Abstract
The facultative intracellular bacterium Francisella tularensis is capable of causing systemic infections in various hosts, including mice and humans. The liver is a major secondary site of F. tularensis infection, but hepatic immune responses to the pathogen remain poorly defined. Immune protection against the pathogen is thought to depend on the cytokine gamma interferon (IFN-γ), but the cellular basis for this response has not been characterized. Here we report that natural killer cells from the livers of naïve uninfected mice produced IFN-γ when challenged with live bacteria in vitro and that the responses were greatly increased by coactivation of the cells with either recombinant interleukin-12 (IL-12) or IL-18. Moreover, the two cytokines had strong synergistic effects on IFN-γ induction. Neutralizing antibodies to either IL-12 or IL-18 inhibited IFN-γ production in vitro, and mice deficient in the p35 subunit of IL-12 failed to show IFN-γ responses to bacterial challenge either in vitro or in vivo. Clinical isolates of highly virulent type A Francisella tularensis subsp. tularensis organisms were comparable to the live attenuated vaccine strain of Francisella tularensis subsp. holarctica in their ability to induce IL-12 and IFN-γ expression. These findings demonstrate that cells capable of mounting IFN-γ responses to F. tularensis are resident within the livers of uninfected mice and depend on coactivation by IL-12 and IL-18 for optimum responses.
Francisella tularensis is a small, gram-negative, facultative intracellular bacterium that causes zoonotic infections and leads to cutaneous, pulmonary, gastrointestinal, or systemic tularemia. Routes of transmission include tick bites, ingestion of contaminated food or water, contact with infected carcasses, or exposure to contaminated aerosols. Fewer than 20 viable type A (Francisella tularensis subsp. tularensis) organisms can cause lethal disease (11), and secondary colonization of the liver, spleen, and lungs by F. tularensis is common (13, 22, 40). Most research on immunity to F. tularensis has been performed on mice with the live vaccine strain (LVS), which was produced by the serial passage of a Francisella tularensis subsp. holarctica type B strain. This organism shows attenuated virulence in human beings but is lethal in mice.
Gamma interferon (IFN-γ) is known to play a central role in protective host responses to F. tularensis in both immunized and naïve animals. Mice treated with neutralizing antibodies to IFN-γ (2, 18, 29) or animals lacking a functional IFN-γ gene (16, 19) fail to efficiently clear the organism and/or succumb to challenge doses of F. tularensis that are considerably lower than those that are lethal for wild-type mice. One of the potential roles of IFN-γ in an F. tularensis infection is the activation of macrophages. F. tularensis has been found to replicate within resting macrophages (21), presumably after escaping the phagosome and entering the cytosol (24), but the survival of the pathogen is limited in macrophages activated with IFN-γ (3, 8, 21, 36).
Mice infected with F. tularensis show high levels of circulating IFN-γ, and many infected tissues express mRNA for the cytokine (10, 25). Unlike the lipopolysaccharides of most gram-negative bacterial species, F. tularensis lipopolysaccharide does not appear to induce IFN-γ production (15), and the microbial components recognized by the host remain largely unknown. Interleukin-12 (IL-12) is an important coactivating signal for IFN-γ induction by many microbes (23, 32, 33, 45), including F. tularensis (17), and recombinant IL-12 (rIL-12) has been found to increase the survival of F. tularensis-infected mice, an effect that was dependent on IFN-γ (16, 35). Based on studies of F. tularensis-immunized humans, T cells can be activated by the pathogen to produce IFN-γ (41, 42). However, T cells may not be the only cell type capable of producing the cytokine in response to F. tularensis, especially in naïve animals. Lopez et al. (30) showed that natural killer (NK) cells isolated from the lungs of infected mice, when restimulated in vitro with phorbol ester and ionomycin, expressed intracellular IFN-γ. Gosselin et al. (26) demonstrated that depletion of NK cells from human peripheral blood mononuclear cells decreased IFN-γ production in response to F. tularensis. Taken together, these findings indicate that a variety of lymphocyte subsets may participate in the protective IFN-γ response to F. tularensis and derive coactivating signals from cytokines that include, but may not be limited to, IL-12.
