Abstract
c-Fos is a component of transcription factor AP-1. We show that macrophages lacking c-Fos exhibit enhanced production of proinflammatory cytokines, potentiated NF-κB phosphorylation, and increased cell death following Salmonella enterica serovar Typhimurium infection. Furthermore, mice lacking c-Fos are highly susceptible to infection, suggesting that c-Fos confers resistance to Salmonella infection in mice.
Salmonella enterica serovar Typhimurium infection in mice provides a model to study the pathogenesis of typhoid-like systemic disease caused by gram-negative intracellular parasites. Detailed molecular mechanisms by which host cells, particularly macrophages, respond to and clear Salmonella infections are still obscure. Salmonella activates various Toll-like receptors (TLR) of host cells, such as TLR4 by lipopolysaccharide (LPS) and TLR5 by flagellin. TLR signaling activates transcription factors, including nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), which are responsible for producing proinflammatory cytokines (1, 11).
The transcription factor c-Fos, a component of AP-1, is necessary for osteoclast differentiation. Mice lacking c-Fos (Fos−/− mice) are osteopetrotic due to a lack of osteoclasts (3, 18). In contrast to its role in activating osteoclastogenic or antiosteoclastogenic target genes (9, 17, 20), the function of c-Fos in the innate immune system remains unclear (10). c-Fos and AP-1 family genes are induced in macrophages activated by LPS and gamma interferon (1). Several studies reveal that c-Fos also functions in macrophages (2, 12, 15). Using Fos−/− mice, we observed that the absence of c-Fos leads to enhanced production of tumor necrosis factor alpha (TNF-α), interleukin-12 p40 (IL-12 p40), and IL-6 but decreased production of IL-10 in response to LPS stimulation (13). However, the responses of Fos−/− macrophages or mice to bacterial infection have not been evaluated.
To determine if inflammatory responses of macrophages infected with Salmonella are affected by lack of c-Fos, Fos+/− mice on a 129×C57BL/6 genetic background (18) were backcrossed to C57BL/6 until all progeny carried the Salmonella-sensitive Nramp1 (Slc11a1) allele derived from C57BL/6 (Asp169 mutation) (16). We then prepared macrophage colony-stimulating factor (M-CSF)-dependent macrophages (MDMs) from wild-type and Fos−/− littermates and infected them with Salmonella enterica serovar Typhimurium strain χ3306 (4). We harvested both cells and culture supernatants at various time points and measured mRNA and protein levels of TNF-α, IL-12 p40, and IL-6. We observed that both mRNA and protein levels of IL-12 p40 and TNF-α in Fos−/− MDMs were significantly higher than those seen for wild-type MDMs after Salmonella infection, while levels of IL-6 production were comparable between wild-type and Fos−/− MDMs (Fig. 1).
FIG. 1.
Proinflammatory cytokine production by MDMs after Salmonella infection. MDMs were generated by culturing nonadherent cells in the presence of 10 ng/ml M-CSF (R&D) for 4 days as described previously (8). Adherent cells were replated at 1 × 105 cells per well in 48-well plates. MDMs from wild-type (WT) and Fos−/− (KO) mice were infected with Salmonella enterica serovar Typhimurium strain χ3306 at an MOI of 10 for 30 min. Infected cells were washed and fed with fresh medium containing 25 μg/ml gentamicin to kill extracellular bacteria. Cellular mRNA (left panels) and supernatant protein levels (right panels) of TNF-α, IL-12 p40, and IL-6 were measured by quantitative reverse transcription-PCR (RT-PCR) and enzyme-linked immunosorbent assay, respectively. Bars represent means ± standard deviations (SD) (n = 3 for each time point).
