Abstract
The 42-kDa processed fragment of Plasmodium falciparum merozoite surface protein 1 (MSP-142) is a prime candidate for a blood-stage malaria vaccine. Merozoite surface protein 8 contains two C-terminal epidermal growth factor (EGF)-like domains that may function similarly to those of MSP-142. Immunization with either MSP-1 or MSP-8 induces protection that is mediated primarily by antibodies against conformation-dependent epitopes. In a series of comparative immunogenicity and efficacy studies using the Plasmodium yoelii rodent model, we tested the ability of recombinant P. yoelii MSP-8 (rPyMSP-8) to complement rPyMSP-1-based vaccines. Unlike MSP-1, PyMSP-8-dependent protection required immunization with the full-length protein and was not induced with recombinant antigens that contained only the C-terminal EGF-like domains. Unlike PyMSP-8, the immunogenicity of the PyMSP-1 EGF-like domains was low when present as part of the rPyMSP-142 antigen. Immunization with a mixture of rPyMSP-142 and rPyMSP-8 further inhibited the antibody response to protective epitopes of rPyMSP-142 and did not improve vaccine efficacy. To improve PyMSP-1 immunogenicity, we produced a chimeric antigen containing the EGF-like domains of PyMSP-1 fused to the N terminus of PyMSP-8. Immunization with the chimeric rPyMSP-1/8 antigen induced high and comparable antibody responses against the EGF-like domains of both PyMSP-1 and PyMSP-8. This enhanced MSP-1-specific antibody response and the concurrent targeting of MSP-1 and MSP-8 resulted in improved, nearly complete protection against lethal P. yoelii 17XL malaria. Unexpectedly, immunization with rPyMSP-1/8 failed to protect against challenge infection with reticulocyte-restricted P. yoelii 17X parasites. Overall, these data establish an effective strategy to improve the efficacy of P. falciparum MSP-based vaccines.
Morbidity and mortality due to Plasmodium falciparum and Plasmodium vivax, the two predominant human malaria parasites, result during the asexual development and replication of these protozoan parasites within erythrocytes (RBCs) (44). To reduce this burden on nearly half of the world's population, several malaria vaccine strategies are being pursued (28, 40, 53). Blood-stage vaccines are being developed to reduce parasite load and/or prevent life-threatening complications of malaria once parasites are replicating within RBCs. The single most feasible strategy for blood-stage malaria is to immunize with subunit vaccines that induce high titers of antibodies that neutralize extracellular merozoites and prevent the invasion of erythrocytes (2, 25, 31, 40). The multiple receptor-ligand interactions and alternate redundant pathways involved in the merozoite invasion of RBCs combined with the polymorphism of vaccine candidate antigens present a challenge for vaccine design (2, 25, 26).
P. falciparum merozoite surface protein 1 (MSP-1) (PfMSP-1) emerged during the 1980s as a viable blood-stage vaccine target. MSP-1 is an abundant component of the merozoite surface coat, is conserved across plasmodial species, and is essential for parasite growth (2, 25, 26, 31, 48). During schizont maturation and segmentation, MSP-1 is synthesized as a ∼195-kDa precursor protein that is proteolytically processed to form a multisubunit complex expressed on the surface of merozoites (33, 39, 42). MSP-142, the 42-kDa glycosylphosphatidylinositol-anchored component, is further cleaved near the time of invasion, leaving only a 19-kDa C-terminal domain on the merozoite surface (5). MSP-119 contains two highly conserved epidermal growth factor (EGF)-like domains, which are targets of protective antibodies and the major focus of the MSP-1 vaccine development effort (6, 11-13, 17-19, 24, 29, 30, 36, 37, 49, 60). However, by replacing the MSP-119 coding regions of P. falciparum with those of Plasmodium chabaudi, O'Donnell et al. (48) previously demonstrated that it is the conserved spatial structure of the MSP-1 EGF-like domains, not their primary amino acid sequence, that is essential for parasite growth.
Vaccines based on the two major alleles of PfMSP-142 that include both MSP-133 and MSP-119 subcomponents are currently in clinical trials (1, 47, 58). The MSP-133 processed fragment does not appear to be a primary target of neutralizing antibodies but can provide a source of parasite-specific T-cell epitopes. In previous studies involving nonhuman primates, the induction of high titers of protective antibodies required PfMSP-1-based vaccines that were formulated with complete Freund's adjuvant (6, 12, 19, 36, 37, 60). In those efficacy studies, alternate adjuvants with a potential for use in human subjects were tested but proved to be ineffective for PfMSP-1-based vaccines. Phase I safety and immunogenicity trials of PfMSP-142 (3D7 allele) formulated with AS02A (GlaxoSmithKline Biologicals), an oil-in-water emulsion containing both QS21 and 3-deacylated monophosphoryl lipid A, have been completed using malaria-naïve U.S. volunteers and semi-immune Kenyan adults (47, 58). However, the immunogenicity data and the relatively low activity of elicited antibodies in growth inhibition assays suggest that further improvements in the adjuvant, antigen conformation, and/or antigen allele may be required.
In an immunization-based screen to identify antigens that could complement MSP-1-based vaccines, our laboratory cloned and characterized merozoite surface protein 8 (MSP-8) in the rodent malaria parasite Plasmodium yoelii (7, 8). In P. yoelii, MSP-8 is a 46-kDa glycosylphosphatidylinositol-anchored protein that is expressed on the surface of trophozoites and merozoites and, like MSP-1, contains two C-terminal EGF-like domains. P. yoelii MSP-8 (PyMSP-8) expression is detectable throughout the entire erythrocytic life cycle, reaching a peak during trophozoite development (55). This pattern of expression overlaps with but is distinct from that of PyMSP-1. The putative receptor for PyMSP-8 on RBCs is sensitive to trypsin digestion but is resistant to treatment with chymotrypsin or neuraminidase. Most significantly, mice immunized with full-length recombinant PyMSP-8 (rPyMSP-8) are protected against lethal P. yoelii 17XL malaria (7, 56).
MSP-8 in P. falciparum (3, 4, 21, 22, 52) and P. vivax (51) has also been studied, and orthologues are present in the genome sequences of Plasmodium knowlesi, Plasmodium berghei, and P. chabaudi (www.PlasmoDB.org). MSP-8 is highly conserved throughout its protein sequence among P. falciparum isolates, with the C-terminal 250 amino acids, including the double EGF-like domains, being invariant (4). Unlike MSP-1, MSP-8 is not essential for the in vitro growth of P. falciparum blood-stage parasites (3, 21). However, it appears that the two EGF-like domains of MSP-1 and MSP-8, although quite different in primary sequence, are functionally similar. Chimeric P. falciparum merozoites whose MSP-1 EGF-like domains were replaced with those from MSP-8 of P. berghei showed no visible in vitro growth phenotype in comparison to nontransgenic, parental P. falciparum parasites (22).
In addition to increasing subunit vaccine immunogenicity, the above-mentioned data suggest that for a blood-stage malaria vaccine, it may be advantageous to simultaneously target the EGF-like domains of both MSP-1 and MSP-8. We tested this hypothesis. In this study, our initial efforts showed that coimmunization with a mixture of MSP-142 and full-length MSP-8 unexpectedly reduced the immunogenicity of MSP-142 and did not improve vaccine efficacy. However, this was not the case following immunization with a single chimeric antigen containing MSP-119 fused to full-length MSP-8. Herein, we present data demonstrating that immunization with a recombinant MSP-1/8 chimeric antigen (i) increases the immunogenicity of protective MSP-119 epitopes and (ii) dramatically increases protective efficacy against lethal blood-stage P. yoelii malaria.
MATERIALS AND METHODS
Mice and parasites.
Male BALB/cByJ mice, 5 to 6 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals were housed in the Animal Care Facility of Drexel University College of Medicine under specific-pathogen-free conditions. The lethal 17XL and nonlethal 17X strains of P. yoelii were originally obtained from William P. Weidanz (University of Wisconsin, Madison, WI).
Expression and purification of PyMSP-1 and PyMSP-8 recombinant proteins. (i) rPyMSP-142 and rPyMSP-8.
