Abstract
CD97 is a member of the adhesion family of G protein-coupled receptors. Alternatively spliced forms of CD97 bind integrins α5β1 and αvβ3, decay accelerating factor, or dermatan sulfate. CD97 is expressed on myeloid cells at high levels and a variety of other cell types at lower levels. Little is known about the physiological function of CD97. To begin dissecting the function of CD97, we evaluated the immune response of CD97 null mice to systemic infection by Listeria monocytogenes. CD97 null mice were significantly more resistant to listeriosis than matched wild-type mice. A major determinant of the difference in survival appeared to be the comparatively more robust accumulation of granulocytes in the blood and in infected livers of CD97 null mice within 18 h of inoculation, correlating with a decrease in the number of bacteria. CD97 null mice also displayed a mild granulocytosis in the nonchallenged state. Because there is a strong suggestion that CD97 functions in an adhesive capacity, we examined the migratory properties of granulocytes in CD97 null mice. In chimeric animals, CD97 null and wild-type granulocytes migrated similarly, as determined by inflammation-induced emigration from the bone marrow and accumulation in the peritoneum. Granulocyte development in the bone marrow of CD97 null mice was comparable to that of wild-type mice, and CD97 deficiency did not appear to stimulate granulocytosis secondary to peripheral inflammation and resultant granulocyte colony-stimulating factor induction, unlike various other models of adhesion deficiencies. Our results suggest that CD97 plays a role in peripheral granulocyte homeostasis.
CD97 is a member of the adhesion family of G-protein-coupled receptors (GPCR) (2). Members of this family are composed of related seven-transmembrane subunits, associated with extracellular regions that contain one or more adhesion motifs atop an extended stalk region. The presence of adhesion motifs in combination with a GPCR suggests that proteins in this family will couple signaling with adhesion to extracellular matrix or other cells.
Almost all of the family members are proteolytically processed from a single polypeptide to form the noncovalently associated extracellular and seven-transmembrane subunits (3, 15). The extracellular domains are usually large and contain repeats such as epidermal growth factor (EGF)-like, cadherin, thrombospondin, and laminin motifs that are assumed to determine their functional specificity. Thus, receptors in this family have structurally distinct adhesion motifs associated with GPCRs, which themselves are most similar to the class B family. It seems likely that adhesion per se, independent of the specific adhesion motif, is the initiating event for further coupled signaling. Despite the fact that adhesion family G-protein-coupled receptors are one of the largest GPCR families, comprised of 33 members in humans, relatively little is known about their mechanism of action or physiological functions (2).
CD97 belongs to a subclass of receptors, predominantly expressed by hematopoietic cells, whose only known adhesion motifs are EGF-like repeats (24). The human genes include CD97, EMR1 (F4/80 in the mouse), EMR2, EMR3, EMR4 (FIRE in the mouse), and ETL. The mouse genome is missing EMR2 and EMR3, probably because the duplication of the region on chromosome 19 that led to EMR2 and EMR3 occurred after the divergence of mice and men. Recently it has been reported that F4/80, expressed on CD1d+ antigen-presenting cells, is required for the differentiation of CD8+ T reg cells, functioning as either an adhesion molecule and/or a signaling receptor (26).
CD97 is produced in alternatively spliced forms that contain between three and five fibrillin class 1 type EGF-like repeats. To date, low-affinity interactions with various splice variants of the EGF-like repeats have been identified. The variant containing EGF-like repeats 1, 2, and 5 interacts with CD55, a ubiquitously expressed, cell surface complement regulatory protein (19, 21). The fourth EGF-like repeat, which is contained in only the largest variant, interacts in multimerized form with chondroitin sulfate (36). No morphological or physiological response has been observed in CD97-expressing cells upon binding to either CD55 or chondroitin sulfate, and thus, the physiological role of these interactions is unknown. We recently have demonstrated that purified human CD97 binds integrins α5β1 and αvβ3 in an RGD-dependent manner (39). The binding of CD97 to these integrins on endothelial cells is of sufficiently high affinity to stimulate endothelial cells migration and invasion in vitro and angiogenesis in vivo (39).
CD97 is expressed at high levels in hematopoietic cells, including constitutively high levels in myeloid cells and activation-induced high levels in T cells (15, 23). As hematopoietic cells are continuously circulating, one important function of adhesion is to regulate migration patterns. Antibodies directed against CD97 appear to inhibit localization of granulocytes to inflammatory sites in vivo following systemic administration or pretreatment of adoptively transferred granulocytes (25). These data have been interpreted to suggest that CD97 plays an essential role in granulocyte migration. We have used an alternative genetic approach by evaluating, in CD97 null mice, host defense, responsiveness to acute inflammation, and direct measurements of in vitro and in vivo granulocyte migration. Based on the previous results with anti-CD97 antibodies, the expectation was that granulocyte migration would be impaired in CD97 null mice.
Intravenous administration of the facultative intracellular bacterium, Listeria monocytogenes, has been a widely used model, revealing important roles for a sequence of innate and acquired cellular immunity in resistance to listeriosis (6, 30, 40). Neutrophils, monocytes, NK cells, and T cells contribute to host resistance (4, 5, 9, 10). The variety of genetic alterations, particularly null mutations, that impacts host survival underscores the complexity of the cellular functions and interactions leading to sterile immunity (31).
Following systemic introduction of L. monocytogenes, the majority of bacteria localize to the liver and spleen (17). The innate immune system is important for the early control of bacterial amplification. Because epithelial hepatocytes and splenic hematopoietic cells appear to support replication of bacteria in the liver and spleen, respectively, the contribution of distinct innate immune populations and functions in the two organs may be somewhat different (5). Kupffer cells in the liver play an important role in early entrapment of bacteria and initiation of antimicrobial responses through soluble mediators (16, 18). Granulocytes and probably recruited inflammatory monocytes have been found to play a critical role in reducing the bacterial burden in the liver during the first 3 days postinfection (5). Although granulocytes most likely contribute to bacterial reduction in the spleen, a population of monocyte-derived dendritic cells, which produce tumor necrosis factor (TNF) and nitric oxide synthase 2, additionally contribute to controlling bacterial growth in the spleen (35).
In light of prior data concerning the function of CD97, we evaluated its role with respect to the in vivo migratory capacity of granulocytes in response to inflammation induced by Listeria monocytogenes or individual cytokines. Contrary to expectations, we present evidence that CD97 null granulocytes accumulate normally in inflamed lungs and peritoneal cavities and emigrate from bone marrow similarly to wild-type granulocytes. Unexpectedly, we found that CD97 null mice demonstrated increased resistance to systemic L. monocytogenes, and such resistance appears, at least in part, attributable to mild basal and inflammation-induced granulocytosis. In contrast to the granulocytosis induced in integrin and selectin-deficient strains that is secondary to peripheral inflammation and elevated granulocyte colony-stimulating factor (G-CSF) levels, CD97 deficiency does not appear to invoke such a reactive response but instead affects peripheral granulocyte homeostasis through a distinct mechanism.
MATERIALS AND METHODS
Generation of CD97-deficient mice by homologous recombination.
