Abstract
Pyrin domain (PYD) proteins have recently emerged as important signaling molecules involved in the development of innate immunity against intracellular pathogens through activation of inflammatory mediator pathways. ASC is the central adaptor protein, which links pathogen recognition by PYD-containing pathogen recognition receptors, known as PYD-Nod-like receptors (NLR), PAN, PYPAF, NALP, Nod, and Caterpiller proteins, to the activation of downstream effectors, including activation of caspase-1 and NF-κB. Activation of these effectors occurs when specific protein complexes, known as inflammasomes, are formed. PYD signal transduction leads to inflammasome assembly and activation of specific effector proteins. It is modulated by a cellular PYD-only protein (cPOP1), which binds to ASC and interferes with the recruitment of ASC to activated PYD-NLRs. Here we describe the identification and characterization of a second cellular POP (cPOP2), which shows highest homology to the PYD of PAN1. cPOP2 binds to ASC and PAN1, thereby blocking formation of cryopyrin and PAN1-containing inflammasomes, activation of caspase-1, and subsequent processing and secretion of bioactive interleukin-1β. Existence of a second cPOP provides additional insights into inflammasome formation and suggests that POPs might be a common regulatory mechanism to “fine-tune” the activity of specific PYD-NLR family protein-containing inflammasomes.
Pyrin domain (PYD) proteins are important signaling molecules involved in host defense against pathogens through activation of inflammatory mediator pathways. Humans have at least 23 proteins containing a PYD (12, 31, 33, 34). Pathogens are recognized by the leucine-rich region (LRR) of PYD-containing pathogen recognition receptors, known as PYD-Nod-like receptors (NLR), pyrin- and NACHT domain-containing proteins (PAN), pyrin domain-containing Apaf1-like proteins (PYPAF), NACHT-LRR- and pyrin domain-containing proteins (NALP), nucleotide-binding oligomerization domain-containing proteins (Nod), and Caterpiller proteins. Activation of these proteins is hypothesized to be achieved through displacement of the intramolecular interaction of the LRR with the NACHT (NAIP, CIITA, HET-E, and TP1) domain. Activated PYD-NLR family proteins recruit and oligomerize the PYD-containing adaptor protein ASC (apoptosis-associated speck-like protein containing a caspace recruitment domain; TMS1) (5, 24) into cytoplasmic structures, referred to as the inflammasomes, where downstream effector activation occurs (22). Caspase-1-mediated processing of pro-interleukin (IL)-1β and activation of the transcription factor NF-κB have been described as effectors of PYD-mediated signal transduction, and enforced oligomerization of ASC is sufficient for their activation.
Hereditary mutations in PYD family proteins can cause dysregulated recruitment of ASC, which leads to autoinflammatory disorders, such as periodic fever syndromes. Specifically, mutations in pyrin (marenostrin) account for familial Mediterranean fever, whereas mutations in the PYD-NLR family member cryopyrin (CIAS1, PYPAF1, NALP3, CLR1.1) have been linked to familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and chronic infantile neurological cutaneous and articular syndrome (3).
The PYD belongs to the death domain fold (DDF) domain family, which also includes the caspase recruitment domain (CARD), the death domain (DD), and the death effector domain (DED). CARD and DED interactions are also regulated by CARD-only proteins (COPs) and DED-only proteins, respectively. These small proteins are composed of a single CARD or one or two DED domains and include the CARD-only proteins COP (Pseudo-ICE), ICEBERG and INCA (9, 11, 17, 18), or the DED-only proteins PED (PEA-15), FLIP-s, and viral FLIP (4, 16). In general, DDF-only proteins can function as dominant-negative inhibitors for particular signaling pathways by competing for critical binding partners, thereby interrupting signal transmission to downstream effector proteins. Inflammasome formation, upon pathogen infection, is regulated by cellular and viral pyrin-only proteins (vPOPs), which interfere with the PYD-PYD interaction between ASC and PYD-NLR family proteins, thereby impairing host defense by blocking downstream effectors (13, 29; A. Dorfleutner, S. J. McDonald, N. B. Bryan, K. N. Funya, J. C. Reed, X. Shi, D. C. Flynn, Y. Rojanasakul, and C. Stehlik, submitted for publication). Here we report the identification and characterization of a second cellular POP, cPOP2, which impairs PYD-mediated activation of pro-caspase-1 and subsequent processing of pro-IL-1β.
MATERIALS AND METHODS
Plasmids and strains.
The complete open reading frame of cPOP2 was cloned from a pooled cDNA library by high-fidelity PCR (PfuUltra; Stratagene) using the primer pair 5′-GCGAATTCATGGCATCTTCTGCAGAGCTG-3′ and 5′-CCGCCTCGAGTTAAGGTGGGGGCATCACACA-3′. The PCR product was digested with EcoRI and XhoI restriction enzymes and cloned into pcDNA3 (Invitrogen) expression plasmids, which were modified to contain an NH2-terminal myc, hemagglutinin (HA), or Flag epitope tag. The authenticity of cPOP2 was confirmed by DNA sequence analysis. Expression constructs for ASC, ASC-PYD, ASC-CARD, cPOP1, cryopyrin (R260W), PAN1, pro-IL-1β, and pro-caspase-1 were described previously (2, 28-30).
Cell culture and transfection.
HEK293N, HEK293T, and Jurkat cells were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (HEK293) and RPMI 1640 medium (Jurkat cells) and supplemented with 4 mM l-glutamine, 1.5 g/liter sodium bicarbonate, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, and 10% fetal bovine serum. HEK293 cells were transfected using Polyfect (QIAGEN) or the calcium precipitation method, and Jurkat cells were transfected using Lipofectamine Plus (Invitrogen).
Reverse transcriptase PCR (RT-PCR) reaction.
RNA was isolated from cells using the TRIzol reagent (Invitrogen), according to the manufacturer's instructions. Total RNA (5 μg) was subjected to DNase I (Invitrogen) treatment and reverse transcribed using the Superscript II enzyme (Invitrogen) into first-strand cDNA, as suggested by the manufacturer, followed by PCR amplification with GoTaq polymerase (Promega) using the cPOP2-specific primer pair 5′-ATGGCATCTTCTGCAGAGCTG-3′ and 5′-TTAAGGTGGGGGCATCACACA-3′ (294 bp) and the β-actin-specific primer pair 5′-GACGATGATATTGCCGCACT-3′, 5′-GATACCACGCTTGCTCTGAG-3′ (533 bp). A negative-control experiment with pooled RNA samples, in which the RT was omitted during the RT-PCR step, was also performed. PCR products were excised from agarose gels, and sequences were verified.