For the PyMSP-142 gene construct, a 1,080-bp fragment derived from the 3′ end of the P. yoelii 17XL MSP-1 gene (nucleotides 4378 to 5453; GenBank accession no. J04668) (38) was PCR amplified from P. yoelii 17XL genomic DNA using oligonucleotide primers 5′-GAACATATGCCAGAAAAAGATATT-3′ and 5′-TGAGGATCCCATTTAGCTGGAAGA-3′. To facilitate subcloning, NdeI and BamHI restriction sites were incorporated into the 5′ and 3′ primers, respectively. The amplified fragment was gel purified, digested with NdeI and BamHI, and ligated into NdeI/BamHI-digested pET-15b (Novagen, Madison, WI). Using the pET/T7 RNA polymerase expression system with the Escherichia coli BL21(DE3)(pLysS) host strain, a 42-kDa recombinant antigen that represents the C terminus of PyMSP-1, minus the hydrophobic anchor sequence, was produced. This recombinant PyMSP-142 contains 20 plasmid-encoded amino acids fused to its N terminus, including six histidine residues. rPyMSP-142 was purified from an insoluble fraction of bacterial lysate by Ni-nitrilotriacetic acid (NTA) affinity chromatography under denaturing conditions (9). To promote the formation of disulfide bonds, the denaturant was gradually removed by dialysis in the presence of reduced and oxidized glutathione (10:1 ratio) (54) as previously reported for rPyMSP-8 (7, 56) as well as MSP-142 and apical membrane antigen 1 (AMA-1) of P. chabaudi (9, 15). The expression and purification of full-length rPyMSP-8 from P. yoelii 17XL followed a similar protocol and has been previously described in detail (7, 55, 56).
(ii) GST-PyMSP-119 and GST-PyMSP-8C.
Previously published protocols for the expression and purification of the C-terminal portion of PyMSP-1 fused to Schistosoma japonicum glutathione S-transferase (GST) (GST-PyMSP-119) were followed (16). A similar approach for the generation of a comparable construct based on PyMSP-8, namely, GST-PyMSP-8C, was taken. Briefly, a 417-bp fragment encoding the 3′ end of the PyMSP-1 gene (nucleotides 5044 to 5460; GenBank accession no. J04668) (38) was PCR amplified from P. yoelii 17XL genomic DNA using oligonucleotides 5′-CCCGAATTCACATAGCCTCAATAGCTTTAA-3′ and 5′-CCCGAATTCTCCCATAAAGCTGGA-3′ as primers. Similarly, a 393-bp fragment encoding the C terminus of the PyMSP-8 gene (nucleotides 1021 to 1413; accession no. AY005132) (7) was PCR amplified using oligonucleotide primers 5′-ATGGATCCATAACTATACTTAATTTAGCAAATGGT-3′ and 5′-GGGAATTCAACTTGAACAATAAATACCATCTCC-3′. Each amplified fragment was inserted into the EcoRI site of the pGEX/2T expression vector (Amersham Biosciences, Piscataway, NJ), and the correct orientation was determined by restriction enzyme digestion. Fusion proteins were expressed using E. coli XL-1 Blue as the host strain (Stratagene, La Jolla, CA). Recombinant GST-PyMSP-119 and GST-PyMSP-8C were purified from the soluble lysate of isopropyl-β-d-thiogalactopyranoside (IPTG)-induced bacterial cells by affinity chromatography using a glutathione agarose column (Amersham Biosciences) as previously described (16, 17). For control immunizations, nonfused GST was purified as described above from E. coli transformed with the pGEX/2T vector containing no inserted DNA.
The concentrations of recombinant proteins were determined using the bicinchoninic acid protein assay (Pierce Biotechnology Inc., Rockford IL). Protein purity and conformation were assessed by Coomassie blue staining following sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) run under both reduced and nonreduced conditions. Corresponding immunoblots were probed with the monoclonal antibody mAb302 (41) for recombinant PyMSP-1 antigens and with rabbit anti-PyMSP-8 serum (7) for GST-PyMSP-8C. The yields of purified rPyMSP-142 and GST-PyMSP-119 were approximately 12 and 4 mg per liter of induced bacterial culture, respectively. The yields of purified rPyMSP-8 and GST-PyMSP-8C were both ∼1.5 mg per liter of induced bacterial culture.
Construction and expression of recombinant PyMSP-1/8 chimeric antigen.
A 414-bp fragment encoding PyMSP-119 was PCR amplified from plasmid pGEX/PyMSP-119 described above using oligonucleotides 5′-GCCCATATGCACATAGCCTCAATAGCTTTAAAC-3′ and 5′-CCCCATATGACCACCACCACCTCCCATAAAGCTGGAAGAACT-3′ as primers. A five-residue glycine spacer was incorporated into the 3′ primer. To facilitate subcloning, NdeI restriction sites were added to both the 5′ and 3′ primers. The amplified fragment was gel purified, digested with NdeI, and ligated into NdeI-digested plasmid pET-15/PyMSP-8. Plasmids with inserts were digested by EcoRV to examine the orientation of the inserted fragment. For expression, a clone with the correct orientation was transformed into Origami(DE3)(pLysS) E. coli cells, a K-12 derivative with mutations in both the thioredoxin reductase (trxB) and glutathione reductase (gor) genes to facilitate disulfide bond formation (Novagen, Madison, WI). The deduced amino acid sequence of the chimeric rPyMSP-1/8 protein contains 20 vector-encoded residues at the N terminus, including a six-His tag joined to PyMSP-119, followed by five glycine residues and then the full-length PyMSP-8. The predicted molecular mass of rPyMSP1/8 is 59.4 kDa.
Purification of recombinant PyMSP-1/8.
At the mid-log phase of growth, rPyMSP-1/8 expression was induced by the addition of IPTG to a final concentration of 1 mM, and incubation continued for 3 h at 37°C. Bacteria were harvested, washed, resuspended in lysis buffer (50 mM Tris-HCl [pH 8.0], 500 mM NaCl, 10 mM EDTA), and treated with lysozyme (0.5 mg/ml) for 2 h. Following lysis, viscosity was reduced by a brief sonication. The rPyMSP-1/8 antigen was purified from a soluble fraction of lysed bacteria obtained following centrifugation for 30 min at 40,000 × g. A 30% ammonium sulfate fraction of the initial lysate was dialyzed into a solution containing 20 mM Tris-HCl (pH 8.0) and 500 mM NaCl at 4°C overnight. The fraction was recovered from dialysis and cycled over a nickel-chelate affinity chromatography column at 4°C (Ni-NTA Superflow matrix; QIAGEN, Inc., Valencia, CA) under nondenaturing conditions. The column was washed and eluted, and fractions were examined by SDS-PAGE. Fractions containing rPyMSP-1/8 were combined and dialyzed into renaturation buffer (50 mM Tris-HCl [pH 8.3], 500 mM NaCl, 3 mM reduced glutathione, 0.3 mM oxidized glutathione) containing 4 M guanidine-HCl. The concentration of guanidine-HCl was gradually removed by dialysis in the presence of reduced and oxidized glutathione (54). Following dialysis into a solution containing 20 mM Tris-HCl (pH 8.0) and 500 mM NaCl, rPyMSP-1/8 was further purified on a second Ni-NTA affinity column under nondenaturing conditions. The final protein concentration and purity were determined as described above. The yield of purified rPyMSP-1/8 was approximately 0.5 to 1 mg per liter of induced bacterial culture.
Immunizations and experimental infections.
Groups of 4 to 10 BALB/cByJ mice were immunized subcutaneously (two sites) with various recombinant MSP proteins formulated with Quil A (25 μg; Accurate Chemical and Scientific Corporation, Westbury, NY) as an adjuvant. For each immunization, doses of recombinant antigen per mouse varied from 1 to 50 μg as indicated in Table 1 and the corresponding figure legends. For the combined formulation, rPyMSP-142 and rPyMSP-8 were mixed in saline with the adjuvant just prior to injection. Control groups were immunized with nonfused GST formulated with Quil A or with Quil A alone. In all experiments, mice received three immunizations at 3-week intervals with the same dose of antigen and adjuvant used for the priming immunization. Two weeks following the last immunization, all mice were challenged by intraperitoneal injection of 1 × 105 P. yoelii 17XL or P. yoelii 17X parasitized RBCs obtained from a donor mouse. Blood parasitemia was monitored by the enumeration of parasitized erythrocytes in thin tail blood smears stained with Giemsa stain. In accord with Institutional Animal Care and Use policy, P. yoelii 17XL infections were considered lethal when parasitemia exceeded 50%, at which time animals were euthanized.
