2 min. Coverslips were mounted as described above.
[3H]thymidine incorporation assay.
Lymph node cells were isolated from C57BL/6 mice that were infected with wild-type or transgenic L. major parasites in the footpad. At 1, 2, and 3 weeks postinfection, cells (5 × 105) from each group were added to a 96-well round-bottom plate and stimulated with 25 μg of soluble Leishmania antigen (SLA) for 72 h. After 72 h, 1 μCi of [3H]thymidine (MP Biomedicals, Inc., Irvine, CA) was added to each well and mixed with the stimulated and unstimulated lymphocytes for 6 h. Proliferation was measured by using a Cell Harvester 96 (Tomtec, Hamden, CT) and a 1450 Microbeta Trilux liquid scintillation and luminescence counter (EG&G Wallac, Finland). SLA was prepared as previously described (39).
Cytokine measurement.
Gamma interferon (IFN-γ) and interleukin-4 (IL-4) were measured from 100-μl supernatants of SLA-stimulated lymphocytes by sandwich ELISA using capture (clone R4-6A2) and detection (clone XMG1.2) anti-IFN-γ antibodies and capture (clone 11B11) and detection (clone BVD6-24G2) anti-IL-4 antibodies (BD Pharmingen, San Diego, CA).
Isolation of cells from infected mouse ears.
Ears infected with wild-type or transgenic L. major MCP-1 parasites were excised, soaked in 70% ethanol, and air dried for 5 to 10 min. The ears were then split into ventral and dorsal portions and placed in Liberase (5 mg/ml; Roche, Indianapolis, IN) diluted 1:100 in PBS for 2 h at 37°C as previously described (3). The ears were put into a 50 μM Medicon homogenizer (BD Biosciences) with 1 ml of PBS and homogenized in BD's Medimachine (BD Biosciences) for 1 min. The liquefied, homogenized ears were then passed through a 50-μm-pore-size syringe Filcon filter (BD Biosciences) and centrifuged at 300 × g for 10 min. The cells isolated from the ears were then resuspended in PBS containing 5% FBS and 5 mM EDTA and labeled with antibodies for flow cytometry.
Flow cytometry.
Cells isolated from ears were labeled with the following antibodies: phycoerythrin-conjugated anti-mouse F4/80 (clone BM8; eBioscience, San Diego, CA), PerCp-Cy5.5-conjugated anti-mouse CD11b (clone M1/70), and FITC-conjugated anti-mouse Gr-1 (clone RB6-8C5; BD Pharmingen). MC-21 (rat anti-mouse CCR2) (22) was provided by Matthias Mack (University of Munich, Munich, Germany) and used along with the secondary antibody FITC-conjugated goat anti-rat immunoglobulin (BD Biosciences) to identify CCR2-positive cells. Cells were sorted by using BD FACSAria (BD Biosciences) at Johns Hopkins Bloomberg School of Public Health.
Staining sorted, infected CCR2-positive macrophages.
Sorted CCR2-positive macrophages were cytospun by using Cytospin 4 (Thermo Shandon, Pittsburgh, PA) at 600 rpm for 6 min. Cells were fixed with methanol and then stained in 1:20 diluted Giemsa stain (Sigma-Aldrich) for 20 to 30 min. The number of parasites per 100 CCR2-positive macrophages was counted with a maximum of 10 parasites per macrophage as a limit for heavily infected macrophages.
MCP-1 coactivation in vitro studies.
Mice were intraperitoneally injected with 1 ng of recombinant MCP-1 (Peprotech, Inc., Rocky Hill, NJ) or PBS. Three days later peritoneal macrophages were isolated from both groups and plated as described above. Both sets of macrophages were either unstimulated or treated with 100 U of IFN-γ, 1 ng of MCP-1, or both IFN-γ and MCP-1 for 10 h. The cells were then washed twice with warm PBS, and fresh D10 was added. Infection was done at an MOI of 10:1 of wild-type L. major parasites for 2 h. Staining was performed as described above. Intracellular survival was analyzed at 0 and 72 h postinfection.
RESULTS
Generation of transgenic MCP-1-secreting Leishmania parasites.
MCP-1-secreting Leishmania parasites were engineered by using SOE-PCR (13, 14), a technique that led to the construction of a hybrid PCR product containing the L. major gp63 signal sequence attached to the murine MCP-1/JE gene (Fig. 1A). This product was inserted into the multiple cloning site of pIR1SAT, a Leishmania expression plasmid (Fig. 1B). Wild-type L. major and L. amazonensis were transfected with linearized pIR1SAT-MCP-1/JE (Fig. 1C).
Transgenic L. major MCP-1 and L. amazonensis MCP-1 transcribed MCP-1 mRNA during the promastigote stage of development (Fig. 2A). Wild-type-nontransfected parasites did not transcribe MCP-1 mRNA as expected. Axenically grown amastigotes of transgenic L. amazonensis MCP-1 also transcribed murine MCP-1 (Fig. 2A). The level of MCP-1 produced by these parasites during the extracellular promastigote stage of development was measured by ELISA. Transgenic L. major MCP-1 and L. amazonensis MCP-1 secreted 161 ± 14.6 and 199 ± 51 pg/ml, respectively (Fig. 2B). Wild-type parasites produced no detectable MCP-1, as expected. The two clones represented in Fig. 2B secreted the highest level of MCP-1 of the parasites tested. In order to verify that the transgenic parasites did not lose their ability to secrete MCP-1 in vivo, BALB/c mice were infected with transgenic parasites, and 4 weeks later the parasites were isolated from footpads and screened for MCP-1 production. Despite the absence of antibiotic selective pressure in the mouse, the transgenic parasites isolated from the infected footpads continued to secrete high levels of MCP-1 after isolation (Fig. 2C). This expression in the absence of selection is consistent with the stable integration of the linearized constructs into the Leishmania genome.