Coimmunoprecipitation assay.
Immunoprecipitation assays were performed following transient expression of the HA-tagged PYD of ASC (ASC-PYD) and the myc-tagged cPOP2; the myc-tagged PYD of PAN1 (PAN1-PYD) and the Flag-tagged cPOP2; and PAN1 and the myc-tagged cPOP2 into HEK293T cells. At 36 h posttransfection, cells were lysed in isotonic lysis buffer (150 mM NaCl, 20 mM Tris-HCl [pH 7.4], 10% glycerol, 0.2% NP-40) supplemented with protease and phosphatase inhibitors. Clarified lysates were subjected to immunoprecipitation using agarose-conjugated anti-myc or anti-Flag antibodies (Santa Cruz Biotechnology) for 4 h at 4°C. Following extensive washing in lysis buffer, bound immune complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting using horseradish peroxidase (HRP)-conjugated anti-HA (Santa Cruz Biotechnology) and anti-myc (Santa Cruz Biotechnology) antibodies or anti-PAN1 antibodies (Imgenex) in conjunction with an ECL detection system (Amersham-Pharmacia). Where indicated, cell lysates (5% volume) were included alongside immune complexes. Alternatively, lysates were directly analyzed by immunoblotting after normalization for total protein content.
In vitro protein interaction assay.
cPOP2 was expressed as a glutathione S-transferase (GST) fusion protein in Escherichia coli BL-21 cells (Stratagene) and affinity purified using glutathione (GSH)-Sepharose (Amersham-Pharmacia). GST-cPOP2 or the GST control (50 ng) immobilized on 10 μl GSH-Sepharose was blocked in a mixture containing 142.4 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH 7.4), 0.5 mM EGTA, 1 mM EDTA, 0.2% Nonidet P-40, and 1 mM dithiothreitol and supplemented with protease inhibitors and 1 mg/ml bovine serum albumin (BSA) for 30 min at room temperature. Beads were washed twice and incubated with in vitro-translated and biotinylated (Promega) ASC and PAN1-PYD proteins overnight at 4°C as described above, except that the buffer was supplemented with protease inhibitors and 0.5 mg/ml BSA. Bound proteins were washed extensively, separated by SDS-PAGE, immunoblotted with streptavidin-HRP, and detected with ECL-Plus (Amersham-Pharmacia).
Direct yeast two-hybrid interaction.
The EGY48 strain of Saccharomyces cerevisiae (MAT trp1 ura3 his leu2::plexApo6-leu2) was maintained in yeast extract-peptone-dextrose medium. cPOP2 and the PYD of several NLR family proteins, pyrin, and ASC were cloned into pJG4-5 and pGilda yeast expression vectors under the GAL1 promoter. Plasmids were cotransformed by the LiCl method with pSH1834, which contains eight repeats of a LexA promoter to drive expression of the β-galactosidase reporter gene, in EGY48. Transformed yeast cells were plated onto leucine-deficient Burkholder's minimal medium containing 2% galactose and 1% raffinose and supplemented with appropriate amino acids, and the interaction of the proteins was scored by leucine complementation-dependent growth. Colonies were then replicated onto Burkholder's minimal medium plates containing leucine and 2% glucose, and β-galactosidase filter assays were performed and scored according to the time required to yield a blue color.
Confocal microscopy analysis.
HEK293T cells were seeded onto collagen type I-coated coverslips in six-well dishes and transiently transfected the following day with pcDNA3 expression plasmids for myc-tagged cPOP2, myc-tagged cPOP1, Flag-tagged ASC, and HA-tagged PAN1 as indicated, using the calcium phosphate precipitation method. At 36 h posttransfection, cells were fixed in 3.7% formaldehyde; permeabilized with 0.5% saponin (Sigma); blocked with 0.5% saponin, 1.5% BSA, and 1.5% normal goat serum (Zymed); and immunostained with rabbit polyclonal anti-myc antibodies (1:500; Santa Cruz Biotech), mouse monoclonal anti-Flag M2 antibodies (1:3,500, Sigma), rat polyclonal anti-HA antibodies (1:2,500; Roche Applied Science), and secondary Alexa Fluor 488 and 546 conjugated antibodies (1:200; Molecular Probes) in phosphate-buffered saline supplemented with 0.5% saponin, 1.5% BSA, and 1.5% normal goat serum (Zymed). Nuclei were visualized by incubation with ToPro-3 (Molecular Probes), and the actin cytoskeleton was stained with Alexa Fluor 488- or 546-conjugated phalloidin. After three washes with PBS, supplemented with 0.5% saponin, samples were mounted with Vectashield (Vectorlabs), and images were acquired by confocal laser-scanning microscopy (Zeiss LSM510).
IL-1β secretion assay.
HEK293N cells were transiently transfected with pcDNA3 expression plasmids for mouse pro-IL-1β, myc-tagged pro-caspase-1, HA-tagged ASC, myc-tagged cryopyrin, untagged PAN1, and Flag-tagged cPOP2 in 24-well plates using the Polyfect transfection reagent (QIAGEN). At 24 h posttransfection, the culture medium was replenished with 0.5 ml of fresh culture medium. At 36 h posttransfection, IL-1β secreted into the culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (BD Biosciences-Pharmingen) and a Genios multimode plate reader (Tecan). Assays were performed in triplicate, and data were normalized for cell number, as determined by crystal violet assay.
RESULTS
Identification of a second cellular PYD-only protein.
We recently characterized human POP1, which is highly similar to the PYD of ASC, and showed that it interferes with the PYD-PYD interaction between ASC and cryopyrin to block activation of NF-κB (29). POP1 represents the first endogenous regulator of the PYD signal transduction pathway. More recently, we and others identified poxviral POP homologues that impair host defense by preventing inflammasome formation, which prompted us to suggest renaming POP1 to cPOP1 (7a, 13). Poxviruses are large double-stranded DNA viruses, which encode virulence factors that enable them to evade the host immune responses upon infection, and are known to frequently hijack proteins from the host (25, 27).
