Abstract
RNA localization is an important means of post-transcriptional regulation of gene expression in many eukaryotic cell types. In neurons, select RNAs are delivered to postsynaptic dendritic microdomains, a mechanism that is considered a key underpinning in the administration of long-term synaptic plasticity. BC1 RNA is a small untranslated RNA that interacts with translation initiation factors and functions as a translational repressor by targeting assembly of 48 S initiation complexes. BC1 RNA is specifically and rapidly transported to dendrites where it is found concentrated in postsynaptic microdomains. The cytoskeletal infrastructure underlying dendritic localization of BC1 RNA has not been investigated. We now report that the dendritic delivery of BC1 RNA is dependent on intact microtubules. In two neuronal cell types, hippocampal neurons and sympathetic neurons in primary culture, disruption of microtubules abolished dendritic localization of BC1 RNA. In contrast, disruption of actin filaments had no significant effect on the somatodendritic distribution of BC1 RNA. It is concluded that the long-range dendritic delivery of BC1 RNA is supported by microtubules. At the same time, a role for actin filaments, while unlikely for long-range BC1 delivery, is not ruled out for short-range local translocation and anchoring at dendritic destination sites.
Keywords: dendrites, RNA transport, microtubules, localized RNAs, untranslated RNAs
Introduction
Local protein synthesis at the synapse is increasingly seen as one of the molecular mechanisms that contribute to long-term synaptic plasticity (see recent reviews).1-7 A key requisite for this mechanism is the targeted delivery of select RNAs to postsynaptic microdomains in dendrites.7,8 Cis-acting dendritic targeting elements (DTEs) have been identified in a number of neuronal RNAs, and a major current challenge lies in establishing how such DTEs encode transport along the dendritic cytoskeleton.7,8
BC1 RNA9,10 is a dendritic untranslated RNA (utRNA)11 that is concentrated at synapses.9,12 BC1 RNA is a translational repressor that has been implicated in the local control of translation in synapto-dendritic domains.2,13-15 A cis-acting DTE, contained within the 65 5′-most nucleotides, is responsible for dendritic delivery of the RNA.16 BC1 RNA is transported into dendrites at an average speed of 390 μm/s,16 a rate suggesting involvement of fast molecular motors. Fast transport rates have subsequently also been reported for other dendritic RNAs,17 thus raising the question as to which cytoskeletal components are used to support such transport.7 Here, we report that the long-range delivery of BC1 RNA into dendrites is dependent on an intact microtubular cytoskeleton.
Results
As many localized RNAs associate with microtubules or actin filaments,18 we asked whether dendritic delivery of BC1 RNA was dependent on either of these cytoskeletal components. To address this question, we worked with two neuronal primary culture systems: sympathetic neurons in culture and hippocampal neurons in culture.
The somatodendritic distribution of BC1 RNA was examined in cultured neurons after application of the cytoskeleton-disrupting agents cytochalasin D (actin filaments), colchicine (microtubules), or nocodazole (microtubules).19-21 The effectiveness of these agents in our experimental system was verified using fluorescent-conjugated phalloidin to visualize actin filaments, and antibodies specific for β-tubulin to visualize microtubules, as described20-22 (data not shown). Cytoskeletal disrupting agents were applied for limited periods of time at low concentrations that have previously been shown to ensure effectiveness and specificity in neurons.23,24
In control experiments, we first confirmed that BC1 RNA was localized to somata and dendrites in cultured sympathetic neurons (Figure 1(a) and (b)) as well as in cultured hippocampal neurons (Figure 2(a) and (b)). Labeling extended to distal dendritic segments, as has been reported.25 We next used the actin filament-disrupting agent cytochalasin D and found that the somatodendritic BC1 labeling pattern was indistinguishable from that of untreated cells (Figure 1(c) and (d) for sympathetic neurons; Figure 2(c) and (d) for hippocampal neurons). In clear contrast, after microtubules were depolymerized with nocodazole, the BC1 labeling signal remained restricted to neuronal somata (Figures 1(e) and (f) and 2(e) and (f)). Analogous results were obtained when colchicine was used instead of nocodazole (Figures 1(g) and (h) and 2(g) and (h)). These results indicate that the dendritic delivery of BC1 RNA is dependent on microtubules, but not on actin filaments.