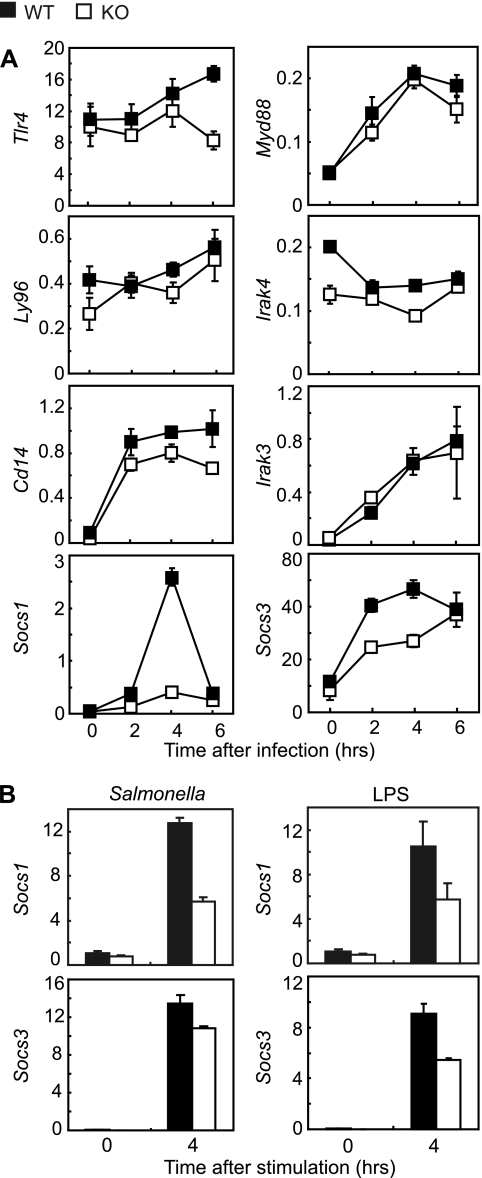
To gain insight into molecular mechanisms underlying the observed hyperresponsiveness of Fos−/− MDMs, we quantified mRNA levels of Tlr4 (encoding TLR4), Ly96 (MD-2), Cd14 (CD14), Myd88 (MyD88), Irak4 (IRAK-4), Irak3 (IRAK-M), Socs1 (suppressor of cytokine response-1), and Socs3 at 0, 2, 4, and 6 h after infecting MDMs with Salmonella. Strikingly, Socs1 and Socs3 induction was impaired in Fos−/− MDMs (Fig. 2A). Consistently, when peritoneal macrophages (PMs) were infected with Salmonella or stimulated with LPS purified from Salmonella, induction of Socs1 and Socs3 mRNA was lower in Fos−/− PMs than in wild-type PMs (Fig. 2B). SOCS-1 negatively regulates TLR signaling by mediating degradation of Mal (MyD88-adaptor like, also called TIRAP), an adaptor molecule that relays TLR signaling (7). One might predict that impaired Socs1 induction in Fos−/− MDMs would result in sustained activation of Mal followed by potentiated phosphorylation of the p65 subunit of NF-κB, which enhances proinflammatory cytokine production. We did observe potentiated p65 phosphorylation but not sustained Mal activation (Fig. 3). Therefore, at present, it is unknown how p65 phosphorylation is elevated in the absence of c-Fos.
FIG. 2.
Induction of Socs1 and Socs3 mRNA is suppressed in Fos−/− macrophages in response to Salmonella infection. (A) TLR signaling-associated transcripts in MDMs. MDMs were infected with Salmonella as described for Fig. 1. Cellular mRNA levels of Tlr4, Ly96 (Md2), Cd14, Myd88, Irak4, Irak3, Socs1, and Socs3 were measured by quantitative RT-PCR from triplicate cultures. The transcripts are relative to Gapdh. Bars represent means ± standard errors of the means. WT, wild type; KO, Fos−/−. (B) Socs1 and Socs3 transcripts in PMs. Peritoneal cells were plated at 1 × 105 cells per well in 48-well plates and cultured overnight, and adherent cells were used as PMs (duplicate cultures for each genotype). Cells were infected with Salmonella as described for Fig. 1 or stimulated with 100 ng/ml LPS (S. enterica serovar Minnesota Re595; Sigma) for 4 h, and Socs1 and Socs3 mRNA levels were measured by quantitative RT-PCR from duplicate cultures. The transcripts are relative to Gapdh. Bars represent means ± standard errors of the means.
FIG. 3.
Western blotting of NF-κB p65 and Mal after Salmonella infection. MDMs prepared from wild-type (WT) or Fos−/− (KO) mice in the presence of 30 ng/ml M-CSF were plated at 4 × 106 cells per 6-cm dish and infected with Salmonella as described for Fig. 1. Total cell lysates were harvested, and Western blotting was performed as previously described (8) using mouse monoclonal anti-phospho (Ser536) NF-κB p65 (phospho-p65) (7F1; Cell Signaling), rabbit polyclonal anti-NF-κB p65 (F-6; Santa Cruz), rabbit polyclonal anti-TIRAP (Mal/TIRAP) (Abcam), and anti-actin (I-19; Santa Cruz).