In addition to infecting the numerous resident macrophages (Kupffer cells) found in the liver, hepatocytes support productive replication of F. tularensis (13). Hepatotoxicity and severe liver dysfunction can result, which probably contributes substantially to the morbidity and mortality seen for systemic tularemia. Despite the importance of hepatic damage in this disease, relatively little is known about the cellular immune responses to F. tularensis in the liver. In the present study we have used a cell culture approach to characterize the lymphocytes from the livers of naïve mice that produce IFN-γ and to identify the coactivating cytokine signals that are required for this response. We have also asked whether or not the immune response to the attenuated live vaccine strain, LVS, differs from responses to virulent type A strains (F. tularensis subsp. tularensis). In particular, we wished to determine whether type A strains have evolved mechanisms for inhibiting the production of IFN-γ as a means of evading protective immunity in the mouse.
MATERIALS AND METHODS
Reagents.
The following fluorochrome-labeled antibodies were used in this study: allophycocyanin (APC)-conjugated mouse anti-mouse NK1.1 (BD Biosciences, San Jose, CA), R-phycoerythrin-Cy7 (PE-Cy7)-conjugated hamster anti-mouse CD3ɛ (BD Biosciences), and PE-conjugated rat anti-mouse IFN-γ (BD Biosciences). Neutralizing antibodies included rat anti-mouse IL-12 (p40/p70) (BD Biosciences), rat anti-mouse IL-18 (Medical & Biological Laboratories, Woburn, MA), rat anti-mouse IL-23 (eBioscience, San Diego, CA), and rat anti-mouse IL-6 (BioLegend, San Diego, CA). Normal rat IgG2a(κ) (BD Biosciences) served as an isotype control for the neutralization studies. Recombinant cytokines included mouse IL-12 (R&D Systems, Minneapolis, MN), granulocyte-macrophage colony-stimulating factor (R&D Systems), and IL-18 (Invitrogen, Carlsbad, CA).
Mice.
Six- to 10-week-old wild-type female C57BL/6J (B6) mice and IL-12p35-deficient B6.129S1-Il12atm1Jm/J male mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and maintained on a cycle of 12 h of light and 12 h of darkness with food and water ad libitum. All protocols were approved by the University of Kansas Medical Center Animal Care and Use Committee.
Growth of F. tularensis LVS and conditions of infection.
The LVS of F. tularensis subsp. holarctica was obtained from Jeannine Petersen (Centers for Disease Control and Prevention, Fort Collins, CO). A small sample from frozen stock was streaked onto a chocolate agar plate (Remel, Lenexa, KS) and incubated at 37°C for 3 days. An isolated colony was then spread across a second plate, and a lawn of bacteria was grown overnight at 37°C. The entire lawn of growth was inoculated into a small starter culture in supplemented Mueller-Hinton broth prepared as described previously (4, 20). After overnight incubation at 37°C, the culture was diluted approximately 1:10 in supplemented Mueller-Hinton broth and incubated at 37°C for as long as 48 h until it reached an A600 of 0.3 to 0.4. Aliquots were stored frozen without glycerol at −70°C for as long as 3 months. For use in vitro, aliquots of the bacteria were thawed rapidly at 37°C, washed once with Dulbecco's phosphate-buffered saline (DPBS), and resuspended in tissue culture medium without antibiotics. For in vivo experiments, the aliquots of bacteria were similarly thawed and washed but were resuspended in DPBS for injection. Mice were challenged by the intraperitoneal (i.p.) route with F. tularensis LVS, which results in colonization of the liver within 12 h and significant IFN-γ production in the liver within 15 h (data not shown).
F. tularensis subsp. tularensis strains.
F. tularensis clinical isolates KU49 and KU54 were acquired from patient specimens collected at the University of Kansas Medical Center between 1986 and 1990 and stored at −70°C in rabbit blood. For the current study, the strains were grown in the same fashion as described above for F. tularensis LVS. To identify their subspecies, a sample of DNA from each strain was prepared and analyzed by PCR by Guillermo Madico (Boston University). The following species- or subspecies-specific primers were used: hyp4 (F. tularensis subsp. tularensis), pdpDh (F. tularensis subsp. holarctica), hypR (Francisella tularensis subsp. mediasiatica and F. tularensis subsp. tularensis), pdpDn (Francisella novicida), and hyp6 (F. tularensis subsp. tularensis and Francisella philomiragia). Strains KU49 and KU54 were designated type A (F. tularensis subsp. tularensis) based on the amplification of PCR products with primers hyp4, hypR, and hyp6 but not with primer pdpDh or pdpDn.
Preparation and culture of HMC.
