Abstract
Regulation of the synthesis of Vi polysaccharide, a major virulence determinant in Salmonella enterica serotype Typhi, is under the control of two regulatory systems, ompR-envZ and rscB-rscC, which respond to changes in osmolarity. Some serotype Typhi strains exhibit overexpression of Vi polysaccharide, which masks clinical detection of lipopolysaccharide O antigen. This variation in Vi polysaccharide and O antigen display (VW variation) has been observed since the initial studies of serotype Typhi. In this study, we report that rpoS plays a role in this increased expression in Vi polysaccharide. We constructed a variety of isogenic serotype Typhi mutants that differed in their expression levels of RpoS and examined the role of the rpoS product in synthesis of Vi polysaccharide under different osmolarity conditions. Vi polysaccharide synthesis was also examined in serotype Typhi mutants in which the native promoter of the rpoS was replaced by an araCPBAD cassette, so that the expression of rpoS was arabinose dependent. The RpoS− strains showed increased syntheses of Vi polysaccharide, which at low and medium osmolarities masked O antigen detection. In contrast, RpoS+ strains showed lower syntheses of Vi polysaccharide, and an increased detection of O antigen was observed. During exponential growth, when rpoS is unstable or present at low levels, serotype Typhi RpoS+ strains overexpress the Vi polysaccharide at levels comparable to those for RpoS− strains. Our results show that RpoS is another regulator of Vi polysaccharide synthesis and contributes to VW variation in serotype Typhi, which has implications for the development of recombinant attenuated Salmonella vaccines in humans.
Salmonella enterica serotype Typhi is a facultative intracellular pathogen that causes typhoid enteric fever exclusively in humans and is among the organisms causing the most costly human infections in terms of both morbidity and mortality (51). The mechanism responsible for the virulence of serotype Typhi is different from those of other serovars of Salmonella, and in this regard, serotype Typhi produces the virulence capsular (Vi) polysaccharide, which is an important virulence determinant during infection (27). Vi polysaccharide is a polymer of α-1→4-galacturonic acid with an N-acetyl group at position C-2 and a variable O acetylation at C-3 (69). Virtually all strains isolated from blood or bone marrow samples from patients with acute typhoid fever and from bile samples or feces from those who carry serotype Typhi in the gallbladder are found to express Vi polysaccharide antigen when tested in clinical microbiology laboratories (29, 39, 55). Vi-positive (Vi+) strains were shown to be more virulent than Vi− mutant strains in experiments conducted with human volunteers (27), and the Vi+ strains were resistant to complement-mediated killing and phagocytosis (61) and survived in human serum (20). In addition, the Vi+ strain but not the Vi− mutant strain can multiply in the human macrophage cell line THP-1 and the mouse macrophage-like cell line J774.1 (22).
The genes required for the biosynthesis of the capsular antigen Vi are located in a 133.5-kb chromosomal region called Salmonella pathogenicity island 7 (SPI-7). Locus viaB, which encodes Vi polysaccharide in serotype Typhi, consists of 10 genes: tviBCDE for Vi polysaccharide biosynthesis; vexABCDE for the export of the Vi antigen; tviA, which is activated by unlinked regulators rcsB-rcsC (1, 28); and ompR-envZ, which are themselves controlled by osmolarity levels (53).
The Vi polysaccharide is expressed or overexpressed in media with low or medium osmolarities, respectively, and often covers and masks the lipopolysaccharide (LPS) O antigen (1, 71). Strains of serotype Typhi Ty2 grown in media with medium osmolarities (446 mosmol, ∼170 mM NaCl [71]) exhibit high-level production of Vi antigen. When the Vi antigen is expressed, the bacteria are less adherent to and invasive into epithelial cells (71) but are more resistant to killing by macrophages (22). In high-osmolarity media (676 mosmol, ∼300 mM of NaCl [71] or more, as in the intestinal lumen), the Vi antigen is not expressed (1, 53, 80), exposing the somatic antigen and liberating secretion proteins, which are stockpiled inside the bacterial cell when Vi polysaccharide is expressed at the bacterial cell surface (1). In this stage, serotype Typhi is more invasive into epithelial cells but is less resistant to killing by macrophages (22). Therefore, the Vi antigen of serotype Typhi is a negative factor for invasion but a positive factor for survival and multiplication inside macrophages (22). Since the Vi polysaccharide can block the access of antibodies to the underlying O antigen, sometimes agglutination with Salmonella somatic D1 antiserum cannot be demonstrated until the bacterial cells are boiled to remove the Vi polysaccharide (11).
From initial studies on serotype Typhi, variations in Vi and O antigen detection have been observed. Observations recorded by Kauffman (33) and confirmed by Felix and Pitt (14) demonstrated the concept of VW variation in Vi and O antigen relationships (74). Serotype Typhi strains inagglutinable with O antisera and agglutinable only with Vi antisera are called V-form strains, while serotype Typhi strains that lack the Vi antigen and agglutinate only with O antisera are called W-form strains (74). The VW form, which is referenced as the most common form observed in clinical laboratories, is identified when both Vi and O antigen are detected by agglutination with the respective antisera (74). Coincident with the early work of Felix and Pitt (14), and since verified by others (16, 17, 22, 75), most virulent strains of serotype Typhi were the VW form.
In Salmonella, the rpoS gene encodes an alternative sigma factor (σs/RpoS) that is the master regulator in the general stress response and is required for survival under extreme conditions (including osmotic and oxidative stress, acid shock, and transition to stationary phase) and for virulence of S. enterica serotype Typhimurium (6, 13, 36, 47, 48). RpoS controls the expression of the serotype Typhimurium virulence plasmid genes, spvRABCD (13, 47). In addition, RpoS regulates chromosomal genes required for colonization of Peyer's patches and for persistence in mice (6, 47). Serotype Typhi does not contain a virulence plasmid, and the role of rpoS in the virulence of this serotype is unknown. However, rpoS might also contribute to the virulence of this serotype because RpoS− strains of serotype Typhi are less cytotoxic than RpoS+ strains, but RpoS− strains survive better inside resting THP-1 macrophages without apoptosis induction and have higher capacities for resistance in the macrophage (34).
We deduced from our observations that there is a correlation between Vi polysaccharide overexpression and the allele state of the rpoS gene. We report here that rpoS is involved in the subtle overexpression of Vi polysaccharide at osmolarities lower than 667 mosmol resulting from the addition of 300 mM NaCl to culture media. We presume that the rpoS gene influences the VW form described in earlier studies. Furthermore, we discuss the implication of the effect of rpoS on the synthesis of Vi antigen in serotype Typhi Ty2 and serotype Typhi ISP1820, which are the most useful parental strains in designing live serotype Typhi vaccines.
MATERIALS AND METHODS
Salmonella strains and conditions of culture.
Salmonella strains used in this study are listed in Table 1. The strains were routinely cultured at 37°C in LB medium (Bacto tryptone, 10 g/liter; Bacto yeast extract, 5 g/liter; NaCl, 10 g/liter) (44). Media were solidified with 1.5% (wt/vol) agar. When required, media were supplemented with chloramphenicol (Cm; 25 μg/ml), ampicillin (Ap; 100 μg/ml), 2,6-diaminopimelic acid (DAP; 50 μg/ml), l-arabinose (Ara; 0.2% [wt/vol]), or sucrose (5% [wt/vol]). The osmolarities of the LB agar plates were changed by adding NaCl (0, 10, 85, 150, 300, 400, and 500 mM) (71) or sucrose. The final pHs of the media were adjusted to 7.0 with NaOH before the autoclaving.
TABLE 1.
Salmonella strains and relevant characteristics
| Strain | Relevant characteristics | Origin | Source or reference |
|---|---|---|---|
| χ3769 serotype Typhi Ty2 | Wild-type RpoS− Aps Cys− V form | 15 | |
| χ3744 serotype Typhi ISP1820 | Wild-type RpoS+ Aps Cys− Trp− | 70 | |
| V-W form | |||
| χ8438 serotype Typhi Ty2 | RpoS+ Aps Cys− VW form | χ3769 | This study |
| χ9060 serotype Typhi Ty2a | rpoSΩAp RpoS− Apr Cys− V form | χ8438 | This study |
| χ9061 serotype Typhi ISP18201 | rpoSΩAp RpoS− Apr Cys− Trp− V form | χ3744 | This study |
| χ9066 serotype Typhi ISP18202 | ΔPrpoS183::TTaraC PBADrpoS Cys− Trp− | χ3744 | This study |
| VW form induced by arabinose | |||
| χ9067 serotype Typhi Ty2b | ΔPrpoS183::TTaraC PBADrpoS | χ3769 | This study |
| RpoS− Cys− V form | |||
| χ9068 serotype Typhi Ty2b | ΔPrpoS183::TTaraC PBADrpoS Cys− | χ8438 | This study |
| VW form induced by arabinose | |||
| χ9197 serotype Typhi Ty2 | ΔtviABCDE10 Cys− Vi− Vi-phageS RpoS− W form | χ3769 | This study |
| χ9198 serotype Typhi ISP1820 | ΔtviABCDE10 Cys− Trp− Vi− Vi-phageS RpoS+ W form | χ3744 | This study |
| χ9336 serotype Typhi Ty2 | ΔtviABCDE10 Cys− Vi− Vi-phageS RpoS+ W form | χ8438 | This study |
| χ4991 serotype Typhimurium UK-1 | rpoSΩAp RpoS− Apr | 47 | |
| χ7213 E. coli K-12 | thi-1 thr-1 leuB6 fhuA21 lacY1 glnV44 ΔasdA4 recA1 RP42-Tc::Mu [λpir] Kmr | 62 |
Mutants generated by P22HTint transduction.