TABLE 1.
Protection induced by immunization with PyMSP-1 and PyMSP-8 recombinant antigensa
| Antigen | Dose (μg/immunization)b | % Parasitemia on day 8 | Survival (no. of surviving mice/ total no. of mice)c |
|---|---|---|---|
| rPyMSP-8 | 1 | 16.9 ± 16.0d | 3/5e |
| 5 | 5.6 ± 3.3d | 5/5e | |
| 10 | 2.1 ± 2.2d | 9/10e | |
| 25 | 4.6 ± 3.1d | 5/5e | |
| GST-PyMSP-8C | 10 | 52.4 ± 17.9 | 0/14 |
| 25 | 47.9 ± 17.4 | 0/5 | |
| 50 | 39.6 ± 4.0 | 0/5 | |
| rPyMSP-142 | 1 | 25.0 ± 23.4d | 0/5 |
| 5 | 26.9 ± 22.3d | 1/5 | |
| 10 | 40.6 ± 18.5 | 1/9 | |
| 25 | 29.0 ± 20.8d | 3/10e | |
| GST-PyMSP-119 | 10 | 24.0 ± 23.1d | 6/15e |
| 25 | 8.5 ± 10.9d | 2/4e | |
| GST control | 10 | 64.5 ± 17.8 | 0/5 |
| 25 | 52.2 ± 15.1 | 0/5 | |
| 50 | 46.0 ± 22.5 | 0/4 | |
| Quil A control | 47.7 ± 16.8 | 0/28 |
Data compiled from six immunization and challenge experiments (four to five mice/group/experiment).
All animals were immunized three times at 21-day intervals with the indicated amount of each recombinant antigen formulated with Quil A as an adjuvant (25 μg/dose).
Clearance of P. yoelii 17XL blood-stage parasites, with maximum parasitemia not exceeding 50% in any animal.
Significantly reduced compared to Quil A controls (analysis of variance, P < 0.05).
Significantly different compared to Quil A controls, considering survival period and percent mortality (Mantel-Haenszel log rank test, P < 0.05).
ELISA.
Approximately 2 to 3 days prior to P. yoelii challenge infection, a small volume of serum was collected from immunized mice, and the titers of antigen-specific antibodies were measured by enzyme-linked immunosorbent assay (ELISA). For mice immunized with rPyMSP-142, titers were determined using wells coated with rPyMSP-142 or GST-PyMSP-119. For mice immunized with rPyMSP-8, titers were determined using wells coated with rPyMSP-8 or GST-PyMSP-8C. For mice immunized with the combination of rPyMSP-142 and rPyMSP-8 or with the chimeric rPyMSP-1/8, titers were determined using wells coated with each of the four recombinant MSP antigens. Antigen-coated wells (0.25 μg/well) were washed and blocked for 1 h with Tris-buffered saline (25 mM Tris-HCl [pH 8.0], 150 mM NaCl) containing 5% nonfat dry milk. Serial twofold dilutions of each serum sample in Tris-buffered saline-0.1% Tween 20 containing 1% bovine serum albumin were added to antigen-coated wells and incubated for 1 h at room temperature. Bound antibodies were detected using horseradish peroxidase-conjugated rabbit antibody specific for mouse immunoglobulin G (Zymed Laboratories, South San Francisco, CA). The mean absorbance of sera from adjuvant control mice (n = 5) was subtracted as the background. Titer was defined as the dilution of serum that yielded an optical density at 405 nm of 0.5. Hyperimmune sera obtained from mice that were infected three times with P. yoelii 17X were pooled and included in each assay as an internal reference to normalize the data between assays.
Statistical analysis.
The statistical significance of differences in antibody responses and in mean parasitemia between groups was calculated by analysis of variance. The significance of differences in lethality between groups was determined by the Mantel-Haenszel log rank test (GraphPad Prism 4.0; GraphPad Software Inc., San Diego, CA) considering percent survival and day of death postchallenge in nonsurviving animals.
RESULTS
Comparison of PyMSP-1 and PyMSP-8 vaccine efficacy against P. yoelii 17XL challenge infection.
Immunization with full-length rPyMSP-8 formulated with the Ribi adjuvant system, complete Freund's adjuvant and interleukin-12, or Quil A as an adjuvant induces comparable protection against P. yoelii 17XL malaria (7, 56). An initial series of immunization and challenge studies was conducted to compare the relative efficacy of immunization with various PyMSP-1 and PyMSP-8 recombinant antigens at several antigen doses. Quil A was selected as an adjuvant based on a large set of PyMSP-8 immunogenicity and efficacy data (56) and minimal adjuvant toxicity at the site of injection. Recombinant MSP antigens included rPyMSP-142, full-length rPyMSP-8, and two GST fusion proteins, GST-PyMSP-119 and GST-PyMSP-8C, that contained only the C-terminal EGF-like domains of PyMSP-1 and PyMSP-8, respectively. Protein purity and conformation were assessed by SDS-PAGE and immunoblot analysis. Coomassie-blue-stained gels of these recombinant MSP antigens run under reducing and nonreducing conditions are shown in Fig. 1. The corresponding immunoblots of nonreduced antigens to further evaluate conformation are shown using mAb302 specific for PyMSP-1 (Fig. 2A) and polyclonal rabbit antiserum raised against refolded rPyMSP-8 (Fig. 2B). Based on relative migration on SDS-polyacrylamide gels and the presence of a single, dominant reactive band on immunoblots, the recombinant PyMSP-1 and PyMSP-8 antigens appeared to be properly folded. BALB/cByJ mice were immunized and boosted twice with each MSP vaccine formulation prior to P. yoelii 17XL challenge infection. The results of six efficacy trials are summarized in Table 1.
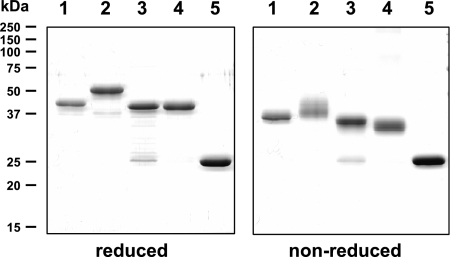
FIG. 1.
Purified recombinant PyMSP-1 and PyMSP-8 antigens. Coomassie-blue-stained SDS-polyacrylamide gels (12%) run under reducing (left panel) or nonreducing (right panel) conditions and containing (lane 1) rPyMSP-142 (3 μg), (lane 2) rPyMSP-8 (3 μg), (lane 3) GST-PyMSP-119 (3 μg), (lane 4) GST-PyMSP-8C (3 μg), and (lane 5) GST are shown. Molecular mass markers (M) are indicated.
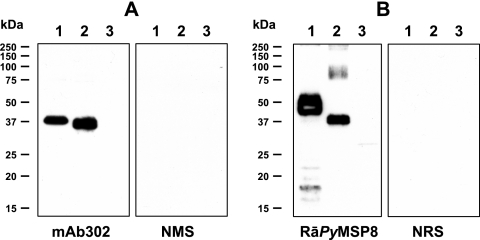
FIG. 2.
Immunoblot analysis of purified recombinant PyMSP-1 and PyMSP-8 antigens. (A) Immunoblots of 12% SDS-polyacrylamide gels containing (lane 1) rPyMSP-142 (0.1 μg), (lane 2) GST-PyMSP-119 (0.1 μg), and (lane 3) GST (0.1 μg) were probed with mAb302 that recognizes epitopes associated with the EGF-like domains of PyMSP-1 or with normal mouse serum (NMS). (B) Immunoblots of 12% SDS-polyacrylamide gels containing (lane 1) rPyMSP-8 (0.1 μg), (lane 2) GST-PyMSP-8C (0.1 μg), and (lane 3) GST (0.1 μg) were probed with polyclonal rabbit serum raised against refolded rPyMSP-8 or normal rabbit serum (NRS). Molecular mass markers are indicated.