Figure 1.
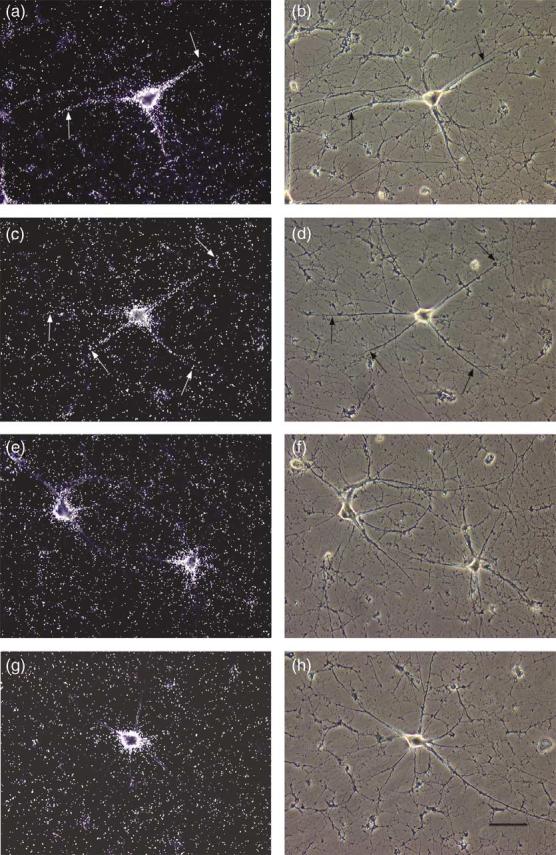
Somato-dendritic distribution of BC1 RNA in sympathetic neurons in culture: effects of cytoskeletal disrupting agents. Dark field photomicrographs are shown in the left column, corresponding phase contrast photo-micrographs in the right column. (a) Untreated culture (control). Substantial BC1 labeling is evident both in the soma and along dendrites (arrows). (c) Culture treated with cytochalasin D. A strong BC1 labeling signal is seen both in the soma and along dendrites (arrows), similar to the untreated control (a). (e) Culture treated with nocodazole. The BC1 labeling signal is restricted to the soma. (g) Culture treated with colchicine. The BC1 labeling signal remains restricted to the soma, similar to neurons treated with nocodazole (e). The scale bar represents 80 μm.
Figure 2.

Somato-dendritic distribution of BC1 RNA in hippocampal neurons in culture: effects of cytoskeletal disrupting agents. Dark field photomicrographs are shown in the left column, corresponding phase contrast photo-micrographs in the right column. (a) Untreated neurons exhibit a strong BC1 labeling signal in somata and along dendrites (arrows). (c) Neurons treated with cytochalasin D also show BC1 labeling in somata and along dendrites (arrows). (e) In neurons treated with nocodazole, the BC1 labeling signal is restricted to somata. (g) Neurons treated with colchicine exhibit a BC1 labeling signal that remains restricted to somata, similar to cells treated with nocodazole (e). The scale bar represents 40 μm.
The entire data set was next subjected to quantitative analysis (Figure 3). The profile of somatodendritic BC1 distribution was established by imaging of BC1 signal intensities along the dendritic extent in 50 μm intervals. Results from both sympathetic neurons (Figure 3(a)) and hippocampal neurons (Figure 3(b)) are shown. Statistical analyses confirmed that disruption of microtubules with either nocodazole or colchicine resulted in severely reduced BC1 levels in proximal and distal dendrites. In contrast, disruption of actin filaments with cytochalasin D did not produce any significant reduction of dendritic BC1 levels. It is also noteworthy that none of the cytoskeleton-disrupting agents caused any significant change in somatic BC1 levels in either sympathetic or hippocampal neurons (Figure 3). This result indicates that treatment with these drugs in the concentration ranges chosen did not adversely affect basal BC1 expression levels (i.e. relevant determinants such as BC1 transcription, processing, or stability). In summary, the data therefore suggest that microtubules but not actin filaments are required for maintaining steady-state levels of BC1 RNA along dendrites.