FIG. 2.

Transgenic parasites express murine MCP-1. (A) Total RNA was isolated from wild-type and transgenic MCP-1 parasites during promastigote development. Murine MCP-1/JE was amplified from the cDNA of transgenic L. major MCP-1 (Lm-Tg) and transgenic L. amazonensis MCP-1 (La-Tg) parasites but not from the cDNA of wild-type L. major (Lm-WT) or wild-type L. amazonensis (La-WT). Axenically grown L. amazonensis MCP-1 and wild-type L. amazonensis amastigotes were also analyzed. MCP-1/JE was amplified from transgenic amastigotes but not from the wild type. The gene gp63 was amplified from the cDNA as a loading control. (B) A total of 5 × 106 transgenic L. major, wild-type L. major, transgenic L. amazonensis, or wild-type L. amazonensis parasites was added to an ELISA plate coated with monoclonal goat anti-mouse MCP-1 antibody. After 24 h, the levels of MCP-1 production were determined. (C) MCP-1 production by transgenic L. major MCP-1 parasites grown in culture (Tg-C, □) was determined by ELISA. MCP-1 production by transgenic parasites isolated from two different mice infected 4 weeks prior (Tg-M1 and Tg-M2, ▨) or wild-type L. major parasites (WT-M3, ▪) is also shown. **, P < 0.01.
The presence of parasite-derived MCP-1 was examined in L. major MCP-1-infected BMDM from MCP-1 KO mice. Parasite-derived MCP-1 was detected in the cytoplasm of transgenic MCP-1-infected BMDM (Fig. 3A) but not in wild-type-infected macrophages (Fig. 3B), confirming that the transgenic parasites are capable of secreting MCP-1 during their intracellular stage of development. MCP-1 was not detected in the supernatants of transgenic MCP-1-infected BMDM (MCP-1 KO) by ELISA during various time points up to 96 h postinfection (Fig. 3C). However, considerable amounts of MCP-1 (318 ± 3 pg/ml) were detected in the supernatant after the transgenic MCP-1-infected macrophages were lysed at 96 h postinfection. Macrophages infected with wild-type parasites did not release MCP-1 after lysing as expected (Fig. 3C). These results suggest that the parasite-derived MCP-1 does not leak out of the infected cells and must be released at the same time the amastigotes are released when the infected cells burst.
FIG. 3.

Parasite-derived MCP-1 expressed during intracellular growth. (A and B) BMDM from MCP-1 KO mice were infected with transgenic (A) or wild-type (B) L. major parasites at an MOI of 10:1. After 48 h, the infected cells were washed, fixed with methanol, and stained with separate polyclonal antibodies against Leishmania and murine MCP-1. Nuclei were counterstained with Hoechst stain. (C) BMDM were infected as described above. Supernatants were collected at 0 and 96 h (96-S) postinfection. In addition, cells infected for 96 h were lysed (96-L) using 0.01% Triton X plus 5 mM MgCl2 in H2O. An MCP-1 ELISA was used to detect the level of MCP-1. **, P < 0.01.
The metabolic activity of the transgenic parasites was evaluated and compared to wild-type parasites by measuring their ability to reduce 3-(4,5-dimethylthiazol-2-ly)-2,5-diphenlytetrazolium bromide (MTT) to formazan. Transgenic L. major MCP-1 was able to reduce MTT to formazan, and the rate and extent of MTT reduction was not different between wild-type and transgenic parasites (data not shown).
To verify that the transgenic parasites were as infective as the wild-type parasites, we infected resting resident peritoneal macrophages separately with either wild-type or transgenic promastigotes and measured the intracellular survival 72 h later. The resting resident peritoneal macrophages that were used in this assay express the CCR2 receptor and are easily infected by Leishmania. Equal numbers of infected macrophages were found at 0 h (data not shown). At 72 h, there was an equivalent number of wild-type L. major (Fig. 4A) and L. major MCP-1 (Fig. 4B) present inside the macrophages. Similar results were obtained with the amastigote form. The numbers of wild-type and transgenic amastigotes present inside infected macrophages were similar at 1 h postinfection (data not shown), and these numbers remained similar at 72 h postinfection (Fig. 4C and D).
FIG. 4.
Transgenic parasites efficiently infect monolayers of resident macrophages. Peritoneal macrophages (105) were infected at an MOI of 10:1 for 2 h with wild-type (Lm-WT) (A) or transgenic (Lm-Tg) (B) L. major promastigotes. After a 72-h incubation, monolayers were washed, fixed, and stained with a polyclonal antibody to Leishmania. Nuclei were counterstained with propidium iodide. The experiment was also performed using lesion-derived amastigotes from either wild-type (C) or transgenic (D) L. major-infected CCR2 KO mice. The amastigotes were used at an MOI of 10:1 and added for 1 h before washing. Cells were fixed and stained after 72 h of incubation.
Lack of lesion development in mice infected with transgenic L. major MCP-1.
