Abstract
The Escherichia coli maltose-binding protein (MBP) is used to increase the stability and solubility of proteins in bacterial protein expression systems and is increasingly being used to facilitate the production and delivery of subunit vaccines against various pathogenic bacteria and viruses. The MBP tag is presumed inert, with minimum effects on the bioactivity of the tagged protein or its biodistribution. However, few studies have characterized the immunological attributes of MBP. Here, we analyze the phenotypic and functional outcomes of MBP-treated dendritic cells (DCs) and show that MBP induces DC activation and production of proinflammatory cytokines (interleukin-1β [IL-1β], IL-6, IL-8, tumor necrosis factor alpha, and IL-12p70) within 24 h and strongly increases Iκβ phosphorylation in treated cells. Interestingly, phosphorylation of Iκβ was largely abrogated by the addition of anti-human Toll-like receptor 4 (TLR4) antibodies, indicating that MBP activates signaling for DC maturation via TLR4. Consistent with this hypothesis, MBP activated the TLR4-expressing cell line 293-hTLR4A but not control cultures to secrete IL-8. The observed data were independent of lipopolysaccharide contamination and support a role for TLR4 in mediating the effects of MBP. These results provide insight into a mechanism by which MBP might enhance immune responses to vaccine fusion proteins.
Escherichia coli maltose-binding protein (MBP) is a high-affinity maltose/maltodextrin-binding protein (approximately 42 kDa) responsible for the capture and transport of maltodextrins from the periplasmic space in gram-negative bacteria (2). Recombinant proteins are commonly fused to MBP to improve their yield and to facilitate their purification (13). MBP confers the additional benefits of enhanced stability and solubility to passenger proteins, possibly by the formation of stable sequestered intermediate fusion partners that permit proteins to eventually regain their native conformation (4). More recently, MBP was utilized as a chaperone component in various experimental subunit vaccines against pathogenic bacteria (6, 9) and viruses (3, 15-17). The enhanced immunogenicity of recombinant protein-MBP vaccines has been demonstrated in animal models against pathogens such as dengue virus (15, 16) and Plasmodium falciparum (7). MBP is presumed to lengthen the half-life and increase the solubility of the inoculum protein in immunized animals, hence optimizing the interactions of the vaccine with the cellular arm of the immune system. However, its direct effect on the immune system remains largely unexplored. To further understand how MBP fusions influence immune responses to vaccine preparations, we studied their effects on human dendritic cells (DCs) in vitro. The induction and maintenance of antigen-specific responses require the participation of host antigen-presenting cells, primarily DCs because of their superior capacity for acquiring and processing antigens, and the expression of high levels of costimulatory molecules that drive T-cell activation (8). DCs possess a number of antigen-sensing strategies, including Toll-like receptors (TLR), and become activated when they receive a maturation stimulus. This process is associated with phenotypic and functional changes, including the upregulation of molecules required for effective priming of naïve T cells and the production of cytokines (5).
Here, we show that MBP provides a potent proinflammatory stimulus, inducing cytokine secretion and maturation of human DCs in vitro. MBP induced the activation of the nuclear factor-κB (NF-κB) pathway by phosphorylation of the inhibitory molecule Iκ-B, a process that may occur via TLR4 in DCs. The data presented here may challenge the idea that MBP passively augments the antigenicity of fusion proteins and highlight the potential adjuvant properties of MBP in the enhancement of immune responses.
MATERIALS AND METHODS
Reagents.
E. coli MBP was purchased from New England Biolabs (Ipswich, MA). The protein was produced from an E. coli strain that carries the MBP expression vector pMAL-c2 and consists of MBP preceded by methionine, with the final 4 amino acids replaced by 23 residues encoded by the polylinker of pMAL-c2. Lipopolysaccharide (LPS; E. coli 055:B5) was obtained from Sigma-Aldrich (Saint Louis, MO). Anti-TLR2 (TL2.1) and -TLR4 (HTA125) were obtained from eBioscience (San Diego, CA). The 293-hTLR4A cell line was acquired from InvivoGen (San Diego, CA).