We mined the human genome for additional cellular POPs. Since cPOP1 is most homologous to the PYD of ASC (29), we applied the PYD of other PYD-containing proteins for the database mining. Using the PYD of PAN1 (PYPAF2, NALP2, NBS1, CLR19.9) (a PYD-NLR protein), we identified a potential PYD-containing open reading frame, carried on a single exon on chromosome 3q28, which recently was also predicted by NCBI using GNOMON (accession no. XR_001234). The deduced amino acid sequence revealed a potential POP, similar to cPOP1 and several poxviral POPs, which all bind to ASC by PYD-PYD interaction (Fig. 1A). This protein, which we named cPOP2, shows 68% sequence similarity (61% sequence identity) to the PYD of PAN1 (Fig. 1B). High sequence similarity of cPOP2 to the PYD of PAN7 (PYPAF3, NALP7, NOD12, CLR19.4) and ASC (Fig. 1C) is also detectable. Several conserved amino acid residue patches are present in all known POPs (Fig. 1D).
FIG. 1.
The human genome encodes a second cellular PYD-only protein. (A) Schematic domain representation of cPOP2 and other known viral and cellular POPs, ASC, and PYD-NLR family proteins. Myxoma virus (MV; M013L), rabbit Shope fibroma virus (SFV; gp013L), swinepox virus (SPV; SPV14L), Yaba-like disease virus (YLDV; 18L), mule deer poxvirus (DPV; DPV83gp024). (B) Clustal W alignment of cPOP2 and the most similar PYD of PAN1 (PYPAF2, NALP2, NBS1, CLR19.9). (C) cPOP2 and the PYDs of the three most similar PYD-containing proteins, PAN1, PAN 7 (PYPAF3, NALP7, NOD12, CLR19.4), and ASC. (D) cPOP2 with other known POPs. Dark gray and light gray shading indicates identical and similar (conserved) amino acid residues, respectively. The α-helices, as determined for the PYD of ASC, are marked above the sequences (e.g., H1, H2, etc.) (19).
cPOP2 is expressed in human cells.
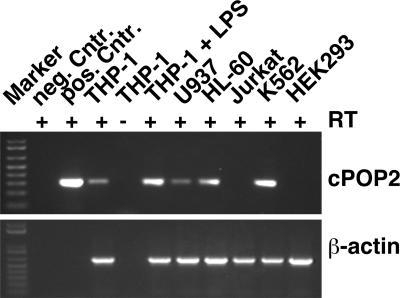
Because we predicted cPOP2 from the human genome, we investigated whether cPOP2 is actually expressed in human cells. Due to the lack of a specific antibody, we performed RT-PCR analysis using cPOP2-specific primers and RNA isolated from various human cell lines. The presence of a cPOP2-specific transcript of 294 bp in several cell lines confirmed that cPOP2 is indeed transcribed and that it encodes a predicted protein of approximately 11 kDa (Fig. 2). Since cPOP2 is encoded on a single exon, we ensured that all potential genomic DNA contamination of the isolated RNA was removed by treatment of the RNA with DNase I, prior to cDNA synthesis. We also performed a negative-control cDNA synthesis reaction from THP-1 RNA in the absence of the RT, which did not yield a cPOP2-specific PCR product, verifying that no genomic DNA contamination had occurred. We performed a PCR using as a template a plasmid-encoding cPOP1 for a negative control and a plasmid-encoding cPOP2 for a positive control. Because cPOP2-specific transcripts were not found in all cell lines tested, we also verified the quality of the cDNA template by performing the PCR using β-actin-specific primers, which amplified a 533-bp product. PCR products were excised from agarose gels and confirmed by DNA sequencing to correspond to cPOP2.
FIG. 2.
cPOP2 is expressed in human cell lines. cPOP2-specific transcripts were detected in several human cell lines by RT-PCR, which was performed with cPOP2-specific primers and primers specific for β-actin. RT-PCR was also performed using pooled RNA from THP-1 cells treated with 600 ng/ml LPS (E. coli serotype O111:B4) for 2, 4, 6, 12, and 24 h (THP-1 + LPS). A cPOP1 expression plasmid (pcDNA3-myc-cPOP1) was included as a negative control, and a cPOP2 expression plasmid (pcDNA3-myc-cPOP2) was used as a positive control. Pooled RNA not incubated with reverse transcriptase during the RT-PCR step was also used as a negative control.
cPOP2 interacts with ASC and PAN1.
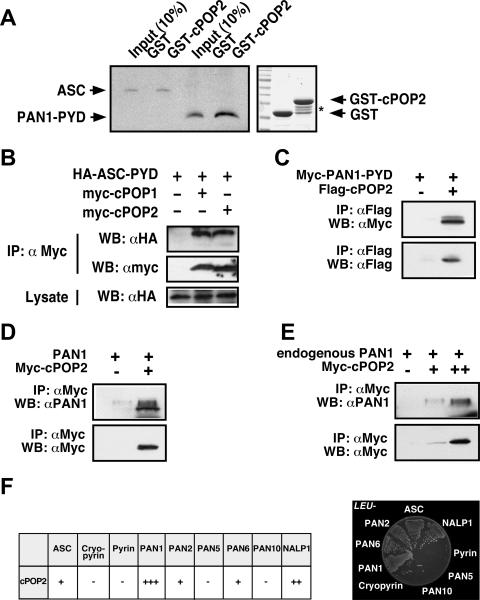
cPOP1 and vPOPs regulate the activation of PYD-dependent immune effectors by PYD-PYD interaction with the central adaptor protein ASC. cPOP2 further shows highest sequence similarity to the PYD of PAN1, a PYD-NLR protein. The ability of cPOP2 to bind to ASC and PAN1 was therefore investigated by an in vitro glutathione S-transferase pull-down assay using ASC and PAN1 as prototypical examples. A recombinant cPOP2 protein was expressed as a GST fusion protein in E. coli BL21 and affinity purified, whereas the PYDs of PAN1 and ASC were generated by in vitro translation. These in vitro interaction studies confirmed that cPOP2 is capable of interacting not only with ASC but also with the PYD of PAN1 (Fig. 3A). No interaction was detectable between ASC and the PYD of PAN1 with the GST control, demonstrating the specificity of these interactions (Fig. 3A).