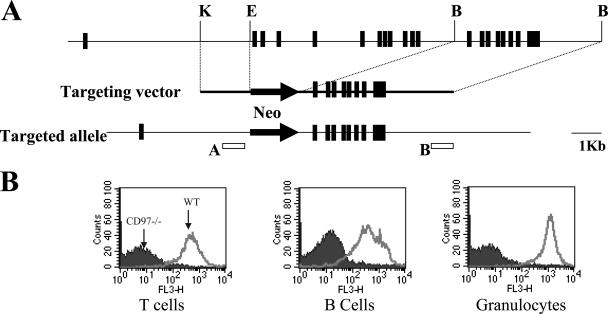
Genomic clones encoding murine CD97 were isolated from a 129/SvJ-derived lambda library (Stratagene, San Diego, CA) using a human CD97 cDNA probe (15) and mapped by a combination of PCR, restriction digest, and sequence analyses using genomic sequences corresponding to the CD97 locus on mouse chromosome 8 (http://www.ensembl.org/Mus_musculus/geneview?gene=ENSMUSG00000002885). A gene-targeting vector derived from pPNT was constructed by replacing 11.8 kb of genomic sequence containing exons 2 to 12, corresponding to nucleotides 88 to 1186, of the murine CD97 cDNA (32) with a PGKneo cassette. TC1 embryonic stem (ES) cells (11) were electroporated with linearized targeting vector, pPNT-CD97, and clones carrying a targeted mutation in CD97 were identified by genomic Southern blot analyses (Fig. 1A). Two clones were injected into C57BL/6 blastocysts, and germ line transmission was obtained at high frequency with both clones. Subsequently, one line carrying a disrupted CD97 locus on the C57BL/6 background was produced by at least 9 generations of backcrossing to C57BL/6 mice (National Cancer Institute, Frederick, MD).
FIG. 1.
Generation of CD97−/− mice. (A) Mutagenesis strategy. Genomic locus maps of the wild-type and targeted alleles are shown above and below a map of the targeting construct, respectively. The probes shown labeled A and B were used to probe, respectively, EcoRI-digested (wt 13 kb and targeted 2.5 kb) and EcoRV-digested (wt 13 kb and targeted 10.4 kb) genomic DNA to identify targeted ES cells. (B) Flow cytometry analysis of femoral bone marrow cells and spleen cells isolated from wild-type and CD97−/− mice. Cells were stained with biotinylated anti-mouse CD97 1A2 (20), followed by streptavidin-cy-chrome and FITC-Gr-1, PE-B220, and PE-CD4 plus CD8 for the identification of bone marrow granulocytes and splenic B and T cells, respectively.
Mice.
Sex- and age-matched pathogen-free adult C57BL/6 mice (CD45.2; National Cancer Institute, Frederick, MD) or littermates were used as control wild-type mice in this study. CD97−/− mice were bred in NCI animal care facilities, and genotypes were routinely confirmed by PCR. Congenic B6.SJL CD45a(Ly5a)/NAi (CD45.1) mice were from Taconic (Hudson, NY). This work was done under a protocol approved by the Animal Care and Use Committee of the National Institutes of Health.
Bacteria and survival studies.
Log-phase cultures of L. monocytogenes (strain EGD) were grown in brain-heart infusion broth (BD Difco Laboratories), and aliquots were frozen at −80°C until used. The final concentration of viable bacteria was enumerated by counts on brain heart infusion agar plates (Difco laboratories). For survival studies, mice were injected intravenously with 5 × 104 L. monocytogenes CFU diluted in 100 μl phosphate-buffered saline (PBS), and mice were monitored twice daily for as long as 14 days. For long-term survival studies, mice were injected with 2.5 × 104 bacteria and monitored for at least 90 days.
Determination of CFU in the liver and spleen.
Mice were infected with 5 × 104 L. monocytogenes and sacrificed at different time points thereafter. The liver and spleen were homogenized in distilled water, and numbers of CFU were determined by plating three serial dilutions of normalized organ homogenates on brain heart infusion agar plates.
Histology and immunohistochemistry.
Liver and splenic tissue samples from mice infected with L. monocytogenes were fixed with Protocal formalin (Fisher Scientific Co. LLC), and sections were stained with hematoxylin and eosin. For Gr-1 labeling in the liver, specimens were fixed in IHC zinc solution (BD Biosciences), 3- to 5-μm sections were made, and endogenous peroxidase was quenched with 0.2% H2O2 in methanol. Sections were first incubated with rat anti-mouse Gr-1 followed by rabbit anti-rat immunoglobulin G conjugated to horseradish peroxidase (BD Biosciences, San Diego, CA) according to the manufacturer's protocol. Granulocytes in liver sections were enumerated by counting Gr-1-positive cells in four randomly taken high-power fields (×400 magnification) for each mouse. Five mice from each experimental group were analyzed.
In vivo depletion of granulocytes.
Mice were injected intraperitoneally with 120 μg of anti-Gr-1 monoclonal antibody (MAb), 1 day before L. monocytogenes infection (33). RB6-8C5 ascites, used for in vivo depletion, was a generous gift from Alan Sher (NIAID, NIH). Granulocyte depletion was monitored by differential blood counts.
Differential mononuclear cell determinations in peripheral blood, peritoneal exudates, lung, spleen, and bone marrow.
Peripheral blood was collected by cardiac puncture of the left ventricle. Early analyses examining total and differential leukocyte counts in peripheral blood (Table 1) were determined using an Abbott cell-dyn 3500 automatic cell counter (Abbott Diagnostics) operated by the Department of Laboratory Medicine, Clinical Center, NIH. These data were confirmed by flow cytometric analysis. Data obtained for chimeric animals were wholly obtained using flow cytometry (see below). Peritoneal exudates were collected by lavage of the peritoneal cavity with 10 ml PBS containing 5 mM EDTA. Bone marrow cells were collected by gently exuding the bone marrow plug from dissected femurs with PBS. Total lung tissues were harvested, minced, and incubated with Liberase (Roche, Indianapolis, IN) for 1 h at 37°C. Lung cells were collected, and the numbers of lung leukocytes were counted. For spleen cells, single-cell suspensions were produced, red blood cells were lysed with ACK buffer, and washed cells were stained with fluorescent antibodies.
TABLE 1.
Peripheral leukocyte counts after L. monocytogenes infectiona
| Cell type | Leukocyte count (103/ml) for mouse type on day:
|
|||||
|---|---|---|---|---|---|---|
| 0
|
1
|
2
|
||||
| wt | CD97−/− | wt | CD97−/− | wt | CD97−/− | |
| Granulocytes | 624 ± 125 | 1,051 ± 335b | 1,675 ± 180 | 2,410 ± 536b | 1,039 ± 227 | 2,265 ± 1,109 |
| Monocytes | 417 ± 138 | 570 ± 335 | 917 ± 195 | 1,548 ± 339b | 816 ± 196 | 946 ± 550 |
| Lymphocytes | 7,786 ± 2,077 | 7,994 ± 1,561 | 1,549 ± 695 | 1,351 ± 426 | 664 ± 257 | 745 ± 499 |
| Leukocytes | 8,979 ± 1,798 | 9,753 ± 2,128 | 4,398 ± 777 | 5,586 ± 1,193 | 2,606 ± 172 | 4,023 ± 2,140 |
Mice were injected with 5 × 104 CFU L. monocytogenes in the tail vein. After 1 and 2 days of infection, animals were sacrificed and differential leukocyte counts were determined. Data are means ± standard deviations of results from 4 to 10 mice per group.
P < 0.05.
Flow cytometric analysis.