Generation of human dendritic cells.
DCs were generated from positively isolated CD14+ cells (Miltenyi Biotech, Auburn, CA) from peripheral blood mononuclear cells collected from normal, healthy, and consenting donors under a protocol approved by the Walter Reed Army Institute of Research Review Board. Enriched CD14+ cells were cultured for 7 days in complete RPMI (1% l-glutamine, 1% penicillin-streptomycin, 1% sodium pyruvate, 1% essential amino acids, and 10% heat-inactivated fetal calf serum) in the presence of 100 ng/ml recombinant human granulocyte-macrophage colony-stimulating factor (Leukine; Immunex, Seattle, WA) and 50 ng/ml recombinant human interleukin-4 (IL-4; R&D Systems, Minneapolis, MN) at 37°C, 5% CO2, and 95% relative humidity.
Cytokine quantification.
DCs (1 × 106 cells/ml) were plated in six-well plates in complete RPMI medium. Cells were stimulated with 10 ng/ml or 1 μg/ml MBP or left untreated. After incubation for 24, 48, or 72 h, the medium was collected and centrifuged to pellet the cells. Cell-free medium was transferred to fresh tubes and frozen until assayed. Fifty microliters from each sample medium was used to measure inflammatory cytokine production using a cytometric bead array (CBA) kit that utilizes flow cytometry to detect multiple soluble analytes in a particle-based immunoassay (BD Pharmingen, San Diego, CA). Analysis was done by FACScan using CBA software (BD Pharmingen).
Flow cytometry.
For cell surface CD80 and major histocompatibility complex class II (MHC-II) detection, unexposed dendritic cells or cells exposed to various concentrations of MBP for 24 h were stained with phycoerythrin (PE)-conjugated CD80 (L307.4), MHC-II (G46-6) antibodies, or isotype-matched, PE-labeled antibody controls, all purchased from BD PharMingen. In some experiments, DCs were stimulated with MBP and LPS in the presence or absence of 5 μg/ml polymyxin B. Cell surface staining of TLR4 was performed using PE anti-human Toll-like receptor 4 (HTA125) from eBioscience. In a comparison of isotype-matched controls from different manufacturers, clone GI55-178 from BD Biosciences was found to be the most appropriate nonbinding control. This antibody is an immunoglobulin G2a (IgG2a) specific for trinitrophenol, a hapten not expressed on human cells. Staining was performed in the presence of nonimmune human IgG to block nonspecific FcγR binding. Samples were analyzed on a FACSCalibur using Cell Quest software (Becton Dickinson).
Western blot analysis.
DCs were treated with 1 μg/ml MBP or LPS in the presence or absence of 5 μg/ml polymyxin B prior to being seeded in 48-well plates at 4 × 105 to 5 × 105 cells per well. In some experiments, DCs were treated with MBP fusion proteins MBP-YF env (MBP fused with the envelope protein of yellow fever virus) and MBP-DV env (MBP fused to envelope of dengue virus), containing approximately 100 amino acids of the envelope protein of yellow fever virus and dengue virus, respectively. Both fusion proteins have previously been tested as experimental vaccines in animal models (16). Cells were incubated at 37°C for 60 min, collected in 1.5-ml centrifuge tubes, and washed twice. Cell lysates were prepared in a Triton X-100-based buffer in the presence of the protease inhibitor leupeptin and the phosphatase inhibitors NaVO3 and Na pyrophosphate. Protein determination was obtained using the bicinchoninic acid protein detection system (Bio-Rad, Hercules, CA). Up to 10 μg of protein per sample was resolved in acrylamide denaturing gels and transferred to nitrocellulose membranes. Membranes were blotted using anti-phospho IκΒ-α from Cell Signaling (Beverly, MA). Detection of phosphorylated IκΒ was used to determine the level of NF-κΒ pathway activation. For loading controls, membranes were stripped off of their bound antibodies by using Restore stripping buffer (Pierce, Rockford, IL) and blotted again using anti-β-actin (Cell Signaling). Bands were visualized using SuperSignal chemiluminescence substrate from Pierce. When used, 20 μg/ml anti-TLR2 and 20 μg/ml anti-TLR4 [both mouse IgG2a(κ)] were added to cultures 30 min before treatments.