Figure 3.
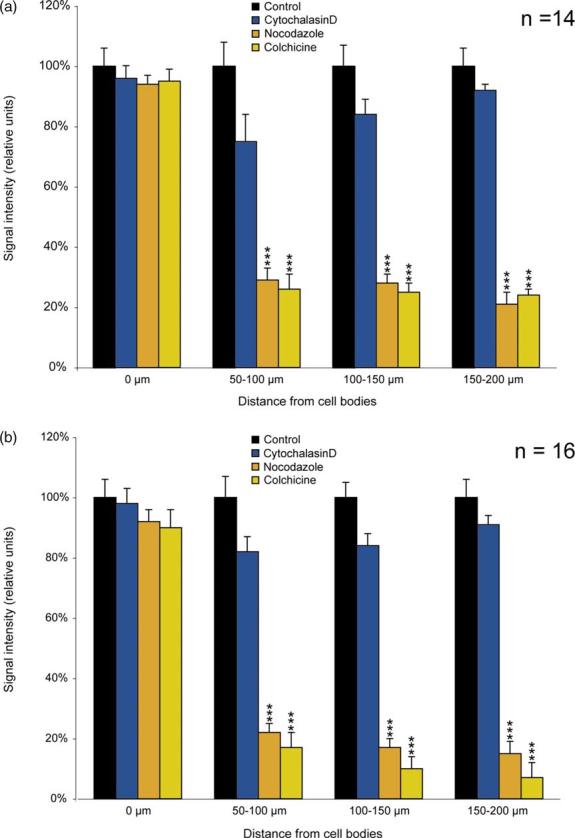
Somato-dendritic distribution of BC1 RNA after cytoskeletal disruption: quantitative analysis. (a) Sympathetic neurons in culture; (b) hippocampal neurons in culture. Signal intensities were quantified in cell bodies and dendrites and are expressed in relative units as percentage of control (i.e. untreated neurons). In neurons treated with cytochalasin D (blue), the distribution of BC1 RNA in cell bodies and dendrites is not significantly different from controls (black) either in somata or in dendrites (one-way ANOVA, p>0.05). In neurons treated with nocodazole (gold) or colchicine (yellow), BC1 levels in somata (0 μm) are similar to controls (one-way ANOVA, p>0.05). In dendrites, in contrast, BC1 levels are significantly decreased both in sympathetic and hippocampal neurons after treatment with nocodazole or colchicine (one-way ANOVA, p<0.001; Scheffe's multiple comparison post hoc analysis (comparison with signal intensity in control dendrites): ***p<0.001 for nocodazole and colchicine in all dendritic segments examined (50–100 μm, 100–150 μm, 150–200 μm)). n, number of cells analyzed.
Discussion
RNA transport and localization have been observed in diverse eukaryotic cell types.7 Whenever examined, these mechanisms have been shown to be mediated by cytoskeletal components, typically by microtubule and/or actin filament systems, depending on cell type and subcellular delivery distances.7,18,26,27 Both systems are in place in neuronal processes, and microtubules have received particular attention as they are essential for the fast, long-range transport of various cargoes in axons.28
BC1 RNA is a small untranslated RNA that regulates gene expression at the level of translation.13,15 BC1 RNA is a prominent RNA species in postsynaptic dendritic microdomains,12 and the combined data suggest a role of the RNA in the regulation of local synaptic protein repertoires and thus in the long-term modulation of synaptic strength.2 BC1 RNA has previously been shown to be rapidly transported along dendrites,16 a result to indicate involvement of the cytoskeleton and fast molecular motors.