Biotinylated hamster anti-mouse CD97 antibody, clone 1A2, was the generous gift of Jorg Hamman (University of Amsterdam, The Netherlands) (20). Streptavidin-conjugated fluorescein isothiocyanate (FITC), 7-amino-actinomycin D (7AAD), and rat anti-mouse CD16/CD32 (clone 2.4G2) in addition to FITC-, phycoerythrin (PE)-, allophycocyanin (APC)-, or PE-Cy7-conjugated MAbs specific for Gr-1 (clone RB6-8C5), CD11b, CD3, CD4, CD8, CD45.1, and CD45.2 were purchased from BD Biosciences. Cells from peripheral blood, peritoneal exudates, spleen, and lung were harvested as above, and 1 × 106 cells/sample in 50 μl PBS with 2% fetal calf serum (fluorescence-activated cell sorter [FACS] staining buffer) were used for staining. Cells were blocked with 2.4G2 antibody (1 μg per 1 × 106 cells), incubated with appropriately diluted antibodies, and washed twice with FACS buffer. Cell preparations were run on a FACSCalibur flow cytometer (BD Biosciences), using propidium iodide (Sigma-Aldrich) to identify dead cells. Flow cytometric data were analyzed with Flowjo software (Treestar, San Carlos, CA).
Total and differential leukocyte counts in peripheral blood, spleen, peritoneal exudates, and lung were determined using FACS analysis and enumeration of total leukocytes. Granulocytes were identified by their characteristic front scatter/side scatter and Gr-1 and CD11b double-positive staining. The number of granulocytes was calculated as (total leukocyte count × proportion of Gr-1+ CD11b+ cells). Total cell numbers in peripheral blood, peritoneal exudates, and bone marrow were determined using a hemacytometer. Bone marrow granulocytes were subdivided into three populations of double-positive cells: CD11bint Gr-1int, CD11blo Gr-1hi, and CD11bhi Gr-1hi (38). Gr-1hi CD11bhi cells are mature peripheral granulocytes and Gr-1int CD11bint cells are mitotic progenitors of all granulocytes, whereas Gr-1hi CD11blo cells represented an intermediate stage of granulocyte maturation. Gr-1lo CD11bhi cells are defined as monocytes.
Production of mixed bone marrow chimeras.
The mice used were CD97−/− (CD45.2), which had been backcrossed to C57BL/6 mice for a minimum of 13 generations, and B6/Ly5.2 (CD45.1). Donor mice were euthanized, and bone marrow cells were harvested by flushing both femurs and tibias with PBS containing 2% fetal calf serum under sterile conditions. Erythrocytes were depleted with ACK buffer, and subsequently, the leukocytes were washed twice with PBS and resuspended. Recipient B6/Ly5.2 mice were lethally irradiated with 900 rad. Six hours later, approximately 2 × 107 unfractionated bone marrow cells from B6/Ly5.2 and CD97−/− mice, mixed in a 1:1 ratio, in 200 μl PBS were delivered intravenously through the tail vein of each recipient mouse. Recipient mice were housed under pathogen-free conditions before and after bone marrow transplantation. After bone marrow transplantation, the mice were maintained on antibiotics. Mice were used for experiments after 6 to 8 weeks of bone marrow reconstitution. Bone marrow and blood differentiation were analyzed by flow cytometry. We analyzed the relative composition of CD45.1 and CD45.2 cells in the bone marrow. The ratio of CD97 null (CD45.2) to wild-type (wt) (CD45.1) cells for granulocytes was 3.05 ± 0.59 (n = 15) and that for all remaining cells, which are mainly lymphocytes, was 1.413 ± 0.14 (n = 15). The significance or reasons for the apparent preferential growth of CD97 null granulocytes under conditions of competitive reconstitution is not clear, but it did not appear to result from greater numbers of preexisting myeloid CFU. Reconstitution of irradiated CD97 null or wt recipients with wholly CD97 null or wt bone marrow demonstrated no significant difference in myeloid CFU within the developing hematopoietic systems of the chimeras (not shown).
Analysis of subsets in mixed chimeras.
Six to eight weeks after transplantation, recipient mice were intraperitoneally injected with 2 μg KC or G-CSF or 2 × 105 CFU L. monocytogenes in 200 μl PBS. Four or 24 h later, peritoneal exudates, bone marrow, and blood were colleted for flow cytometric analysis. The distribution of leukocyte subsets with respect to genotype in chimeric animals was determined by direct immunofluorescence using a laser flow cytometer LSR II (BD Bioscience) and analyzed with FlowJo software (Treestar, San Carlos, CA). The fluorescent labeling procedure was the same as above. Forward and side scatter as well as 7AAD exclusion were used to discriminate dead cells. Peripheral blood, peritoneal exudates, and bone marrow cells were stained with anti-CD45.2-FITC and anti-CD45.1-PE to determine the origin of the cells. Staining with Gr-1 and anti-CD11b antibodies was used to distinguish myeloid cells.
Real-time PCR for liver KC and MIP-2.
Livers were removed and stored frozen in RNAlater (QIAGEN Inc.) buffer. Total RNA was prepared using an RNeasy Midi kit from QIAGEN according to the manufacturer's instructions, and cDNA was prepared using Superscript RT II (Invitrogen). Expression of KC and macrophage inflammatory protein 2 (MIP-2) was quantified by real-time reverse transcription (RT)-PCR using SYBR green (Applied Biosystems) and an ABI Prism 7900HT sequencer (Applied Biosystems). PCR was performed using the following primers and cycling conditions: hypoxanthine phosphoribosyltransferase, GTT GGA TAC AGG CCA GAC TTT GTT G (forward) and GAT TCA ACT TGC GCT CAT CTT AGG C (reverse); MIP-2, GTG AAC TGC GCT GTC AAT GC (forward) and CGC CCT TGA GAG TGG CTA TG (reverse); KC, TGT CAG TGC CTG CAG ACC AT (forward) and CCT CGC GAC CAT TCT TGA GT (reverse). The initial incubation of cDNA was at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Samples were normalized to hypoxanthine phosphoribosyltransferase, and the relative expression of MIP-2 and KC was calculated by comparison with expression in livers of uninfected wild-type mice.
Cytokines and chemokines.
Cytokine levels for TNF-α, gamma interferon (IFN-γ), interleukin-1β (IL-1β), IL-10, IL-12 (BD Pharmigen), and G-CSF (R&D) were determined by following the manufacturer's instructions. Liver homogenates were prepared by placing the whole liver in 10 ml distilled water, homogenizing on high speed for 6 min using a Stomacher no. 80 (Sewar LTD), and harvesting supernatants after centrifugation at 12,000 × g for 10 min. Stromal cell-derived factor 1α (SDF-1α) was assayed in bone marrow extracellular fluids using Quantikine M kits (R&D Systems) according to the manufacturer's instructions. The detection limits were 5 pg/ml for G-CSF. Bone marrow extracellular fluid was obtained by flushing each femur with 1 ml of ice-cold PBS without serum and harvesting supernatants after centrifugation at 400 × g for 5 min. Basal serum levels of granulocyte-macrophage colony-stimulating factor, IL-6, IL-17, MIP-1α, SDF-1β, TNF-α, and monocyte chemoattractant protein 1 were measured in a cytokine multiplex assay analysis by Pierce. Granulopoiesis was stimulated in mice using recombinant human G-CSF (Peprotech), diluted in PBS, and administered by daily subcutaneous injection at a dose of 5 μg/mouse per day.
Apoptosis assay.