Reverse transcription (RT)-PCR.
DCs were isolated and cultured as described above, after which they were collected and washed twice in ice-cold phosphate-buffered saline. RNA from approximately 5 × 105 cells was isolated using the RNA aqueous system (Ambion, Austin, TX). One microgram of RNA was reverse transcribed with a combination of random hexamers and oligo(dT) primers at 42°C using an iScript cDNA synthesis kit (Bio-Rad). A reverse transcription was also done in the absence of reverse transcriptase as a control for genomic contamination. The resulting cDNA was used as a template to amplify human TLR2 (hTLR2) (forward, GGC CAG CAA ATT ACC TGT GT; reverse, TTC TCC ACC CAG TAG GCA TC [298 bp]) and TLR4 (forward, TGA GCA GTC GTG CTG GTA TC; reverse, CAG GGC TTT TCT GAG TCG TC [167 bp]). PCR was done using TaqMan Gold (ABI, Foster City, CA) Amplification went for 35 cycles at a 55°C annealing temperature.
IL-8 assay.
A human 293-TLR clone stably transfected with the plasmid pUNO-hTLR4A was used to determine TLR activation by assessing IL-8 production. Cells were plated in 96-well round-bottom plates at 2 × 105 cells/well in complete 10% fetal calf serum and Dulbecco modified Eagle medium. 293-hTLR4 cells were kept in the antibiotic blasticidin (10 μg/ml) for the duration of the experiments. After 1 h of incubation in medium alone, cells were treated (in duplicate) with 1 μg/ml LPS or MBP or left untreated. Cell-free medium was collected after 24 h and frozen at −80°C until assayed. IL-8 was measured in an IL-8 enzyme-linked immunosorbent assay (R&D Systems) following the manufacturer's recommendations.
RESULTS
MBP induces maturation of DCs and cytokine secretion.
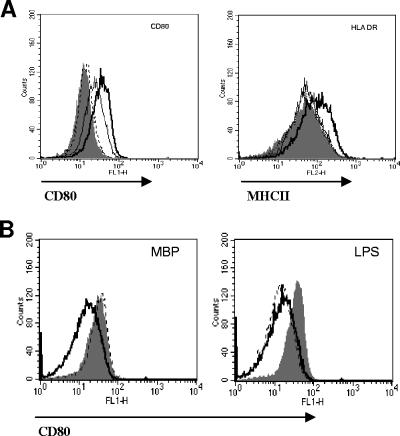
To test whether MBP had a direct effect on DCs, we examined the phenotype of DCs exposed to MBP at doses ranging from 1 ng/ml to 1 μg/ml of MBP and found increased surface expression of CD80 and MHC-II (Fig. 1A). DC activation was dose dependent; we detected increased CD80 expression at concentrations as low as 10 ng/ml of MBP. For MHC-II expression, 1 μg/ml of MBP was required. We tested for potential endotoxin contaminants in the MBP preparations by high-pressure liquid chromatography and a Limulus amebocyte lysate assay. High-pressure liquid chromatography showed a single peak, and the Limulus amebocyte lysate showed minimal endotoxin levels (less than 6 endotoxin units/ml). To further discount potential LPS contamination in the MBP solutions, DCs were stimulated with MBP or LPS in the presence or absence of the LPS-binding antibiotic polymyxin B at 5 μg/ml (Fig. 1B) and an increase of CD80 surface expression was measured by cytometry. As Fig. 1B shows, the addition of polymyxin B did not influence MBP-induced upregulation of CD80 on DCs. However, as expected, polymyxin B abrogated the upregulation of CD80 by LPS at 1 μg/ml (the equivalent of up to 10,000 endotoxin units/ml). The results were the same when MHC-II expression was measured (not shown).