We now report that the maintenance of steady-state BC1 levels along the proximo-distal dendritic extent, and thus the long-range dendritic delivery of the RNA, is dependent on intact microtubules. This result is consistent with the notion that microtubules are required whenever RNA is moved over substantial distances in large cells or in cells with highly elongated processes. (In contrast, in other eukaryotic cells such as fibroblasts, myoblasts, neuroblasts, and yeast cells, among others, an extensive actin cytoskeleton may be adequate to serve as the predominant if not sole infrastructure for short-range RNA delivery and localization.)18,29 In neurons, furthermore, the large distances involved make it imperative that RNA transport be fast in order to be efficient, with microtubule-based molecular motors being obvious candidates to meet this criterion.28 Long-range BC1 transport in neurons is indeed rapid, as has been shown both in dendritic16 and axonal30 model systems. In axons, furthermore, long-range BC1 transport has previously been shown to be microtubule-dependent,30 suggesting that common principles may be at work in dendritic and axonal RNA transport.
The decrease of dendritic BC1 levels after application of microtubule-disrupting agents invites an obvious question: what happens to preexisting dendritic BC1 RNA, i.e. RNA that was en route or localized at the time of drug action? Several scenarios can be envisioned. It is possible that a sudden release of BC1 RNA from microtubules, confronting dendrites with high concentrations of an RNA with prominent double-stranded motifs, triggers a PKR-like response31 aimed at eliminating such RNAs. Alternatively or in addition, BC1 RNA may undergo natural turnover or recycling. BC1 RNA appears to be constitutively delivered to dendrites; one would therefore have to postulate that in order to maintain steady-state concentrations at dendritic destinations, equal amounts of the RNA will have to be removed from such domains. Such elimination could be in the form of on-site degradation or of retrograde (in this case microtubule-independent) transport to the soma. Experimental evidence for either scenario is lacking at this time, and further work will be necessary to address this issue and to differentiate between the proposed scenarios.
In axonal BC1 delivery, long-range microtubule-dependent transport is succeeded by a second step, one that is dependent on actin filaments, in which the RNA is radially translocated to cortical destination sites.30 Our present finding that long-range dendritic delivery of BC1 RNA requires the participation of microtubules is certainly compatible with the possibility that actin filaments may be involved in steps subsequent to proximo-distal transport, e.g. in the local translocation of the RNA into dendritic spines where it has been shown to accumulate.12 However, given current spatial resolution limits, it is quite unlikely that we could have detected such short-range localization of BC1 RNA in dendrites. Thus, for BC1 RNA and other dendritic RNAs, the cytoskeletal requirements for local translocation, positioning, and final anchoring steps in postsynaptic dendritic microdomains remain to be elucidated.
Materials and Methods
Cell culture
Primary cultures of sympathetic or hippocampal neurons were generated as described.32,33 To depolymerize microtubules, hippocampal neurons were treated with nocodazole (5 μg/ml) or colchicine (10 μg/ml; Sigma, St. Louis, MO) in culture medium at 37 °C for 1 h20,23 before fixation (4% formaldehyde made from paraformaldehyde). Cytochalasin D (5 μg/ml; Sigma) was added to the cell medium for 2.5 h to disrupt microfilaments.23 For sympathetic neurons, the following concentrations were used (incubation for 3 h): nocodazole, 10 μg/ml; colchicine, 20 μg/ml; cytochalasin D, 10 μg/ml.
In situ hybridization
Plasmid pMK1, containing the 3′ segment of BC1 RNA (nt 93–152), was used to generate RNA probes for the detection of BC1 RNA in cell cultures.9
In situ hybridization was performed as described.25,34 Specimens on cover-slips were post-fixed with 4% formaldehyde in phosphate-buffered saline (PBS) (freshly prepared from paraformaldehyde). High-stringency washes were performed at 50 °C. Probe specificity was verified by sense-strand control experiments that were performed in parallel. Specimens were examined with a Nikon Microphot-FXA microscope using dark-field and phase-contrast optics. Digital images were acquired either with a Sony DKC-5000 3CCD camera or with a Photometrics CoolSnapHQ cooled CCD camera; images were subsequently processed with Adobe Photoshop. MetaMorph software (Universal Imaging Corporation, Downingtown, PA) was used for image analysis.