Mutants generated by conjugation and allele exchange.
Characterization of serotype Typhi strains.
The serotype Typhi strains were characterized for type I fimbriae in static broth cultures (35, 49) and for motility in motility medium (bioMérieux, Marcy I'Etoile, France). The presence of LPS was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis when visualized by silver staining (23, 72). Plasmid profiles were verified by alkaline lysis and agarose gel (0.5%) electrophoresis (31, 63). Fermentation patterns of various carbohydrates and production of H2S were determined by using the API 20E system (bioMérieux, Marcy I'Etoile, France). The serotype Typhi RpoS− strains gave API 20E code number 640454057 (a good identification as serotype Typhi [97.4%] and Salmonella spp. [1.8%]), whereas the RpoS+ strains gave API 20E code number 440454057 (an excellent identification as serotype Typhi [99.9%] and S. enterica serovar Choleraesuis [0.1%]). The expression levels of the Vi, O9 (serotype Typhi-specific), D1 (O1, O9, and O12), and Hd antigens of serotype Typhi strains were evaluated by the growth of strains on LB agar plates with different osmolarities after 18 to 24 h.
Construction of serotype Typhi Ty2 RpoS+.
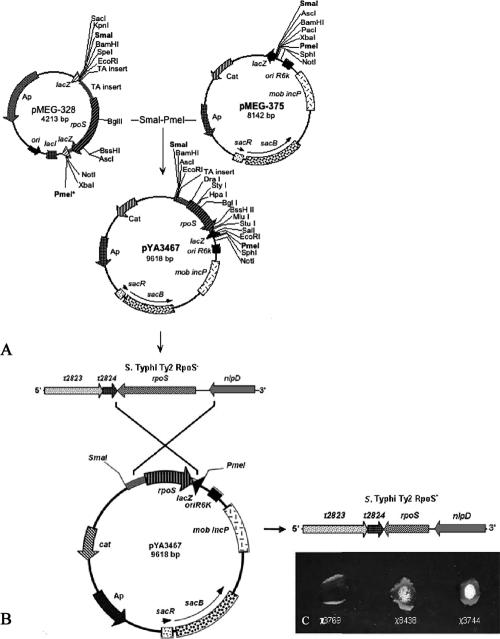
The recombinant suicide vector pYA3467 (9.6 kb), carrying the rpoS gene from serotype Typhi ISP1820 (993 bp) between flanking regions (338 bp 5′ and 83 bp 3′) of the rpoS (σs2/RpoS−) allele in serotype Typhi Ty2 (χ3769), was constructed as follows (Fig. 1A). The rpoS allele from serotype Typhi Ty2 represents a frameshift mutation caused by a guanosine insertion at position 1313 that modified the amino acid sequence of the C-terminal part of σs/RpoS, resulting in a protein of 384 amino acids rather than 330 (58). The SmaI/PmeI fragment, harboring 338 bp of the 5′-end upper region, 993 bp of the rpoS gene, an extra 83 bp of the 3′-end region, and ∼93 bp of multiple cloning sites (a total of ∼1,450 bp), was inserted into the SmaI/PmeI sites of pMEG-375 to generate pYA3467 (Fig. 1A). To construct serotype Typhi Ty2 RpoS+, the suicide plasmid pYA3467 was conjugationally transferred from Escherichia coli χ7213 (32) to serotype Typhi Ty2 strain χ3769 in LB broth supplemented with DAP (67). Strains containing single-crossover plasmid insertions (serotype Typhi Ty2 rpoS::pYA3467) (Fig. 1B) were isolated on LB agar plates containing Cm without DAP. Loss of the suicide vector after the second recombination between homologous regions (i.e., allelic exchange) was selected by using the sacB-based sucrose sensitivity counterselection system (18). The colonies were screened for Cms and for positive catalase activity evidence by bubble production after addition of H2O2 (Fig. 1C). In particular, rpoS mutants are impaired in the ability to resist H2O2 during stationary phase. Indeed, two of the catalases produced by Salmonella (KatE and KatN) are expressed in stationary phase under the control of rpoS (3, 60). To observe the presence of rpoS mutations, we added H2O2 to the bacterial colony mass removed from LB agar. The extent of bubbling indicated absence or reduction of catalase production in strains with differing rpoS genotypes (57). The presence of the RpoS+ insertion in χ8438 serotype Typhi Ty2 RpoS+ was confirmed by a catalase activity assay (Fig. 1C).
FIG. 1.
Construction of suicide vector pYA3467 and generation of RpoS+ strain in serotype Typhi Ty2. (A) Construction of suicide vector pYA3467 (9,618 bp) used to generate the serotype Typhi Ty2 RpoS+ strain. (B) Crossover between suicide vector pYA3467 and chromosome of serotype Typhi Ty2. (C) Catalase test for determination of RpoS activity.
Construction of serotype Typhi rpoS mutants.
Strains χ9060 and χ9061 were constructed by P22HTint-mediated transduction of χ8438 and χ3744, respectively, to introduce the inactive rpoS allele (rpoSΩAp; truncated RpoS) from χ4991 (47). The bacteriophage P22HTint was propagated in the serotype Typhimurium χ4991 donor strain by standard methods (65). Plaque assays were performed to determine phage titers. The concentrated phage lysate was prepared, using χ4991 as the host, as described by Santander and Robeson (64). Serotype Typhi was infected with a multiplicity of infection of 10 (phage/recipient). Transductants were selected on LB agar containing Ap. Green indicator plates (54) and Evans blue uridine indicator plates (68) were used to confirm that transductants were phage-free. The serotype Typhi rpoSΩAp strains were screened for Apr and absence of bubbling after addition of H2O2 and expression of a truncated RpoS protein by Western blot analysis (see Fig. 3).
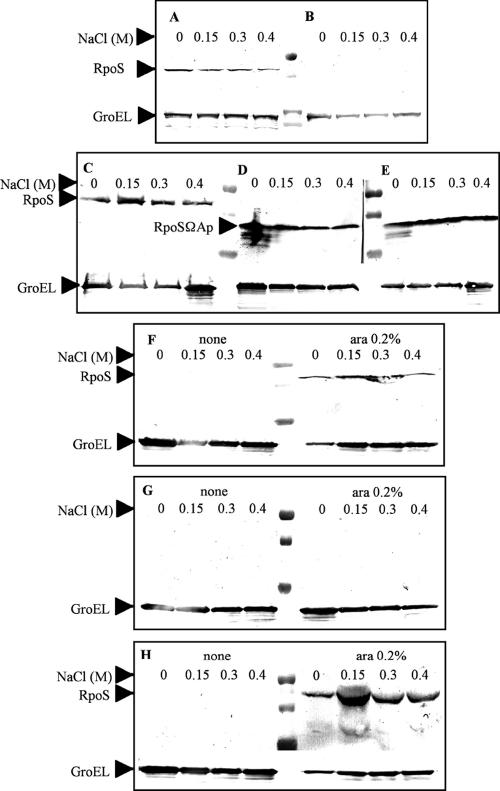
FIG. 3.
Evaluation of RpoS expression at different osmolarities by Western blot analysis. (A) χ3744 serotype Typhi ISP1820 RpoS+. (B) χ3769 serotype Typhi Ty2 RpoS−. (C) χ8438 serotype Typhi Ty2 RpoS+. (D) χ9060 serotype Typhi Ty2 rpoSΩAp RpoS−. (E) χ9061 serotype Typhi ISP1820 rpoSΩAp RpoS−. (F to H) Assays conducted in the absence and presence of arabinose (0.2%). (F) χ9066 serotype Typhi ISP1820 RpoS+ ΔPrpoS183::TTaraCPBADrpoS. (G) χ9067 serotype Typhi Ty2 RpoS− ΔPrpoS183::TTaraCPBADrpoS. (H) χ9068 serotype Typhi Ty2 RpoS+ ΔPrpoS183::TTaraCPBADrpoS.