FIG. 1.
DC activation by MBP. (A) DCs were treated with 10 ng/ml (dotted line), 100 ng/ml (thin black line), or 1 μg/ml (thick black line) of MBP for 24 h, and surface expression changes of CD80 (left) and MHC-II (right) were compared to those of untreated controls (solid histograms). (B) DCs were exposed to 10 μg/ml of MBP (left) or 1 μg/ml of LPS (right) for 24 h in the presence (dotted line) or absence (solid histogram) of 5 μg/ml of polymyxin B. Changes of CD80 surface expression were compared to those of untreated controls (solid black line). The data are representative of two independent experiments.
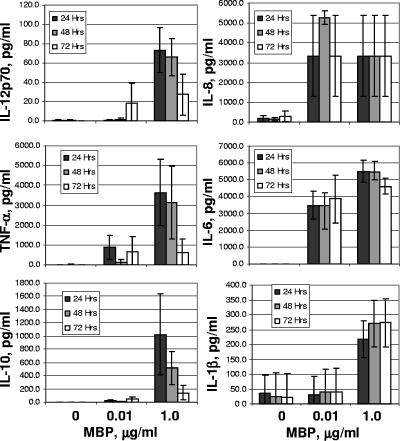
In separate experiments, we measured levels of IL-12p70, tumor necrosis factor alpha, IL-10, IL-8, IL-6, and IL-1β in supernatants collected from MBP-stimulated DC cultures (Fig. 2). MBP significantly enhanced DC cytokine production compared to untreated controls. Cytokine production was time and dose dependent; secretion of IL-12p70, tumor necrosis factor alpha, and IL-10 peaked at 24 h in cultures stimulated with 1 μg/ml MBP, whereas induction of IL-6, IL-8, and IL-1β required lower concentrations of MBP and continued robustly up to 72 h.
FIG. 2.
Cytokine secretion by MBP-treated DCs. DCs were treated with MBP (1 μg/ml or 0.01 μg/ml) or left untreated. Media were collected after 24, 48, or 72 h (hrs) and assayed by CBA for cytokine secretion. The evaluation of cytokines was limited to those shown. The data shown are averages for three experiments ± standard errors of the means. TNF-α, tumor necrosis factor alpha.
MBP activates Iκβ.
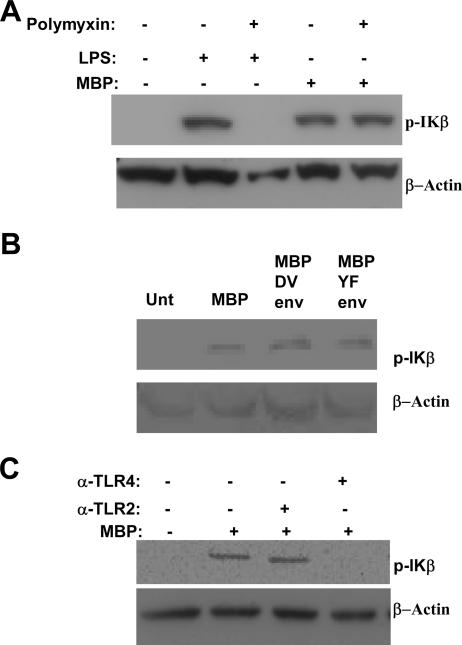
The cytokine secretion profiles of MBP-stimulated DCs suggested that MBP might function as a proinflammatory agent. To study this possibility, we measured changes in IκΒ activation in MBP-treated DCs. Signaling through the NF-κB pathway is associated with proinflammatory events resulting in increased levels of Iκβ phosphorylation. Resting unphosphorylated IκΒ is associated with the transcription factor NF-κΒ in the cytoplasm of the cells. Phosphorylation of IκΒ results in the release and consequent nuclear translocation of NF-κΒ (1). Treatment with 100 ng/ml (data not shown) or 1 μg/ml (Fig. 3A) MBP resulted in a marked increase in IκΒ phosphorylation after 1 h compared to untreated samples. IκΒ phosphorylation was also detected in DC cultures treated with 1 μg/ml MBP-YF Env and MBP-DV Env fusion proteins (Fig. 3B). To discount the possibility that LPS contaminants induced IκΒ phosphorylation, DCs were exposed to either MBP or MBP preincubated with polymyxin B at 5 μg/ml. As Fig. 3A shows, the addition of polymyxin B did not reverse the effect of MBP, indicating that MBP was responsible for activating the NF-κΒ pathway in these experiments. In contrast, a reversal of LPS-induced activation of IκΒ was detected after preincubation of LPS with polymyxin B (Fig. 3A).