Bone marrow cells were sterile harvested into minimal essential medium (Invitrogen) with 10% fetal bovine serum. Cells were either used immediately or cultured at 1 × 106 cells/ml for 16 to 24 h. The collected cells were washed and counted. For analysis, cells were resuspended in PBS to 1 × 107 cells/ml and 0.5 ml (5 × 106 cells) was incubated with 10 μl mouse BD Fc block for 5 min at 4°C, followed by 10 μl FITC-conjugated anti-Gr-1 monoclonal antibody for 15 min at room temperature. Cells were then washed twice in PBS and resuspended in 100 μl annexin V binding buffer. Then 5 μl of annexin V was added, and cells were incubated for 15 min at room temperature. Cells were then diluted with 400 μl annexin V binding buffer, and 5 μl of 7AAD was added. Following 5 min of incubation at room temperature, cells were analyzed by flow cytometry to determine the number of granulocytes undergoing apoptosis. Cells stained individually with either Gr-1 antibody, annexin V, or 7AAD were used as controls. All staining reagents were purchased from BD Biosciences Pharmingen.
Granulocyte chemotaxis assay.
Bone marrow leukocytes were harvested, washed, and counted. Cells were resuspended in RPMI-0.1% bovine serum albumin to 1 × 107 cells/ml, and 300 μl of cells was added to the top of a 3-μm-pore transwell chamber (Corning). The lower chamber contained 500 μl of either KC (PeproTech) at 10 or 100 ng/ml or SDF-1 (PeproTech) at 25 or 100 ng/ml. Cells were allowed to migrate for 1 h at 37°C and 5% CO2, and cells that had migrated into the lower chamber were counted. The percentage of granulocytes migrated was determined by flow cytometric analysis of cells stained with anti Gr-1 antibody.
Listeria killing assay.
Bactericidal/bacteriostatic activity was assessed by determining the Listeria CFU existing after incubation with inflammatory peritoneal cells, essentially as described previously (8). Peritoneal exudate cells (PEC), collected 4 h after injection of 1 ml of 3% thioglycolate, were composed of approximately 90% granulocytes and 5% macrophages. Assays were performed in either 17- by 100-mm tubes or 30-mm plates using 2 × 106 PEC and 1 × 106 Listeria monocytogenes. In some instances, a 30-min preincubation of granulocytes with 100 ng/ml recombinant mouse TNF (R&D Systems) was performed.
Statistical analysis.
Statistical significance was assessed using a two-sided Student's t test and Prism software. In all cases, a threshold P value of <0.05 was considered the minimum level of significance.
RESULTS
Creation of CD97 null mice.
To create a null allele for CD97, we replaced exons 2 through 12 with PGKneo in the same orientation as CD97 transcription (Fig. 1A). This disruption eliminates amino acids 11 to 376, deleting half of the leader sequence and introducing several stop codons after the ATG and before exon 13. Also, in the targeted construction, adjacent exons 1 and 13 are out of frame relative to one another, assuming that the predicted splice junctions are used. PCR amplification of cDNA extracted from granulocytes, using primers from the 3′ untranslated region of CD97, demonstrated that transcripts are produced. However, it was anticipated that even if transcripts are produced and correctly spliced, no functional protein would be expressed. Animals heterozygous for the targeted CD97 allele were established using standard methods and subsequently backcrossed onto the C57BL/6 background for at least 9 generations before being used in the experiments reported here. To verify proper inactivation of CD97, various hematopoietic cell types, including T cells, B cells, NK cells, macrophages, and granulocytes were stained with CD97-specific antibodies. There was no CD97-specific reactivity with any cell type assayed, as shown for granulocytes, T cells, and B cells (Fig. 1B).
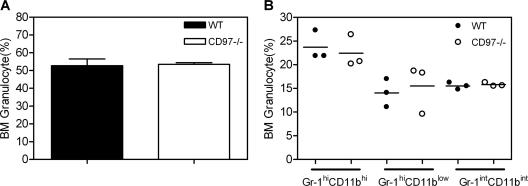
Mice with the targeted CD97 allele were born at the expected Mendelian frequency, thrived normally, and were fertile. Complete necropsies on male and female CD97 null adult mice revealed a mild peripheral blood granulocytosis (Table 1) and no other remarkable findings. To evaluate whether the peripheral granulocytosis resulted from hyperplasia of developing granulocytes in the bone marrow, the relative composition of Gr-1int-hi/Mac-1int-hi cells was determined (Fig. 2). Gr-1+ cells, which encompass mature and developing granulocytes as well as rare bone marrow monocytes, were comparable in wild-type and CD97 null mice, suggesting that the peripheral granulocytosis did not originate from increased granulocytes in the bone marrow.
FIG. 2.
Normal bone marrow granulocyte development in CD97−/− mice. Bone marrow cells were labeled with MAbs specific for mouse CD11b and Gr-1 and analyzed by flow cytometry. (A) Total granulocytes include Gr-1hi CD11bhi cells (mature peripheral granulocytes), Gr-1int CD11bint cells (mitotic progenitors of all granulocytes), and Gr-1hi CD11blo cells (granulocytes at an intermediate stage of maturation). (B) The relative proportion of granulocytes at each stage of development is similar for wt and CD97−/− mice (n = 3). Data are pooled from two separate experiments.
CD97 null mice are more resistant to Listeria monocytogenes infection.
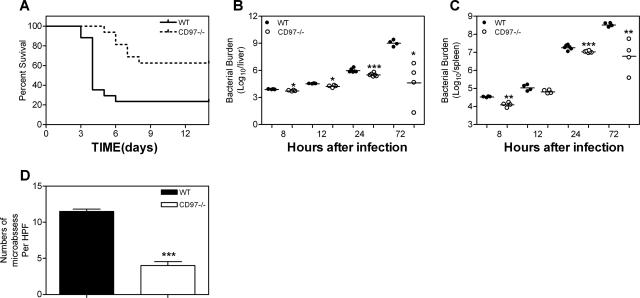
To begin examining the role of CD97 in immune function, CD97 null and wild-type mice were infected via intravenous administration with 5 × 104 CFU of L. monocytogenes (approximately three times the 50% lethal dose). Shown in Fig. 3A, CD97 null mice were significantly more resistant to the mortality induced by such an acute infection, which resulted in 80% mortality for wild-type mice and only 40% mortality for CD97-deficient mice. To evaluate acquired immunity, CD97 null and wt mice were infected with lower doses (2.5 × 104 CFU) of Listeria. CD97 null mice developed normal long-term immunity, as demonstrated by survival beyond 3 months postinfection of 5/8 wt and 7/8 CD97 null mice.
FIG. 3.
Enhanced resistance to L. monocytogenes infection in CD97−/− mice. (A) Mice were injected with 5 × 104 CFU in the tail vein (−/−, n = 16; +/+, n = 17). P = 0.003 for +/+ versus −/− (log rank test). (B and C) Bacterial burden. CFU from whole livers (B) and spleens (C) were determined 8, 12, 24, and 72 h p.i. with 5 × 104 CFU L. monocytogenes. The day 3 result is a representative result from two experiments. Each symbol indicates CFU from an individual animal. The mean value is indicated by a solid bar. (D) The average number of microabscesses per high-power field (HPF) ± standard deviation. Two fields were counted for each of five individual mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (CD97−/− compared to wild-type mice).