Image analysis
To measure BC1 levels in neuronal somata, images were analyzed with MetaMorph. To calculate the extent of dendritic BC1 labeling, cells were analyzed under a 40x objective ruler bar (at 410× magnification). Signal intensities were established as silver grain counts in three consecutive 50 μm units of dendritic extent: 50–100 μm, 100–150 μm, and 150–200 μm. Distances were measured from the center of the soma; initial dendritic segments (0–50 μm) were not considered for analysis. One dendrite was analyzed per cell.
Analysis of variance (one-way ANOVA) and Scheffe's multiple comparison post hoc analysis were performed on data sets obtained from somata and dendrites, using SPSS for Windows version 11.0 (SPSS, Chicago, IL).
Acknowledgements
This work was supported, in part, by Istituto Pasteur–Fondazione Cenci Bolognetti (M. C.), by CNR and Istituto Pasteur–Fondazione Cenci Bolognetti (to A.I.), by the New York City Council Speaker's Fund for Biomedical Research (to I.A.M.), and by National Institutes of Health grant NS46769 (to H.T.).
Abbreviations used
- DTEs
dendritic targeting elements
- utRNA
untranslated RNA
- PBS
phosphate-buffered saline
Footnotes
Present address: M. Cristofanilli, Department of Ophthalmology, New York University School of Medicine, New York, NY 10016, USA.
References
- 1.Richter D, editor. Cell Polarity and Subcellular RNA Localization. Springer; Berlin: 2001. [Google Scholar]
- 2.Wang H, Tiedge H. Translational control at the synapse. Neuroscientist. 2004;10:456–466. doi: 10.1177/1073858404265866. [DOI] [PubMed] [Google Scholar]
- 3.Richter JD, Lorenz LJ. Selective translation of mRNAs at synapses. Curr. Opin. Neurobiol. 2002;12:300–304. doi: 10.1016/s0959-4388(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 4.Wells DG, Richter JD, Fallon JR. Molecular mechanisms for activity-regulated protein synthesis in the synapto-dendritic compartment. Curr. Opin. Neurobiol. 2000;10:132–137. doi: 10.1016/s0959-4388(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 5.Job C, Eberwine J. Localization and translation of mRNA in dendrites and axons. Nature Rev. Neurosci. 2001;2:889–898. doi: 10.1038/35104069. [DOI] [PubMed] [Google Scholar]
- 6.Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- 7.Kindler S, Wang H, Richter D, Tiedge H. RNA transport and local control of translation. Annu. Rev. Cell Dev. Biol. 2005;21:223–245. doi: 10.1146/annurev.cellbio.21.122303.120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith R. Moving molecules: mRNA trafficking in mammalian oligodendrocytes and neurons. Neuroscientist. 2004;10:495–500. doi: 10.1177/1073858404266759. [DOI] [PubMed] [Google Scholar]
- 9.Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Dendritic location of neural BC1 RNA. Proc. Natl Acad. Sci. USA. 1991;88:2093–2097. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosius J, Tiedge H. Neural BC1 RNA: dendritic localization and transport. In: Lipshitz HD, editor. Localized RNAs. R.G. Landes; Austin, TX: 1995. pp. 289–300. [Google Scholar]
- 11.Brosius J, Tiedge H. RNomenclature. RNA Biol. 2004;1:81–83. doi: 10.4161/rna.1.2.1228. [DOI] [PubMed] [Google Scholar]
- 12.Chicurel ME, Terrian DM, Potter H. mRNA at the synapse: analysis of a preparation enriched in hippocampal dendritic spines. J. Neurosci. 1993;13:4054–4063. doi: 10.1523/JNEUROSCI.13-09-04054.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Iacoangeli A, Popp S, Muslimov IA, Imataka H, Sonenberg N, et al. Dendritic BC1 RNA: functional role in regulation of translation initiation. J. Neurosci. 2002;22:10232–10241. doi: 10.1523/JNEUROSCI.22-23-10232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondrashov AV, Kiefmann M, Ebnet K, Khanam T, Muddashetty RS, Brosius J. Inhibitory effect of naked neural BC1 RNA or BC200 RNA on eukaryotic in vitro translation systems is reversed by poly(A)-binding protein (PABP) J. Mol. Biol. 2005;353:88–103. doi: 10.1016/j.jmb.