Livers were aseptically removed from mice and washed once in Hanks' buffered saline solution (HBSS) containing 10 mM HEPES. The liver pieces were then transferred to a digestion buffer (HBSS, 10 mM HEPES, 80 μg/ml DNase [Roche, Indianapolis, IN], 50 μg/ml Liberase [Roche]) and minced finely with scissors. The liver suspension was transferred to a stomacher bag (Seward, Thetford, United Kingdom), which was sealed and incubated for 15 min at 37°C with shaking. The bag was then placed in an aerosol-free tissue homogenizer (Stomacher 80 Biomaster; Seward), and mechanical homogenization was continued for 15 min at room temperature. Digestion was stopped by a threefold dilution with HBSS lacking Ca2+ and Mg2+ but containing 10 mM HEPES and 10% fetal bovine serum (FBS). The liver cell suspension was transferred to a tube, and a solution of 28% Percoll was carefully added beneath the cell suspension. Tubes were then centrifuged at room temperature (1,200 × g for 25 min) to isolate the mononuclear cells. The supernatant containing debris and Percoll was discarded, and the pellet was resuspended in prewarmed ACK lysis buffer (Cambrex, Walkersville, MD). Incubation was continued at 37°C for 3 min to lyse contaminating erythrocytes. The hepatic mononuclear cells (HMC) were washed at 300 × g for 10 min in complete tissue culture medium (RPMI 1640, 10 mM HEPES, 10% FBS), counted, and plated in 96-well microtiter plates at a density of 150,000 or 200,000 cells per well. In experiments involving anti-cytokine antibodies, the antibodies were added to the HMC cultures just before the addition of F. tularensis. Cells were challenged with live F. tularensis organisms for 2 h at 37°C under a humid atmosphere of 5% CO2, and gentamicin was then added at a final concentration of 50 μg/ml. In some cases, recombinant cytokines were added with the antibiotic. The cultures were then incubated at 37°C under 5% CO2 for 24 h, and culture supernatant fluids were collected and stored at −70°C until the assay.
Preparation and staining of cells for flow cytometry analysis.
Hepatic mononuclear cells from infected mice that were to be analyzed by flow cytometry were prepared as described above with the following modifications. Brefeldin A (GolgiPlug; BD Biosciences) at a concentration of 0.2 μl per ml was added to all buffers used for preparing the cells. After erythrocyte lysis with ACK buffer, the HMC were washed, resuspended in a tissue culture medium containing penicillin (100 U/ml), streptomycin (100 μg/ml), and GolgiPlug (1 μl per ml of medium), and incubated at 37°C (5% CO2) for 3 h in petri dishes. For staining of HMC activated in vitro, brefeldin A was added for the final 5 h of a 15-h culture. The cells were then washed, resuspended in staining buffer (DPBS with 2% calf serum), and incubated for 20 min on ice with anti-CD16/CD32 (1 μg/106 cells) to block FcγII/III receptors. Then fluorochrome-conjugated antibodies for cell surface markers were added (0.5 μg/106 cells), and the cells were incubated for an additional 20 min on ice. After two washes with staining buffer, the cells were fixed with Cytofix/Cytoperm (BD Biosciences) for 20 min and washed with Perm/Wash buffer (BD Biosciences). Then PE-conjugated anti-IFN-γ was added (0.5 μg/106 cells) to detect intracellular cytokines, and the cells were incubated for an additional 20 min on ice. The cells were analyzed on a BD LSR II system using BD FACSDiva software.
Preparation of bone marrow-derived macrophages.
Bone marrow-derived macrophages (BMMφ) were differentiated in vitro in the presence of L929 cell-conditioned medium as previously described (37, 46). Specifically, bone marrow cells were flushed from the femurs of mice with HBSS containing 10 mM HEPES, centrifuged, and resuspended in macrophage growth medium (Dulbecco's modified Eagle medium, 10 mM HEPES, 7% L929 cell-conditioned medium, 10% FBS, penicillin, and streptomycin). The cells were then incubated in tissue culture flasks at 37°C under 5% CO2 for 6 to 14 days. The culture medium was replaced with fresh medium if the cells were grown beyond 7 days. More than 95% of the resulting cells expressed surface F4/80 as determined by flow cytometry. When used to supplement HMC cultures, adherent macrophages were dislodged from the tissue culture flasks by treatment with 0.25% trypsin-EDTA for about 5 min, washed, resuspended in fresh macrophage growth medium, and incubated overnight to allow attachment to the wells of 96-well plates. The growth medium was replaced with complete tissue culture medium prior to addition of HMC to these cultures.
