Abstract
The adhC gene from 11 strains of Neisseria gonorrhoeae was distinguished from its homologue in Neisseria meningitidis by the presence of a premature stop codon caused by a single base insertion. Mutational analysis showed that NADH S-nitrosoglutathione oxidoreductase activity was associated with adhC in Neisseria meningitidis but not in Neisseria gonorrhoeae.
Neisseria gonorrhoeae and Neisseria meningitidis are closely related obligate human pathogens (3) with conservation of the majority of genes (1, 7). While both organisms are associated with mucosal tissues, meningococcus is associated primarily with the nasopharynx, where it is carried asymptomatically. In rare cases, N. meningitidis causes invasive disease resulting in meningitis and septicemia. Gonococcus usually inhabits the urogenital tract, but it can also infect the throat or rectum. Gonococcus is typically linked to inflammation and purulent discharge but may be carried asymptomatically. Unlike N. meningitidis, gonococcus is not usually associated with septicemia. The differences in the interaction with the human host that the two bacteria exhibit in both colonization and disease states lead to the expectation that the bacteria should also exhibit genetic and biochemical differences. An example of differences between the two bacterial species is in their defenses against oxidative stress (11).
Recently, we described a regulon in N. gonorrhoeae which is controlled by NmlR, a transcription factor of the MerR family (6). NmlR controls the expression of adhC, which encodes a class III alcohol dehydrogenase, an enzyme which is conserved from bacteria to mammals and is known to protect cells against nitrosative stress by catalyzing the NADH-dependent reduction of S-nitrosoglutathione (GSNO) (2, 5, 9). It was postulated the AdhC might be part of a defense system that protected N. gonorrhoeae from killing by nitric oxide (6). AdhC in bacteria is usually encoded by a single gene. However, in the N. gonorrhoeae genome (strain FA1090), this gene is annotated as two open reading frames, adhC1 and adhC2. In view of the presence of phenotypically silent genes (pseudogenes) in bacteria that arise from mutational events, we investigated the gonococcal adhC locus in more detail and compared it to the adhC gene in N. meningitidis. The results suggest that there are critical differences between meningococcus and gonococcus in the way that they metabolize glutathione and S-nitrosoglutathione.
The adhC gene from all gonococcal strains contains a premature stop codon.
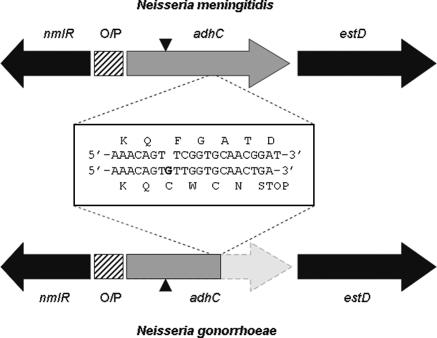
The genetic organization of the nmlR-adhC loci in N. gonorrhoeae FA1090 and N. meningitidis MC58 is shown in Fig. 1. The meningococcal adhC gene is a single locus of 1,137 nucleotides. However, the gonococcal adhC gene is interrupted by a stop codon arising from a single base pair insertion at nucleotide 764 (Fig. 1). The single base insertion causes a frameshift mutation, with the complete adhC coding sequence continuing in an alternate reading frame terminating at the same position as the meningococcal adhC coding sequence (Fig. 1). This nucleotide sequence is unlikely to be expressed or to encode a functional polypeptide. Thus, it appears that the adhC locus in gonococcus is likely to produce a single truncated gene product. To determine whether this altered sequence is conserved across different N. gonorrhoeae strains, the adhC gene was sequenced in wild-type strain 1291 as well as 10 clinical isolates representing a broad spectrum of infection sites, geographical locations, and isolation dates (P. M. Power et al., submitted for publication). Sequence data identified the presence of the single base pair insertion in each strain, consistent with N. gonorrhoeae strain FA1090.
FIG. 1.
Comparison of the adhC genes of Neisseria meningitidis and Neisseria gonorrhoeae. The inset shows a nucleotide and amino acid sequence alignment which contains the frameshift mutation (G insertion) in N. gonorrhoeae that leads to a premature stop codon. The light-gray region after the frameshift indicates the remainder of the full-length adhC open reading frame continuing in an alternate reading frame. ▾ and ▴ indicate the insertion of the kanamycin resistance cassette into the unique AvaI restriction site for adhC mutant strains. O/P, operator/promoter.
Mutation of the adhC gene in N. gonorrhoeae is not correlated with the loss of NADH GSNO oxidoreductase activity.