FIG. 3.
Activation of IκΒ by MBP. (A) DCs were treated with 1 μg/ml MBP for 60 min in the presence or absence of 5 μg/ml polymyxin B and Iκβ activation determined by Western blotting of whole-cell lysates. Control cultures were treated with 1 μg/ml LPS. Membranes were blotted against the phosphorylated (activated) form of IκΒ. (B) DCs were left untreated (Unt) or were treated for 60 min with 1 μg/ml of MBP or MBP-YF Env and MBP-DV Env fusion proteins. Membranes were blotted using anti-phospho IκΒ antibody (Ab). (C) DCs were pretreated with 20 μg/ml anti-hTLR2 (α-TLR2) and 20 μg/ml anti-hTLR4 (α-TLR4) prior to the addition of 1 μg/ml MBP for 60 min. Membranes were blotted using anti-phospho IκΒ Ab. In all experiments, membranes were stripped and reblotted with anti-human β-actin as a loading control.
Activation of IκΒ is dependent on TLR4.
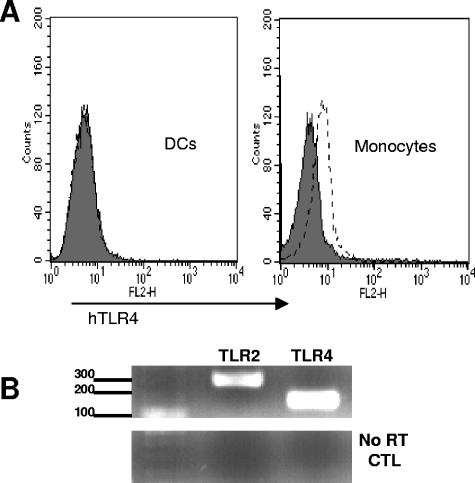
NF-κΒ induction is a common pathway downstream from TLR; therefore, we explored the involvement of TLR in mediating the effects of MBP. Since MBP is a bacterial product, TLR2 and TLR4 were selected because of their involvement in microbial cell wall component recognition (10, 12, 19). Figure 3C shows IκΒ phosphorylation of MBP-treated DCs pretreated with a neutralizing amount of the anti-hTLR2 or anti-hTLR4 antibody. As shown, pretreatment of DCs with anti-hTLR4 but not anti-hTLR2 antibody eliminated IκΒ phosphorylation. These results were not isotype dependent, as both blocking antibodies are mouse IgG2a(κ). These results strongly suggest that TLR4 mediates, at least partially, the activating effects of MBP. At the level of surface expression, TLR4 protein was expressed at low levels on control monocyte cultures but expression on DCs was too low to be detected by flow cytometry (Fig. 4A), confirming previous descriptions of low surface TLR4 density on immature DCs (20). An examination of TLR message by RT-PCR showed high expression levels of both TLR2 and TLR4 mRNAs in DCs (Fig. 4B).