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Iacoangeli A, Lin D, Williams K, Denman RB, Hellen CUT, Tiedge H. Dendritic BC1 RNA in translational control mechanisms. J. Cell Biol. 2005;171:811–821. doi: 10.1083/jcb.200506006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muslimov IA, Santi E, Homel P, Perini S, Higgins D, Tiedge H. RNA transport in dendrites: a cis-acting targeting element is contained within neuronal BC1 RNA. J. Neurosci. 1997;17:4722–4733. doi: 10.1523/JNEUROSCI.17-12-04722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiedge H. RNA reigns in neurons. Neuron. 2005;48:13–16. doi: 10.1016/j.neuron.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol. Cell. 2005;97:97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JA. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goslin K, Birgbauer E, Banker G, Solomon F. The role of cytoskeleton in organizing growth cones: a microfilament-associated growth cone component depends upon microtubules for its localization. J. Cell Biol. 1989;109:1621–1631. doi: 10.1083/jcb.109.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matus A, Bernhardt R, Bodmer R, Alaimo D. Microtubule-associated protein 2 and tubulin are differently distributed in the dendrites of developing neurons. Neuroscience. 1986;17:371–389. doi: 10.1016/0306-4522(86)90253-8. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira A, Busciglio J, Caceres A. An immunocytochemical analysis of the ontogeny of the microtubule-associated proteins MAP-2 and Tau in the nervous system of the rat. Brain Res. 1987;431:9–31. doi: 10.1016/0165-3806(87)90191-x. [DOI] [PubMed] [Google Scholar]
- 23.Shan J, Munro TP, Barbarese E, Carson JH, Smith R. A molecular mechanism for mRNA trafficking in neuronal dendrites. J. Neurosci. 2003;23:8859–8866. doi: 10.1523/JNEUROSCI.23-26-08859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis F, Roy S, Brady ST, Black MM. Transport of neurofilaments in growing axons requires microtubules but not actin filaments. J. Neurosci. Res. 2005;79:442–450. doi: 10.1002/jnr.20399. [DOI] [PubMed] [Google Scholar]
- 25.Muslimov IA, Banker G, Brosius J, Tiedge H. Activity-dependent regulation of dendritic BC1 RNA in hippocampal neurons in culture. J. Cell Biol. 1998;141:1601–1611. doi: 10.1083/jcb.141.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloc M, Zearfoss NR, Etkin LD. Mechanisms of subcellular mRNA localization. Cell. 2002;108:533–544. doi: 10.1016/s0092-8674(02)00651-7. [DOI] [PubMed] [Google Scholar]
- 27.Kloc M, Etkin LD. RNA localization mechanisms in oocytes. J. Cell Sci. 2005;118:269–282. doi: 10.1242/jcs.01637. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu. Rev. Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 29.Jansen RP. RNA-cytoskeletal associations. FASEB J. 1999;13:455–466. [PubMed] [Google Scholar]
- 30.Muslimov IA, Titmus M, Koenig E, Tiedge H. Transport of neuronal BC1 RNA in Mauthner axons. J. Neurosci. 2002;22:4293–4301. doi: 10.1523/JNEUROSCI.22-11-04293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman RJ. The double-stranded RNA-activated protein kinase PKR. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. pp. 503–527. [Google Scholar]
- 32.Higgins D, Lein PJ, Osterhout DJ, Johnson MI. Tissue culture of mammalian autonomic neurons. In: Banker G, Goslin K, editors. Culturing Nerve Cells. MIT Press; Cambridge, MA: 1991. pp. 177–205. [Google Scholar]
- 33.Goslin K, Banker G, Asmussen H. Rat hippocampal neurons in low density cultures. In: Banker K, Goslin K, editors. Culturing Nerve Cells. 2nd edit. MIT Press; Cambridge, MA: 1998. pp. 339–370. [Google Scholar]
- 34.Tiedge H. The use of UV light as a cross-linking agent for cells and tissue sections in in situ hybridization. DNA Cell Biol. 1991;10:143–147. doi: 10.1089/dna.1991.10.143. [DOI] [PubMed] [Google Scholar]