FIG. 3.
cPOP2 associates with ASC and PYD-NLR family proteins. (A) In vitro binding between cPOP2, ASC, and PAN1. ASC and the PYD of PAN1 were in vitro translated, labeled with biotin, and subjected to in vitro GST pull-down assays using GST-POP2 and a GST control immobilized on GSH-Sepharose, as indicated. Protein complexes were separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes, and bound proteins were visualized by immunoblotting with streptavidin-HRP and ECL-Plus (Amersham Pharmacia Biotech) detection. The recombinant GST-cPOP2 and GST proteins are visualized by Coomassie blue staining. The asterisk denotes several degradation products of cPOP2, which did not affect this assay. (B) In vivo binding between cPOP2 and ASC. HEK293T cells were transiently transfected with the HA-tagged PYD of ASC (ASC-PYD), myc-tagged cPOP1, and myc-tagged cPOP2, as indicated. At 36 h posttransfection, clarified and normalized cell lysates were subjected to coimmunoprecipitation, using immobilized anti-myc antibodies (Santa Cruz Biotechnology). Immune complexes were separated by SDS-PAGE, transferred onto PVDF membranes, and probed with anti-HA antibodies directly conjugated to HRP and detected with ECL (Amersham Pharmacia Biotech). Membranes were stripped and reprobed with anti-myc-HRP antibodies. Five percent of the total lysate was also immunoblotted with HA-HRP antibodies to control for ASC-PYD expression. IP, immunoprecipitation; WB, Western blot. (C, D, E) In vivo binding between cPOP2 and PAN1. HEK293T cells were transiently transfected with the myc-tagged PYD of PAN1 (PAN1-PYD) and Flag-tagged cPOP2 (C) or full-length, untagged PAN1 and myc-tagged cPOP2 (D), as indicated. At 36 h posttransfection, clarified and normalized cell lysates were subjected to coimmunoprecipitation, using immobilized anti-Flag antibodies (Sigma) (C) or immobilized anti-myc antibodies (Santa Cruz Biotechnology) (D). Immune complexes were separated by SDS-PAGE, transferred onto PVDF membranes, and probed with anti-myc antibodies directly conjugated to HRP (Santa Cruz Biotechnology) (C) or with anti-PAN1 antibodies (Imgenex) and secondary anti-rabbit antibodies conjugated to HRP (Amersham Pharmacia Biotech) and detected with ECL and ECL-Plus (Amersham Pharmacia Biotech) (D). Membranes were stripped and reprobed with anti-Flag-HRP antibodies (Sigma) (C) or anti-myc HRP antibodies (D). Jurkat cells were transiently transfected with small (+) and large (++) amounts of myc-tagged cPOP2, and cleared lysates were coimmunoprecipitated at 36 h posttransfection, using immobilized anti-myc antibodies (Santa Cruz Biotechnology) (E). Immune complexes were separated by SDS-PAGE, transferred onto PVDF membranes and probed with anti-PAN1 antibodies (Imgenex) and secondary anti-rabbit HRP antibodies (Amersham Pharmacia), and detected with ECL-Plus (Amersham Pharmacia Biotech). Membranes were stripped and reprobed with anti-myc-HRP antibodies (Santa Cruz Biotechnology). (F) Direct interaction screen in yeast between cPOP2, ASC, and PYD-NLR family proteins. Yeast two-hybrid assays were performed, scoring for activation of reporter genes encoding leucine (LEU2) and β-galactosidase (left panel). Plasmid combinations that resulted in growth on leucine-deficient media within 4 days were scored as positive (right panel). The β-galactosidase activity of each colony was also tested by filter assay and scored according to the time required to yield a blue color. cPOP2 was fused to the DNA binding domain of LexA, and the PYD family proteins were fused to the activation domain of B42. The scoring was obtained by considering also the reciprocal experiments, where cPOP2 was fused to the activation domain of B4, and the PYD family proteins were fused to the DNA binding domain of LexA. In all cases, there was good agreement between data obtained by both experiments and also between the leucine and the β-galactosidase assays. Note that all plasmids contained only the PYD. Results for PAN2 (PYPAF4, NALP4, CLR19.5), PAN5 (NALP10, PYNOD, NOD8, CLR11.1), PAN6 (PYPAF7, NALP12, Monarch-1, CLR19.3), PAN10 (PYPAF6, NALP11, NOD17, CLR19.6), NAC (CARD7, DEFCAP, NALP1, CLR17.1), and cryopyrin (CIAS1, PYPAF1, NALP3, CLR1.1) are shown.
We next investigated whether the interaction between cPOP2 and ASC or PAN1 can also occur in vivo. The HA-tagged PYD of ASC (ASC-PYD) was coexpressed with myc-tagged cPOP2 (or as a control, cPOP1) in HEK293T cells by transient cotransfection. Coimmunoprecipitation experiments of cleared protein lysates with anti-myc Sepharose demonstrated the presence of HA-tagged ASC-PYD in immune complexes containing myc-tagged cPOP1 or cPOP2 but not in control immune complexes. These findings indicated that cPOP2 can form complexes with ASC in vivo, reminiscent of cPOP1 (Fig. 3B). To test the interaction between cPOP2 and PAN1, we first coexpressed the myc-tagged PYD of PAN1 (PAN1-PYD) and Flag-tagged cPOP2 into HEK293T cells. Coimmunoprecipitation experiments of cleared protein lysates with anti-Flag Sepharose demonstrated the presence of myc-tagged PAN1-PYD in immune complexes containing Flag-tagged cPOP2 but not in control immune complexes (Fig. 3C). Similarly, coexpression of untagged full-length PAN1 with myc-tagged cPOP2 in HEK293T cells followed by coimmunoprecipitation of cleared protein lysates further confirmed specific binding of cPOP2 to PAN1 (Fig. 3D). To test the interaction of cPOP2 and PAN1 also under endogenous conditions, we transiently transfected large and small amounts of myc-tagged cPOP2 into Jurkat cells, because no antibody specific for cPOP2 is currently available and Jurkat cells express PAN1 (2). Coimmunoprecipitation of myc-containing immune complexes from cleared protein lysates demonstrated the presence of endogenous PAN1 in a dose-dependent manner (Fig. 3E). These experiments demonstrated that cPOP2 is capable of binding to ASC and PAN1 and that the binding is mediated by PYD-PYD interaction.