Immunization with full-length rPyMSP-8 reproducibly protected mice against lethal P. yoelii 17XL malaria. In all groups of rPyMSP-8-immunized mice, mean parasitemia on day 8 of infection was significantly reduced (P < 0.05) relative to that of adjuvant controls, indicative of the delay in ascending parasitemia (Table 1). Furthermore, 90 to 100% of rPyMSP-8-immunized mice (5-, 10-, and 25-μg doses) survived a lethal P. yoelii 17XL challenge infection. Significant protection was noted following immunization with even as little as 1 μg of antigen/mouse/immunization. In contrast to full-length rPyMSP-8, immunization with GST-PyMSP-8C provided no protection against P. yoelii 17XL malaria, even with high antigen doses (50 μg/immunization). rPyMSP-142-immunized mice exhibited a significant but modest reduction in day 8 parasitemia and a 20 to 30% survival rate (P < 0.05), but protection was variable with respect to antigen dose. In contrast to MSP-8, immunization with GST-PyMSP-119 (10 to 25 μg/dose) partially protected (P < 0.05) mice to a level similar to that achieved with rPyMSP-142 (25 μg/dose). In mice immunized with recombinant PyMSP-1 or PyMSP-8 antigens, protection could not be readily correlated with the prechallenge titer of antibodies against the immunizing antigen (data not shown). No protection was ever observed in mice immunized with only the GST carrier protein formulated with Quil A or with Quil A alone. These data indicate that for any combined MSP-1 and MSP-8 vaccine formulation, immunization with full-length rPyMSP-8 will be required. In addition, rPyMSP-8-induced protection against lethal P. yoelii malaria was consistently better than that achieved by immunization with comparable doses of rPyMSP-142 or GST-PyMSP-119 when utilizing Quil A as an adjuvant.
Combined immunization with rPyMSP-142 and rPyMSP-8 does not improve protection against P. yoelii 17XL infection.
To test if a combined antigen formulation could improve overall vaccine efficacy, BALB/cByJ mice were immunized with rPyMSP-142 (10 μg), rPyMSP-8 (10 μg), or a combination of rPyMSP-142 and rPyMSP-8 (10 μg plus 10 μg) formulated with Quil A as adjuvant. As shown in Fig. 3A, rPyMSP-8-immunized mice were protected against an otherwise lethal P. yoelii 17XL challenge infection, with peak parasitemia occurring 12 to 16 days postchallenge, with a mean of 16.3% ± 14.6%. All mice immunized with Quil A alone developed fulminant, unremitting parasitemia by day 8 of infection. Immunization with rPyMSP-142 at 10 μg/dose did not afford any significant level of protection. Somewhat unexpectedly, no increase in protection was observed in mice immunized with the combination of rPyMSP-142 and rPyMSP-8. In fact, only three animals immunized with the combination controlled a lethal P. yoelii 17XL infection. Through day 14 of infection, the mean peak parasitemia in mice immunized with both rPyMSP-142 and rPyMSP-8 was 39.5% ± 13.0%, significantly higher than that in mice immunized with rPyMSP-8 only (P < 0.05). Two animals from the same group showed a delay in the onset of patent parasitemia but were sacrificed on day 14 when parasitemia exceeded 50%. Thus, protection against P. yoelii 17XL malaria induced by immunization with rPyMSP-8 alone was actually better than that achieved by immunization with the combination of rPyMSP-142 and rPyMSP-8.
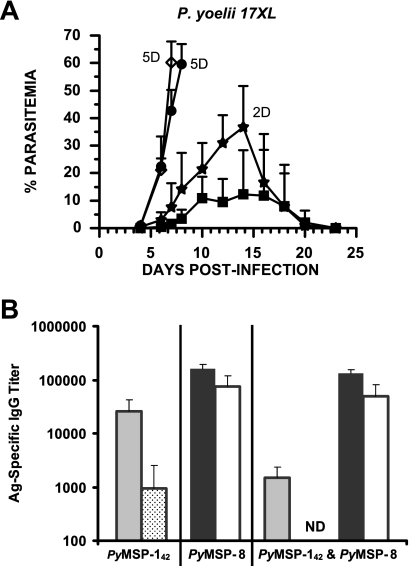
FIG. 3.
Coimmunization with rPyMSP-142 and rPyMSP-8 does not improve vaccine efficacy. (A) BALB/cByJ mice (n = 5) were immunized with rPyMSP-142 (•) (10 μg), rPyMSP-8 (▪) (10 μg), or rPyMSP-142 and rPyMSP-8 (★) (10 μg each) formulated with Quil A as an adjuvant or with Quil A alone (⋄). Two weeks following the third immunization, mice were challenged with 1 × 105 P. yoelii 17XL parasitized erythrocytes. The resulting parasitemia was monitored by enumerating parasitized RBCs in thin tail blood smears stained with Giemsa stain. D refers to the number of deceased animals at each time point. (B) Prechallenge antibody titers (means ± standard deviations) against rPyMSP-142 (░⃞), GST-PyMSP-119 (▩), rPyMSP-8 (▪), and GST-PyMSP-8C (□) determined by ELISA are shown. Immunization groups are indicated along the x axis. ND, none detected.
To determine if there were differences in the immunogenicities of rPyMSP-142 and rPyMSP-8 formulated alone or in combination, antibodies present in prechallenge immunization sera specific for rPyMSP-142 and rPyMSP-8 were measured by ELISA. To assess the response to conformational epitopes associated with the double EGF-like domains of each antigen, immunization-induced antibodies that were reactive with GST-PyMSP-119 and GST-PyMSP-8C were also measured. As shown in Fig. 3B, immunization with rPyMSP-8 induced a high level of antibodies against rPyMSP-8, a significant portion of which also bound to its C-terminal EGF-like domains. The overall antibody response induced by immunization with rPyMSP-142 was also strong but significantly less than that observed for rPyMSP-8 (P < 0.01). Distinct from that observed with PyMSP-8, a smaller proportion of the total anti-PyMSP-142 antibody induced recognized the EGF-like domains of PyMSP-119. Most importantly, the response to PyMSP-142 was markedly inhibited in mice immunized with the combination of rPyMSP-142 and rPyMSP-8, with little or no antibodies to protective PyMSP-119 epitopes detected (P < 0.05). These data are indicative of a significant level of competition between the two MSP vaccine antigens when formulated in combination and of the immunodominance of rPyMSP-8 over rPyMSP-142.
Production and analysis of a chimeric PyMSP-119 and PyMSP-8 vaccine.
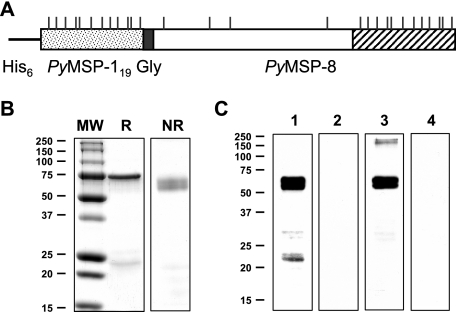
Immunization with rPyMSP-8 promoted a strong antibody response to B-cell determinants associated with its C-terminal EGF-like domains. This was not the case with rPyMSP-142, creating an obstacle for the combined antigen immunization. To focus the antibody response on the protective EGF-like domains of PyMSP-1 while continuing to provide malaria-specific T-cell help, a chimeric antigen gene was constructed (Fig. 4A). The expressed recombinant protein contained the protective EGF-like domains of MSP-1 (PyMSP-119) linked to the N terminus of full-length PyMSP-8 via a glycine spacer. The conformation of the purified chimeric antigen was assessed based on its migration on SDS-polyacrylamide gels (reduced versus nonreduced) and its corresponding immunoblot reactivity with mAb302, which recognizes a conformational epitope on the first EGF-like domain of PyMSP-119, and with polyclonal rabbit antibodies raised against refolded rPyMSP-8. As shown in Fig. 4B, chimeric rPyMSP-1/8 migrated as a predominant band of ∼60 kDa in the presence of 2-mercaptoethanol (reduced) and as a faster-migrating doublet in the absence of 2-mercaptoethanol (nonrecombinant). As shown by immunoblot analysis, the 60-kDa rPyMSP-1/8 was strongly reactive with the PyMSP-119-specific mAb302 and with polyclonal rabbit anti-refolded rPyMSP-8 serum (Fig. 4C). Higher-molecular-weight aggregates of refolded rPyMSP-1/8 run under nonreducing conditions were minimal. A smaller band of ∼22 kDa was detected by immunoblot using mAb302 under nonreducing conditions. This was not detected by the rabbit anti-PyMSP-8 serum, suggesting that the fragment is an N-terminal cleavage product containing PyMSP-119. Overall, the data indicate that for a high proportion of chimeric rPyMSP-1/8 proteins, the conformational epitopes of PyMSP-119 and PyMSP-8 appear to be intact.