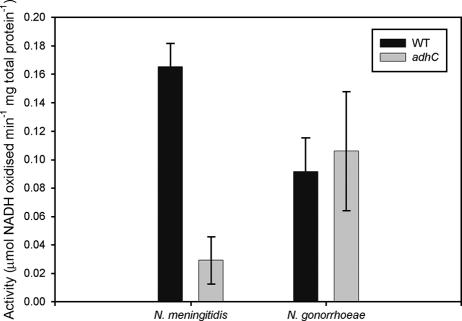
To determine whether the N. gonorrhoeae adhC gene encoded an active enzyme, we mutated the gene in N. gonorrhoeae 1291 and N. meningitidis MC58 ¢3 (an acapsulate version of MC58) by marker exchange mutagenesis. The N. gonorrhoeae 1291 adhC gene was amplified with primers adhC-KO-F1 (5′-CAAGGAAAGGCGTTCTTCAATGGT-3′) and adhC-KO-R1 (5′-TTTGCCGTTGGTAGGAAAATGCTC-3′), cloned into pGEM-T Easy (Promega), and interrupted by the insertion of the kanamycin resistance cassette from pUC4kan into the unique AvaI site (Fig. 1). The resulting plasmid, pGEM-T:adhC:kan, was linearized with XmnI and used to transform N. gonorrhoeae 1291 and N. meningitidis MC58¢3. Correct insertion into the chromosome was verified by PCR using primers external to the construct used for mutagenesis: adhC-F-check (5′-AGCAAGCAACGGATTAGAGC-3′) and adhC-R-check (5′-GAGGCTTGGCGATAAAATAGG-3′). GSNO reductase assays were then performed on wild-type and adhC mutant cell lysates. Strains were grown overnight on brain heart infusion agar (Acumedia) with 10% Levinthal's base at 37°C in 5% CO2. Medium for N. gonorrhoeae was also supplemented with IsoVitaleX (Becton Dickinson). Cells were resuspended in 1 ml phosphate-buffered saline and lysed by subjecting the suspension to five freeze-thaw cycles. Cell debris was removed by centrifugation for 15 min at 18,000 × g and the solutions sterilized by passing them through a 0.22-μm filter (Millipore). The total protein concentration of the supernatant was determined spectrophotometrically using the following equation: protein (mg/ml) = 1.55 × A280 − 0.76 × A260 (8). The GSNO reductase activity of cell lysates was then determined using a method similar to that described by Liu et al. (9). NADH (0.2 mM; Roche), 1 mM GSNO (prepared according to the method described by Sahoo et al. [10]), and 200 μg total protein were combined in a 1-ml reaction mixture, and the decrease in absorbance at 340 nm was measured. GSNO reductase activity was expressed as μmol NADH oxidized per minute per mg total protein. The N. meningitidis adhC mutant was found to exhibit much lower GSNO reductase activity than the wild type (Fig. 2). There was a background of NADH GSNO oxidoreductase activity in N. gonorrhoeae, but the adhC mutant exhibited activity similar to that of its wild-type counterpart (Fig. 2). This indicates that the GSNO reductase activity in N. gonorrhoeae is not associated with the adhC gene and must arise from a distinct and thus-far-unidentified enzyme(s).
FIG. 2.
GSNO reductase activity of N. meningitidis and N. gonorrhoeae wild-type and adhC strains. Error bars indicate ±1 standard deviation from the mean. Experiments were conducted using triplicate cultures and repeated at least three times. The data shown are representative results. There is a statistically significant difference in activity between N. meningitidis wild-type (WT) and adhC mutant strains (P = 0.001); there is no statistically significant difference between N. gonorrhoeae wild-type and adhC mutant strains (P = 0.633), as determined using Student's t test.
Expression of the neisserial adhC gene in Escherichia coli.
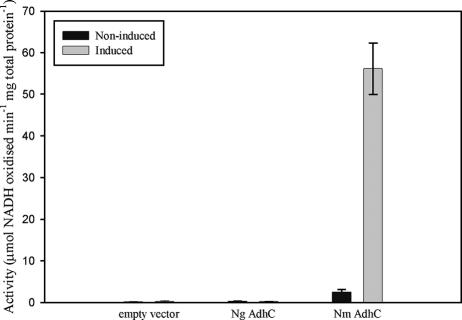
To further investigate the properties of N. gonorrhoeae and N. meningitidis AdhC, the adhC gene of each was cloned into the expression vector pPROEX (Life Technologies) under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter. adhC was PCR amplified using primers adhC-F1 (5′-AACCATGGAAATGAAACAAACCG-3′) and adhC-R1 (5′-GGGTCGACCTTAGTAATGAATAA-3′) and cloned into pPROEX by using NcoI and SalI. The resulting plasmids, pPROEX:Ng-adhC and pPROEX:Nm-adhC, were transformed into competent E. coli BL21 (DE3) cells. E. coli was grown at 37°C in LB containing 100 μg ml−1 ampicillin. Gene expression was induced by adding 0.5 mM IPTG to 50 ml of exponentially growing cells and shaking (180 rpm) at 22°C for 5 h. Noninduced samples were treated as described above but without IPTG addition. Cells were harvested by centrifugation at 4,470 × g (Universal 16R) for 10 min and resuspended in 10 ml phosphate-buffered saline before being broken by sonication. Clarified lysates were prepared, and GSNO reductase activity in E. coli was determined as previously described. Induction of N. meningitidis adhC expression was found to result in >20-fold increased GSNO reductase activity compared with the noninduced sample (Fig. 3). In contrast, induction of N. gonorrhoeae adhC had no effect on activity (Fig. 3) and further analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting revealed that most of the N. gonorrhoeae AdhC protein expressed in E. coli formed insoluble inclusion bodies (data not shown).