POPs can impair PYD-mediated signal transduction by binding to the PYD of ASC but may also block the PYD of PYD-NLR family proteins, such as PAN1, to prevent recruitment of ASC by PYD-PYD interaction. To investigate this possibility, we performed direct yeast two-hybrid interactions between cPOP2 and several PYD-NLR family proteins. cPOP2 was fused to the DNA-binding domain of LexA, whereas the PYDs of PYD-NLR family proteins were fused to the activation domain of B42, because we expect that the interaction will occur between the PYDs of PYD-NLRs and cPOP2, as shown for ASC and PAN1 (Fig. 3F). Results were confirmed by performing a reciprocal experiment. This screen identified several PYD-NLR family proteins as potential binding partners of cPOP2. cPOP2 displays highest sequence similarity to the PYD of PAN1 (Fig. 1B), which consistently conferred the strongest growth of yeast on selective plates and yielded the highest β-galactosidase activity. Also, the PYDs of PAN2 (PYPAF4, NALP4, CLR19.5), PAN6 (PYPAF7, NALP12, Monarch-1, CLR19.3), NALP1 (NAC, CARD7, DEFCAP, CLR17.1), and ASC, which all show significant similarities to cPOP2, were identified as potential cPOP2-interacting proteins. The PYDs of pyrin, cryopyrin (CIAS1, PYPAF1, NALP3, CLR1.1), PAN5 (NALP10, PYNOD, NOD8, CLR11.1), and PAN10 (PYPAF6, NALP11, NOD17, CLR19.6) did not interact with cPOP2 under these conditions, demonstrating the specificity and selectivity of the identified cPOP2 interactions.
cPOP2 colocalizes with PAN1 and the central PYD-containing adaptor protein ASC.
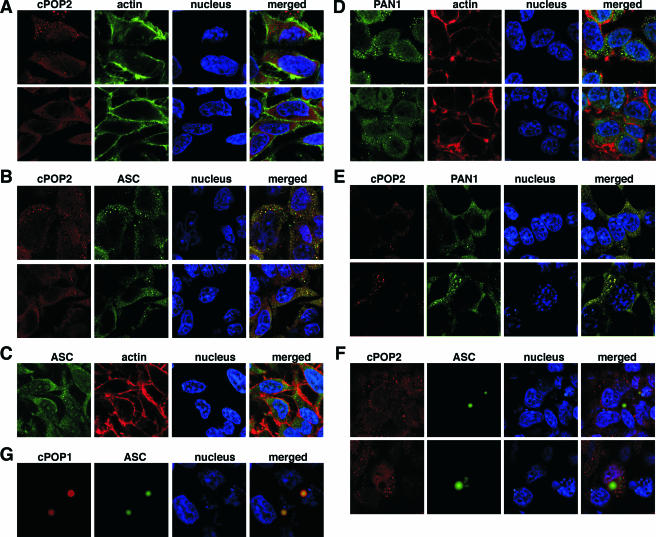
cPOP1 and vPOPs show a distinct intracellular localization to punctate structures in the cytoplasm and to the nucleus (7a, 13, 29). When coexpressed with the central adaptor protein ASC, cPOP1 is recruited into ASC-formed specks. In contrast, this redistribution into specks was not observed with vPOPs, which instead colocalized with ASC in smaller, cytoplasmic vesicular structures (7a, 29). Because cPOP2 is capable of binding to ASC and PAN1, we used immunofluorescence to investigate whether cPOP2 can also colocalize with ASC or PAN1, upon transient coexpression in HEK293 cells. cPOP2 localized primarily to vesicular cytoplasmic structures and the nuclei of cells (Fig. 4A). Coexpression with ASC (Fig. 4C) resulted in partial colocalization of cPOP2 with ASC to these vesicular structures in the cytoplasm (Fig. 4B).
FIG. 4.
cPOP2 colocalizes with ASC and PAN1. Localization of epitope-tagged proteins was analyzed following transient transfection into HEK293T cells. (A) Myc-tagged cPOP2 was immunostained with a rabbit polyclonal anti-myc antibody and visualized with an Alexa Fluor 546-conjugated anti-rabbit antibody. Actin was visualized with Alexa Fluor 488-conjugated phalloidin, and the nucleus was stained with ToPro-3. Shown from left to right are myc-tagged cPOP2 (red), actin (green), the nucleus (blue), and a merged image. (B) Myc-tagged cPOP2 and Flag-tagged ASC and were immunostained with rabbit polyclonal anti-myc and mouse monoclonal anti-Flag antibodies and visualized with Alexa Fluor 546- and 488-conjugated anti-rabbit and anti-mouse antibodies, respectively. The nucleus was stained with ToPro-3. Shown from left to right are myc-tagged cPOP2 (red), Flag-tagged ASC (green), the nucleus (blue), and a merged image. (C) Flag-tagged ASC was immunostained with a mouse monoclonal anti-Flag antibody and visualized with Alexa Fluor 488-conjugated anti-mouse antibodies. The nucleus was stained with ToPro-3. Shown from left to right are Flag-tagged ASC (green), actin (red), the nucleus (blue), and a merged image. (D) HA-tagged PAN1 was immunostained with a rat polyclonal anti-HA antibody and visualized with Alexa Fluor 488-conjugated anti-rat antibodies. The nucleus was stained with ToPro-3. Shown from left to right are HA-tagged PAN1 (green), actin (red), the nucleus (blue), and a merged image. (E) Myc-tagged cPOP2 and HA-tagged PAN1 were immunostained with rabbit polyclonal anti-myc and rat polyclonal anti-HA antibodies and visualized with Alexa Fluor 546- and 488-conjugated anti-rabbit and anti-rat antibodies, respectively. The nucleus was stained with ToPro-3. Shown from left to right are myc-tagged cPOP2 (red), HA-tagged PAN1 (green), the nucleus (blue), and a merged image. (F) Myc-tagged cPOP2 and Flag-tagged ASC were immunostained as described for panel B, and a representative cell that showed an ASC-formed speck was imaged. Shown from left to right are myc-tagged cPOP2 (red), Flag-tagged ASC (green), the nucleus (blue), and a merged image. (G) Myc-tagged cPOP1 and Flag-tagged ASC were immunostained as described above, and a representative cell that showed an ASC-formed speck was imaged. Shown from left to right are myc-tagged cPOP1 (red), Flag-tagged ASC (green), the nucleus (blue), and a merged image.