ELISA.
IFN-γ, IL-12p40, and IL-12p70 were each measured by an enzyme-linked immunosorbent assay (ELISA) using OptEIA paired antibody sets from BD Biosciences. Mouse serum samples were diluted at least fivefold in sample buffer prior to the assay. ELISA plates were read using a Biotek EL340, and the results were analyzed with the DeltaSOFT II program. Unless otherwise indicated, intersample error values within the ELISAs were determined by the software using a response-error relationship. For the data shown in Fig. 5 and Table 1, the standard deviations refer to variability between animals within each group. The limits of detection of the ELISA for IFN-γ and IL-12p70 were approximately 5 pg/ml for cell culture supernatant fluids and 25 pg/ml for serum samples.
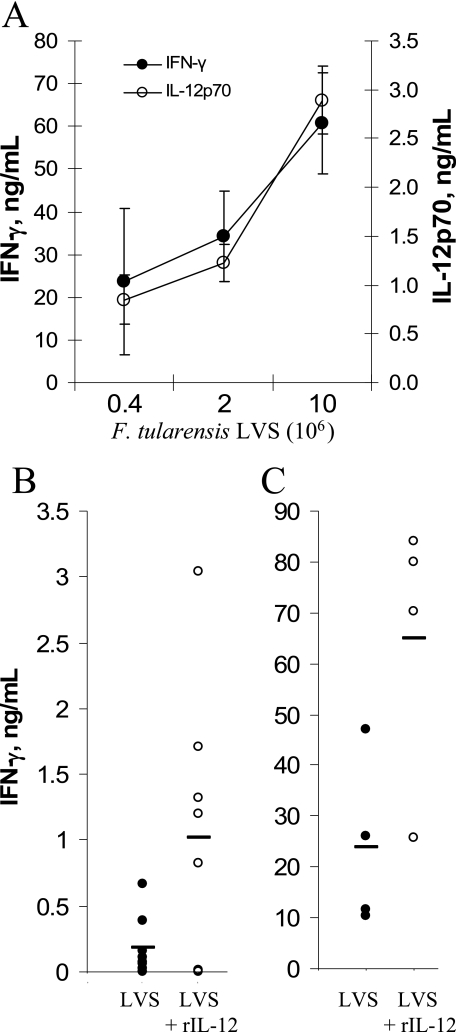
FIG. 5.
Effects of rIL-12 on IFN-γ responses to F. tularensis LVS in vivo. (A) Wild-type B6 mice were injected i.p. with the indicated doses of F. tularensis LVS, and their sera were collected 16 h later for analysis of IFN-γ or IL-12p70. (B and C) At two different F. tularensis LVS doses, one group of mice was injected with 0.5 μg of rIL-12 just prior to bacterial challenge. Either 5 × 104 CFU was given to 4 mice per group (B) or 4 × 105 CFU was given to 8 mice per group (C). In both cases, the result for each animal is shown, with horizontal lines representing the mean of each group. The groups receiving F. tularensis LVS plus rIL-12 (open circles) were significantly different from the groups receiving the same dose of F. tularensis LVS alone (P < 0.05).
TABLE 1.
IL-12p70 and IFN-γ responsesa to F. tularensis in IL-12p35-deficient mice
| Genotype | Concn (ng/ml) of the following cytokine in serumb:
|
% IFN-γ+ cellsc | |
|---|---|---|---|
| IL-12p70 | IFN-γ | ||
| IL-12p35+/+ | 2.9 ± 0.70 | 57 ± 1.4 | 6.9 ± 2.4 |
| IL-12p35−/− | 0.028 ± 0.022 | 0.26 ± 0.07 | 0.2 ± 0.1 |
Values are means ± standard deviations for each group of mice. Four animals were used in each group.
Mice were injected i.p. with 106 CFU of F. tularensis LVS, and sera were collected 16 h later. The concentrations of IL-12p70 and IFN-γ were determined by ELISA. The levels of IL-12p70 and IFN-γ in normal mouse serum were below the limit of detection (25 pg/ml).
HMC were also isolated from these infected mice and analyzed by flow cytometry (see Fig. 6).
Statistics.