To evaluate a kinetic parameter associated with survival, we determined the extent of bacterial growth that had occurred in the livers and spleens of CD97 null and wild-type mice, 8, 12, 24, and 72 h postinfection (p.i.) (Fig. 3B and C). These data showed a relatively greater containment of bacterial growth at all time points in the CD97-deficient animals. At the earliest time point of 8 h, there was already a measurable decrease in the number of bacteria recovered from the spleens and livers of CD97 null mice. Within 24 h p.i., there were four- and twofold-fewer CFU, respectively, in the livers and spleens of CD97 null mice than in those of wild-type mice, suggesting that relative resistance to L. monocytogenes was established early in the infection. By 72 h p.i., there were approximately 4 and 2 logs fewer CFU, respectively, in the livers and spleens of CD97 null mice, consistent with their lower mortality rate. Local replication of bacteria can be observed as microabscesses, which contain a focus of dying hepatocytes and infiltrating granulocytes. In agreement with fewer CFU in CD97 null livers, we observed threefold-fewer microabscesses 72 h p.i. in null mice than in wild-type mice (Fig. 3D). To address the relative inhibition of Listeria growth by CD97 wild-type and null granulocytes, we assayed the number of Listeria CFU existing after in vitro incubation with peritoneal granulocytes. Inflammatory PEC inhibited the growth of Listeria by approximately 50% following a 2-h incubation in either the absence or presence of TNF preactivation. This growth-inhibitory activity was not significantly different for CD97 null and wild-type granulocytes (not shown).
CD97 null mice display enhanced numbers of granulocytes in the liver and blood at early times after systemic L. monocytogenes infection.
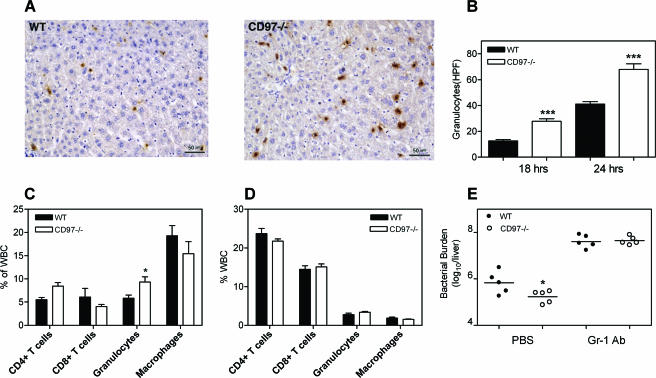
Because granulocytes are important for containing early bacterial replication and previous reports have suggested that CD97 is required for granulocyte recruitment, we measured by immunocytochemistry the extent of granulocyte presence in the livers of CD97 null and wild-type mice at 0, 8, 18, and 24 h p.i. Granulocytes in the liver were rarely observed at 0 or 8 h p.i. for either null or wild-type mice (not shown), most likely due to the insensitivity of histological assays. By 18 and 24 h p.i., there were threefold- and twofold-greater numbers, respectively, of Gr-1+ cells in the livers of null mice than in those of wild-type mice (Fig. 4A and B). At these time points, Gr-1+ cells were scattered single cells mostly within the vascular sinuses, although a few small microabscesses, indicative of granulocytes entering the liver parenchyma, were also apparent.
FIG. 4.
Increased granulocyte emigration contributes to a protective effect in CD97−/− mice. (A) Enumeration of Gr-1+ cells in the livers of infected animals 18 h p.i. with 5 × 104 CFU L. monocytogenes given intravenously. Liver tissues were collected, fixed, subsequently sectioned, and stained with anti-Gr-1 antibody. Representative sections from wild-type and CD97-deficient mice are shown. (B) Average number of granulocytes per high-power field (HPF) ± standard deviation at 18 h p.i. (n = 5) and 24 h p.i. (n = 2). Four fields (×400 magnification) from each sample were counted. Lung (C) and spleen (D) leukocytes (WBC) were harvested and labeled with fluorescent MAbs against mouse CD4, CD8, Gr-1, and CD11b 24 h after the intravenous injection of 5 × 104 CFU L. monocytogenes. (E) Effect of in vivo depletion of granulocytes on L. monocytogenes bacterial growth 24 h p.i. Mice were treated with 120 μg anti-Gr-1 MAb 24 h prior to intravenous infection with 5 × 104 CFU L. monocytogenes. CFU in livers were determined at 24 h p.i. Each symbol represents CFU in an individual animal. Horizontal lines indicate the mean CFU. *, P < 0.05; ***, P < 0.001 (CD97−/− compared to the wild type).
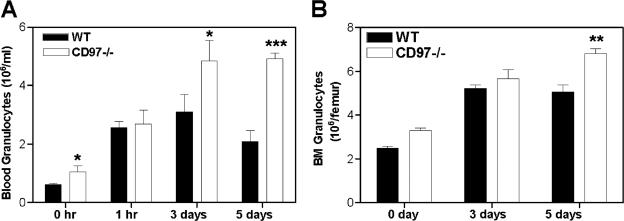
We compared the numbers of granulocytes in the peripheral blood of mice before and after L. monocytogenes infection. As discussed earlier, CD97 null mice were found to have approximately twofold-more basal granulocytes than matched wild-type mice (Table 1). Prior to infection, the total number of monocytes and lymphocytes in the two types of mice were similar. At 1 day p.i., CD97 null and wild-type mice demonstrated a two- to threefold increase in blood granulocytes and monocytes relative to uninfected animals, and there was an approximately 50% greater number of granulocytes and monocytes in the blood of CD97 null mice than in that of wild-type mice, consistent with the sinusoidal granulocytosis observed in the liver.
To determine the systemic response to listeriosis, we also evaluated the distribution of leukocyte subsets in the lung (Fig. 4C) and spleen (Fig. 4D) 24 h after intravenous L. monocytogenes infection. Listeria infection elicited granulocytic migration to the lung. There were slightly more granulocytes relative to total leukocytes in the lungs and similar levels of granulocytes in the spleens of CD97 null compared to wild type mice. These data indicate that the peripheral blood and liver sinusoid granulocytosis in CD97 null mice did not result from an abnormal redistribution of granulocytes from other organs.
Monocyte-, NK-, and T-cell-derived inflammatory cytokines and chemokines are expressed similarly in CD97-deficient and wild-type animals.
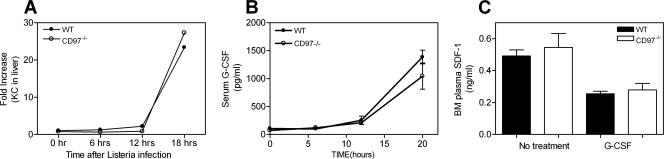
Monocyte lineage-derived cells and their induced products, TNF and inducible nitric oxide synthase, play an important role in resistance to Listeria (31). We have determined the levels of inflammatory cytokines produced in the livers of null and wild-type mice after 24 h of L. monocytogenes infection. As shown in Table 2, there were no significant differences in the levels of TNF-α, IL-1β, IFN-γ, IL-12, or IL-10 between CD97 null and wild-type animals, suggesting that Kupffer cells, which initially respond to the presence of Listeria, and recruited inflammatory monocytes are activated equivalently in wild-type and CD97 null animals (18). These results also suggest that granulocytes are not major contributors to the production of those cytokines measured at 24 h, since cytokines do not vary as does the granulocyte number between wild-type and CD97-deficient animals. Using quantitative RT-PCR, we observed, in the liver, similar induction kinetics and levels of RNA for two major granulocyte chemokines, KC (Fig. 5A) and MIP-2 (not shown), both of which engage the CXCR2 receptor and have been shown previously to play a significant role in Listeria-initiated granulocyte recruitment (12).