FIG. 3.
GSNO reductase activity of E. coli BL21 (DE3) cells carrying the pPROEX overexpression vector with either N. meningitidis (Nm) or N. gonorrhoeae (Ng) adhC cloned downstream of its IPTG-inducible promoter. Cells carrying empty vector were used as a negative control. Error bars indicate ±1 standard deviation from the mean. Experiments were conducted using triplicate cultures and repeated at least three times. The data shown are representative results.
The results presented herein indicate that N. gonorrhoeae adhC may be a pseudogene. It is already established that in N. gonorrhoeae, the ggt gene, encoding a γ-glutamyl transpeptidase, is also a pseudogene, although the meningococcal homologue encodes an active enzyme (12). Like the gonococcal ggt gene, the adhC gene is transcriptionally active (6) but phenotypically silent. The present results may relate to differences in the way that the two species handle nitric oxide. We note that γ-glutamyl transpeptidase is able to accelerate the decomposition of GSNO by hydrolyzing the γ-glutamyl moiety (4). Similarly, an active AdhC protein in this bacterium would accelerate the removal of GSNO. We speculate that selective pressure for the loss of a functional ggt and adhC in N. gonorrhoeae is associated with a distinctive mechanism of handling nitric oxide that is suited to an interaction with the human host which allows the bacterium to respond to the acute inflammatory response. The setting in which AdhC has an important role for survival of N. meningitidis has not yet been identified.
Acknowledgments
This work was supported by program grant 284214 from the National Health and Medical Research Council of Australia to M.P.J. and A.G.M.
A.J.P. thanks the University of Queensland for a postgraduate scholarship.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263-277. [DOI] [PubMed] [Google Scholar]
- 2.Fernández, M. R., J. A. Biosca, and X. Pares. 2003. S-Nitrosoglutathione reductase activity of human and yeast glutathione-dependent formaldehyde dehydrogenase and its nuclear and cytoplasmic localisation. Cell. Mol. Life Sci. 60:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guibourdenche, M., M. Y. Popoff, and J. Y. Riou. 1986. Deoxyribonucleic acid relatedness among Neisseria gonorrhoeae, N. meningitidis, N. lactamica, N. cinerea and “Neisseria polysaccharea”. Ann. Inst. Pasteur Microbiol. 137B:177-185. [DOI] [PubMed] [Google Scholar]
- 4.Hogg, N., R. J. Singh, E. Konorev, J. Joseph, and B. Kalyanaraman. 1997. S-Nitrosoglutathione as a substrate for gamma-glutamyl transpeptidase. Biochem. J. 323:477-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen, D. E., G. K. Belka, and G. C. Du Bois. 1998. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 331:659-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd, S. P., A. J. Potter, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2005. NmlR of Neisseria gonorrhoeae: a novel redox responsive transcription factor from the MerR family. Mol. Microbiol. 57:1676-1689. [DOI] [PubMed] [Google Scholar]
- 7.Klee, S. R., X. Nassif, B. Kusecek, P. Merker, J. L. Beretti, M. Achtman, and C. R. Tinsley. 2000. Molecular and biological analysis of eight genetic islands that distinguish Neisseria meningitidis from the closely related pathogen Neisseria gonorrhoeae. Infect. Immun. 68:2082-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layne, E. 1957. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 3:447-454. [Google Scholar]
- 9.Liu, L. M., A. Hausladen, M. Zeng, L. Que, J. Heitman, and J. S. Stamler. 2001. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490-494. [DOI] [PubMed] [Google Scholar]
- 10.Sahoo, R., T. Dutta, A. Das, S. S. Ray, R. Sengupta, and S. Ghosh. 2006. Effect of nitrosative stress on Schizosaccharomyces pombe: inactivation of glutathione reductase by peroxynitrite. Free Radic. Biol. Med. 40:625-631. [DOI] [PubMed] [Google Scholar]
- 11.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi, H., and H. Watanabe. 2005. A gonococcal homologue of meningococcal gamma-glutamyl transpeptidase gene is a new type of bacterial pseudogene that is transcriptionally active but phenotypically silent. BMC Microbiol. 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]