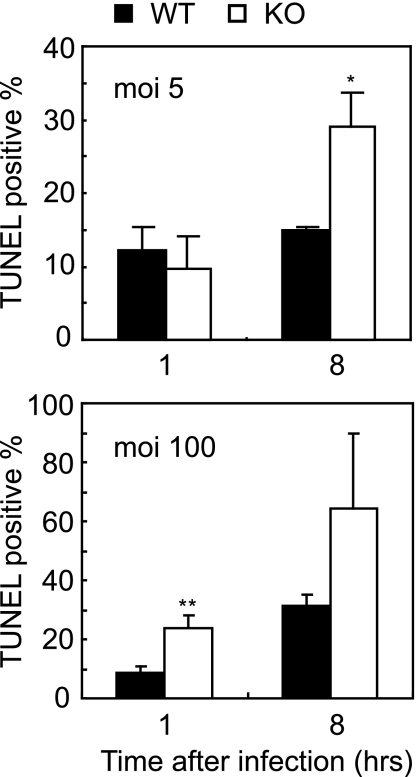
Salmonella can induce cell death in macrophages via diverse mechanisms (5). We therefore compared the frequencies of apoptosis for wild-type and Fos−/− MDMs after Salmonella infection. MDMs of each genotype were infected with Salmonella, and after 1 or 8 h, apoptotic cells were stained by a terminal deoxynucleotidyltransferase-mediated biotin-dUTP nick end labeling (TUNEL) method. At a low multiplicity of infection (MOI) of 5, Fos−/− cultures contained a greater abundance of TUNEL-positive cells than did wild-type controls 8 h after infection (Fig. 4, upper panel). At a high MOI (100), increased apoptosis in Fos−/− MDMs was apparent 1 h after infection (Fig. 4, lower panel).
FIG. 4.
Induction of apoptosis by Salmonella infection. MDMs prepared as described for Fig. 3 were infected at 3 × 105 cells per 24 wells as in Fig. 1 at an MOI of 5 (upper panel) or 100 (lower panel) for 1 h. After an additional 1 or 8 h of incubation, cells were stained by TUNEL (Promega), and the percentages of TUNEL-positive cells relative to total DAPI (4′,6′-diamidino-2-phenylindole)-positive nuclei were calculated. Error bars represent means ± SD (n = 3 each).  , P < 0.05 versus wild-type control;
, P < 0.05 versus wild-type control; 
 , P < 0.01 versus wild-type control.
, P < 0.01 versus wild-type control.
To determine whether Fos−/− mice are resistant or susceptible to Salmonella infection, we orally infected wild-type and Fos−/− mice with 3.3 × 107 CFU/g body weight of Salmonella. We sacrificed infected mice on day 3 and measured levels of TNF-α, IL-12 p40, and IL-6 in sera. Consistent with in vitro data (Fig. 1), TNF-α and IL-12 p40 levels were greatly elevated in Fos−/− compared to those in wild-type mice (Fig. 5A). On the other hand, IL-6 levels after infection were similar for both genotypes (Fig. 5A). We next quantified Salmonella CFU in these mice on day 3. Fos−/− mice showed significantly higher numbers of CFU in blood, spleen, and liver than did wild-type mice (Fig. 5B). Finally, we observed that while wild-type mice survived infection, all Fos−/− mice died within 7 days (Fig. 5C). These data show that lack of c-Fos results in increased proliferation of Salmonella, elevated production of proinflammatory cytokines, and death.
FIG. 5.
Fos−/− mice are susceptible to Salmonella infection. (A and B) Wild-type (WT) and Fos−/− (KO) mice (n = 4 for each genotype) were inoculated orally with 3.3 × 107 CFU/g body weight of Salmonella. Serum cytokine levels (A) and CFU in blood, liver, and spleen (B) were determined 3 days after infection. Error bars represent means ± SD (n = 3 each).  , P < 0.05 versus wild-type control;
, P < 0.05 versus wild-type control; 
 , P < 0.01 versus wild-type control. Cont., control; Infec., infected. (C) Survival of mice was monitored for 1 week after infection as described for panel A. n = 4 for each genotype.
, P < 0.01 versus wild-type control. Cont., control; Infec., infected. (C) Survival of mice was monitored for 1 week after infection as described for panel A. n = 4 for each genotype.
We have shown that serum levels of TNF-α and IL-12 p40 are greatly elevated in Fos−/− mice after Salmonella infection, most likely due to potentiated NF-κB p65 phosphorylation. Currently, we cannot explain why c-Fos loss does not affect IL-6 induction after Salmonella infection, while it does after LPS stimulation (13). Increased levels of proinflammatory cytokines, particularly TNF-α, cause profound endotoxic shock in Fos−/− mice (13). Consistently, mice lacking TNF-α or mice injected with a neutralizing anti-TNF-α antibody show increased survival after endotoxic shock (14). On the other hand, increased mortality observed for Fos−/− mice is associated with excessive proliferation of Salmonella in various organs. Since increased proinflammatory cytokines are reported to promote bacterial proliferation in vitro (6), elevated proinflammatory cytokines might enhance bacterial proliferation in Fos−/− mice, thereby increasing lethality. Alternatively, elevated proinflammatory cytokines may directly enhance apoptotic cell death of macrophages and other immune cells (19), allowing bacterial proliferation. We cannot formally exclude the possibility, however, that elevated proinflammatory cytokines help clear bacteria in Fos−/− mice after limited infection at lower doses.