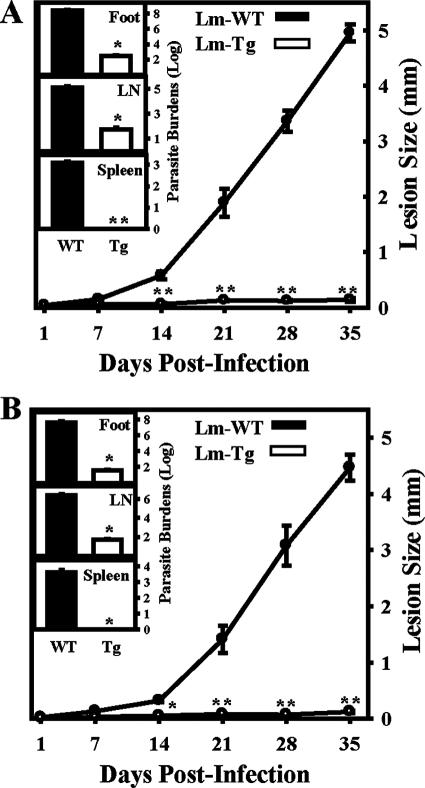
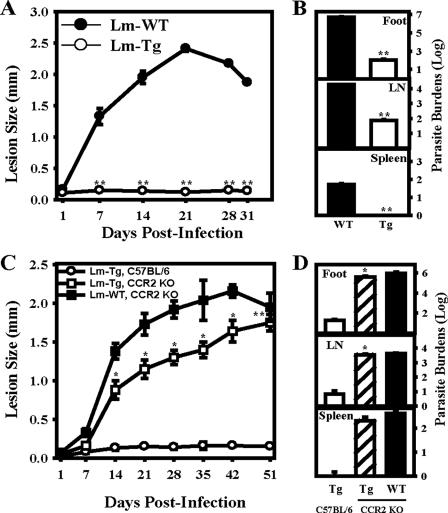
We infected BALB/c mice in the hind footpads with 105 transgenic L. major- MCP-1 or wild-type L. major parasites and monitored lesion development over 35 days. The transgenic L. major MCP-1 caused little to no detectable lesions, with the mean peak swelling of only 0.14 ± 0.03 mm (Fig. 5A). In contrast, wild-type L. major-infected mice developed progressively larger lesions, which reached an average diameter of 4.95 ± 0.15 mm. When parasites were isolated from the foot, lymph node, and spleen after 35 days, there were significantly fewer parasites in the feet of the transgenic L. major MCP-1-infected mice (274 ± 38 parasites) than in the wild-type-infected mice (2.17 × 108 ± 6 × 107 parasites) (Fig. 5A, inset). There were also fewer parasites in the lymph nodes (54 ± 23 compared to 1.22 × 105 ± 3.7 × 104), and no transgenic parasites were detected in the spleen. In these initial infectivity experiments, L. major parasites that were transfected with the empty plasmid (L. major pIR1SAT) were also used. They caused lesions that were comparable in size to the those of the wild-type-infected mice and had comparable numbers of parasites (data not shown).
FIG. 5.
Lack of lesion development in transgenic L. major-infected mice. (A) Lesion sizes of BALB/c mice infected with 105 wild-type L. major (Lm-WT) parasites in the footpad (•) were compared to those of BALB/c mice infected with transgenic L. major (Lm-Tg, ○). Lesions were measured in weekly intervals. Parasite burdens (inset) were determined for wild-type (▪) and transgenic (□) L. major on day 35 postinfection by limiting dilution assays. Parasite burden isolation was performed on footpads, lymph nodes (LN), and spleens. (B) MCP-1 KO mice on a BALB/c background were infected with wild-type (•) or transgenic (○) L. major as described above. Lesion size and parasite burdens were determined as described above. For the experiments in both panels A and B, error bars represent the standard error of the mean of three separate experiments done with a minimum of five mice per group. *, P < 0.05; **, P < 0.01.
An identical infection was performed in mice lacking the MCP-1/JE gene (21). Similar to what was observed in BALB/c mice, the MCP-1 KO mice developed essentially no lesions (0.12 ± 0.02 mm) by day 35 after infection with L. major-MCP-1 (Fig. 5B). The mice also had minimal to no parasites in the foot (37 ± 13), lymph nodes (50 ± 15), and spleen (0 ± 0) (Fig. 5B, inset). Infection of the MCP-1 KO mice with wild-type L. major resulted in substantial lesion development (4.47 ± 0.43 mm) and higher numbers of parasites in the foot (4.43 × 107 ± 1.45 × 107), lymph nodes (2.66 × 106 ± 8.8 × 105), and spleen (4,300 ± 1914). These results suggest that the parasite-derived MCP-1 was sufficient to induce the healing phenotype that was observed after infection with transgenic L. major MCP-1.
Lesion differences in transgenic infected C57BL/6 and CCR2 KO mice.
To evaluate the infectivity of our transgenic parasites in a more resistant strain of mouse, we proceeded with infections in C57BL/6 mice. These mice were infected in the hind footpad with 5 × 106 transgenic L. major MCP-1 or wild-type L. major parasites, and lesion development was monitored over the course of 31 days. The transgenic parasites caused essentially no lesions (0.12 ± 0.01 mm), whereas the wild-type-infected mice developed lesions that reached maximum size on day 21 (2.41 ± 0.04 mm) (Fig. 6A). Consistent with the healing phenotype of these mice, the lesions had diminished to 1.87 ± 0.02 mm by day 31 postinfection. Mice infected with transgenic parasites contained fewer parasites in the foot (108 ± 23), lymph nodes (74 ± 13), and spleen (0 ± 0) than did the wild-type-infected mice (foot [4.85 × 106 ± 5.5 × 105], lymph node [1.9 × 104 ± 6.54 × 102], and spleen [52 ± 7]) (Fig. 6B). Thus, mice that are relatively resistant to infection with wild-type L. major parasites show significantly higher resistance to infection with the transgenic L. major MCP-1 parasites.
FIG. 6.
Lesion differences in transgenic infected C57BL/6 and CCR2 KO mice. (A and B) C57BL/6 mice were infected with 5 × 106 wild-type (Lm-WT, •) or transgenic (Lm-Tg, ○) L. major, and lesion development was measured at various time points (indicated in panel A). (C and D) CCR2 KO mice were similarly infected with wild-type (▪) or transgenic (□) L. major and compared to infections with transgenic L. major in C57BL/6 mice (○). Parasite numbers were quantitated at day 31 for C57BL/6 mice (B) and at day 51 for the CCR2 KO mice (D). Error bars represent the standard error of the mean of two (panel C) or three (panel A) separate experiments done with a minimum of five mice per group. *, P < 0.05; **, P < 0.01.