ASC forms inflammasomes, which require activation of a specific PYD-NLR family member by pathogen-associated molecular patterns. cPOP2 shows highest similarity to the PYD of PAN1, which is one of the PYD-NLRs that recruits ASC to form a specific inflammasome (1, 2). Transient expression of PAN1 in HEK293 cells showed a very similar localization to punctate cytoplasmic structures and the nucleus (Fig. 4D). Coexpression with cPOP2 resulted in efficient colocalization of both proteins to these punctate cytoplasmic structures, but neither of the proteins localized any longer to the nucleus (Fig. 4E).
ASC can also form characteristic cytoplasmic structures, referred to as specks, which are large, perinuclear aggregates (24). ASC-interacting proteins, including cPOP1 and vPOPs, are frequently recruited into these structures (7a, 29). cPOP2 was not recruited into ASC-formed specks but was excluded from these structures (Fig. 4F). Also, PAN1 was not recruited into ASC-formed specks under these conditions (data not shown). In contrast, cPOP1 efficiently colocalized with ASC to these specks (Fig. 4G).
cPOP2 interferes with PYD-mediated activation of caspase-1.
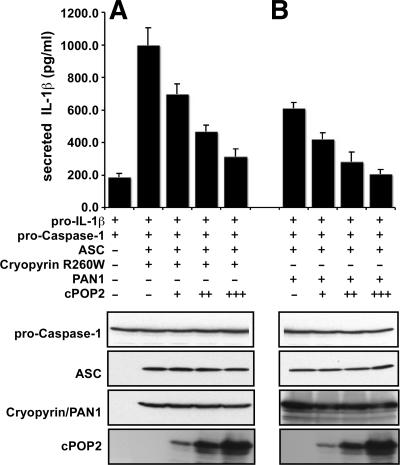
The best-characterized effector protein downstream of the PYD signal transduction pathway is caspase-1. Pro-caspase-1 is activated upon recruitment to inflammasomes by CARD-CARD interactions mediated by ASC. Interaction of cPOP1 and vPOPs with ASC impairs PYD-mediated activation of downstream effectors by preventing association with upstream PYD-NLR family proteins (7a, 29). Because cPOP2 associates with inflammasome-associated proteins, we investigated the effect of cPOP2 interaction with ASC or PAN1 on PYD-mediated activation of caspase-1. The PYD signal transduction pathway was reconstituted in HEK293N cells, which are deficient in endogenous components of this pathway. Coexpression of a constitutively active cryopyrin (R260W) (an NLR family member), ASC, pro-caspase-1, and pro-IL-1β leads to activation of the PYD-mediated signal transduction pathway, which can be measured by secretion of bioactive IL-1β. The disease-associated cryopyrin mutant R260W interacts more efficiently with ASC and promotes ASC oligomerization, activation of pro-caspase-1, and subsequently IL-1β secretion, even in the absence of a ligand (8). Coexpression of cPOP2 with ASC and cryopyrin (R260W) impaired ASC-mediated activation of caspase-1 in a dose-dependent manner, as measured by secretion of bioactive IL-1β, which is processed from pro-IL-1β by caspase-1 (Fig. 5A). As previously reported, coexpression of ASC and PAN1 also induces activation of pro-caspase-1 (1, 2). Coexpression of cPOP2 with ASC and PAN1 also impaired activation of caspase-1 by the PAN1-containing inflammasome in a dose-dependent manner (Fig. 5B). These results demonstrate a potential role for cPOP2 in modulating activation of caspase-1 by specific inflammasomes.
FIG. 5.
cPOP2 inhibits caspase-1-mediated processing of pro-IL-1β. HEK293N cells were transiently transfected in triplicate with expression constructs for pro-caspase-1, murine pro-IL-1β, ASC, cPOP2, and cryopyrin (R260W) (A) or PAN1 (B) (small dose, +; medium dose, ++; and large dose, +++), as indicated. At 36 h posttransfection, secreted IL-1β was measured by ELISA (BD Pharmingen) from normalized culture supernatants using a standard curve generated with recombinant IL-1β. Data are presented as picograms per milliliter of secreted IL-1β (mean ± standard deviation; n = 3). Transfected cells were also directly lysed in Laemmli buffer and analyzed by SDS-PAGE immunoblotting for the expression of all constructs, except pro-IL-1β, which was measured by ELISA.
DISCUSSION
PYD-containing proteins have emerged as important mediators of inflammatory responses during host defense. The PYD signal transduction pathway is triggered by the recognition of specific pathogen-associated molecular patterns by PYD-containing pathogen recognition receptors (PYD-NLR family proteins). Active PYD-NLRs recruit the adaptor protein ASC to form specific inflammasomes, where activation of pro-caspase-1 occurs (1, 22). To date, 16 human PYD-NLR family members that form inflammasomes, including NALP1, cryopyrin, and PAN1, have been recognized (31). However, the exact molecular mechanism is currently poorly understood. Specific ligands have thus far been identified only for cryopyrin and NALP1 (1, 14, 15, 21-23, 32). Activation of PYD-NLR family proteins results in oligomerization and recruitment of the adaptor protein ASC via PYD-PYD interactions (22). Subsequently, ASC links pathogen recognition to downstream effector activation, including caspase-1-mediated processing and secretion of IL-1β, IL-18, and possibly IL-33 (20, 32, 35). Due to the key role of the proinflammatory cytokine IL-1β, its generation and receptor binding are regulated at several key areas in mammalian cells, including CARD-only proteins (COPs) and IL-1RA (6, 7).