FIG. 4.
Design, production, and analysis of a chimeric PyMSP-1 and PyMSP-8 vaccine. (A) Cartoon of the chimeric PyMSP-1/8 expression construct with sequences encoding the double EGF-like domains of PyMSP-119 (stippled box) followed by a five-glycine spacer (filled box) and the full-length PyMSP-8 including the C-terminal EGF-like domains (open and hatched boxes). Cysteine residues are indicated by vertical lines. (B and C) Coomassie-blue-stained SDS-polyacrylamide gel (12%) of purified rPyMSP1/8 (3 μg/lane) under reducing (R) or nonreducing (NR) conditions (B) and the corresponding immunoblot analysis of nonreduced rPyMSP-1/8 probed with (lane 1) PyMSP-119-specific mAB302, (lane 2) normal mouse serum, (lane 3) rabbit anti-rPyMSP-8 serum, or (lane 4) normal rabbit serum (C). Molecular weight markers (MW) (in thousands) are indicated.
Immunization with the chimeric rPyMSP-1/8 vaccine markedly enhances protection against P. yoelii 17XL malaria.
To compare immunogenicities and protective efficacies, groups of BALB/cByJ mice were immunized with rPyMSP-8 (10 μg) or with an equimolar dose of the chimeric rPyMSP-1/8 antigen (14 μg) formulated with Quil A as an adjuvant or with adjuvant alone. Two weeks after the third immunization, prechallenge serum samples were collected, and immunization-induced antibodies were quantitated by ELISA. Groups of mice were subsequently challenged with P. yoelii 17XL, the lethal strain that invades both normocytes and reticulocytes, or with nonlethal P. yoelii 17X parasites that preferentially invade reticulocytes.
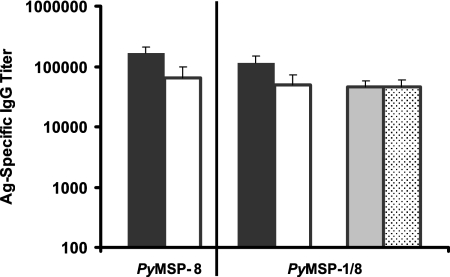
As shown in Fig. 5, immunization with rPyMSP-8 or rPyMSP-1/8 induced a high and comparable level of immunoglobulin G that recognized full-length PyMSP-8. In both groups of immunized animals, a high proportion of the total anti-PyMSP-8 antibody also recognized epitopes on the C-terminal EGF-like domains, as measured by reactivity with GST-PyMSP-8C. Most importantly, an equally high titer of antibodies was induced by rPyMSP-1/8 immunization, which recognized the protective EGF-like domains of PyMSP-1 as measured by ELISA using rPyMSP-142- or GST-PyMSP-119-coated plates. The quantity of antibodies recognizing PyMSP-119 epitopes that was induced by immunization with the chimeric rPyMSP-1/8 was ∼50-fold greater than that induced by immunization with rPyMSP-142 alone (Fig. 3B) (P < 0.01). Due to this marked improvement in PyMSP-119 immunogenicity, the previous problem with competition between MSP-1 and MSP-8 in mice immunized with an admixture of rPyMSP-142 and rPyMSP-8 was eliminated.
FIG. 5.
MSP-specific antibody response induced by immunization with the chimeric rPyMSP-1/8. BALB/cByJ mice (n = 5) were immunized and boosted twice with rPyMSP-8 or the chimeric rPyMSP-1/8 formulated with Quil A as an adjuvant. Serum samples were collected 2 weeks following the third immunization. Prechallenge antibody titers (means ± standard deviations) against rPyMSP-142 (░⃞), GST-PyMSP-119 (▩), rPyMSP-8 (▪), and GST-PyMSP-8C (□) determined by ELISA are shown. Immunization groups are indicated along the x axis. Ag, antigen; IgG, immunoglobulin G.
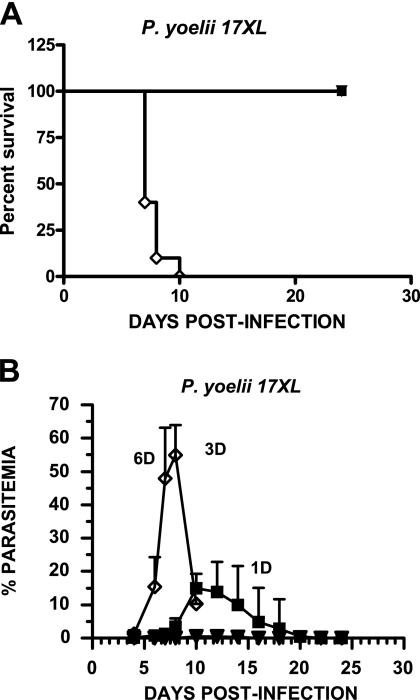
As shown in Fig. 6A, all mice immunized with either rPyMSP-8 or rPyMSP-1/8 survived lethal P. yoelii 17XL challenge, with all adjuvant control animals succumbing to infection by day 10. As measured by reductions in peak parasitemia in immunized and protected mice, the efficacy of immunization with chimeric rPyMSP-1/8 was markedly improved relative to that of rPyMSP-8. As shown in Fig. 6B, all mice immunized with rPyMSP-1/8 cleared P. yoelii 17XL parasites from circulation, with a remarkably low mean peak parasitemia of only 0.9% ± 0.8%. Mice immunized with rPyMSP-8, on the other hand, developed a significantly higher parasitemia, reaching a mean peak of 18.8% ± 7.7% between days 10 and 14 of infection before final parasite clearance (P < 0.01). Combined, the data clearly indicate that immunization with the chimeric rPyMSP-1/8 vaccine provided high, nearly complete protection against P. yoelii 17XL malaria. This level of protection could not be achieved by immunization with rPyMSP-142 alone, rPyMSP-8 alone, or a mixture of rPyMSP-142 and rPyMSP-8.
FIG. 6.
Chimeric PyMSP-1/8 vaccine induces solid protection against lethal P. yoelii 17XL malaria. BALB/cByJ mice (n = 10) were immunized with rPyMSP-8 (▪) (10 μg) or rPyMSP1/8 (▾) (14 μg) formulated with Quil A as an adjuvant or with Quil A alone (⋄). Two weeks following the third immunization, mice were challenged with 1 × 105 P. yoelii 17XL parasitized erythrocytes. Percent survival (A) and mean parasitemia (B) in each experimental group are plotted versus days postinfection. Parasitemia was monitored as described above. Similar data were obtained in two replicate experiments.
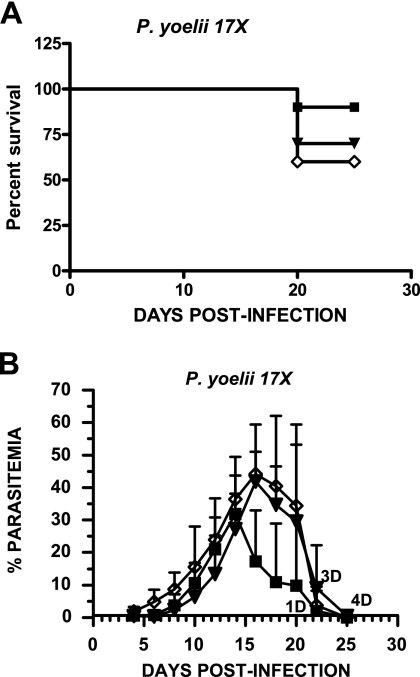
In contrast, mice immunized with rPyMSP-8 or chimeric rPyMSP-1/8 showed little or no reduction in parasitemia following challenge with the nonlethal, reticulocyte-restricted P. yoelii strain 17X (Fig. 7). Peak parasitemia in rPyMSP-8-immunized animals reached a mean of 34.7% ± 10.9%, somewhat lower than that of 47.9% ± 14.8% for Quil A control mice (P < 0.05). However, the mean peak parasitemia in rPyMSP-1/8-immunized mice of 44.4% ± 11.6% was not different from that of adjuvant controls (P > 0.05). Late during infection, animals in each experimental group that had not cleared parasites and were anemic were sacrificed. The difference in mortality between groups was not significant (P > 0.05). The inability to protect mice against reticulocyte-restricted blood-stage parasites using a highly efficacious vaccine that suppresses the growth of blood-stage parasites in mature RBCs must be further investigated.