Construction of serotype Typhi mutants with regulatable expression of rpoS.
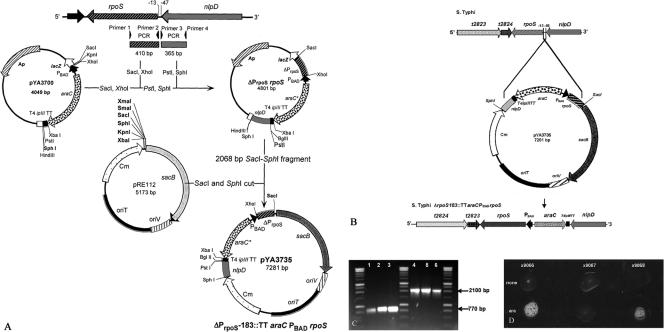
The recombinant suicide vector pYA3735 (7.3 kb), carrying a TTaraCPBAD (1,329-bp) insertion between flanking regions (410 bp 5′ and 365 bp 3′) deleting a 35-bp promoter sequence (rpoS −13 to −48), was constructed as follows (Fig. 2A). PCR primers 5′-TGCGAGCTCCTCGACTGCACGGATAAGCCCC-3′ (primer 1) and 5′-CCGCTCGAGAGGAGCCACCTTATGCAGATTA-3′ (primer 2) were designed to amplify the rpoS flanking region (410 bp). A SacI site was included in primer 1 (underlined), and an XhoI site was included in primer 2 (underlined). The nlpD flanking region (365 bp) was amplified, mediated by primers 5′-CATCTGCAGCTGGTTCCGCCGCTTTA TCGCTG-3′ (primer 3) and 5′-ACATGCATGCGGATGGCAAAGTGATCGAAA-3′ (primer 4). A PstI site was included in primer 3 (underlined), and an SphI site was included in primer 4 (underlined). The flanking regions were amplified from serotype Typhi Ty2 χ3769 and were cloned into pYA3700 between the TTaraCPBAD insertion (Fig. 2A). The resultant plasmid (designated pΔPrpoSrpoS, 4,801 bp), carrying the TTaraCPBAD cassette between the flanking regions, was digested with SacI and SphI to yield a 2,068-bp fragment that was cloned into pRE112 digested with SacI and SphI (12). The resulting suicide vector was designated pYA3735. To construct serotype Typhi ΔPrpoS183::TTaraCPBADrpoS mutants, the suicide plasmid pYA3735 was conjugationally transferred from Escherichia coli χ7213 (32) to serotype Typhi strains in LB supplemented with DAP (67). The strains containing single-crossover plasmid insertions were isolated on LB agar plates containing Cm without DAP (Fig. 2B). Loss of the suicide vector after the second recombination between homologous regions (i.e., allelic exchange) was selected by using the sacB-based sucrose sensitivity counterselection system (18). The colonies were screened for Cms and for positive catalase activity with H2O2 (bubble production) only when grown in the presence of 0.2% Ara (Fig. 2D). The presence of the ΔPrpoS183::TTaraCPBADrpoS insertion in serotype Typhi strains was confirmed by PCR amplification of DNA fragments bigger than those amplified from the parental strains. The PrpoS primer set (primers 1 and 4) amplified 770- and 2,100-bp DNA fragments from the chromosomal DNA templates of serotype Typhi (parental) and the serotype Typhi (ΔPrpoS183::TTaraCPBADrpoS) mutant, respectively (Fig. 2C). The regulation of the rpoS gene by araCPBAD was confirmed using a catalase activity assay for cells grown with and without 0.2% Ara (Fig. 2D).
FIG. 2.
Construction of suicide vector pYA3735 and generation of serotype Typhi with regulated rpoS expression (ΔPrpoS183::TTaraCPBADrpoS). (A) Construction of suicide vector pYA3735 (7,281 bp) used to generate serotype Typhi ΔPrpoS183::TTaraCPBADrpoS. (B) Crossover between suicide vector pYA3735 and chromosome of serotype Typhi. (C) Agarose gel (0.8%), a PCR product from chromosomal DNA from wild-type (770-bp) and ΔPrpoS183::TTaraCPBADrpoS (2,100-bp) mutants. Lanes: 1, χ3744 serotype Typhi ISP1820 RpoS+; 2, χ3769 serotype Typhi Ty2 RpoS−; 3, χ8438 serotype Typhi Ty2 RpoS+; 4, χ9066 serotype Typhi ISP1820 RpoS+ ΔPrpoS183::TTaraCPBADrpoS; 5, χ9067 serotype Typhi Ty2 RpoS− ΔPrpoS183:: TTaraCPBADrpoS; 6, χ9068 serotype Typhi Ty2 RpoS+ ΔPrpoS183::TTaraCPBADrpoS. (D) Catalase test for detection of ΔPrpoS183::TTaraCPBADrpoS without and with growth in the presence of 0.2% Ara.
Construction of serotype Typhi Vi− strains.
Additionally, serotype Typhi Vi− strains (ΔtviABCDE10) were constructed as controls. Deletion of the tviA, tviB, tviC, tviD, and tviE genes (ΔtviABCDE10) in the viaB loci of the serotype Typhi strains was performed by allelic exchange. The recombinant suicide vector pYA4009 (5.9 kb), carrying the flanking regions (361 bp 5′ and 422 bp 3′), was constructed as follows. PCR primers 5′-ACATGCATGCGAACGGTATTACTGTCAGTCACAAG-3′ (primer 1) and 5′-TGCGACCTCATGAAAAAAATCATCATATTACTA-3′ (primer 2) were designed to amplify the upstream tviA flanking region (361 bp). An SphI site was included in primer 1 (underlined), and a SacI site was included in primer 2 (underlined). The vexA flanking region (422 bp) was amplified by primers 5′-TGCGAGCTCGAAGTCTCCTTATGCTGAAATAAC-3′ (primer 3) and 5′-TCCCCCGGGCAGATTATTTCAAATACGATTAGG-3′ (primer 4). A SacI site was included in primer 3 (underlined), and a SmaI site was included in primer 4. The flanking regions were amplified from serotype Typhi Ty2 χ3769 and were cloned into pRE112 digested with SphI and SmaI (12). The resulting suicide vector was designated pYA4009. The tviABCDE10 mutant strains were constructed by allelic exchange and the sucrose sensitivity counterselection system (18), as described above. The deletion was confirmed by PCR and corroborated by the sensitivity of the mutant to the Vi-II phage and the lack of agglutination with Vi antisera.
Agglutination assays.
Agglutination tests were performed on glass microscope slides by mixing 50 μl of antisera against Vi, O9, D1 (O1, O9, and O12), and Hd (Difco Laboratories, Detroit, MI) with suspensions of single colonies. The reactions were visualized by phase contrast microscopy at 10× magnification. When the assays for O9 and D1 antigens were negative, the cells were boiled for 20 min and cooled prior to addition of O antisera for detection of O antigens (39, 53).
Western blot analysis.
The strains were grown in 3 ml of LB medium with different NaCl concentrations at 37°C with aeration. The samples were collected when the absorbance values reached 1.5 (optical density at 600 nm, 1.5; ∼1 × 109 CFU/ml), which corresponds to stationary growth phase, known to stimulate rpoS expression. One milliliter of culture was collected and centrifuged at 10,500 × g for 10 min. The supernatant was discarded, and the total bacterial proteins were suspended in 150 μl of loading buffer and boiled for 10 min. The total proteins were normalized by a nanodrop spectrophotometer (ND-1000; NanoDrop) at 25 mg/μl, separated by 10% (wt/vol) sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes (63). Fat-free milk powder solution (5% [wt/vol]) was used for blocking. The membrane was incubated with a primary anti-mouse RpoS monoclonal antibody (1:1,000) (Neoclone), anti-rabbit Hd polyclonal (1:5,000) (Difco), or anti-rabbit GroEL polyclonal (1:10,000) (Sigma) for 1 h at room temperature, washed three times with phosphate-buffered saline supplemented with Tween (0.05%), and then incubated with a 1:10,000 dilution of alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Sigma) or anti-rabbit immunoglobulin G (Sigma). Color was developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate.
Rocket immune electrophoresis.