FIG. 4.
TLR expression in DCs. (A) Cell surface expression of TLR4 on untreated DCs (left) and control monocyte cultures (right). Shaded histograms indicate cells stained with isotype controls; open histograms represent cells stained with anti-TLR4 monoclonal antibody. (B) RT-PCR analysis of TLR2 and TLR4 mRNAs in untreated DCs. The bottom panel shows results for the “no RT” controls. The experiment for which the results are shown was one of two showing identical results. CTL, cytotoxic T lymphocyte.
MBP induces IL-8 secretion in a human TLR4-expressing cell line.
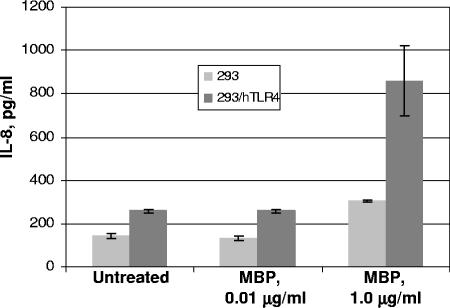
To test the possibility that MBP acts as a TLR4 ligand, the TLR4-expressing cell line 293-hTLR was incubated with various concentrations of MBP and TLR4 activation assessed by evaluating IL-8, which is produced upon TLR stimulation. We found that the addition of 1 μg/ml of MBP for 24 h induced the secretion of significant amounts of IL-8 (Fig. 5). By contrast, minimal amounts of IL-8 were secreted by MBP-exposed control 293 cell cultures.
FIG. 5.
TLR4-dependent production of IL-8. 293-hTLR4A cells or control 293 cells lacking TLR4 expression were treated with stated amounts of MBP or left untreated. Media were collected after 24 h and assayed for IL-8 by enzyme-linked immunosorbent assay. Histograms are means ± standard deviations for duplicate experiments.
DISCUSSION
The use of MBP as a chaperone for vaccine proteins generates robust humoral and cellular immune responses in immunized animals (7, 12, 14, 16, 17). It is believed that MBP augments the antigenicity of the carrier protein in formulations by creating a “depot” effect that facilitates the interaction between the antigen and antigen-presenting cells and/or binds and transports antigens to secondary lymphoid organs (4). In addition to this role, we show here that MBP can also provide intrinsic maturation stimuli to DCs and, as such, may potentiate antigen-presenting functions in immunized animals. In our experiments, the phenotypic changes detected on MBP-exposed DCs were equivalent to the effects detected with LPS, a potent inducer of DC maturation. Importantly, treated DCs produced large amounts of proinflammatory cytokines, which implies that the addition of MBP to vaccines might promote TH1-type responses and associated immunoglobulin G2 antibody. Consistent with this, MBP fusion proteins elicited gamma interferon from spleen mononuclear cells in response to scrub typhus (14).
Our data suggest that MBP could be considered a ligand for the pattern recognition receptor TLR4; however, this finding requires further investigation because although TLR4 message was easily detected in DCs, cell surface expression was too low to be detected by flow cytometry, raising the question of whether there was sufficient expression to account for the MBP response. Similar to LPS, for which there is ample evidence for the involvement of TLR4-mediated signaling (11), MBP induced the activation of the NF-κB signaling pathway via TLR4, presumably leading to proinflammatory cytokine secretion. The LPS molecule is a complex consisting of a polysaccharide, a core oligosaccharide, and a highly conserved lipid A, the portion of the complex believed to bind TLR4 (10); however, MBP is a monomeric structure with no reported lipid moiety (18). Whether MBP directly binds TLR4 remains to be determined, and the involvement of other receptors akin to the CD14 coreceptor for LPS cannot be ruled out. It is also possible that TLR4 functions as a membrane “sensor,” responding to alterations in DC membranes following the uptake of MBP. This argument is supported by the observation that in contrast to TLR2, which responds to a diverse number of bacteria and bacterial products, including gram-positive organisms, spirochetes, mycoplasma, and mycobacteria, TLR4 is more restrictive in its ligands (10).