TABLE 2.
Cytokine levels in liver homogenates 24 h p.i. with L. monocytogenesa
| Mouse type | Level (pg/ml) of cytokine:
|
||||
|---|---|---|---|---|---|
| TNF-α | IL-1β | IFN-γ | IL-12 | IL-10 | |
| wt | 1,855 ± 275 | 9,205 ± 1,011 | 8,987 ± 1,497 | 8,122 ± 1,080 | 3,571 ± 307 |
| CD97−/− | 1,706 ± 382 | 8,213 ± 1,445 | 6,778 ± 2,189 | 7,471 ± 1,117 | 3,459 ± 292 |
Mice were injected with 5 × 104 CFU L. monocytogenes in the tail vein, and after 24 h, whole-liver homogenates were made and cytokine levels were assayed by enzyme-linked immunosorbent assay. Data are means ± standard deviations of results from 6 mice per group.
FIG. 5.
Granulocyte chemotactic factor expression is similar in CD97 +/+ and −/− mice. (A) Quantitation of KC RNA in the livers of wild-type and CD97−/− mice at various times p.i. with L. monocytogenes. (B) G-CSF levels in the blood of mice before and after infection with 5 × 104 CFU L. monocytogenes. (0 h, n = 6; 6 and 12 h, n = 3; 20 h, n = 11). (C) The levels of SDF-1 in bone marrow supernatants were measured before (n = 4) and after (n = 5) 5 days of G-CSF treatment.
To determine whether the reduced bacterial replication observed in the livers of CD97 null mice following systemic administration could be attributable to a Gr-1+ population of cells, anti-Gr-1 antibody was administered prior to infection (7). Granulocytes, “inflammatory” monocytes, but not tissue-resident monocytes, and plasmacytoid dendritic cells express Gr-1 (14). As shown in Fig. 4E, depletion of Gr-1+ cells markedly increased the CFU in the livers of CD97 null and wild-type mice and, importantly, eliminated the threefold differential in CFU which is normally seen. Coupled with the finding that monocyte-derived inflammatory cytokines are similar in wt and CD97 null livers following systemic infection, we favor the interpretation that increased granulocyte numbers in CD97 null mice are at least in part responsible for enhanced resistance to Listeria.
Granulopoietic cytokines and chemokines are normal in CD97 null mice.
One identifiable consequence of the genetic ablation of CD97 is a basal and inflammation-induced elevation of peripheral blood granulocyte numbers. A more severe basal granulocytosis and associated resistance to L. monocytogenes has been observed in other mice deficient in leukocyte or endothelial adhesion molecules such as integrins or selectins (13, 22, 28). In these models, granulocytosis is associated with significantly elevated G-CSF, secondary to IL-17 and IL-23 dysregulation, which is hypothesized to result from insufficient granulocyte trafficking to the periphery. G-CSF is a principal cytokine regulating steady-state granulocyte production and egress of granulocytes from the bone marrow (1). Shown in Fig. 5B, CD97 null and wild-type mice had comparable basal levels of G-CSF and showed similar kinetics and levels of G-CSF induction following infection. G-CSF induces release of mature granulocytes from the bone marrow indirectly through the generation of a trans-acting signal, one important component of which is the decrease of SDF-1 in the bone marrow compartment (27, 34). G-CSF stimulated a decrease in bone marrow plasma SDF-1, which was similar in wild-type and CD97-deficient samples (Fig. 5C). These results suggest that the granulocytosis in CD97 null mice is not secondary to G-CSF or SDF-1 dysregulation. Likewise, similar basal serum concentrations for IL-6, IL-17, and granulocyte-macrophage colony-stimulating factor were observed in wild-type and CD97-deficient mice (not shown). Other cytokines tested in basal serum (MIP-1α, SDF-1β, TNF-α, and monocyte chemoattractant protein 1) were below the level of detection in both strains of mice.
An additional factor that is expected to influence granulocyte accumulation is the rate of granulocyte turnover or apoptosis. We have evaluated the extent of apoptosis and the rate of survival for bone marrow granulocytes cultured in vitro (see Materials and Methods). Although we observed approximately 50% loss of survival after culturing for 16 h, wild-type and CD97 null granulocytes expressed apoptosis markers and survived comparably (not shown).
The in vitro and in vivo migratory properties of CD97 null granulocytes are comparable to those of wild-type granulocytes.
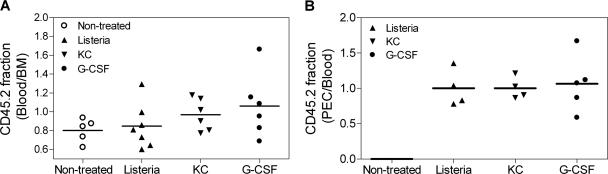
Previous data using in vivo antibody treatment directed against CD97 has suggested a necessary role for CD97 in granulocyte migration (25). We determined that in vitro chemotaxis toward either KC or SDF-1 by fresh or cultured bone marrow granulocytes was similar between CD97 null and wild-type strains (not shown, see Materials and Methods). To directly compare the migratory properties of CD97 null and wt granulocytes in vivo, we produced mixed bone marrow chimeras using congenic CD45 markers. Lethally irradiated C57BL/6 (CD45.1) host mice were reconstituted with an equal mixture of CD97 null (CD45.2) and wt (CD45.1) bone marrow. We determined that the absolute number of granulocytes in the blood stabilized by 4 to 5 weeks after reconstitution (not shown). The migration of granulocytes from bone marrow to blood and subsequently into inflamed tissue in the mixed chimeric mice was determined after the intraperitoneal injection of inflammatory stimuli. Four hours after the introduction of KC, G-CSF, or L. monocytogenes, the average increase in the absolute number of blood granulocytes was 2, 5, and 2.5 times greater than that of untreated controls, respectively.
The fraction of granulocytes composing each genotype in the bone marrow, blood, and peritoneal cavity was determined, and the relative migration of CD97 null and wt granulocytes was compared. If migration of the two genotypes is equivalent, the relative composition of CD45.2 and CD45.1 cells will be maintained and a ratio of the percent composition between the anatomical compartments (bone marrow, blood, or peritoneum) will equal 1. The ratio of the fraction of CD45.2 cells in the blood relative to the fraction in the bone marrow following no treatment or 4 h after the induction of an inflammatory response is shown for individual animals in Fig. 6A. The mean of the ratios (0.92 ± 0.24) is not significantly different from 1.0. Thus, we conclude that CD97 null and wt granulocytes migrated from the bone marrow to the blood in proportion to their composition in the bone marrow, suggesting similar intrinsic migratory ability. Also, granulocytes migrated into an inflamed peritoneal cavity proportionally to their composition in blood (Fig. 6B). Thus, the granulocytosis in CD97 null mice most likely does not result from an enhancement of bone marrow emigration to the blood. In addition, these data show that granulocyte migration into the inflamed peritoneum is similar for the two genotypes.
FIG. 6.