The measurement of Vi polysaccharide expression was visualized by rocket immune electrophoresis (38, 73) adapted for Vi antigen. The strains were grown under the same conditions described above for the Western blot assays. One milliliter of culture was centrifuged at 10,500 × g for 10 min. The supernatant was discarded, and the total bacterial pellet was washed one time and resuspended in 150 μl of barbital buffer (60 mM, pH 8.6) (Sigma). The cells were disintegrated by sonication on ice until the suspension became clarified. Total protein concentration was used to normalize the samples (25 mg/μl). Agarose (1% [wt/vol]) was prepared in barbital buffer, melted, and adjusted to 55°C. The Vi antiserum (Sigma) was mixed with the warm agarose to make 1% Vi antiserum (vol/vol) gels. The gel was prepared in mini plates (Bio-Rad) 1.5 mm thick. After being cooled, the gel was placed in a Gel-bond membrane (3 M). Holes with 3-mm diameters were made 10 mm from the bottom of the gel, with 3 mm of distance between them. An aliquot of 20 μl of disrupted bacteria was placed in the holes. The electrophoresis was run from the negative to the positive pole at 10 V/cm for 3 h in barbital buffer. Paper filters were used as conducting bridges. Following the run, the gels were removed from the membrane and placed in NaCl (0.2 M) overnight. The gel was washed with distilled water for 1 h and placed back in the Gel-bond membrane. The gels were dried at 30°C and stained with a solution of Coomassie blue (0.5%) in acetic acid-methanol-water (1:5:5 [vol/vol/vol]) at 37°C for 10 min. The excess stain was removed by washing the gels with the same solution without Coomassie blue. Purified serotype Typhi Vi polysaccharide vaccine (Aventis Pasteur, Lyon, France) was used as a standard for determination of the Vi antigen synthesis of the serotype Typhi strains grown at different osmolarities.
Evaluation of Vi antigen expression in serotype Typhi during growth.
The strains were grown in LB media with 150 mM of NaCl with aeration at 37°C. The growth was monitored by measurement of absorbance at 600 nm. The samples, taken at different times, were normalized to an optical density at 600 nm of 0.8. Then, 1 ml of culture in duplicate (proteins and Vi antigen samples) was centrifuged at 10,500 × g for 10 min. The supernatant was discarded, and the sample was frozen at −70°C overnight. Western blot assays were performed for RpoS and GroEL as explained above. Evaluation of Vi antigen was determined by rocket immune electrophoresis as described above.
RESULTS
Strain construction.
Since in preliminary studies we observed that production of Vi antigen in serotype Typhi strains differed depending on the RpoS phenotype, we constructed a variety of strains that differed in their RpoS expression levels to further understand the relationship between this sigma factor, Vi capsule production, and osmolarity. To be sure that the results observed with serotype Typhi Ty2 strain χ3769, which has an rpoS mutation (57), were due to the rpoS mutation, we first restored this strain to the RpoS+ phenotype to yield χ8438. We then generated RpoS− derivatives of the RpoS+ Ty2 (χ8438) and ISP1820 (χ3744) strains by insertion of rpoSΩAp and selection for the Ap cassette to yield χ9060 and χ9061, respectively. The strains were Apr and catalase negative and presented an expression of a truncated RpoS due to the Ap insertion cassette (Fig. 3). rpoS mutants with regulatable expression of rpoS were also constructed by deleting PrpoS and replacing it with an araCPBAD cassette. The presence of RpoS+, rpoSΩAp, and ΔPrpoS183::TTaraCPBADrpoS mutations was verified by testing for resistance to Ap, sensitivity to Cm, and formation of bubbles from H2O2 by PCR and by Western blot analysis as described in Materials and Methods. All mutants conserved the phenotypes of the original strains, including smooth LPS, fimbrial type I production, antigenic composition, and auxotrophic markers.
RpoS expression.
Western blot assays were used to determine the RpoS expression levels during stationary phase growth under different osmolarity conditions. The expression levels of RpoS were relatively the same under all osmolarity conditions (Fig. 3). RpoS− strains χ3769 and χ9067 did not show expression of RpoS. The rpoSΩAp strains, which express a truncated RpoS, showed lower-molecular-weight proteins than wild-type RpoS, as expected (Fig. 3D to E). The strains with regulatable rpoS showed expression of RpoS only when grown in the presence of 0.2% arabinose (Fig. 3F to H; see also Table 3).
TABLE 3.
Synthesis of Vi polysaccharide (μg of Vi/500 mg of total serotype Typhi protein) of serotype Typhi strains grown on LB agar (pH 7) supplemented with different amounts of NaCl at 37°Ca
| NaCl concn (mM) | Vi antigen concn (μg/mg) for indicated strain
|
||||
|---|---|---|---|---|---|
| χ3769 RopS− | χ3744 RpoS+ | χ8438 RpoS+ | χ9060 RpoS− | χ9061 RpoS− | |
| 0 | 14.01 ± 0.01 | 3.74 ± 0.03 | 0.31 ± 0.4 | 10.59 ± 0.04 | 9.61 ± 0.06 |
| 150 | 15.48 ± 0.03 | 11.08 ± 0.05 | 8.14 ± 0.09 | 14.01 ± 0.05 | 13.52 ± 0.03 |
| 300 | 14.01 ± 0.06 | 5.70 ± 0.04 | 5.20 ± 0.07 | 14.01 ± 0.03 | 13.03 ± 0.08 |
| 400 | 10.10 ± 0.06 | 0.80 ± 0.2 | 0.80 ± 0.3 | 14.01 ± 0.07 | 13.03 ± 0.05 |
The rocket lengths were transformed to the Vi antigen concentrations by the following equation: Vi (μg) = 4.8916 × (rocket length [cm]) − 5.0643. The standard deviations correspond to two independent experiments.
Effect of rpoS on Vi synthesis.
Since the osmolarity of the growth media influences the synthesis of Vi antigen, we examined strains of different rpoS genotypes in media with different osmolarities for levels of Vi antigen synthesis. The RpoS+ strains grown at osmolarities less than 676 mosmol (∼300 mM NaCl [71]) showed overexpression of Vi antigen sufficient to cover the somatic O antigen. The phenotypically O antigen-negative strains were boiled, and the O antigen was subsequently detected in all cases. When RpoS− strains were grown in media with 300 mM NaCl (676 mosmol) or more, the expression of Vi polysaccharide was less or null and the O antigen was exposed. Under this condition (>300 mM NaCl), RpoS+ strains have the same synthesis of the Vi antigen and the O antigens are exposed (Table 2). The RpoS+ strains and regulatable rpoS mutants grown with Ara (0.2%) did not overexpress the Vi polysaccharide, and both Vi and O antigens were detected at osmolarities lower than 676 mosmol. However, at 676 mosmol or higher osmolarities, Vi synthesis was almost null in these strains (Table 2).
TABLE 2.
O9 and Vi slide agglutination reactions of serotype Typhi strains grown on LB agar (pH 7) supplemented with different amounts of NaCl at 37°C overnight (18 to 24 h)a
| Strain | Reaction for indicated antigen and NaCl concn (mM)a
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
10
|
85
|
150
|
300
|
400
|
500
|
||||||||
| O9 | Vi | O9 | Vi | O9 | Vi | O9 | Vi | O9 | Vi | O9 | Vi | O9 | Vi | |
| χ3769 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ | ++ | +++ | +++ | + | +++ | − |
| χ3744 RpoS+ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | − | +++ | − | +++ | − |
| χ8438 RpoS+ | ++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | +++ | − | +++ | − | +++ | − |
| χ9060 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ | ++ | +++ | +++ | + | +++ | − |
| χ9061 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ | ++ | +++ | +++ | + | +++ | − |
| χ9066 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ | ++ | +++ | +++ | ± | +++ | − |
| χ9067 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ | ++ | +++ | +++ | + | +++ | − |
| χ9068 RpoS− | − | +++ | − | +++ | − | +++ | − | +++ | ++ | +++ | +++ | + | +++ | − |
| χ9066 RpoS+b | ++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | +++ | − | +++ | − |
| χ9067 RpoS−b | − | +++ | − | ++ | − | +++ | − | +++ | − | +++ | +++ | − | +++ | − |
| χ9068 RpoS+b | ++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | + | +++ | − | +++ | − |
O9 agglutination reactions were carried out without prior boiling of cells. The degrees of agglutination ranged from not detectable (−) to weak (+) to strong (+++); ± and ++ indicate intermediate degrees.
Strains grown with 0.2% arabinose to induce rpoS (see text for details).
To make sure that the effects of osmolarity and rpoS genotype/expression on Vi antigen synthesis and detection of O antigen were not due to a consequence of using NaCl, identical evaluations were made using sucrose to alter osmolarity. These results are essentially identical to those presented in Table 2, with the exception that osmolarities higher than 676 mosmol did not result in substantial Vi antigen synthesis for masking O antigen detection (Table 2), whereas sucrose did cause Vi antigen synthesis that masked O antigen detection (data not shown). This effect of sucrose was more pronounced in strains that were RpoS− than in strains that were RpoS+.