Student's t test was used to determine whether there were any significant differences between groups (P < 0.05). Each experiment was performed at least twice, and the results of a representative experiment are shown.
RESULTS
Mouse hepatic mononuclear cells produce IFN-γ in response to F. tularensis LVS challenge in vitro.
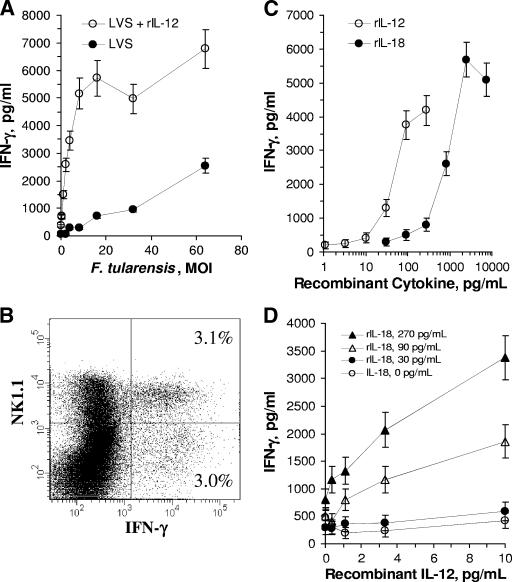
To characterize IFN-γ responses to F. tularensis LVS, mononuclear leukocytes were prepared from the livers of naïve uninfected mice by enzymatic digestion and Percoll density centrifugation. The cells were then challenged in vitro with viable F. tularensis LVS at various multiplicities of infection (MOI). Analysis of IFN-γ in the culture supernatant fluids collected 24 h later indicated a dose-dependent response to bacterial challenge (Fig. 1A). The identity of the IFN-γ-producing cells in these cultures was investigated by flow cytometry. Half the cells that expressed intracellular IFN-γ were NK1.1 positive (Fig. 1B), even though only 19% of the total HMC population expressed the NK1.1 cell surface marker. Of the NK1.1+ cells, approximately 17% were activated for IFN-γ expression. These results are similar to the results obtained when HMC of mice infected with F. tularensis LVS were analyzed in this fashion (see below) and indicate that NK1.1+ cells contribute substantially to the IFN-γ response in the liver.
FIG. 1.
Effects of recombinant coactivating cytokines on IFN-γ production by F. tularensis LVS-stimulated HMC in vitro. (A) Hepatic mononuclear cells from B6 mice were cultured with F. tularensis LVS at the indicated MOI in the presence or absence of recombinant IL-12 (50 pg/ml), and IFN-γ production was measured 24 h later by ELISA. Cells incubated in the absence of bacteria produced 68 pg of IFN-γ per ml. (B) HMC activated in vitro at an MOI of 66 were stained with APC-conjugated anti-NK1.1 and PE-conjugated anti-IFN-γ. (C) Comparison of the effects of rIL-12 and rIL-18 in F. tularensis LVS-challenged HMC cultures (MOI, 30). (D) Synergistic effects of rIL-12 and rIL-18 on IFN-γ production by HMC challenged at an MOI of 30.
IL-12 and IL-18 provide coactivating signals for the hepatic IFN-γ response to F. tularensis LVS.
Given the importance of IL-12 in the survival of F. tularensis LVS-infected mice (discussed above), we tested the effects of rIL-12 on the IFN-γ response by hepatic lymphocytes. Interleukin-12 decreased the number of bacteria required to elicit this response and significantly increased total IFN-γ production at all MOI tested (Fig. 1A). Surprisingly, the concentration of rIL-12 necessary for this effect was less than 50 pg/ml (Fig. 1C).
Recombinant mouse IL-18 also coactivated HMC for IFN-γ production in response to F. tularensis LVS (Fig. 1C), although the concentration of rIL-18 required for this effect was approximately 10-fold higher than that required for a similar effect by rIL-12. Combinations of rIL-12 and rIL-18 had potent synergistic effects (Fig. 1D). For example, in the presence of rIL-18 at a concentration of 90 pg/ml, as little as 1 pg/ml of rIL-12 coactivated HMC for high levels of IFN-γ production.