A similar study was performed in CCR2 KO mice on a C57BL/6 background. Lesion progression was measured over a 51-day period. Infection of CCR2 KO mice with either transgenic L. major MCP-1 or wild-type parasites resulted in similar lesion progression (Fig. 6C). On day 51, wild-type parasites caused lesions of 1.95 ± 0.18 mm, whereas transgenic parasites caused lesions of 1.75 ± 0.11 mm. The number of parasites on day 51 in the transgenic L. major-infected CCR2 KO foot (4.16 × 105 ± 1 × 105), lymph nodes (3475 ± 664), and spleen (210 ± 84) were comparable to the number of parasites in CCR2 KO mice infected with wild-type L. major parasites; foot (6.2 × 105 ± 2 × 105), lymph nodes (4250 ± 342), and spleen (425 ± 53) (Fig. 6D). Parallel infection of C57BL/6 mice with transgenic parasites caused essentially no lesions (0.15 ± 0.01 mm), as previously described with fewer parasites in the foot, lymph node, and spleen. These observations suggest that the presence of CCR2, the receptor for MCP-1, on cells that are involved in the immune response against the transgenic parasites is required for the healing phenotype that we observe.
Lack of adaptive immunity in mice infected with transgenic parasites.
We were interested in determining whether the avirulent transgenic parasites could stimulate T-cell responses. C57BL/6 mice were used in these studies because previous reports have shown that they are an excellent model for studying immunological memory and protection against L. major (44). Lymphocytes isolated from the draining lymph nodes of mice infected with transgenic L. major MCP-1 on weeks 1, 2, and 3 postinfection proliferated poorly in response to SLA stimulation (Fig. 7A, open bars), whereas mice infected with wild-type parasites exhibited strong proliferative responses (Fig. 7A). These SLA-stimulated lymphocytes from transgenic L. major MCP-1-infected mice secreted virtually no IFN-γ (Fig. 7B) or IL-4 (Fig. 7C), whereas lymphocytes isolated from wild-type-infected mice secreted significantly higher levels of IFN-γ (Fig. 7B) and IL-4 (Fig. 7C). There was essentially no spontaneous lymphocyte proliferation or cytokine production from either group in the absence of SLA stimulation (data not shown). These data suggest that the adaptive immune response plays a minimal role in the clearance of transgenic MCP-1 parasites.
FIG. 7.
Lack of adaptive immune response in transgenic L. major-infected mice. (A) C57BL/6 mice were infected with 5 × 104 wild-type L. major (Lm-WT) or transgenic L. major (Lm-Tg) parasites in the footpad. Popliteal lymph nodes were isolated on weeks 1, 2, and 3 postinfection. The proliferation of 5 × 105 lymph node cells from mice infected with wild-type (▪) or transgenic (□) L. major was measured by determining the [3H]thymidine incorporation after stimulation with SLA. (B and C) IFN-γ (B) or IL-4 (C) levels were measured by ELISA after stimulation of the lymph node T cells from mice infected with wild-type (▪) or transgenic (□) L. major. (D) C57BL/6 mice were vaccinated with 5 × 104 viable wild-type or transgenic L. major parasites in the right footpad. Footpad lesions were monitored for 5 weeks. After 5 weeks, when the lesions had resolved, 105 wild-type L. major parasites were injected into the ears of unprotected (▴), wild-type L. major-protected (•), and transgenic L. major-protected (○) C57BL/6 mice. Ears were monitored over the next 5 weeks for lesion development. (E and F) Parasite burdens in the ears (E) and lymph nodes (LN) (F) were measured at 5 weeks. The data are expressed as means plus the standard errors of the mean of three experiments with a minimum of five mice per group. *, P < 0.05; **, P < 0.01.
This lack of adaptive immunity was confirmed in a vaccination study wherein C57BL/6 mice were infected in the right footpad with 5 × 104 transgenic L. major MCP-1 or wild-type parasites. After 5 weeks when the lesions were resolving, these mice, along with an unprotected C57BL/6 group, were challenged in the ear with 105 wild-type L. major parasites as previously described (44). The ears were monitored over the course of the next 5 weeks for lesion development (Fig. 7D). The unprotected group of mice developed progressive lesions as expected (Fig. 7D), with substantial numbers of parasites in the ears (Fig. 7E) and lymph nodes (Fig. 7F). Mice vaccinated with wild-type parasites were more resistant, developing smaller lesions (Fig. 7D) with few detectable parasites in the ears (Fig. 7E) and lymph nodes (Fig. 7F). Mice vaccinated with transgenic L. major MCP-1, however, developed large lesions (Fig. 7D, open circles) with high numbers of parasites in the ears (Fig. 7E) and lymph nodes (Fig. 7F). By week 5, these lesions had begun to spread over the entire length of the ear and were necrotic (data not shown). Thus, prior infection of mice with avirulent transgenic L. major MCP-1 parasites did not provide any protection against subsequent challenge with wild-type parasites. In fact the mice developed larger lesions with high levels of parasites.
The control of transgenic parasite infection coincides with an increased migration of CCR2-positive macrophages.
We quantitated the migration of CCR2-positive (CD11b-positive, F4/80-positive, Gr-1-negative) macrophages into the ears of mice infected with transgenic L. major MCP-1 parasites during early stages of the infection. BALB/c mice were infected in the ears with either transgenic L. major MCP-1 or wild-type parasites. On various days postinfection, cells were isolated from ears to identify cells migrating into the lesions. At day 7, there was a significant increase in the mean number of CCR2-positive macrophages (41,633 ± 5,994 [4.8% of the total ear cells]) in the ears of mice infected with transgenic parasites (Fig. 8A) compared to those infected with wild-type parasites (27,831 ± 5,799 [3.02% of the total ear cells]) (Fig. 8B). This influx was transient, and by day 14 the number of macrophages had begun to recede to levels similar to wild-type parasites. There was no difference in the amount of cell migration between the two groups on day 21 postinfection (data not shown). In addition, the number of neutrophils was the same in both groups during early time points (data not shown).