Pickard et al. (53) showed that in serotype Typhi vaccine strains, Vi antigen synthesis is regulated by the ompB locus and is dependent on osmolarity. These authors also observed that serotype Typhi Ty2 and serotype Typhi ISP1820 have different levels of Vi antigen synthesis when these strains were grown at different osmolarities. We observed the same results in these different strains of serotype Typhi (Table 2). However, Pickard et al. (53) explained the differences among the Vi antigen syntheses in these strains by the different times of isolation. On the other hand, an rpoS frameshift mutation in serotype Typhi Ty2 has been previously reported (58). These references and the results reported in this study suggest that the rpoS allelic state, one of the major differences between serotype Typhi Ty2 and serotype Typhi ISP1820, is responsible for the subtle down-regulation of Vi polysaccharide synthesis at osmolarities lower than 676 mosmol (300 mM of NaCl).
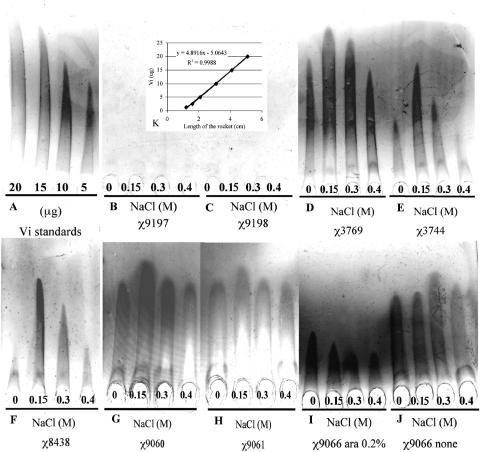
The rocket immune electrophoresis assays indicated that RpoS+ strains down-regulated Vi antigen expression. The major production of Vi polysaccharide in RpoS+ strains was at 150 mM of NaCl, even in the presence of RpoS, but these strains never exhibited expression levels higher than those of RpoS− strains (Fig. 4 and Table 3). The RpoS− strains demonstrated overexpression of Vi polysaccharide at all osmolarities tested. However, RpoS− strains showed regulation of Vi polysaccharide in response to osmolarity changes (Fig. 4 and Tables 2 and 3), but with a substantial increase in Vi synthesis (Fig. 4 and Table 3). These results indicated that RpoS down-regulated the Vi polysaccharide expression in serotype Typhi.
FIG. 4.
Evaluation of the effect of RpoS on the synthesis of Vi polysaccharide in serotype Typhi. (A) Standards of Vi antigen. (B) Negative control, χ9197 serotype Typhi Ty2 RpoS− ΔtviAEBCD10. (C) Negative control, χ9198 serotype Typhi ISP1820 RpoS+ ΔtviAEBCD10. (D) χ3744 serotype Typhi ISP1820 RpoS+. (E) χ3769 serotype Typhi Ty2 RpoS−. (F) χ8438 serotype Typhi Ty2 RpoS+. (G) χ9060 serotype Typhi Ty2 rpoSΩAp. (H) χ9061 serotype Typhi ISP1820 rpoSΩAp. (I) Noninduced χ9066 serotype Typhi ΔPrpoS183::TTaraCPBADrpoS RpoS. (J) χ9067 serotype Typhi Ty2 RpoS− ΔPrpoS183::TTaraCPBADrpoS RpoS induced by 0.2% arabinose. The strains were grown in LB media with 0, 0.15, 0.3, and 0.4 mM of NaCl. (K) Relation between Vi antigen concentration (μg) and length of rocket (cm).
Both RpoS+ and RpoS− strains showed regulation of Vi polysaccharide in response to changes in osmolarity, suggesting that the repression of Vi polysaccharide synthesis in response to osmolarity is not under the control of RpoS (1, 53). However, RpoS may help in the repression of Vi polysaccharide at high and low osmolarities, conditions under which rpoS is induced (6, 43).
Effects of rpoS on Vi antigen synthesis and Hd detection.
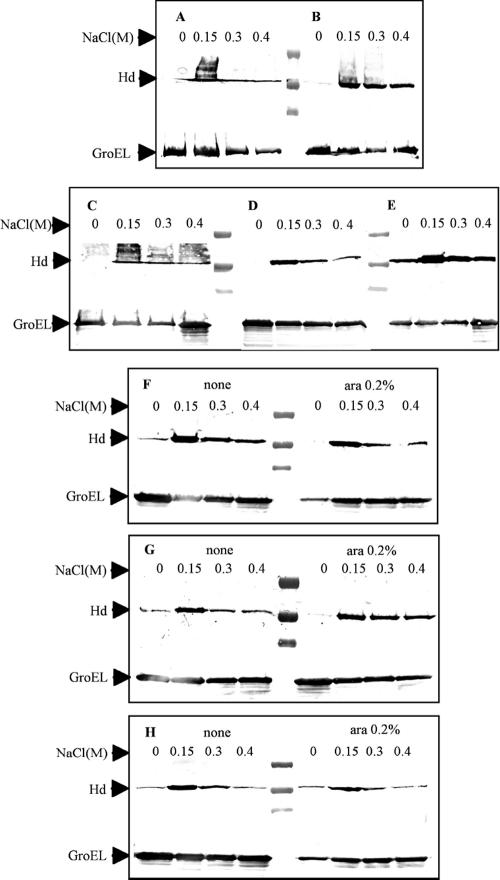
The fliC gene is present only in serotype Typhi strains and encodes one type of flagellin, designated Hd (1). Serotype Typhi GIFU10007 appeared to require intrinsic, intact motility for invading cultured epithelial cells, as nonmotile mutants were not invasive (42). The production of the Hd flagellin in serotype Typhi, as well as that of Vi antigen, is modulated by the RcsB-RcsC regulatory system in response to changes in the osmolarity of the growth medium (1). We examined Hd synthesis in strains of different rpoS genotypes in media with different osmolarities. Synthesis of the Hd flagellar antigen in RpoS+ strains was detected at all osmolarities tested. In contrast, the Hd flagellar antigen was not detected in RpoS− strains at osmolarities lower than 10 mM of NaCl (Table 4). At osmolarities higher than 10 mM NaCl, the Hd antigen was detected with a strong reaction in RpoS− strains, the same as in RpoS+ strains. However, when the RpoS− strains were grown at 0 and 10 mM NaCl and then boiled, the Hd antigen was detected. These results show that the Vi antigen also covers and masks the Hd antigen at low osmolarities in RpoS− strains. These results collectively suggest that the rpoS gene causes a subtle down-regulation of Vi polysaccharide synthesis in serotype Typhi.
TABLE 4.
Hd flagellar antigen slide agglutination reactions of serotype Typhi strains grown on LB agar (pH 7) supplemented with different amounts of NaCl at 37°C overnight (18 to 24 h)a
| Strain | Reaction for indicated NaCl concn (mM)
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 85 | 150 | 300 | 400 | 500 | |
| χ3769 RpoS− | − | − | +++ | +++ | +++ | +++ | +++ |
| χ3744 RpoS+ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| χ8438 RpoS+ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| χ9060 RpoS− | − | − | +++ | +++ | +++ | +++ | +++ |
| χ9061 RpoS− | − | − | +++ | +++ | +++ | +++ | +++ |
| χ9066 RpoS− | − | ± | +++ | +++ | +++ | +++ | +++ |
| χ9067 RpoS− | − | − | +++ | +++ | +++ | +++ | +++ |
| χ9068 RpoS− | − | − | +++ | +++ | +++ | +++ | +++ |
| χ9066 RpoS+b | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| χ9067 RpoS−b | − | + | +++ | +++ | +++ | +++ | +++ |
| χ9068 RpoS+b | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
The degrees of agglutination ranged from not detectable (−) to weak (+) to strong (+++); ± and ++ indicate intermediate degrees.
Strains grown with 0.2% arabinose to induce rpoS (see text for details).
Although we observed similar results for masking detection of the Hd flagellar antigen with growth in media by adding sucrose to increase osmolarity, this masking in RpoS− strains was observed at much higher concentrations when sucrose was used (data not shown) than when NaCl was used to alter osmolarity (Table 4).
Flagellar synthesis is dependent on the rcsB-rcsC loci, which decrease the syntheses of flagella at low osmolarities (1). Western blot analysis showed that Hd flagellin synthesis was decreased under low-osmolarity conditions, as expected (Fig. 5). Similar results were obtained for rpoS arabinose-inducible mutants in the presence or absence of arabinose (Fig. 5). Expression levels of flagella were considerably lower when the strains were grown at 0 mM of NaCl, but flagella were still detected by agglutination in RpoS+ strains. However, in RpoS− strains, flagella were not detected, due to overexpression of the Vi polysaccharide (Table 4).
FIG. 5.