A direct in vivo comparison of CD97 null and wt granulocytes with respect to their accumulation in various anatomical compartments following the administration of an inflammatory stimulus. Chimeric animals were either left untreated or injected intraperitoneally with L. monocytogenes, KC, or G-CSF. Four hours later, peritoneal exudate cells, peripheral blood, and bone marrow were collected and processed to determine the number of CD45.1 (wt) and CD45.2 (CD97 null) granulocytes. Each data point represents an individual animal and was compiled from the results of at least three independent experiments. (A) The relative migration from bone marrow to blood is expressed as the ratio of the fraction of CD45.2 cells in the blood over the bone marrow. The mean of the ratios shown is 0.92, which is not significantly different from 1.0. (B) The ratio of the fraction of CD45.2 cells in the blood relative to the peritoneal cavity is shown. Peritoneal cells from untreated mice were not examined.
To further verify and characterize the granulocytosis phenotype in CD97 null mice with an agent that stimulates a less pleiotropic response than Listeria, we induced granulocyte production and differentiation in mice with chronic administration of G-CSF. Following G-CSF administration, there were, after 3 and 5 days, respectively, 50% and 250% more mature granulocytes in the blood of CD97 null mice than in that of wild-type mice (Fig. 7A). Consistent with the blood granulocytosis phenotype that was observed in both the resting and Listeria-challenged CD97 null animals, there was a relatively greater increase in blood granulocytes compared to bone marrow granulocytes in response to G-CSF (Fig. 7A and B). Because the bone marrow emigration response to G-CSF was shown to be similar in CD97-deficient and wild-type mice (Fig. 6A), these data independently confirm the localization of the CD97 null granulocytosis to the peripheral blood compartment.
FIG. 7.
G-CSF-stimulated granulocytosis. Granulocyte numbers were assayed using FACS analyses as detailed in Materials and Methods. (A) Mobilization and granulopoietic response to G-CSF administration. Mice (n = 5) were treated daily with G-CSF subcutaneously, and the absolute number of granulocytes in the blood was determined either 1 h after the initial treatment or 4 h after treatment on days 3 and 5. (B) The absolute number of bone marrow granulocytes was assayed on days 3 and 5 after G-CSF administration as described above for panel A.
DISCUSSION
We have produced and characterized a CD97 null mouse strain with the goal of gaining insight into the function of CD97. An obvious phenotypic consequence of CD97 deletion in the resting immune system is a mild granulocytosis. In addition, CD97 null mice demonstrated increased resistance to listeriosis, and we favor the explanation that such resistance is, at least in part, secondary to an enhanced inflammation-induced granulocytosis. The increased resistance to Listeria resulted from a decrease in bacterial burden that was observable by 8 h, a time frame dependent upon innate immune function. Kinetic analyses of Listeria growth in the liver have shown a role for GR-1+ cells in the containment of bacterial growth between 10 min and 6 h after intravenous injection (17), and we observed that the differential sensitivity of wild-type and CD97 null mice to Listeria was dependent upon a GR-1+ cell. Since we were unable to find evidence for enhanced, Listeria-induced activation of monocyte lineage or natural killer cells as measured by typical cytokine or chemokine release, it is reasonable to suggest that the increased concentration of granulocytes in the blood of CD97 null mice contributes to enhanced resistance.
These studies also address a prior suggestion that CD97 function is necessary for granulocyte migration to inflammatory sites in the colon and lung, based upon the in vivo use of monoclonal antibodies to mouse CD97 (25). As evidenced by the studies presented here, the absence of CD97 does not impede migration of granulocytes to sites of inflammation in the lungs (Fig. 4C) or the peritoneal cavity (Fig. 6B). Therefore, CD97 is not absolutely required for migration of granulocytes, and it seems unlikely that antibody treatment inhibits migration as a result of blocking CD97 ligand interactions. Rather, it is possible that anti-CD97 antibodies have an agonistic function that impedes migration. Technical artifacts such as capture of antibody-bound cells by the reticuloendothelial system or complement-dependent lysis are also possible explanations for the inhibition of granulocyte accumulation in vivo following treatment with antibodies directed toward CD97.
What is the mechanism of granulocytosis in CD97-deficient mice? Recent evidence has been presented demonstrating homeostatic regulation of neutrophil production, which is dependent upon granulocyte migration to peripheral tissues. The phagocytosis by macrophages and dendritic cells of apoptotic granulocytes in tissues curbs secretion of IL-23, which in turn controls IL-17 and G-CSF production (37). Thus, mice deficient in adhesion molecules, such as integrins, produce granulocytes that do not migrate readily to some peripheral organs, such as skin (29, 37). Consequently, these strains display granulocytosis secondary to increased IL-17 and G-CSF blood cytokine levels. We found no evidence of enhanced basal or inflammation-induced G-CSF (Fig. 5B) or basal IL-17 levels (not shown) in the blood of CD97-deficient mice. Various other granulopoietic cytokines were normal as well. Therefore, granulocytosis induced by CD97 deficiency most likely occurs through a distinct mechanism compared to integrin and/or selectin deficiencies. A CD97-dependent mechanism is probably independent of feedback regulation resulting from adhesion and migration of granulocytes into peripheral tissues.
Is granulocytosis a result of a migratory defect involving the bone marrow-peripheral blood axis? Using mixed bone marrow chimeras to directly evaluate inflammation-induced migration from the bone marrow to the blood, we have shown that CD97 null and wild-type granulocytes emigrate similarly (Fig. 6). Another mechanism that could lead to mild granulocytosis is a prolonged half-life of CD97-deficient granulocytes in the blood. An increased half-life could result from decreased apoptosis and/or an attenuated homing to bone marrow of senescent granulocytes. However, we were unable to observe cell-autonomous differences in the in vitro apoptotic response of wild-type and CD97-deficient, cultured bone marrow granulocytes. Additionally, there were no differences in the ability of cultured, CXCR4-expressing granulocytes to migrate toward SDF-1 (not shown) nor were there differences in the amount of constitutively expressed SDF-1 in the bone marrow (Fig. 5C). SDF-1 has been proposed to regulate the homing of senescent granulocytes to bone marrow (27). Finally, we cannot eliminate the possibility that immune response genes linked to CD97 from the 129-derived ES cells have had an effect on granulocyte homeostasis.
In summary, we have shown here that CD97 deficiency leads to peripheral blood granulocytosis, which has a significant biological consequence, as demonstrated by increased resistance to systemic Listeria monocytogenes. It has not been possible to establish a demonstrable difference in the migration, survival, or development of granulocytes from CD97 null mice, and therefore, it is possible that CD97 contributes small differences to more than one determinant in granulocyte homeostasis. As more is learned about the ligands and signaling components that act in the CD97 pathway, we expect that CD97 can be combined with other loci to investigate this important physiological process in mouse models.
Acknowledgments
We thank Anthony Wynshaw-Boris and the core facilities of the National Human Genome Research Institute for generating the chimeric CD97 null mice. We thank Charles Scanga (NIAID, NIH) for performing quantitative PCR analyses of KC and MIP-2 RNA levels and Susan Sharrow (NCI, NIH) for assistance with FACS analysis.
We acknowledge the support of the intramural research program CCR, NCI (T.W., L.T., M.H., R.L., Y.W., and K.K.) and NIAID (J.-L.G., H.W., U.S., and P.M.M.)