FIG. 8.
CCR2-positive macrophage migration into lesions. BALB/c mice were infected with 5 × 104 transgenic L. major MCP-1 (Lm-Tg) (A) or wild-type L. major (Lm-WT) (B) parasites. Cells were isolated from infected ears on day 7 postinfection and labeled with antibodies to identify CD11b-positive, F4/80-positive, Gr-1-negative (data not shown), CCR2-positive macrophages by flow cytometry. The percentages and cell numbers are based on the mean percentages of three separate experiments using the R1 gate. (C) BALB/c mice were infected with 5 × 104 wild-type L. major (•) or transgenic L. major (○) parasites in the ear. Parasite burdens in infected ears were enumerated at 2 h and at days 3, 7, 14, and 21 postinfection. (D) Total cells were isolated from infected ears on days 7, 14, and 21 postinfection, and the CD11b-positive, F4/80-positive, Gr-1-negative, CCR2-positive macrophages were sorted. The numbers of parasites per 100 CCR2-positive macrophages isolated from wild-type L. major (▪)- or transgenic L. major (□)-infected mice were compared. (E and F) Sorted CCR2-positive macrophages were cytospun and Giemsa stained to examine macrophage infectivity. Macrophages from day 21 ears infected with transgenic (E) or wild-type (F) L. major were compared. *, P < 0.05; **, P < 0.01.
To determine the kinetics of disease resolution, we infected BALB/c mice in the ears with either wild-type or transgenic parasites. The number of parasites in infected ears was quantitated at various time points postinfection. Significant differences in parasite loads between wild-type and transgenic L. major MCP-1-infected mice began to appear as early as day 7 postinfection (Fig. 8C). The number of parasites in the transgenic L. major MCP-1-infected ears decreased from 1,292 ± 84 on day 7 to only 467 ± 9 on day 14. In contrast, the number of wild-type parasites increased from 1.2 × 104 ± 2 × 103 on day 7 to 2.94 × 105 ± 1.6 × 104 parasites on day 14 (Fig. 8C). At this time, parasites began to disseminate into the lymph nodes of the wild-type mice, but there was no dissemination into the lymph nodes in the transgenic L. major-infected mice (data not shown). To evaluate the level of macrophage infectivity in wild-type L. major and transgenic L. major MCP-1-infected BALB/c ears, total cells were isolated on days 7, 14, and 21 postinfection. The cells were sorted to isolate a population of CD11b-positive, F4/80-positive, Gr-1-negative, CCR2-positive macrophages. Macrophages isolated from transgenic L. major-infected ears contained very few parasites, and the low level of parasites remained relatively constant over the entire observation period (Fig. 8D). By day 21, CCR2-positive macrophages from transgenic L. major-infected ears contained few to no intact parasites (Fig. 8E). However, macrophages isolated from wild-type L. major-infected ears contained high numbers of parasites per 100 macrophages, and this number progressively increased over time (Fig. 8D). By day 21, the CCR2-positive macrophages from wild-type ears were heavily infected with high numbers of parasites (Fig. 8F).
The kinetics of disease resolution and the levels of CCR2-positive macrophage infectivity were analyzed in wild-type and transgenic L. major-infected C57BL/6 ears, as well as in CCR2 KO ears. Similar to the observations in BALB/c mice, there were significantly fewer transgenic L. major parasites in the ears of C57BL/6 mice by as early as day 7 (Fig. 9A). On day 21, the CCR2-positive macrophages from wild-type-infected C57BL/6 ears were heavily infected with parasites (Fig. 9B), whereas those from transgenic L. major-infected mice were not (Fig. 9C).
FIG. 9.
Parasite levels in the ears of C57BL/6 and CCR2 KO mice. (A) Parasite numbers in the ears of C57BL/6 mice were determined at various time points after infection with 5 × 104 wild-type L. major (Lm-WT, •) or transgenic L. major (Lm-Tg, ○) parasites. (B and C) Intracellular parasites within sorted CCR2-positive macrophages isolated from the ears of C57BL/6 mice, 21 days after infection with wild-type (B) or transgenic (C) L. major parasites. (D) Parasite numbers in the ears of CCR2 KO mice were determined at weekly intervals after infection with 5 × 104 Lm-WT (•, not visible) or transgenic L. major parasites (○). (E and F) Intracellular parasites within macrophages isolated from the ears of CCR2 KO mice, 21 days after infection with wild-type (E) or transgenic (F) L. major parasites. **, P < 0.01.
In contrast to the C57BL/6 mice, infection of CCR2 KO mice with either wild-type or transgenic parasites resulted in the increased parasite accumulation in the ears over the 21-day observation period (Fig. 9D). At this time, macrophages from the infected CCR2 KO mice contained high levels of wild-type (Fig. 9E) or transgenic (Fig. 9F) parasites in them.
Recruited CCR2-positive macrophages and MCP-1 coactivation.
To determine how the recruited CCR2-positive macrophages were capable of killing intracellular parasites, mice were injected intraperitoneally with recombinant MCP-1 to recruit restrictive CCR2-positive macrophages to the peritoneum. These cells were isolated and stimulated in vitro under various conditions. Intracellular killing of wild-type parasites was enhanced when MCP-1 recruited macrophages were activated with both MCP-1 and IFN-γ in vitro (Table 1) . Optimal parasite killing appeared to require both recruitment and stimulation since the removal of either diminished the killing (Table 1). This suggests that transgenic parasites expressing MCP-1 both recruit and stimulate a restrictive population of macrophages to efficiently kill Leishmania parasites.