Evaluation of Hd (serotype Typhi flagella factor) expression at different osmolarities by Western blot analysis. (A) χ3744 serotype Typhi ISP1820 RpoS+. (B) χ3769 serotype Typhi Ty2 RpoS−. (C) χ8438 serotype Typhi Ty2 RpoS+. (D) χ9060 serotype Typhi Ty2 rpoSΩAp RpoS−. (E) χ9061 serotype Typhi ISP1820 rpoSΩAp RpoS−. (F to H) Assays conducted in the absence and presence of arabinose (0.2%). (F) χ9066 serotype Typhi ISP1820 RpoS+ ΔPrpoS183::TTaraCPBADrpoS. (G) χ9067 serotype Typhi Ty2 RpoS− ΔPrpoS183::TTaraCPBADrpoS. (H) χ9068 serotype Typhi Ty2 RpoS+ ΔPrpoS183::TTaraCPBADrpoS.
Evaluation of Vi polysaccharide synthesis in serotype Typhi RpoS+ during growth.
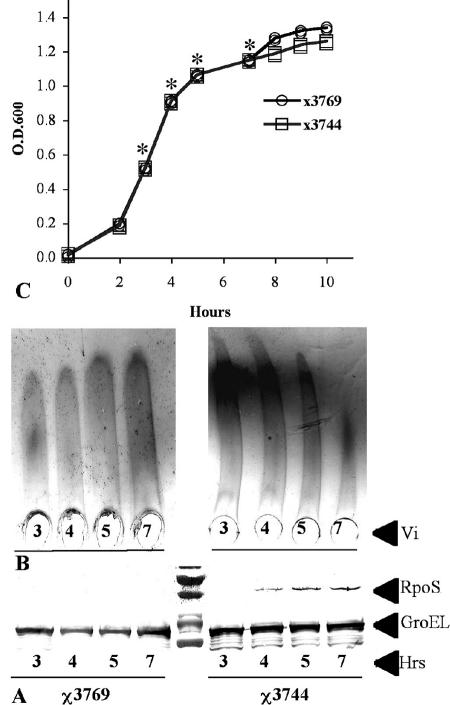
RpoS is a key factor in the stress response during the transition from the exponential growth phase to the stationary growth phase (43). RpoS is expressed at low levels or unstable at exponential growth phase in E. coli (30, 46). Thus, Vi antigen could have different expression levels in exponential phase, when RpoS is not present or is present at low levels, and in stationary phase in serotype Typhi, when RpoS is maximally expressed. We tested the effect of growth phase on the synthesis of Vi antigen in serotype Typhi RpoS+. RpoS was not detected in early exponential phase (Fig. 6) but was detected at different levels from mid-exponential-growth-phase cultures and evidently expressed at stationary phase (Fig. 6), since bubble production in the catalase test was positive only in stationary phase. In concordance with RpoS expression levels, Vi polysaccharide synthesis was decreased (Fig. 6). These results indicated that serotype Typhi RpoS+ overexpressed the Vi antigen during the early growth phase, when RpoS is not expressed or expressed at low levels. This indicates that RpoS is the cause of the VW and V variation in serotype Typhi. RpoS− strains overexpress the Vi polysaccharide without RpoS regulation, which causes a permanent V form. RpoS+ strains exhibit both forms, the V form during the early exponential growth, when RpoS is not expressed or expressed at low levels, and the VW form when RpoS is expressed. On the other hand, SPI-7, where the viaB locus is located, is an unstable genetic element in serotype Typhi (4) and strains that have lost this pathogenic island exhibit the W form (Vi−). Thus, this genetic instability could be the cause of the W form.
FIG. 6.
Evaluation of Vi polysaccharide synthesis in serotype Typhi RpoS+ during growth. (A) RpoS expression during the growth curve. GroEL was used as a control. The strains were grown in LB medium with 150 mM of NaCl. (B) Vi polysaccharide expression during the growth. (C) Growth curve. *, the samples collected at each point were normalized as described in the text.
DISCUSSION
The expression of capsular polysaccharide by some Enterobacteriaceae is highly regulated in response to different environments, being activated by certain signals and suppressed by others (77). Vi capsular expression in serotype Typhi is subject to regulation by at least two separate two-component systems, rcsB-rcsC (in the viaA locus) (1) and ompR-envZ (in the ompB locus) (53). TviA (VipR), a positive regulator that activates its own promoter in the viaB locus, interacts with RcsB, independently of RcsA and Lon protease, to promote optimal transcription of genes involved in Vi antigen synthesis (1, 53, 76, 77). Vi is expressed preferentially at low and medium osmolarities. Such environments might include aqueous environments, which are thought to contain not more than 60 mM NaCl, and certain extracellular environments, such as blood and the cell cytoplasm, where the osmolarities are equivalent to 150 mM of NaCl (310 mosmol) (45, 37, 71). The latter environment is especially relevant to that encountered by serotype Typhi in the infected host. It is possible that this preferential expression of Vi polysaccharide at low and medium osmolarities serves to protect bacterial cells from the complement-mediated actions of the O antigen-specific antibody (61). Recent studies with serotype Typhi Ty2 grown under optimal conditions for Vi polysaccharide expression showed that Vi polysaccharide reduced TLR-dependent interleukin-8 production in human colonic tissue explants, suggesting that the scarcity of neutrophils in intestinal infiltrations of typhoid fever patients is due to the Vi polysaccharide (56). In contrast, RcsB under low-osmolarity conditions, acting in association with the viaB locus-encoded TviA protein, negatively controls the transcription of iagA, invF, and sipB (encoding proteins involved in cell invasion) (1).
At high osmolarities, such as those in the intestinal lumen (with values believed to be equivalent to 300 mM of NaCl and greater [71]), the transcriptions of iagA, invF, and sipB are markedly increased, whereas the transcriptions of genes involved in Vi biosynthesis are markedly decreased (1). We observed that the synthesis of Vi antigen decreased as the osmolarity increased (Table 2 and Fig. 4), and when the osmolarity reached 676 mosmol (300 mM of NaCl), the Vi antigen no longer covered the O antigen (Table 2). Zhao et al. (80) showed that at 300 mM or more of NaCl, serotype Typhi GIFU1007 had null expression of the Vi antigen and exhibited a high invasion index in epithelial cells, together with a high secretion of SipC protein (80). Part of the stress response in the bacterial cell is the induction of the master stress response regulator protein RpoS (6, 43). High osmolarity is one of the environmental signals that induce rpoS (6, 43). In accordance with these findings, our results showed that RpoS down-regulates Vi polysaccharide synthesis, which could have importance for the outcome of infection. On the other hand, serotype Typhi Ty2, which was isolated in 1918 (15) and is RpoS− (58), exhibited maximal adherence to and invasiveness into tissue culture cells when grown in media with 300 mM NaCl (676 mosmol) (71). We observed that the Vi polysaccharide did not cover the O antigen when either RpoS− or RpoS+ strains were grown in media with ≥300 mM of NaCl. However, at osmolarities lower than those produced by 300 mM NaCl (676 mosmol), the Vi antigen was overexpressed and the O antigens were covered and masked in RpoS− strains but not RpoS+ strains, which may affect the invasiveness. In addition, at osmolarities lower than 676 mosmol, adherence to and invasiveness into tissue culture cells have been found to be minimal in serotype Typhi Ty2 (71). On other hand, studies comparing serotype Typhi, serotype Typhimurium, and S. enterica serotype Dublin strains showed that serotype Typhi, in this case ISP1820, is much more adherent to and invasive into Int-407 and murine MODE-K cell lines when grown in media with differing osmolarities, in contrast to the RpoS− serotype Typhi Ty2 strain (79).
Additionally, our results imply that the rpoS gene is responsible for the VW variation in the display of Vi and O antigens in serotype Typhi as described in early studies (15, 74). The VW form, with a dual display of Vi and O antigens, is most commonly displayed by serotype Typhi strains from infected individuals, and the majority of such clinical isolates are also RpoS+ (58).