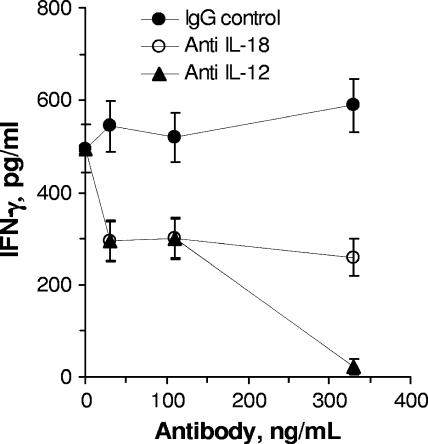
The results reported above show that both rIL-12 and rIL-18 enhanced IFN-γ production by HMC stimulated with F. tularensis LVS but do not establish whether either cytokine is required for IFN-γ production in this system. Therefore, we next determined whether neutralizing antibodies to either mouse IL-12p40, IL-23, or IL-18 inhibited the IFN-γ response to F. tularensis LVS in vitro. As shown in Fig. 2, both anti-IL-12p40 and anti-IL-18 inhibited the IFN-γ response by HMC to F. tularensis LVS. Similar experiments revealed that anti-mouse IL-23 did not significantly block IFN-γ production (data not shown), suggesting that the effect of anti-mouse IL-12p40 was due to its ability to neutralize IL-12 and not IL-23, which also contains the p40 subunit. As shown below, the inhibitory effects of anti-IL-18 were not due to a cross-reaction of the antibody with IL-12 (see Fig. 4C).
FIG. 2.
Effects of neutralizing anti-cytokine antibodies on IFN-γ production by F. tularensis LVS-challenged HMC in vitro. Antibodies were added to HMC cultures just before the addition of F. tularensis LVS (MOI, 48). Antibody-treated cultures were significantly different from the IgG controls at all concentrations (P < 0.05).
FIG. 4.
Supplementation of HMC cultures with BMMφ. (A) A total of 150,000 HMC were added to cultures containing 30,000 BMMφ, and the cultures were challenged with F. tularensis LVS in vitro at the indicated MOI. (B and C) Neutralizing anti-IL-12 or anti-IL-18 was added to cultures just prior to challenge with F. tularensis LVS at an MOI of 61. (B) The IFN-γ values for antibody-treated cultures were significantly different from those for the IgG controls at all antibody concentrations (P < 0.05). (C) The IL-12p70 values for cultures treated with each concentration of anti-IL-12 were significantly different from those for the IgG controls (P < 0.05).
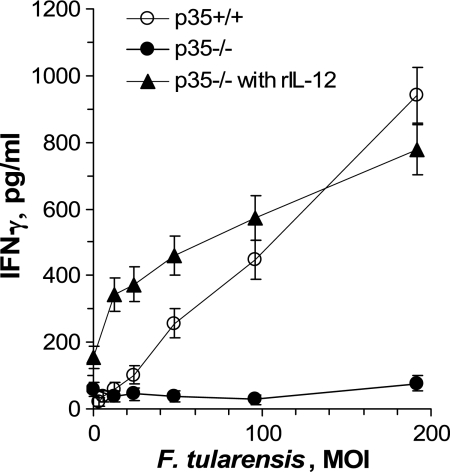
To confirm the necessity for IL-12, HMC were prepared from mice deficient in the unique IL-12 subunit, p35. Compared to HMC from wild-type (p35+/+) mice, cells from p35−/− mice showed essentially no IFN-γ response to F. tularensis LVS (Fig. 3). In addition, the IFN-γ response of p35−/− cells was completely restored when exogenous rIL-12 was added to these cultures. Thus, the only deficiency of the p35−/− cells in this culture system was their inability to produce IL-12.
FIG. 3.
Lack of IFN-γ production in vitro by HMC from mice deficient in IL-12p35. HMC from wild-type B6 mice (p35+/+) or IL-12p35-deficient mice (p35−/−) were challenged in vitro with F. tularensis LVS at the indicated MOI. Cells from the IL-12p35-deficient mice were also challenged with bacteria and rIL-12 (50 pg/ml). Cell culture supernatant fluids were collected after 24 h, and IFN-γ concentrations were measured by ELISA. At MOI greater than 40, the values for the p35−/− cells were significantly different from those for the other two groups (P < 0.05).
Macrophages can provide essential coactivating signals, including IFN-γ-inducing cytokines.