In summary, our study provides evidence that MBP, which has hitherto been considered to play a passive role in vaccine formulations, may have intrinsic adjuvant-like properties and potently activates DCs.
Acknowledgments
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 2.Boos, W., and H. Shuman. 1998. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 62:204-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi, A. H., M. Basu, M. M. McNeal, J. A. Bean, J. D. Clements, and R. L. Ward. 2004. Intranasal administration of an Escherichia coli-expressed codon-optimized rotavirus VP6 protein induces protection in mice. Protein Expr. Purif. 38:205-216. [DOI] [PubMed] [Google Scholar]
- 4.Fox, J. D., R. B. Kapust, and D. S. Waugh. 2001. Single amino acid substitutions on the surface of Escherichia coli maltose-binding protein can have a profound impact on the solubility of fusion proteins. Protein Sci. 10:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang, Q. Z., G. C. Duan, Q. T. Fan, and Y. L. Xi. 2005. Fusion expression of Helicobacter pylori neutrophil-activating protein in E. coli. World J. Gastroenterol. 11:454-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushwaha, A., P. P. Rao, R. P. Suresh, and V. S. Chauhan. 2001. Immunogenicity of recombinant fragments of Plasmodium falciparum acidic basic repeat antigen produced in Escherichia coli. Parasite Immunol. 23:435-444. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia, A., and F. Sallusto. 2001. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr. Opin. Immunol. 13:291-298. [DOI] [PubMed] [Google Scholar]
- 9.Lee, L. H., E. Burg III, S. Baqar, A. L. Bourgeois, D. H. Burr, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect. Immun. 67:5799-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 12.Rico, A. I., G. Del Real, M. Soto, L. Quijada, A. C. Martinez, C. Alonso, and J. M. Requena. 1998. Characterization of the immunostimulatory properties of Leishmania infantum HSP70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice. Infect. Immun. 66:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riggs, P. 2000. Expression and purification of recombinant proteins by fusion to maltose-binding protein. Mol. Biotechnol. 15:51-63. [DOI] [PubMed] [Google Scholar]
- 14.Seong, S.-Y., M.-S. Huh, W.-J. Jang, S.-G. Park, J.-G. Kim, S.-G. Woo, M.-S. Choi, I.-S. Kim, and W.-H. Chang. 1997. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect. Immun. 65:1541-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons, M., G. S. Murphy, and C. G. Hayes. 2001. Short report: antibody responses of mice immunized with a tetravalent dengue recombinant protein subunit vaccine. Am. J. Trop. Med. Hyg. 65:159-161. [DOI] [PubMed] [Google Scholar]
- 16.Simmons, M., G. S. Murphy, T. Kochel, K. Raviprakash, and C. G. Hayes. 2001. Characterization of antibody responses to combinations of a dengue-2 DNA and dengue-2 recombinant subunit vaccine. Am. J. Trop. Med. Hyg. 65:420-426. [DOI] [PubMed] [Google Scholar]
- 17.Simmons, M., W. M. Nelson, S. J. Wu, and C. G. Hayes. 1998. Evaluation of the protective efficacy of a recombinant dengue envelope B domain fusion protein against dengue 2 virus infection in mice. Am. J. Trop. Med. Hyg. 58:655-662. [DOI] [PubMed] [Google Scholar]
- 18.Spurlino, J. C., G. Y. Lu, and F. A. Quiocho. 1991. The 2.3- Å resolution structure of the maltose- or maltodextrin-binding protein, a primary receptor of bacterial active transport and chemotaxis. J. Biol. Chem. 266:5202-5219. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 20.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]