Our identification of cPOP2 in the present study is the first indication of an endogenous inhibitor that controls generation of IL-1β at the initiating step, directly at the level of inflammasome formation. Many of the PYD-containing proteins are inducibly expressed in response to proinflammatory stimuli, which is consistent with their proinflammatory function. Also, cPOP2 was induced at the mRNA level in THP-1 monocytes in response to lipopolysaccharide (LPS) stimulation, emphasizing its role during inflammation. cPOP2 shows high similarity to several PYD-NLR family proteins and ASC. The highest similarity is found to the PYD of PAN1, and cPOP2 is capable of interacting with PAN1. Unlike cPOP1, which is clustered with other PYD-containing proteins, including pyrin and ASC, on chromosome 16, cPOP2 is located on chromosome 3q28 alone. cPOP2 displays 40% similarity with cPOP1. The observation that cPOP2 also interacts with the PYD of PYD-NLR family proteins provides additional insights into inflammasome formation and provides evidence that POPs interact not only with the adaptor ASC but also directly with PYD-NLR family proteins to regulate activation of pro-caspase-1.
cPOP2 localizes to cytoplasmic vesicular structures but is also found diffusely throughout the nucleus, similar to cPOP1 (29). ASC localizes to punctate structures in the cytoplasm, or it forms characteristic cytoplasmic aggregates, referred to as specks, to which ASC-interacting proteins, including cPOP1, are frequently recruited. Some colocalization between cPOP2 and ASC can be observed in these vesicular structures in the cytoplasm, but cPOP2 is not recruited to ASC-containing specks, and specks are rarely formed when cPOP2 is coexpressed with ASC. One explanation might be that cPOP2 impairs the aggregation of ASC with other proteins that are essential for speck formation, such as PYD-NLR family proteins. These differences further suggest a distinct functional mechanism of cPOP1 compared to that of cPOP2. Expression of PAN1 resulted in localization similar to that observed for cPOP2, and both proteins efficiently colocalize when coexpressed. Like cPOP1 and vPOPs, cPOP2 also interacts with ASC, the central adaptor protein of the PYD signal transduction pathway. We identified several PYD-NLR family members as potential binding partners for cPOP2, verified binding to PAN1, and showed by coimmunoprecipitation and GST pull-down experiments that the binding is mediated by PYD-PYD interaction. We also expect that, based on yeast two-hybrid assay results, PAN2, PAN6, and NALP1 are capable of binding to cPOP2. Because we did not test all known PYD-containing proteins, there might be more potential PYD-containing binding partners of cPOP2 (Table 1).
TABLE 1.
Similarity of cPOP2 to known human PYD-containing proteinsa
| Protein | cPOP2 (% sequence similarity) |
|---|---|
| PAN1 | 69 |
| PAN7 | 50 |
| PAN3 | 43 |
| PAN6 | 42 |
| PAN2 | 41 |
| PAN12 | 40 |
| cPOP1 | 40 |
| PAN8 | 38 |
| AIM2 | 38 |
| ASC | 37 |
| Pyrin | 37 |
| PAN5 | 37 |
| IFIX | 35 |
| PAN9 | 34 |
| PAN13 | 34 |
| Cryopyrin | 33 |
| NALP1 | 32 |
| PAN10 | 32 |
| PAN11 | 30 |
| IFI16 | 30 |
| PAN14 | 28 |
| MNDA | 27 |
| PAN4 | 25 |
Separate Clustal W alignments of cPOP2 with the PYD of human PYD-containing proteins were performed to calculate percent sequence similarity (identical and conserved amino acid residues).
Binding of ASC to activated PYD-NLR family proteins results in the formation of inflammasomes, which are required for activation of pro-caspase-1 (1, 22). Activation of pro-caspase-1 is required for the processing, activation, and subsequent secretion of the proinflammatory cytokines pro-IL-1β, pro-IL-18, and pro-IL-33 (10, 26). cPOP2 impaired cryopyrin- and ASC-mediated activation of pro-caspase-1, possibly explained by the impaired formation of a cryopyrin-containing inflammasome in the presence of cPOP2. cPOP2 also impaired activation of pro-caspase-1 from a PAN1-containing inflammasome. Assembly of the cryopyrin-containing inflammasome is probably blocked by binding to the PYD of ASC, whereas the PAN1-containing inflammasome could be inhibited by simultaneously blocking the PYDs of ASC and PAN1, which would impair the recruitment of ASC to PAN1. The lower secretion of IL-1β from PAN1-expressing cells than from cryopyrin (R260W)-expressing cells might be due to the higher activity of the R260W-mutated cryopyrin protein, which is found in patients with autoinflammatory disorders, as opposed to the wild-type PAN1 protein (8). The sequence similarity between cPOP2 and cPOP1 is 40% and is 31 to 36% between cPOP2 and vPOPs. Determination of whether cPOP2 and other POPs function to “fine-tune” inflammasome assembly, prevent formation of specific inflammasomes, or participate in the termination of caspase-1 activation to prevent systemic inflammation will require further investigation.
In summary, our data provide evidence for the existence of a second cellular POP, which binds to the PYD of ASC and PAN1 and potentially to several other PYD-NLR proteins, to impair inflammasome-mediated activation of pro-caspase-1 and subsequent processing and secretion of the proinflammatory cytokine IL-1β. The existence of two different cellular POPs might provide for more versatile regulation of inflammasome assembly and might allow specific PYD-NLR family proteins to be suppressed and others to be spared.
Acknowledgments
This work was supported by Public Health Service grants 5P20-RR016440 (to C.S. and D.C.F.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); 1R21-AI067680, 1R03-AI067806 (to C.S.), and R01-AI56324 (to J.C.R.) from the National Institute of Allergy and Infectious Diseases; and 1R01-GM071723 (to C.S.) from the National Institute of General Medical Sciences. We are also grateful for the support from the Alexander Bland Osborn Cancer Center Endowment (to C.S.).