Editor: R. P. Morrison
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Basu, S., A. Dunn, and A. Ward. 2002. G-CSF: function and modes of action. Int. J. Mol. Med. 10:3-10. [PubMed] [Google Scholar]
- 2.Bjarnadottir, T., R. Fredriksson, P. Hoglund, D. Gloriam, M. Lagerstrom, and H. Schioth. 2004. The human and mouse gene repertoire of the adhesion family of G-protein-coupled receptors. Genomics 84:23-33. [DOI] [PubMed] [Google Scholar]
- 3.Chang, G. W., M. Stacey, M. J. Kwakkenbos, J. Hamann, S. Gordon, and H. H. Lin. 2003. Proteolytic cleavage of the EMR2 receptor requires both the extracellular stalk and the GPS motif. FEBS Lett. 547:145-150. [DOI] [PubMed] [Google Scholar]
- 4.Conlan, J. W., and R. J. North. 1991. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J. Exp. Med. 174:741-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousens, L. P., and E. J. Wing. 2000. Innate defenses in the liver during Listeria infection. Immunol. Rev. 174:150-159. [DOI] [PubMed] [Google Scholar]
- 7.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 8.Czuprynski, C. J., P. M. Henson, and P. A. Campbell. 1984. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J. Leukoc. Biol. 35:193-208. [DOI] [PubMed] [Google Scholar]
- 9.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 158:5297-5304. [PubMed] [Google Scholar]
- 10.Dai, W. J., G. Kohler, and F. Brombacher. 1997. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 158:2259-2267. [PubMed] [Google Scholar]
- 11.Deng, C., A. Wynshaw-Boris, F. Zhou, A. Kuo, and P. Leder. 1996. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911-921. [DOI] [PubMed] [Google Scholar]
- 12.Ebe, Y., G. Hasegawa, H. Takatsuka, H. Umezu, M. Mitsuyama, M. Arakawa, N. Mukaida, and M. Naito. 1999. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol. Int. 49:519-532. [DOI] [PubMed] [Google Scholar]
- 13.Forlow, S. B., J. R. Schurr, J. K. Kolls, G. J. Bagby, P. O. Schwarzenberger, and K. Ley. 2001. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood 98:3309-3314. [DOI] [PubMed] [Google Scholar]
- 14.Gordon, S., and P. R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953-964. [DOI] [PubMed] [Google Scholar]
- 15.Gray, J. X., M. Haino, M. J. Roth, J. E. Maguire, P. N. Jensen, A. Yarme, M. A. Stetler-Stevenson, U. Siebenlist, and K. Kelly. 1996. CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J. Immunol. 157:5438-5447. [PubMed] [Google Scholar]
- 16.Gregory, S. H., L. P. Cousens, N. van Rooijen, E. A. Dopp, T. M. Carlos, and E. J. Wing. 2002. Complementary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J. Immunol. 168:308-315. [DOI] [PubMed] [Google Scholar]
- 17.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157:2514-2520. [PubMed] [Google Scholar]
- 18.Gregory, S. H., and E. J. Wing. 2002. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J. Leukoc. Biol. 72:239-248. [PubMed] [Google Scholar]
- 19.Hamann, J., C. Stortelers, E. Kiss-Toth, B. Vogel, W. Eichler, and R. A. van Lier. 1998. Characterization of the CD55 (DAF)-binding site on the seven-span transmembrane receptor CD97. Eur. J. Immunol. 28:1701-1707. [DOI] [PubMed] [Google Scholar]
- 20.Hamann, J., C. van Zeventer, A. Bijl, C. Molenaar, K. Tesselaar, and R. A. van Lier. 2000. Molecular cloning and characterization of mouse CD97. Int. Immunol. 12:439-448. [DOI] [PubMed] [Google Scholar]
- 21.Hamann, J., B. Vogel, G. M. van Schijndel, and R. A. van Lier. 1996. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz, B. H., J. P. Mizgerd, M. L. Scott, and C. M. Doerschuk. 2001. Mechanisms of granulocytosis in the absence of CD18. Blood 97:1578-1583. [DOI] [PubMed] [Google Scholar]
- 23.Jaspars, L. H., W. Vos, G. Aust, R. A. Van Lier, and J. Hamann. 2001. Tissue distribution of the human CD97 EGF-TM7 receptor. Tissue Antigens 57:325-331. [DOI] [PubMed] [Google Scholar]
- 24.Kwakkenbos, M. J., E. N. Kop, M. Stacey, M. Matmati, S. Gordon, H. H. Lin, and J. Hamann. 2004. The EGF-TM7 family: a postgenomic view. Immunogenetics 55:655-666. [DOI] [PubMed] [Google Scholar]
- 25.Leemans, J. C., A. A. te Velde, S. Florquin, R. J. Bennink, K. de Bruin, R. A. van Lier, T. van der Poll, and J. Hamann. 2004. The epidermal growth factor-seven transmembrane (EGF-TM7) receptor CD97 is required for neutrophil migration and host defense. J. Immunol. 172:1125-1131. [DOI] [PubMed] [Google Scholar]
- 26.Lin, H. H., D. E. Faunce, M. Stacey, A. Terajewicz, T. Nakamura, J. Zhang-Hoover, M. Kerley, M. L. Mucenski, S. Gordon, and J. Stein-Streilein. 2005. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 201:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, C., P. C. Burdon, G. Bridger, J. C. Gutierrez-Ramos, T. J. Williams, and S. M. Rankin. 2003. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19:583-593. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto, M., M. Emoto, Y. Emoto, V. Brinkmann, I. Yoshizawa, P. Seiler, P. Aichele, E. Kita, and S. H. Kaufmann. 2003. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J. Immunol. 170:5228-5234. [DOI] [PubMed] [Google Scholar]
- 29.Mizgerd, J. P., H. Kubo, G. J. Kutkoski, S. D. Bhagwan, K. Scharffetter-Kochanek, A. L. Beaudet, and C. M. Doerschuk. 1997. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J. Exp. Med. 186:1357-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North, R. J., P. L. Dunn, and J. W. Conlan. 1997. Murine listeriosis as a model of antimicrobial defense. Immunol. Rev. 158:27-36. [DOI] [PubMed] [Google Scholar]
- 31.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812-823. [DOI] [PubMed] [Google Scholar]
- 32.Qian, Y. M., M. Haino, K. Kelly, and W. C. Song. 1999. Structural characterization of mouse CD97 and study of its specific interaction with the murine decay-accelerating factor (DAF, CD55). Immunology 98:303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiler, P., P. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and S. H. Kaufmann. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 34.Semerad, C. L., F. Liu, A. D. Gregory, K. Stumpf, and D. C. Link. 2002. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17:413-423. [DOI] [PubMed] [Google Scholar]
- 35.Serbina, N. V., T. P. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 36.Stacey, M., G. W. Chang, J. Q. Davies, M. J. Kwakkenbos, R. D. Sanderson, J. Hamann, S. Gordon, and H. H. Lin. 2003. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 102:2916-2924. [DOI] [PubMed] [Google Scholar]
- 37.Stark, M. A., Y. Huo, T. L. Burcin, M. A. Morris, T. S. Olson, and K. Ley. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22:285-294. [DOI] [PubMed] [Google Scholar]
- 38.Ueda, Y., M. Kondo, and G. Kelsoe. 2005. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 201:1771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, T., Y. Ward, L. Tian, R. Lake, G. L. W. Stetler-Stevenson, and K. Kelly. 2005. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counter receptors on endothelial cells. Blood 105:2836-2844. [DOI] [PubMed] [Google Scholar]
- 40.Wing, E. J., and S. H. Gregory. 2002. Listeria monocytogenes: clinical and experimental update. J. Infect. Dis. 185(Suppl. 1):S18-S24. [DOI] [PubMed] [Google Scholar]