TABLE 1.
Killing of wild-type Leishmania by MCP-1 recruited macrophages
| Macrophage groupa | Treatment | Mean no. ± SDb
|
||
|---|---|---|---|---|
| Infected macrophagesc | Total parasites | Macrophages infected with ≥4 parasites | ||
| Resident peritoneal macrophages | No stimulation | 85 ± 3 | 248 ± 10 | 38 ± 4 |
| IFN-γ (100 U) | 84 ± 4 | 238 ± 22 | 35 ± 4 | |
| IFN-γ (100 U) + MCP-1 (1 ng) | 84 ± 3 | 249 ± 6 | 32 ± 2 | |
| MCP-1 recruited macrophages | No stimulation | 81 ± 3 | 232 ± 18 | 34 ± 3 |
| IFN-γ (100 U) | 84 ± 3 | 235 ± 16 | 33 ± 5 | |
| MCP-1 (1 ng) | 87 ± 2 | 219 ± 8 | 30 ± 3 | |
| IFN-γ (100 U) + MCP-1 (1 ng) | 72 ± 4* | 159 ± 15** | 19 ± 2** | |
A total of 100 macrophages were counted in various fields by fluorescent microscopy.
That is, the mean number counted on three different coverslips. *, Significantly different compared to either unstimulated, IFN-γ-stimulated, or MCP-1-stimulated samples (P < 0.05); **, significantly different compared to either unstimulated, IFN-γ-stimulated, or MCP-1-stimulated samples (P < 0.01).
Macrophages were infected with wild-type L. major parasites.
DISCUSSION
Previous studies by others have demonstrated the presence of MCP-1 during self-healing Leishmania infections (25, 32, 34), but they did not establish the cause-and-effect relationship between MCP-1 production, CCR2-positive cellular recruitment, and disease resolution. To recruit CCR2-positive cells into lesions, we developed transgenic Leishmania organisms that secrete the murine CC chemokine MCP-1. Thus, the transgenic parasites would secrete MCP-1 and thereby take an active role in manipulating immune cell migration into lesions. We hypothesized that the MCP-1-induced cell migration could influence the magnitude and character of the immune response and thereby affect the outcome of disease.
The transgenic parasites caused minimal disease in several strains of mice. BALB/c mice, which are normally susceptible to L. major infection, did not develop lesions when infected in the footpad, nor did these parasites efficiently disseminate to other organs, as did wild-type parasites. These parasites were also attenuated in MCP-1-deficient mice, suggesting that a parasite-derived chemokine was responsible for the attenuated phenotype. Transgenic parasites also failed to cause lesions in C57BL/6 mice that are resistant to L. major infection, even with high doses of transgenic L. major MCP-1. However, mice lacking the CCR2 receptor on the C57BL/6 background developed relatively normal lesions when infected with transgenic parasites. This suggests that the signaling through the CCR2 receptor was required for the healing phenotype.
In all of these strains, the phenotype caused by the transgenic parasites was very dramatic. Although the wild-type parasites caused large lesions with many parasites, the transgenic L. major MCP-1 parasites failed to cause measurable lesions, and the number of parasites in the lesions and visceral organs was dramatically reduced. By all of the criteria available to us, the transgenic parasites that we developed appeared to be as healthy as the wild-type parasites. They grow in culture with the same kinetics and same density. They reduce MTT to formazan, and they invade and persist in resting resident peritoneal macrophages similar to wild-type parasites. Finally, these parasites cause lesions in mice lacking CCR2, the receptor for MCP-1, suggesting that the avirulence of these parasites in wild-type mice is due to the specific expression of murine MCP-1.
We believe that the constitutive expression of MCP-1 by the transgenic parasites is important for the continued recruitment of CCR2-positive cells into lesions. Previous research has shown that wild-type Leishmania parasites induce MCP-1 production (24) as early as 1 h postinfection but that the MCP-1 returns to uninduced levels by 4 h in murine BMDM (31) and by 12 h in human monocytes (2). It is possible that a short burst of MCP-1 production may not be sufficient to recruit or retain enough CCR2-positive cells in lesions during the early course of infection with wild-type parasites.
Several experiments were performed to discover whether the CCR2-positive cells that caused the healing phenotype were part of the innate or adaptive immune response that develops in mice infected with transgenic parasites. We observed that lymph node-derived lymphocytes obtained from mice infected with transgenic parasites could neither proliferate nor secrete IFN-γ and IL-4 in response to Leishmania antigen in contrast to lymphocytes from wild-type-infected mice. This suggested that the transgenic parasites were cleared before an adaptive immune response was generated. This was verified by a follow-up vaccination experiment. Mice that were each infected in the footpad with transgenic parasites were not protected 5 weeks later when reinfected in the ear by wild-type parasites. However, mice previously infected with wild-type parasites in the footpad were completely protected when similarly challenged. These results are consistent with the idea that mice infected with transgenic parasites did not develop a detectable adaptive immune response. We therefore decided to focus on the innate immune response in mice infected with transgenic parasites. In an attempt to identify the early events that could be responsible for these lesion differences, we focused on the cells migrating into infected ears during the first week. On day 7 postinfection, there was a 50% increase in the number of CCR2-positive macrophages migrating into the transgenic MCP-1-infected ears relative to the wild-type-infected ears. The increased CCR2-positive macrophage migration correlated with significant differences in parasite levels between wild-type and transgenic L. major-infected ear lesions in both BALB/c and C57BL/6 mice. These differences in parasite numbers were detected as early as 7 days postinfection, and they continued to increase over time. The CCR2-positive macrophages that were sorted on days 7, 14, and 21 postinfection from transgenic L. major-infected BALB/c and C57BL/6 ears contained few if any parasites, whereas macrophages from wild-type-infected ears were heavily infected. In contrast, there were no differences in parasite levels in macrophages from infected CCR2 KO mice. These data suggest that transgenic L. major MCP-1 may recruit a population of CCR2-positive macrophages that prevents intracellular parasite growth and survival.