Macrophages have been shown to be an important source of IFN-γ-coinducing cytokines, such as IL-12 and IL-18, in a number of infection models. To determine if macrophages provided similar functions for activating hepatic IFN-γ production, BMMφ were prepared and added to HMC cultures. As was seen with the addition of recombinant cytokines, BMMφ significantly lowered the threshold for activation of HMC by F. tularensis LVS and increased the overall level of IFN-γ production in the cultures (Fig. 4A). Neutralizing antibody to either IL-12 or IL-18 inhibited the response (Fig. 4B), suggesting that the BMMφ did not change the nature of the immune response to F. tularensis LVS but instead added to the effects of the macrophages that were already present in the HMC preparations. Analysis of IL-12p70 in these culture supernatant fluids (Fig. 4C) established that the anti-IL-12 antibody did in fact neutralize IL-12 and showed that anti-IL-18 did not affect IL-12 secretion or detection.
IFN-γ responses in F. tularensis LVS-infected mice require IL-12.
To determine if the findings from these cell culture experiments were predictive of hepatic IFN-γ responses during infections, B6 mice were challenged with F. tularensis LVS with or without rIL-12. Sixteen hours later, serum samples were collected and assayed for IFN-γ and IL-12p70. Both serum IFN-γ and IL-12p70 concentrations increased with increasing challenge doses of bacteria (Fig. 5). In agreement with the observations in vitro, the addition of rIL-12 reduced 25-fold the number of bacteria necessary to induce high level IFN-γ production (>50 ng/ml), indicating that rIL-12 acted as a coactivator in vivo. Surprisingly, the serum IL-12p70 levels 16 h postchallenge were actually decreased in the mice that received both F. tularensis LVS and rIL-12 (data not shown).
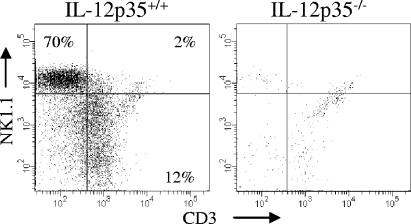
To further ascertain the role of IL-12 in vivo, wild-type and IL-12p35-deficient mice were challenged with F. tularensis LVS, and their HMC were isolated 16 h later. Flow cytometry demonstrated numerous IFN-γ-expressing cells within the livers of wild-type mice but failed to detect significant numbers of IFN-γ+ cells in the livers of infected IL-12p35-deficient mice (Fig. 6; Table 1) The principal IFN-γ-expressing cells present at 16 h in infected wild-type mice were NK cells, although some T cells were activated as well. Analysis of the cytokine concentrations in the sera of the same wild-type and mutant mice also showed that IFN-γ induction by F. tularensis LVS was IL-12p35 dependent (Table 1). Compared to those of p35+/+ mice, the sera of p35−/− mice showed extremely low levels of IFN-γ and, as expected, insignificant amounts of IL-12p70.
FIG. 6.
Requirement of IL-12p35 for the induction of the IFN-γ response in F. tularensis LVS-infected mice. Wild-type (IL-12p35+/+) B6 and IL-12p35−/− mice were injected i.p. with 106 CFU of F. tularensis LVS. Sixteen hours later, HMC were isolated, stained with fluorochrome-labeled antibodies against NK1.1, CD3, and IFN-γ, and analyzed by flow cytometry. Only cells expressing intracellular IFN-γ are shown here.
Liver lymphocyte responses to highly virulent type A F. tularensis subsp. tularensis organisms.
Because strains of F. tularensis subsp. tularensis are typically far more virulent than those of F. tularensis subsp. holarctica, we compared F. tularensis subsp. holarctica LVS to two type A strains isolated from clinical specimens. Mouse HMC were challenged in vitro with each strain, and IL-12p40, IL-12p70, and IFN-γ were measured in culture supernatant fluids 24 h later. While the type A strains induced responses similar to, if not greater than, those induced by the vaccine strain, the doses that were necessary to induce a given response did not differ more than fivefold between the strains (Fig. 7). Two additional clinical isolates of F. tularensis type A showed similar results (data not shown). At a minimum, these findings indicate that virulent type A strains do not derive their pathogenicity from an immune evasion mechanism that disrupts either the IL-12 or the IFN-γ response to the pathogen.
FIG. 7.
IFN-γ and IL-12 production by HMC challenged in vitro with F. tularensis subsp. tularensis clinical isolates KU49 and KU54. Cell culture supernatant fluids were collected after 24 h, and IFN-γ (A), IL-12p70 (B), and IL-12p40 (C) concentrations were measured by ELISA. Cells incubated in the absence of bacteria produced 110 pg of IFN-γ per ml.