Serotype Typhi ISP1820, which is RpoS+, is more virulent in humans than serotype Typhi Ty2, which is RpoS− (70). In concordance, serotype Typhi CVD906, a derivate of the serotype Typhi field isolate ISP1820 with deletion mutations in aroC and aroD, was highly immunogenic but caused fever and other adverse reactions in humans (26), in contrast to the serotype Typhi CVD908 Ty2 ΔaroC ΔaroD strain, which is RpoS− (25). The live typhoid vaccine Ty21a, which is an RpoS−, ΔgalE, and Vi− (59) derivate of serotype Typhi Ty2 (58), has been evaluated in several clinical trials and found to be well tolerated, although only modestly immunogenic, requiring three or four doses to confer protection (40, 41, 66, 78). Furthermore, the live typhoid vaccine Ty21a has yielded poor results when used as a live recombinant serotype Typhi vaccine (RAStyVs) expressing protective antigens against a diversity of pathogens (2, 9, 10, 21, 24, 50). The rpoS mutation could affect the immunogenicities of recombinant vaccines (1). It is thus possible that this poor immunogenicity may be the result of the rpoS mutation in Ty2 and its derivatives rather than Vi antigen deletion. On the other hand, the overexpression of Vi antigen in Ty2 could decrease adherence to and invasion into intestinal tissues necessary to colonize more internal lymphoid effector tissues (2). In serotype Typhimurium, it has been demonstrated that chromosomal RpoS-regulated genes are necessary for invasion into and colonization of the gut-associated lymphoid tissue (47). In accord with this, rpoS mutants of serotype Typhimurium, with or without the virulence plasmid that possesses RpoS-regulated genes that play no role in the colonization of the gut-associated lymphoid tissue (19, 52), exhibit diminished immunogenicities (5, 7). These results collectively imply that serotype Typhi with RpoS+ genotype, with or without the ability to produce the Vi capsular antigen, might be superior to RpoS− strains as a vector in the development of recombinant attenuated Salmonella vaccines for humans (7, 8). This hypothesis requires further testing.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 AI24533, R01 AI057885, and R01 AI056289) and the Bill and Melinda Gates Foundation (grant 37863).
We thank James Wilson (Biodesign Institute at Arizona State University), Vjollca Konjufca (Biodesign Institute at Arizona State University), and Wei Xin (Biodesign Institute at Arizona State University) for helpful advice and Erika Arch (Biodesign Institute at Arizona State University) for her assistance with the manuscript.
Editor: F. C. Fang
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Arricau, N., D. Hermant, H. Waxin, C. Ecobichon, P. S. Duffey, and M. Y. Popoff. 1998. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol. Microbiol. 29:835-850. [DOI] [PubMed] [Google Scholar]
- 2.Black, R. E., M. M. Levine, M. L. Clements, G. Losonsky, D. Herrington, S. Berman, and S. B. Formal. 1987. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J. Infect. Dis. 155:1260-1265. [DOI] [PubMed] [Google Scholar]
- 3.Buchmeier, N. A., S. J. Libby, Y. Xu, P. C. Loewen, J. Switala, D. G. Guiney, and F. C. Fang. 1995. DNA repair is more important than catalase for Salmonella virulence in mice. J. Clin. Investig. 95:1047-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno, S. M., C. A. Santiviago, A. A. Murillo, J. A. Fuentes, A. N. Trombert, P. I. Rodas, P. Youderian, and G. C. Mora. 2004. Precise excision of the large pathogenicity island, SPI7, in Salmonella enterica serovar Typhi. J. Bacteriol. 186:3202-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coynault, C., and F. Norel. 1999. Comparison of the abilities of Salmonella typhimurium rpoS, aroA and rpoS aroA strains to elicit humoral immune responses in BALB/c mice and to cause lethal infection in athymic BALB/c mice. Microb. Pathog. 26:299-305. [DOI] [PubMed] [Google Scholar]
- 6.Coynault, C., V. Robbe-Saule, and F. Norel. 1996. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol. Microbiol. 22:149-160. [DOI] [PubMed] [Google Scholar]
- 7.Curtiss, R., III. 2002. Bacterial infectious disease control by vaccine development. J. Clin. Investig. 110:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtiss, R., III. 2005. Antigen delivery systems: development of live recombinant attenuated bacterial antigen and DNA vaccine delivery vector vaccines, p. 1009-1037. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, J. R. McGhee, and W. Strober (ed.), Mucosal immunology, 3rd edition. Academic Press, San Diego, CA.
- 9.Dilts, D. A., I. Riesenfeld-Orn, J. P. Fulginiti, E. Ekwall, C. Granert, J. Nonenmacher, R. N. Brey, S. J. Cryz, K. Karlsson, K. Bergman, T. Thompson, B. Hu, A. H. Bruckner, and A. A. Lindberg. 2000. Phase I clinical trials of aroA aroD and aroA aroD htrA attenuated S typhi vaccines; effect of formulation on safety and immunogenicity. Vaccine 18:1473-1484. [DOI] [PubMed] [Google Scholar]
- 10.DiPetrillo, M. D., T. Tibbetts, H. Kleanthous, K. P. Killeen, and E. L. Hohmann. 1999. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449-459. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, P. R., and W. H. Ewing. 1972. Identification of enterobacteriaceae, 3rd ed. Burgess Publishing Co., Minneapolis, MN.
- 12.Edwards, R. A., L. H. Keller, and D. M. Schifferli. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149-157. [DOI] [PubMed] [Google Scholar]
- 13.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felix, A., and R. M. Pitt. 1934. A new antigen of B. typhosus. Its relation to virulence and to active and passive immunization. Lancet 227:186-191. [Google Scholar]
- 15.Felix, A., and R. M. Pitt. 1951. The pathogenic and immunogenic activities of Salmonella typhi: in relation to its antigenic constituents. J. Hyg. 49:92-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaines, S., and J. G. Tully. 1961. Comparison of intracerebral and intraperitoneal infective techniques for assaying mouse virulence of Salmonella typhosa strains. Am. J. Hyg. 74:60-66. [DOI] [PubMed] [Google Scholar]
- 17.Gaines, S., J. G. Tully, and W. D. Tigertt. 1961. Enhancement of the mouse virulence of a non-Vi variant of Salmonella typhosa by Vi antigen. J. Immunol. 86:543-551. [PubMed] [Google Scholar]
- 18.Gay, P., D. Le Coq, M. Steinmetz, T. Berkelman, and C. I. Kado. 1985. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 164:918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto, Y., N. Li, H. Yokoyama, and T. Ezaki. 1993. Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella typhi. J. Bacteriol. 175:4456-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hindle, Z., S. N. Chatfield, J. Phillimore, M. Bentley, J. Johnson, C. A. Cosgrove, M. Ghaem-Maghami, A. Sexton, M. Khan, F. R. Brennan, P. Everest, T. Wu, D. Pickard, D. W. Holden, G. Dougan, G. E. Griffin, D. House, J. D. Santangelo, S. A. Khan, J. E. Shea, R. G. Feldman, and D. J. Lewis. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 70:3457-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirose, K., T. Ezaki, M. Miyake, T. Li, A. Q. Khan, Y. Kawamura, H. Yokoyama, and T. Takami. 1997. Survival of Vi-capsulated and Vi-deleted Salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol. Lett. 147:259-265. [DOI] [PubMed] [Google Scholar]
- 23.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohmann, E. L., C. A. Oletta, K. P. Killeen, and S. I. Miller. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J. Infect. Dis. 173:1408-1414. [DOI] [PubMed] [Google Scholar]
- 25.Hone, D. M., A. M. Harris, S. Chatfield, G. Dougan, and M. M. Levine. 1991. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine 9:810-816. [DOI] [PubMed] [Google Scholar]
- 26.Hone, D. M., C. O. Tacket, A. M. Harris, B. Kay, G. Losonsky, and M. M. Levine. 1992. Evaluation in volunteers of a candidate live oral attenuated Salmonella typhi vector vaccine. J. Clin. Investig. 90:412-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornick, R. B., S. E. Greisman, T. E. Woodward, H. L. DuPont, A. T. Dawkins, and M. J. Snyder. 1970. Typhoid fever: pathogenesis and immunologic control. 2. N. Engl. J. Med. 283:739-746. [DOI] [PubMed] [Google Scholar]
- 28.Houng, H. S., K. F. Noon, J. T. Ou, and L. S. Baron. 1992. Expression of Vi antigen in Escherichia coli K-12: characterization of ViaB from Citrobacter freundii and identity of ViaA with RcsB. J. Bacteriol. 174:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jesudason, M. V., G. Sridharan, S. Mukundan, and T. J. John. 1994. Vi-specific latex agglutination for early and rapid detection of Salmonella serotype typhi in blood cultures. Diagn. Microbiol. Infect. Dis. 18:75-78. [DOI] [PubMed] [Google Scholar]
- 30.Jishage, M., A. Iwata, S. Ueda, and A. Ishihama. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 178:5447-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang, H. Y., C. M. Dozois, S. A. Tinge, T. H. Lee, and R. Curtiss III. 2002. Transduction-mediated transfer of unmarked deletion and point mutations through use of counterselectable suicide vectors. J. Bacteriol. 184:307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauffmann, F. 1935. Uber einen neuen serologischen formenwechsel der Typhusbacillen. Z. Hyg. Infektionskr. 117:617-651. [Google Scholar]
- 34.Khan, A. Q., L. Zhao, K. Hirose, M. Miyake, T. Li, Y. Hashimoto, Y. Kawamura, and T. Ezaki. 1998. Salmonella typhi rpoS mutant is less cytotoxic than the parent strain but survives inside resting THP-1 macrophages. FEMS Microbiol. Lett. 161:201-208. [DOI] [PubMed] [Google Scholar]
- 35.Korhonen, T. K. 1979. Yeast cell agglutination by purified enterobacterial pili. FEMS Microbiol. Lett. 6:421-425. [Google Scholar]
- 36.Kowarz, L., C. Coynault, V. Robbe-Saule, and F. Norel. 1994. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J. Bacteriol. 176:6852-6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kugelmass, I. N. 1959. Biochemistry of blood in health and disease. Charles C. Thomas Publisher, Springfield, IL.