One potential scenario that could account for the lack of lesion formation in transgenic L. major-infected mice is that macrophages may become activated by MCP-1, leading to parasite killing. This would be consistent with previous in vitro observations of others (5-7, 23, 33).
A second possibility is that a specific CCR2-positive macrophage subset may be recruited into the lesion, and this subset may be particularly adept at killing intracellular organisms. Recently, a CCR2-positive monocyte population has been identified that migrates into inflammatory sites and is believed to play a role in pathogen clearance (9). In addition, a CCR2-positive Gr-1-positive macrophage population was shown to control toxoplasmosis (26, 36). This population, like our restrictive CCR2-positive macrophage population, expresses CCR2 and controls parasite dissemination. However, the population was also reported to express Gr-1, a marker that we did not detect on our CCR2-positive macrophages.
Our in vitro MCP-1 activation studies using MCP-1 recruited macrophages (Table 1) suggest that both scenarios may be true. We suggest that the transgenic MCP-1-secreting parasites recruit restrictive CCR2-positive macrophages to the site of infection. The MCP-1 produced by the transgenic parasites also helps to activate the CCR2-positive macrophages, making them particularly adept at killing Leishmania parasites.
Others have shown that CCR2 KO mice on a C57BL/6 background infected with L. major developed larger ear lesions with higher numbers of parasites compared to infected CCR2+/+ C57BL/6 mice (38). Our footpad infection results (Fig. 6) are not reflective of these previously published results, probably due to the high dosage of parasites (5 × 106) that were used in the C57BL/6 and CCR2 KO infection experiments. However, when using a lower dosage of parasites (105), our infection resulted in larger lesions in CCR2 KO mice (data not shown) similar to previously published results (38). This increased disease severity in infected CCR2 KO mice was attributed to a lack of Langerhans cell (LC) migration from the ear to the lymph node, thereby affecting antigen presentation to T cells. Other researchers have questioned the role of LCs in antigen presentation during leishmaniasis (35). Although we acknowledge that the lack of DC migration (whether LCs or dermal DCs) contributes to increased lesion progression in wild-type L. major-infected CCR2 KO mice, we would speculate that the increased disease severity could also be attributed to a lack of recruitment of the CCR2-positive macrophages into the infected ear late in the infection.
Previously, it has been shown that L. major induces MCP-1 production in the lymph nodes of C3H mice resistant to L. major but does not induce MCP-1 in BALB/c mice susceptible to L. major (48). In addition, MCP-1 and CCR2 mRNA were detected in the footpads of resistant C57BL/6 mice infected with L. major between 2 and 4 weeks postinfection (15). Based on our findings with the MCP-1 transgenic parasites, it is possible that the MCP-1 produced in resistant mouse strains is the main chemoattractant recruiting a restrictive CCR2-positive macrophage subset to the localized site of infection. These macrophages may assist in resolving the lesions when coactivated with MCP-1, whereas the lack of MCP-1 production may prevent any such CCR2-positive macrophage recruitment from ever occurring in susceptible BALB/c mice. A similar idea can be used to explain the events that lead to self-healing cutaneous leishmaniasis in humans since other groups have already discovered the presence of MCP-1 in self-healing cutaneous lesions and the absence of MCP-1 in the nonhealing diffuse cutaneous leishmaniasis (25, 32, 34). The MCP-1 present in self-healing lesions may recruit and coactivate restrictive CCR2-positive macrophages to the site of infection to kill the parasites and resolve the lesions.
Future studies will attempt to identify subpopulations of CCR2-positive macrophages and biochemically define the macrophage subset recruited by the parasite-derived MCP-1 that is important for early parasite control during leishmaniasis.
Acknowledgments
We thank Patricia Darrah for providing assistance with mouse ear injections. We also thank Barrett Rollins for generously providing the MCP-1 KO mice and Steven Beverley for providing the pIR1SAT cloning vector.