This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Editor: F. C. Fang
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Agostini, L., F. Martinon, K. Burns, M. F. McDermott, P. N. Hawkins, and J. Tschopp. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20:319-325. [DOI] [PubMed] [Google Scholar]
- 2.Bruey, J. M., N. Bruey-Sedano, R. Newman, S. Chandler, C. Stehlik, and J. C. Reed. 2004. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J. Biol. Chem. 279:51897-51907. [DOI] [PubMed] [Google Scholar]
- 3.Brydges, S., and D. L. Kastner. 2006. The systemic autoinflammatory diseases: inborn errors of the innate immune system. Curr. Top. Microbiol. Immunol. 305:127-160. [DOI] [PubMed] [Google Scholar]
- 4.Condorelli, G., G. Vigliotta, A. Cafieri, A. Trencia, P. Andalo, F. Oriente, C. Miele, M. Caruso, P. Formisano, and F. Beguinot. 1999. PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene 18:4409-4415. [DOI] [PubMed] [Google Scholar]
- 5.Conway, K. E., B. B. McConnell, C. E. Bowring, C. D. Donald, S. T. Warren, and P. M. Vertino. 2000. TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res. 60:6236-6242. [PubMed] [Google Scholar]
- 6.Dinarello, C. A. 2005. Blocking IL-1 in systemic inflammation. J. Exp. Med. 201:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 1998. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. N. Y. Acad. Sci. 856:1-11. [DOI] [PubMed] [Google Scholar]
- 7a.Dorfleutner, A., S. J. Talbott, N. B. Bryan, K. N. Funya, J. C. Reed, X. Shi, D. C. Flynn, Y. Rojanasakul, and C. Stehlik. Poxvirus PYRIN-only proteins are modulators of the host immune response.Virus Genes, in press. [DOI] [PMC free article] [PubMed]
- 8.Dowds, T. A., J. Masumoto, L. Zhu, N. Inohara, and G. Nunez. 2004. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279:21924-21928. [DOI] [PubMed] [Google Scholar]
- 9.Druilhe, A., S. M. Srinivasula, M. Razmara, M. Ahmad, and E. S. Alnemri. 2001. Regulation of IL-1beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 8:649-657. [DOI] [PubMed] [Google Scholar]
- 10.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1-11. [DOI] [PubMed] [Google Scholar]
- 11.Humke, E. W., S. K. Shriver, M. A. Starovasnik, W. J. Fairbrother, and V. M. Dixit. 2000. ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell 103:99-111. [DOI] [PubMed] [Google Scholar]
- 12.Inohara, N., and G. Nunez. 2003. NODS: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-381. [DOI] [PubMed] [Google Scholar]
- 13.Johnston, J. B., J. W. Barrett, S. H. Nazarian, M. Goodwin, D. Ricuttio, G. Wang, and G. McFadden. 2005. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23:587-598. [DOI] [PubMed] [Google Scholar]
- 14.Kanneganti, T. D., M. Body-Malapel, A. Amer, J. H. Park, J. Whitfield, L. Franchi, Z. F. Taraporewala, D. Miller, J. T. Patton, N. Inohara, and G. Nunez. 2006. Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 48:36560-36568. [DOI] [PubMed] [Google Scholar]
- 15.Kanneganti, T. D., N. Ozoren, M. Body-Malapel, A. Amer, J. H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, S. Akira, and G. Nunez. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:232-236. [DOI] [PubMed] [Google Scholar]
- 16.Krueger, A., I. Schmitz, S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276:20633-20640. [DOI] [PubMed] [Google Scholar]
- 17.Lamkanfi, M., G. Denecker, M. Kalai, K. D'Hondt, A. Meeus, W. Declercq, X. Saelens, and P. Vandenabeele. 2004. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1beta generation. J. Biol. Chem. 279:51729-51738. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S.-H., C. Stehlik, and J. C. Reed. 2001. COP, a CARD-containing protein and inhibitor of pro-interleukin-1b processing. J. Biol. Chem. 276:34495-34500. [DOI] [PubMed] [Google Scholar]
- 19.Liepinsh, E., R. Barbals, E. Dahl, A. Sharipo, E. Staub, and G. Otting. 2003. The death-domain fold of the ASC PYRIN domain, presenting a basis for PYRIN/PYRIN recognition. J. Mol. Biol. 332:1155-1163. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan, S., K. Newton, D. M. Monack, D. Vucic, D. M. French, W. P. Lee, M. Roose-Girma, S. Erickson, and V. M. Dixit. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213-218. [DOI] [PubMed] [Google Scholar]
- 21.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228-232. [DOI] [PubMed] [Google Scholar]
- 22.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10:417-426. [DOI] [PubMed] [Google Scholar]
- 23.Martinon, F., V. Petrilli, A. Mayor, A. Tardivel, and J. Tschopp. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237-241. [DOI] [PubMed] [Google Scholar]
- 24.Masumoto, J., S. Taniguchi, K. Ayukawa, H. Sarvotham, T. Kishino, N. Niikawa, E. Hidaka, T. Katsuyama, T. Higuchi, and J. Sagara. 1999. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274:33835-33838. [DOI] [PubMed] [Google Scholar]
- 25.Moss, B., and J. L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59-66. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T. K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, D. M. Gorman, J. F. Bazan, and R. A. Kastelein. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479-490. [DOI] [PubMed] [Google Scholar]
- 27.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 28.Stehlik, C., L. Fiorentino, A. Dorfleutner, J. M. Bruey, E. M. Ariza, J. Sagara, and J. C. Reed. 2002. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J. Exp. Med. 196:1605-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stehlik, C., M. Krajewska, K. Welsh, S. Krajewski, A. Godzik, and J. C. Reed. 2003. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappaB and pro-caspase-1 regulation. Biochem. J. 373:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehlik, C., S. H. Lee, A. Dorfleutner, A. Stassinopoulos, J. Sagara, and J. C. Reed. 2003. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J. Immunol. 171:6154-6163. [DOI] [PubMed] [Google Scholar]
- 31.Stehlik, C., and J. C. Reed. 2004. The PYRIN connection: novel players in innate immunity and inflammation. J. Exp. Med. 200:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutterwala, F. S., Y. Ogura, M. Szczepanik, M. Lara-Tejero, G. S. Lichtenberger, E. P. Grant, J. Bertin, A. J. Coyle, J. E. Galan, P. W. Askenase, and R. A. Flavell. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317-327. [DOI] [PubMed] [Google Scholar]
- 33.Ting, J. P., and B. K. Davis. 2005. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu. Rev. Immunol. 23:387-414. [DOI] [PubMed] [Google Scholar]
- 34.Tschopp, J., F. Martinon, and K. Burns. 2003. NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 4:95-104. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto, M., K. Yaginuma, H. Tsutsui, J. Sagara, X. Guan, E. Seki, K. Yasuda, M. Yamamoto, S. Akira, K. Nakanishi, T. Noda, and S. Taniguchi. 2004. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9:1055-1067. [DOI] [PubMed] [Google Scholar]