FIG. 7.
Chimeric PyMSP-1/8 vaccine immunization fails to protect against nonlethal P. yoelii 17X malaria. BALB/cByJ mice (n = 10) were immunized with rPyMSP-8 (▪) (10 μg) or rPyMSP1/8 (▾) (14 μg) formulated with Quil A as an adjuvant or with Quil A alone (⋄). Two weeks following the third immunization, mice were challenged with the nonlethal, reticulocyte-restricted 17X strain of P. yoelii. Percent survival (A) and mean parasitemia (B) in each experimental group are plotted versus days postinfection. Parasitemia was monitored as described above.
DISCUSSION
Antibodies that recognize MSP-1 neutralize merozoites and inhibit invasion of erythrocytes in vitro (5, 13, 24, 49) and reduce parasitemia and disease severity in vivo (6, 11, 12, 17-19, 29, 30, 36, 37, 60). A significant effort has been made to apply this information to the development of a vaccine that is effective against diverse parasites in areas where malaria is endemic to reduce the burden of disease. Preclinical studies and clinical trials of the vaccine candidate PfMSP-142 continue to focus on overcoming issues relating to polymorphism and immunogenicity (40). To minimize the problem with strain variability, it may be possible to immunize with two recombinant PfMSP-142 proteins that represent the two major P. falciparum alleles of MSP-1 (57). Even so, overall efficacy is still a concern. Protection induced in Aotus monkeys by immunization with PfMSP-142 is only partial even when P. falciparum parasites expressing the homologous MSP-1 allelic type are used for the challenge infection (6, 12, 19, 36, 37, 57, 60). This has been attributed, in part, to the rather low immunogenicity of protein subunit vaccines observed in nonhuman primates. Efforts are ongoing to improve adjuvants that can be used safely for the immunization of human subjects within reasonable constraints of antigen dose and immunization frequency (47, 58). There is concern, however, that the titer and/or antiparasitic activity of PfMSP-142 antibodies induced by immunization with this FMP1/AS02A formulation will not be sufficient to protect against P. falciparum malaria.
Merozoite invasion of erythrocytes not only depends on MSP-1 but involves additional distinct receptor-ligand interactions. Targeting additional antigens involved in this process along with PfMSP-1 may improve the overall efficacy of merozoite-neutralizing vaccines. P. falciparum AMA-1 is one such antigen under consideration for use in combination with PfMSP-142 (14, 15, 34, 40, 46, 59). The functions of AMA-1 and MSP-1 during merozoite invasion of RBCs are not fully understood, but both are essential and appear to function independently of one another (26, 27, 45, 48). Testing of combined AMA-1 and MSP-1 vaccines in experimental models has begun (9, 10). While MSP-1 is essential, domain-swapping experiments with transgenic P. falciparum parasites also suggest that proteins other than AMA-1 may be able to partially compensate for the loss or inhibition of some MSP-1 functions (22, 48). This potential for functional redundancy has not been specifically considered in the design of merozoite-neutralizing vaccines.
With issues of immunogenicity and redundancy in mind, we became very interested in testing the ability of MSP-8 to enhance the efficacy of MSP-1-based vaccines. We initially identified MSP-8 as a vaccine antigen of interest based only on two criteria: its immunogenicity and its ability to protect mice against P. yoelii malaria (7, 8). Upon characterization, we learned that PyMSP-1 and PyMSP-8 had similarities in structure and pattern of expression, with each antigen displaying conformational B-cell epitopes that were targets of protective antibodies (2, 25, 31, 56). The domain-swapping experiments described previously by Drew et al. (22) specifically suggested that similar functions may be associated with the C-terminal EGF-like domains of MSP-1 and MSP-8. Combined, these characteristics suggested that the addition of MSP-8 to present MSP-1-based vaccines would be beneficial.
At the start of the study, we decided to select one adjuvant, Quil A, for our comparative immunogenicity studies. This selection was based on our previous vaccine studies with PyMSP-8 (7, 8, 56) and with MSP-142 and AMA-1 vaccines against P. chabaudi (9, 10). We recognize that other adjuvants may be preferable for the immunization of human subjects. In taking this approach, however, we eliminated adjuvant influence as a variable and focused our attention on the inherent immunogenicities of the two antigens when administered individually at various doses or in combination. The basic conclusion from our initial set of studies was that protective B-cell epitopes of PyMSP-8 were not restricted to the C-terminal EGF-like domains of the molecule. While immunization with full-length PyMSP-8 protected against lethal P. yoelii 17XL malaria, immunization with GST-PyMSP-8C could not. While conformational epitopes of PyMSP-8 are required for protection (56), these appear to include, at least in part, residues found outside of the C-terminal double EGF-like domains. This is in contrast to MSP-1 in that protective antibodies recognize epitopes associated primarily with MSP-119, a conclusion supported by extensive data in the literature (11, 17, 18, 29, 30, 41) and data obtained during this study.
The first problem that we encountered concerned differences in the immunogenicities of PyMSP-142 and PyMSP-8. In particular, we noted the less than optimal immunogenicity of protective PyMSP-119 epitopes when present as part of the larger PyMSP-142 recombinant antigen. This may not have been appreciated during previous vaccine studies of MSP-1 in the P. yoelii model, as most studies utilized GST-PyMSP-119 (17, 18, 29, 30). As it is known that the MSP-119 domain contains few CD4+ T-cell epitopes (23, 61), it was concluded that T cells specific for epitopes contained within the GST carrier provided adequate help for the production of high titers of protective antibodies. There are, in fact, very few studies that evaluated the immunogenicities of protective epitopes associated with PyMSP-119 when presented as part of PyMSP-142. However, the situation with the antibody response to PfMSP-142 vaccines may be similar to that observed for PyMSP-142 in the present study. Recent data suggest that immunization of human subjects with PfMSP-142 induced a greater proportion of antibodies that recognized epitopes outside of the critical PfMSP-119 domain (47). In a more quantitative analysis, only about 30% of antigen-specific antibodies induced in Aotus monkeys by PfMSP-142 immunization recognize protective PfMSP-119 epitopes (57). Thus, the antibody responses induced by immunization with recombinant MSP-142 in mice, monkeys, and humans are not optimally focused on the relevant protective epitopes.
The second problem that we encountered was that the immunogenicity of PyMSP-142 was further reduced when mice were immunized with the mixture of PyMSP-142 and PyMSP-8. At present, we cannot rule out the possibility that a direct interaction between PyMSP-142 and PyMSP-8 adversely affected the immunogenicity of PyMSP-1. However, with the combination, the response to PyMSP-8 was unaffected and remained high. This may not have been unexpected, since the potent immunogenicity of PyMSP-8 when present in a crude mixture of P. yoelii blood-stage antigens contributed to its initial identification (7, 8). However, the response to protective epitopes of PyMSP-119 was nearly completely inhibited when animals were immunized with PyMSP-142 and PyMSP-8 formulated in combination. Such competition between vaccine components has been observed with the inclusion of immunodominant thrombospondin-related anonymous protein of P. falciparum in multiple-antigen-containing pre-erythrocytic-stage vaccines (43). Overall, however, few studies have looked at interactions between blood-stage vaccine candidates when administered as part of multiple-antigen vaccine formulations. Our data raise some concerns regarding the immunogenicity of PfMSP-142 when present as part of multistage, multiple-antigen vaccines, as is anticipated (28, 40, 53, 57).
While the parallel PyMSP-142 and PyMSP-8 trials of immunogenicity and efficacy uncovered potential problems, an alternative strategy for a combined MSP-1 and MSP-8 immunization emerged and was tested. By immunization with the rPyMSP1/8 chimeric antigen, we were able to take advantage of the strong immunogenicity of PyMSP-8 while concurrently refocusing the anti-MSP-1 antibody response to the protective epitopes of PyMSP-119. This was possible without relying on the inclusion of epitopes from a heterologous fusion protein, maintaining a malaria-specific T-cell response that could potentially be boosted by natural infection. With the chimeric rPyMSP1/8, the immunogenicity of the PyMSP-8 component was again unchanged. However, the antibody response to PyMSP-119 was dramatically increased over that induced by immunization with PyMSP-142 alone. Most importantly, immunization with rPyMSP1/8 was highly efficacious, with full suppression of P. yoelii 17XL parasitemia shortly after challenge infection and with parasitemia in 7/10 animals not exceeding 1%. Due to the low parasitemia and quick suppression, the number of reticulocytes in circulation remained low. An additional and significant advantage for a comparable P. falciparum MSP-1/8 chimeric vaccine is the high degree of conservation of MSP-8 throughout its sequence among diverse P. falciparum strains (4). This level of conservation is not typical of other malaria vaccine candidate antigens currently under consideration, including PfMSP-142.