This study was supported by National Institutes of Health grant AI055576.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badolato, R., D. L. Sacks, D. Savoia, and T. Musso. 1996. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp. Parasitol. 82:21-26. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman, J. 2003. Current treatment approaches to leishmaniasis. Curr. Opin. Infect. Dis. 16:397-401. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya, S., S. Ghosh, B. Dasgupta, D. Mazumder, S. Roy, and S. Majumdar. 2002. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J. Infect. Dis. 185:1704-1708. [DOI] [PubMed] [Google Scholar]
- 6.Biswas, S. K., A. Sodhi, and S. Paul. 2001. Regulation of nitric oxide production by murine peritoneal macrophages treated in vitro with chemokine monocyte chemoattractant protein 1. Nitric Oxide 5:566-579. [DOI] [PubMed] [Google Scholar]
- 7.Brandonisio, O., M. A. Panaro, I. Fumarola, M. Sisto, D. Leogrande, A. Acquafredda, R. Spinelli, and V. Mitolo. 2002. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1α induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin. Exp. Med. 2:125-129. [DOI] [PubMed] [Google Scholar]
- 8.Daly, C., and B. J. Rollins. 2003. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation 10:247-257. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 10.Gordon, S., and P. R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953-964. [DOI] [PubMed] [Google Scholar]
- 11.Hardison, J. L., W. A. Kuziel, J. E. Manning, and T. E. Lane. 2006. Chemokine CC receptor 2 is important for acute control of cardiac parasitism but does not contribute to cardiac inflammation after infection with Trypanosoma cruzi. J. Infect. Dis. 193:1584-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Held, K. S., B. P. Chen, W. A. Kuziel, B. J. Rollins, and T. E. Lane. 2004. Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology 329:251-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 14.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 15.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapler, G. M., C. M. Coburn, and S. M. Beverley. 1990. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol. 10:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurihara, T., G. Warr, J. Loy, and R. Bravo. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskay, T., A. Diefenbach, M. Rollinghoff, and W. Solbach. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 25:2220-2227. [DOI] [PubMed] [Google Scholar]
- 19.Leiby, D. A., R. D. Schreiber, and C. A. Nacy. 1993. IFN-gamma produced in vivo during the first two days is critical for resolution of murine Leishmania major infections. Microb. Pathog. 14:495-500. [DOI] [PubMed] [Google Scholar]
- 20.Locati, M., and P. M. Murphy. 1999. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu. Rev. Med. 50:425-440. [DOI] [PubMed] [Google Scholar]
- 21.Lu, B., B. J. Rutledge, L. Gu, J. Fiorillo, N. W. Lukacs, S. L. Kunkel, R. North, C. Gerard, and B. J. Rollins. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack, M., J. Cihak, C. Simonis, B. Luckow, A. E. Proudfoot, J. Plachy, H. Bruhl, M. Frink, H. J. Anders, V. Vielhauer, J. Pfirstinger, M. Stangassinger, and D. Schlondorff. 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166:4697-4704. [DOI] [PubMed] [Google Scholar]
- 23.Mannheimer, S. B., J. Hariprashad, M. Y. Stoeckle, and H. W. Murray. 1996. Induction of macrophage antiprotozoal activity by monocyte chemotactic and activating factor. FEMS Immunol. Med. Microbiol. 14:59-61. [DOI] [PubMed] [Google Scholar]
- 24.Matte, C., and M. Olivier. 2002. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. J. Infect. Dis. 185:673-681. [DOI] [PubMed] [Google Scholar]
- 25.Moll, H. 1997. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Inst. Mitt. 99:73-78. [PubMed] [Google Scholar]
- 26.Mordue, D. G., and L. D. Sibley. 2003. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J. Leukoc. Biol. 74:1015-1025. [DOI] [PubMed] [Google Scholar]
- 27.Mosser, D. M., and P. J. Edelson. 1985. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135:2785-2789. [PubMed] [Google Scholar]
- 28.Murdoch, C., and A. Finn. 2000. Chemokine receptors and their role in inflammation and infectious diseases. Blood 95:3032-3043. [PubMed] [Google Scholar]
- 29.Murphy, P. M., M. Baggiolini, I. F. Charo, C. A. Hebert, R. Horuk, K. Matsushima, L. H. Miller, J. J. Oppenheim, and C. A. Power. 2000. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52:145-176. [PubMed] [Google Scholar]
- 30.Peters, W., H. M. Scott, H. F. Chambers, J. L. Flynn, I. F. Charo, and J. D. Ernst. 2001. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 98:7958-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racoosin, E. L., and S. M. Beverley. 1997. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp. Parasitol. 85:283-295. [DOI] [PubMed] [Google Scholar]
- 32.Ritter, U., and H. Korner. 2002. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 24:295-301. [DOI] [PubMed] [Google Scholar]
- 33.Ritter, U., and H. Moll. 2000. Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur. J. Immunol. 30:3111-3120. [DOI] [PubMed] [Google Scholar]
- 34.Ritter, U., H. Moll, T. Laskay, E. Brocker, O. Velazco, I. Becker, and R. Gillitzer. 1996. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 173:699-709. [DOI] [PubMed] [Google Scholar]
- 35.Ritter, U., and A. Osterloh. 30. June 2006. A new view on cutaneous dendritic cell subsets in experimental leishmaniasis. Med. Microbiol. Immunol. [Epub ahead of print.] [DOI] [PubMed]
- 36.Robben, P. M., M. LaRegina, W. A. Kuziel, and L. D. Sibley. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rot, A., and U. H. von Andrian. 2004. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22:891-928. [DOI] [PubMed] [Google Scholar]
- 38.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells: absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 139:221-227. [PubMed] [Google Scholar]
- 40.Scott, P., and T. Scharton. 1994. Interaction between the innate and the acquired immune system following infection of different mouse strains with Leishmania major. Ann. N. Y. Acad. Sci. 730:84-92. [DOI] [PubMed] [Google Scholar]
- 41.Serbina, N. V., W. Kuziel, R. Flavell, S. Akira, B. Rollins, and E. G. Pamer. 2003. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity 19:891-901. [DOI] [PubMed] [Google Scholar]
- 42.Serbina, N. V., T. P. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 43.Sutterwala, F. S., G. J. Noel, R. Clynes, and D. M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabbara, K. S., N. C. Peters, F. Afrin, S. Mendez, S. Bertholet, Y. Belkaid, and D. L. Sacks. 2005. Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection. Infect. Immun. 73:4714-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, P. R., and S. Gordon. 2003. Monocyte heterogeneity and innate immunity. Immunity 19:2-4. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira, M. C., S. R. de Jesus, R. B. Sampaio, L. Pontes-de-Carvalho, and W. L. dos-Santos. 2002. A simple and reproducible method to obtain large numbers of axenic amastigotes of different Leishmania species. Parasitol. Res. 88:963-968. [DOI] [PubMed] [Google Scholar]
- 47.Vester, B., K. Muller, W. Solbach, and T. Laskay. 1999. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect. Immun. 67:3155-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaph, C., and P. Scott. 2003. Interleukin-12 regulates chemokine gene expression during the early immune response to Leishmania major. Infect. Immun. 71:1587-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]