In this study, we report that Fos−/− mice are highly susceptible to Salmonella infection and that Salmonella replicates more vigorously in Fos−/− than in wild-type mice. These findings suggest that c-Fos confers protective immunity against Salmonella in mice.
Acknowledgments
We thank Hidenori Matsui for the Salmonella strain, Latifa Bakiri and Shigeo Koyasu for 129 mouse genomic DNAs, Sadao Komatsu and Yuri Sasaki for technical assistance, and Elise Lamar for critical reading of the manuscript.
Editor: F. C. Fang
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Dillon, S., A. Agrawal, T. Van Dyke, G. Landreth, L. McCauley, A. Koh, C. Maliszewski, S. Akira, and B. Pulendran. 2004. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172:4733-4743. [DOI] [PubMed] [Google Scholar]
- 3.Grigoriadis, A. E., Z. Q. Wang, M. G. Cecchini, W. Hofstetter, R. Felix, H. A. Fleisch, and E. F. Wagner. 1994. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266:443-448. [DOI] [PubMed] [Google Scholar]
- 4.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hueffer, K., and J. E. Galán. 2004. Salmonella-induced macrophage death: multiple mechanisms, different outcomes. Cell. Microbiol. 6:1019-1025. [DOI] [PubMed] [Google Scholar]
- 6.Kanangat, S., G. U. Meduri, E. A. Tolley, D. R. Patterson, C. U. Meduri, C. Pak, J. P. Griffin, M. S. Bronze, and D. R. Schaberg. 1999. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect. Immun. 67:2834-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansell, A., R. Smith, S. L. Doyle, P. Gray, J. E. Fenner, P. J. Crack, S. E. Nicholson, D. J. Hilton, L. A. O'Neill, and P. J. Hertzog. 2006. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7:148-155. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama, K., Y. Takada, N. Ray, Y. Kishimoto, J. M. Penninger, H. Yasuda, and K. Matsuo. 2006. Receptor activator of NF-κB ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J. Immunol. 177:3799-3805. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo, K., J. M. Owens, M. Tonko, C. Elliott, T. J. Chambers, and E. F. Wagner. 2000. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat. Genet. 24:184-187. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo, K., and N. Ray. 2004. Osteoclasts, mononuclear phagocytes, and c-Fos: new insight into osteoimmunology. Keio J. Med. 53:78-84. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 12.Okada, S., S. Obata, M. Hatano, and T. Tokuhisa. 2003. Dominant-negative effect of the c-fos family gene products on inducible NO synthase expression in macrophages. Int. Immunol. 15:1275-1282. [DOI] [PubMed] [Google Scholar]
- 13.Ray, N., M. Kuwahara, Y. Takada, K. Maruyama, T. Kawaguchi, H. Tsubone, H. Ishikawa, and K. Matsuo. 2006. c-Fos suppresses systemic inflammatory response to endotoxin. Int. Immunol. 18:671-677. [DOI] [PubMed] [Google Scholar]
- 14.Riedemann, N. C., R. F. Guo, and P. A. Ward. 2003. Novel strategies for the treatment of sepsis. Nat. Med. 9:517-524. [DOI] [PubMed] [Google Scholar]
- 15.Roy, S., R. Charboneau, K. Cain, S. DeTurris, D. Melnyk, and R. A. Barke. 1999. Deficiency of the transcription factor c-fos increases lipopolysaccharide-induced macrophage interleukin 12 production. Surgery 126:239-247. [PubMed] [Google Scholar]
- 16.Skamene, E., E. Schurr, and P. Gros. 1998. Infection genomics: Nramp1 as a major determinant of natural resistance to intracellular infections. Annu. Rev. Med. 49:275-287. [DOI] [PubMed] [Google Scholar]
- 17.Takayanagi, H., S. Kim, K. Matsuo, H. Suzuki, T. Suzuki, K. Sato, T. Yokochi, H. Oda, K. Nakamura, N. Ida, E. F. Wagner, and T. Taniguchi. 2002. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 416:744-749. [DOI] [PubMed] [Google Scholar]
- 18.Wang, Z. Q., C. Ovitt, A. E. Grigoriadis, U. Möhle-Steinlein, U. Rüther, and E. F. Wagner. 1992. Bone and haematopoietic defects in mice lacking c-fos. Nature 360:741-744. [DOI] [PubMed] [Google Scholar]
- 19.Xaus, J., M. Comalada, A. F. Valledor, J. Lloberas, F. Lopez-Soriano, J. M. Argiles, C. Bogdan, and A. Celada. 2000. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood 95:3823-3831. [PubMed] [Google Scholar]
- 20.Zhao, C., N. Irie, Y. Takada, K. Shimoda, T. Miyamoto, T. Nishiwaki, T. Suda, and K. Matsuo. 2006. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 4:111-121. [DOI] [PubMed] [Google Scholar]