The data presented provide evidence that the potency of MSP-1-based vaccines can be markedly increased by means other than adjuvant selection. We believe that the dramatic increase in the efficacy of the chimeric P. yoelii MSP-1/8 vaccine was due in part to the presence of strong, parasite-specific CD4+ T-cell epitopes resulting in an increase in the immunogenicity of MSP-119 and to the concurrent targeting of both MSP-1 and MSP-8 antigens. Due to slight differences in conformations and/or a direct interaction between MSP-119 and MSP-8, it is also possible that mice immunized with the chimeric P. yoelii MSP-1/8 vaccine produced a population of protective antibodies not elicited by immunization with P. yoelii MSP-142 or PyMSP-8 alone. Drew et al. (21) previously proposed that PfMSP-8 may function in the establishment of the parasitophorous vacuole in early-ring-stage parasites. In that study, PfMSP-8 expression was detected on ring-stage parasites but not on the surface of merozoites by immunofluorescent assay using an antibody raised against the C-terminal EGF-like domains. We can detect MSP-8 on P. falciparum (J. Burns, Jr., et al., unpublished data) and on P. yoelii (7, 55) merozoites but only with antibodies with specificities lying outside of the EGF-like domains. It is possible that the EGF-like domains of MSP-8 are not exposed or are only transiently exposed on merozoites prior to invasion. However, antibodies to other accessible determinants of MSP-8 other than those restricted to the C-terminal EGF-like domains may also contribute to protection, a suggestion supported by our immunization studies comparing PyMSP-8 and GST-PyMSP-8C.
Surprisingly, this highly efficacious, chimeric, MSP-based vaccine that fully suppressed the replication of normocyte-associated parasites provided little control of parasite replication in reticulocytes. Mice immunized with chimeric rPyMSP-1/8 showed no reduction in parasitemia following challenge with the reticulocyte-restricted P. yoelii 17X parasites. We have reported a similar finding for rPyMSP-8-immunized mice that could not be attributed to antigen polymorphism, the loss of MSP-8 expression, or an inability of PyMSP-8 to bind to the surface of uninfected reticulocytes (55, 56). Studies of native PyMSP-1-induced protection against P. yoelii 17X completed many years ago also showed little efficacy (32). We and others have suggested that the localization of parasites to erythropoietic tissues in vivo may be necessary for efficient merozoite invasion of reticulocytes (20, 56). This could partially explain how reticulocyte-restricted parasites might avoid merozoite-neutralizing antibodies. While this remains to be established, it is clear that we do not fully understand the factors that contribute to merozoite invasion of reticulocytes in vivo. These key questions must be addressed to improve MSP-based vaccines against P. falciparum, which can invade mature RBCs or reticulocytes (35, 50), and those aiming to control reticulocyte-restricted P. vivax parasites (25).
Acknowledgments
We thank Amy Cernetich-Ott and Anisha Mistry for technical assistance with these studies.
This work was supported by NIH-NIAID grant R01AI35661.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Angov, E., B. M. Aufiero, A. M. Turgeon, M. Van Handenhove, C. F. Ockenhouse, K. E. Kester, D. S. Walsh, J. S. McBride, M. C. Dubois, J. Cohen, J. D. Haynes, K. H. Eckels, D. G. Heppner, W. R. Ballou, C. L. Diggs, and J. A. Lyon. 2003. Development and pre-clinical testing of a Plasmodium falciparum merozoite surface protein-142 malaria vaccine. Mol. Biochem. Parasitol. 128:195-204. [DOI] [PubMed] [Google Scholar]
- 2.Berzins, K. 2002. Merozoite antigens involved in invasion. Chem. Immunol. 80:125-143. [DOI] [PubMed] [Google Scholar]
- 3.Black, C. G., T. Wu, L. Wang, A. E. Topolska, and R. L. Coppel. 2005. MSP-8 is a non-essential merozoite surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 144:27-35. [DOI] [PubMed] [Google Scholar]
- 4.Black, C. G., T. Wu, L. Wang, A. R. Hibbs, and R. L. Coppel. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 114:217-226. [DOI] [PubMed] [Google Scholar]
- 5.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burghaus, P. A., B. T. Wellde, T. Hall, R. L. Richards, A. F. Egan, E. M. Riley, W. R. Ballou, and A. A. Holder. 1996. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum does not induce protection against challenge infection. Infect. Immun. 64:3614-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, J. M., Jr., C. C. Belk, and P. D. Dunn. 2000. A protective glycosylphosphatidylinositol-anchored membrane protein of Plasmodium yoelii trophozoites and merozoites contains two epidermal growth factor-like domains. Infect. Immun. 68:6189-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns, J. M., Jr., P. D. Dunn, and D. M. Russo. 1997. Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect. Immun. 65:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns, J. M., Jr., P. R. Flaherty, M. M. Romero, and W. P. Weidanz. 2003. Immunization against Plasmodium chabaudi malaria using combined formulations of apical membrane antigen-1 and merozoite surface protein-1. Vaccine 21:1843-1852. [DOI] [PubMed] [Google Scholar]
- 10.Burns, J. M., Jr., P. R. Flaherty, P. Nanavati, and W. P. Weidanz. 2004. Protection against Plasmodium chabaudi malaria induced by immunization with apical membrane antigen-1 and merozoite surface protein-1 in the absence of IFN-γ or IL-4. Infect. Immun. 72:5605-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns, J. M., Jr., W. R. Majarian, J. F. Young, T. M. Daly, and C. A. Long. 1989. A protective monoclonal antibody recognizes an epitope in the C-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J. Immunol. 143:2670-2676. [PubMed] [Google Scholar]
- 12.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, R. T. Yokota, and G. S. N. Hui. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chappel, J. A., and A. A. Holder. 1993. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognize the first growth factor-like domain of merozoite surface protein-1. Mol. Biochem. Parasitol. 60:303-311. [DOI] [PubMed] [Google Scholar]
- 14.Collins, W. E., P. Pye, P. E. Crewther, K. L. Vandenberg, G. G. Galland, A. J. Sulzer, D. J. Kemp, S. J. Edwards, R. L. Coppel, J. S. Sullivan, C. L. Morris, and R. A. Anders. 1994. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51:711-719. [DOI] [PubMed] [Google Scholar]
- 15.Crewther, P. E., M. L. S. M. Matthew, R. H. Flegg, and R. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 18.Daly, T. M., and C. A. Long. 1996. Influence of adjuvant on protection induced by a recombinant fusion protein against malaria infection. Infect. Immun. 64:2602-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darko, C. A., E. Angov, W. E. Collins, E. S. Bergmann-Leitner, A. S. Girouard, S. L. Hitt, J. S. McBride, C. L. Diggs, A. A. Holder, C. A. Long, J. W. Barnwell, and J. A. Lyon. 2005. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 73:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.del Portillo, H. A., M. Lanzer, S. Rodriguez-Malaga, F. Zavala, and C. Fernandez-Becerra. 2004. Variant genes and the spleen in Plasmodium vivax malaria. Int. J. Parasitol. 34:1547-1554. [DOI] [PubMed] [Google Scholar]
- 21.Drew, D. R., P. R. Sanders, and B. S. Crabb. 2005. Plasmodium falciparum merozoite surface protein 8 is a ring-stage membrane protein that localizes to the parasitophorous vacuole of infected erythrocytes. Infect. Immun. 73:3912-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drew, D. R., R. A. O'Donnell, B. J. Smith, and B. S. Crabb. 2004. A common cross-species function for the double epidermal growth factor-like modules of the highly divergent plasmodium surface proteins MSP-1 and MSP-8. J. Biol. Chem. 279:20147-20153. [DOI] [PubMed] [Google Scholar]
- 23.Egan, A., M. Waterfall, M. Pinder, A. Holder, and E. M. Riley. 1997. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence of poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65:3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP119, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galinsky, M. R., A. R. Dluzewski, and J. W. Barnwell. 2005. A mechanistic approach to merozoite invasion of red blood cells: merozoite biogenesis, rupture, and invasion of erythrocytes, p. 113-168. In I. W. Sherman (ed.), Molecular approaches to malaria. ASM Press, Washington, DC.