- 38.Laurel, C. B. 1965. Quantitative estimation of proteins by electrophoresis in agarose gel contains antibodies. Anal. Biochem. 15:45-52. [DOI] [PubMed] [Google Scholar]
- 39.Lesmana, M., R. C. Rockhill, and W. R. Sanborn. 1980. A coagglutination method for presumptive identification of Salmonella typhi. Southeast Asian J. Trop. Med. Public Health 11:302-307. [PubMed] [Google Scholar]
- 40.Levine, M. M., C. Ferreccio, P. Abrego, O. S. Martin, E. Ortiz, and S. Cryz. 1999. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 17(Suppl. 2):S22-S27. [DOI] [PubMed] [Google Scholar]
- 41.Levine, M. M., C. Ferreccio, R. E. Black, and R. Germanier. 1987. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet i:1049-1052. [DOI] [PubMed]
- 42.Liu, S. L., T. Ezaki, H. Miura, K. Matsui, and E. Yabuuchi. 1988. Intact motility as a Salmonella typhi invasion-related factor. Infect. Immun. 56:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowen, P. C., and R. Hengge-Aronis. 1994. The role of the sigma factor sigmas (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 44.Luria, S. E., and J. W. Burrous. 1957. Hybridization between Escherichia coli and Shigella. J. Bacteriol. 74:461-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muffler, A., D. D. Traulsen, R. Lange, and R. Hengge-Aronis. 1996. Posttranscriptional osmotic regulation of the σs subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 178:1607-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nickerson, C. A., and R. Curtiss III. 1997. Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect. Immun. 65:1814-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norel, F., V. Robbe-Saule, M. Y. Popoff, and C. Coynault. 1992. The putative sigma factor KatF (RpoS) is required for the transcription of the Salmonella typhimurium virulence gene spvB in Escherichia coli. FEMS Microbiol. Lett. 78:271-276. [DOI] [PubMed] [Google Scholar]
- 49.Old, D. C., I. Corneil, L. F. Gibson, A. D. Thomson, and J. P. Duguid. 1968. Fimbriation, pellicle formation and the amount of growth of salmonellas in broth. J. Gen. Microbiol. 51:1-16. [DOI] [PubMed] [Google Scholar]
- 50.Orme, I. M. 1995. Prospects for new vaccines against tuberculosis. Trends Microbiol. 3:401-404. [DOI] [PubMed] [Google Scholar]
- 51.Pang, T. 1998. Genetic dynamics of Salmonella typhi—diversity in clonality. Trends Microbiol. 6:339-342. [DOI] [PubMed] [Google Scholar]
- 52.Pardon, P., M. Y. Popoff, C. Coynault, J. Marly, and I. Miras. 1986. Virulence-associated plasmids of Salmonella serotype typhimurium in experimental murine infection. Ann. Inst. Pasteur. Microbiol. 137B:47-60. [DOI] [PubMed] [Google Scholar]
- 53.Pickard, D., J. Li, M. Roberts, D. Maskell, D. Hone, M. Levine, G. Dougan, and S. Chatfield. 1994. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect. Immun. 62:3984-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qadri, A., S. Ghosh, and G. P. Talwar. 1990. Monoclonal antibodies against two discrete determinants on Vi capsular polysaccharide. J. Immunoassay 11:235-250. [DOI] [PubMed] [Google Scholar]
- 56.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Baumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 73:3367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbe-Saule, V., G. Algorta, I. Rouilhac, and F. Norel. 2003. Characterization of the RpoS status of clinical isolates of Salmonella enterica. Appl. Environ. Microbiol. 69:4352-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbe-Saule, V., and F. Norel. 1999. The rpoS mutant allele of Salmonella typhi Ty2 is identical to that of the live typhoid vaccine Ty21a. FEMS Microbiol. Lett. 170:141-143. [DOI] [PubMed] [Google Scholar]
- 59.Robbe-Saule, V., C. Coynault, and F. Norel. 1995. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol. Lett. 126:171-178. [DOI] [PubMed] [Google Scholar]
- 60.Robbe-Saule, V., C. Coynault, M. Ibanez-Ruiz, D. Hermant, and F. Norel. 2001. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigmaS). Mol. Microbiol. 39:1533-1545. [DOI] [PubMed] [Google Scholar]
- 61.Robbins, J. D., and J. B. Robbins. 1984. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J. Infect. Dis. 150:436-449. [DOI] [PubMed] [Google Scholar]
- 62.Roland, K., R. Curtiss III, and D. Sizemore. 1999. Construction and evaluation of a Δcya Δcrp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 43:429-441. [PubMed] [Google Scholar]
- 63.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 64.Santander, J., and J. Robeson. 2002. Isolation and characterization of bacteriophages active against Salmonella enteritidis and their assay on Salmonella pullorum. Acta Microbiol. Soc. Chil. Microbiol. 8:17-22. [Google Scholar]
- 65.Schmieger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75-88. [DOI] [PubMed] [Google Scholar]
- 66.Simanjuntak, C. H., F. P. Paleologo, N. H. Punjabi, R. Darmowigoto, Soeprawoto, H. Totosudirjo, P. Haryanto, E. Suprijanto, N. D. Witham, and S. L. Hoffman. 1991. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 338:1055-1059. [DOI] [PubMed] [Google Scholar]
- 67.Skarmeta, A. M., and J. Robeson. 2000. Probing transfer of an IncP replicon to natural marine bacteria. Rev. Biol. Mar. Oceanogr. 35:121-125. [Google Scholar]
- 68.Sternberg, N. L., and R. Maurer. 1991. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol. 204:18-43. [DOI] [PubMed] [Google Scholar]
- 69.Szu, S. C., and S. Bystricky. 2003. Physical, chemical, antigenic, and immunologic characterization of polygalacturonan, its derivatives, and Vi antigen from Salmonella typhi. Methods Enzymol. 363:552-567. [DOI] [PubMed] [Google Scholar]
- 70.Tacket, C. O., D. M. Hone, R. Curtiss III, S. M. Kelly, G. Losonsky, L. Guers, A. M. Harris, R. Edelman, and M. M. Levine. 1992. Comparison of the safety and immunogenicity of ΔaroC ΔaroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect. Immun. 60:536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tartera, C., and E. S. Metcalf. 1993. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect. Immun. 61:3084-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 73.Tsang, R. S., and A. Wong. 1989. Release of Vi antigens from Salmonella typhi: implications for virulence and diagnosis. FEMS Microbiol. Immunol. 1:437-441. [DOI] [PubMed] [Google Scholar]
- 74.Tully, J. G., and J. A. Currie. 1962. Vi-negative strains of Salmonella typhosa: attempts to induce W-V reversion and the use of non-Vi strains in evaluating typhoid vaccines. J. Bacteriol. 84:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tully, J. G., and S. Gaines. 1961. Comparison of intraperitoneal and intracerebral infective techniques for evaluating typhoid vaccines in mouse protection tests. Am. J. Hyg. 74:259-266. [DOI] [PubMed] [Google Scholar]
- 76.Virlogeux, I., H. Waxin, C. Ecobichon, J. O. Lee, and M. Y. Popoff. 1996. Characterization of the rcsA and rcsB genes from Salmonella typhi: rcsB through tviA is involved in regulation of Vi antigen synthesis. J. Bacteriol. 178:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Virlogeux, I., H. Waxin, C. Ecobichon, and M. Y. Popoff. 1995. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology 141:3039-3047. [DOI] [PubMed] [Google Scholar]
- 78.Wahdan, M. H., C. Serie, Y. Cerisier, S. Sallam, and R. Germanier. 1982. A controlled field trial of live Salmonella typhi strain Ty21a oral vaccine against typhoid: three-year results. J. Infect. Dis. 145:292-295. [DOI] [PubMed] [Google Scholar]
- 79.Weinstein, D. L., B. L. O'Neill, D. M. Hone, and E. S. Metcalf. 1998. Differential early interactions between Salmonella enterica serovar Typhi and two other pathogenic Salmonella serovars with intestinal epithelial cells. Infect. Immun. 66:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao, L., T. Ezak, Z. Y. Li, Y. Kawamura, K. Hirose, and H. Watanabe. 2001. Vi-suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer's patches. Microbiol. Immunol. 45:149-158. [DOI] [PubMed] [Google Scholar]