- 26.Gaur, D., D. C. Mayer, and L. H. Miller. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413-1429. [DOI] [PubMed] [Google Scholar]
- 27.Healer, J., V. Murphy, A. N. Hodder, R. Masciantonio, A. W. Gemmill, R. F. Anders, A. F. Cowman, and A. Batchelor. 2004. Allelic polymorphisms in apical membrane antigen-1 are responsible for evasion of antibody-mediated inhibition in Plasmodium falciparum. Mol. Microbiol. 52:159-168. [DOI] [PubMed] [Google Scholar]
- 28.Heppner, D. G., Jr., K. E. Kester, C. F. Ockenhouse, N. Tornieporth, O. Ofori, J. A. Lyon, V. A. Stewart, P. Dubois, D. E. Lanar, U. Krzych, P. Moris, E. Angov, J. F. Cummings, A. Leach, B. T. Hall, S. Dutta, R. Schwenk, C. Hillier, A. Barbosa, L. A. Ware, L. Nair, C. A. Darko, M. R. Withers, B. Ogutu, M. E. Plohemus, M. Fukuda, S. Pichyangkul, M. Gettyacamin, C. Diggs, L. Soisson, J. Milman, M. C. Dubois, N. Garcon, K. Tucker, J. Wittes, C. V. Plowe, M. A. Thera, O. K. Duombo, M. G. Pau, J. Goudsmit, W. R. Ballou, and J. Cohen. 2005. Towards an RTS,S-based, multi-stage antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine 23:2243-2250. [DOI] [PubMed] [Google Scholar]
- 29.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Good, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 30.Hirunpetcharat, C., P. Vukovic, X. Q. Liu, D. C. Kaslow, L. H. Miller, and M. F. Good. 1999. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J. Immunol. 162:7309-7314. [PubMed] [Google Scholar]
- 31.Holder, A. A. 1996. Preventing merozoite invasion of erythrocytes, p. 77-104. In S. L. Hoffman (ed.), Malaria vaccine development: a multi-immune response approach. ASM Press, Washington, DC.
- 32.Holder, A. A., and R. R. Freeman. 1984. Protective antigens of rodent and human blood stage malaria. Philos. Trans. R. Soc. Lond. B 307:171-177. [DOI] [PubMed] [Google Scholar]
- 33.Holder, A. A., and R. R. Freeman. 1984. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J. Exp. Med. 160:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70:6948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitchen, S. F. 1939. The infection of mature and immature erythrocytes by Plasmodium falciparum and Plasmodium malariae. Am. J. Trop. Med. 19:47-62. [Google Scholar]
- 36.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, K. A. Perdue-Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:325-332. [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis, A. P. 1989. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol. Biochem. Parasitol. 36:271-282. [DOI] [PubMed] [Google Scholar]
- 39.Lyon, J. A., R. H. Geller, J. D. Haynes, and J. L. Weber. 1986. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc. Natl. Acad. Sci. USA 83:2989-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahanty, S., A. Saul, and L. H. Miller. 2003. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J. Exp. Biol. 206:3781-3788. [DOI] [PubMed] [Google Scholar]
- 41.Majarian, W. R., T. M. Daly, W. P. Weidanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131-3137. [PubMed] [Google Scholar]
- 42.McBride, J. S., and H. G. Heidrich. 1987. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 43.McConkey, S. J., W. H. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. M. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. S. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 6:729-735. [DOI] [PubMed] [Google Scholar]
- 44.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell, G. H., A. W. Thomas, G. Margos, A. R. Dluzewski, and L. H. Bannister. 2004. Apical membrane antigen 1, a major malaria merozoite vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect. Immun. 72:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullen, G. E., B. K. Giersing, O. Ajose-Popoola, H. L. Davis, C. Kothe, H. Zhou, J. Aebig, G. Dobrescue, A. Saul, and C. A. Long. 2006. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligonucleotide. Vaccine 24:2497-2505. [DOI] [PubMed] [Google Scholar]
- 47.Ockenhouse, C. F., E. Angov, K. E. Kester, C. Diggs, L. Soisson, J. F. Cummings, A. V. Stewart, D. R. Palmer, B. Mahajan, U. Krzych, N. Tornieporth, M. Delchambre, M. Vanhandenhove, O. Ofori-Anyinam, J. Cohen, J. A. Lyon, D. G. Heppner, and the MSP-1 Working Group. 2006. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine 24:3009-3017. [DOI] [PubMed] [Google Scholar]
- 48.O'Donnell, R. A., A. Saul, A. F. Cowman, and B. S. Crabb. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6:91-95. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-119 are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasvol, G., D. J. Weatherall, and R. J. M. Wilson. 1980. The increased susceptibility of young red cells to invasion by the malarial parasite Plasmodium falciparum. Br. J. Haematol. 45:285-295. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Leal, O., A. Y. Sierra, C. A. Barrero, C. Moncada, P. Martinez, J. Cortes, Y. Lopez, E. Torres, L. M. Salazar, and M. A. Patarroyo. 2004. Plasmodium vivax merozoite surface protein 8 cloning, expression, and characterization. Biochem. Biophys. Res. Commun. 324:1393-1399. [DOI] [PubMed] [Google Scholar]
- 52.Puentes, A., J. García, M. Ocampo, L. Rodriguez, R. Vera, H. Curtidor, R. Lopez, J. Suarez, J. Valbuena, M. Vanegas, F. Guzman, D. Tovar, and M. E. Patarroyo. 2003. P. falciparum: merozoite surface protein-8 peptides bind specifically to human erythrocytes. Peptides 24:1015-1023. [DOI] [PubMed] [Google Scholar]
- 53.Richie, T. L., and A. Saul. 2002. Progress and challenges for malaria vaccines. Nature 415:694-701. [DOI] [PubMed] [Google Scholar]
- 54.Scheele, G., and R. Jacoby. 1982. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J. Biol. Chem. 257:12277-12282. [PubMed] [Google Scholar]
- 55.Shi, Q., A. Cernetich-Ott, M. Lynch, and J. M. Burns, Jr. 2006. Expression, localization and erythrocyte binding activity of Plasmodium yoelii merozoite surface protein-8. Mol. Biochem. Parasitol. 149:231-241. [DOI] [PubMed] [Google Scholar]
- 56.Shi, Q., A. Cernetich, T. M. Daly, G. Galvan, A. B. Vaidya, L. W. Bergman, and J. M. Burns, Jr. 2005. Alteration in host cell tropism limits the efficacy of immunization with a surface protein of malaria merozoites. Infect. Immun. 73:6363-6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh, S., K. Miura, H. Zhou, O. Muratova, B. Keegan, A. Miles, L. B. Martin, A. J. Saul, L. H. Miller, and C. A. Long. 2006. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect. Immun. 74:573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoute, J. A., J. Gombe, M. R. Withers, J. Siangla, D. McKinney, M. Onyango, J. F. Cummings, J. Milman, K. Tucker, L. Soisson, V. A. Stewart, J. A. Lyon, E. Angov, A. Leach, J. Cohen, K. E. Kester, C. F. Ockenhouse, C. A. Holland, C. L. Diggs, J. Wittes, D. G. Heppner, Jr., and the MSP-1 Malaria Vaccine Working Group. 2007. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine 25:176-184. [DOI] [PubMed] [Google Scholar]
- 59.Stowers, A. W., M. C. Kennedy, B. P. Keegan, A. Saul, C. A. Long, and L. H. Miller. 2002. Vaccination of monkeys with recombinant P. falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect. Immun. 70:6961-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratova, D. C. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udhayakumar, V., D. Anyona, S. Kariuki, Y. P. Shi, P. B. Bloland, O. H. Branch, W. Weiss, B. L. Nahlen, D. C. Kaslow, and A. A. Lal. 1995. Identification of T and B cell epitopes recognized by humans in the C-terminal 42 kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J. Immunol. 154:6022-6030. [PubMed] [Google Scholar]