DISCUSSION
Considerable evidence indicates that the production of IFN-γ is a critical protective response in both immunized and naïve mice against the attenuated live vaccine strain of F. tularensis (see the introduction). Lopez et al. (30) have recently demonstrated the potential for a local IFN-γ response to this organism within the mouse lung following intranasal challenge, and we have demonstrated in the current study that the livers of nonimmune mice contain large numbers of lymphocytes with the capacity to produce IFN-γ during systemic infections. Given the density of macrophages in the lung and liver, local IFN-γ production would seem to favor increased intracellular immune elimination of the pathogen, and several in vitro studies indicate that macrophage activation by IFN-γ-producing cells decreases the intracellular survival of the organism (3, 8, 21, 36).
The liver is a key target organ in tularemia because it is a major secondary site of colonization following primary pulmonary and intracutaneous infections, the two most common routes. Even in infections with the relatively less virulent F. tularensis LVS, secondary hepatic infections occur within 3 days following intranasal, intradermal, or intravenous challenge and achieve heavy bacterial burdens in the liver during the subsequent few days (22). This undoubtedly reflects the high rates of bacterial replication within hepatocytes (13). Thus, a local protective innate immune response within the liver itself would be advantageous to the host, and identifying any unique properties for its induction would aid in preventing or treating systemic infections. Here we have demonstrated the role of IL-12 in the expression of IFN-γ during liver infections. We also showed the importance of IL-18 as a coactivator in vitro, which agrees with an earlier report that the coinjection of neutralizing antibodies to both IL-18 and IL-1β decreased the clearance of Francisella novicida in the livers, spleens, and lungs of infected mice (31). The nearly complete lack of an IFN-γ response both in vitro and in the infected IL-12p35-deficient mouse in this study suggests that IL-18 is not by itself a sufficient coactivating signal that can compensate for the absence of IL-12 (Fig. 3; Table 1). Rather, it appears that IL-18 requires the presence of IL-12 to enhance the IFN-γ response. This may reflect the ability of IL-12 to induce expression of the IL-18 receptor, which has been observed in T cells and NK cells (28, 34, 39, 48). In the presence of IL-18, low picogram-per-milliliter concentrations of IL-12 were sufficient to greatly magnify IFN-γ production in vitro (Fig. 1D). The potency of IL-12 in this context suggests that hepatic lymphocytes with the capacity to produce IFN-γ are extremely sensitive to coactivating signals that are locally produced.
Cytokines that are known to coactivate NK cells and/or T cells for responses to microbes include, among others, IL-6 (38, 47), IL-12 (7, 44), IL-18 (5, 14), IL-23 (27), and type I interferons (6). In the present study, antibody neutralization of IL-6 or IL-23 did not inhibit the IFN-γ response to F. tularensis LVS in vitro (data not shown). Recombinant vaccinia virus protein B18R, a soluble interferon receptor that inhibits type I interferon activity (1, 9, 43), also did not inhibit HMC responses to F. tularensis LVS in vitro (data not shown), suggesting that the type I interferons did not provide essential coactivating signals. By contrast, both IL-12 and IL-18 were potent coinducers of IFN-γ in vitro, and mice deficient in the p35 subunit of IL-12 failed to express IFN-γ in vitro or in vivo following F. tularensis LVS challenge. rIL-12 also increased IFN-γ production in infected animals.
Organisms belonging to F. tularensis subsp. tularensis are generally far more virulent in mice than the attenuated F. tularensis subsp. holarctica LVS. While the 50% lethal dose for F. tularensis LVS given to BALB/c mice by the intranasal route is approximately 3 × 103 CFU (16), virulent type A strains show 50% lethal doses of 10 to 20 CFU (11). This raises the possibility that virulent strains evade innate immune elimination in the liver and other organs by inhibiting the production of IFN-γ. Mice lacking the IFN-γ response show increased numbers of LVS organisms in the liver (12) and increased lethality rates (19) compared to wild-type mice. The present study is the first to report that F. tularensis type A strains induced both IL-12 and IFN-γ production by mouse liver lymphocytes in vitro and that this response required MOI comparable to those seen with the attenuated LVS strain. Apparently, none of the four type A strains we tested have evolved the means for inhibiting IFN-γ production.
Acknowledgments
This work was supported by NIH grants R21 AI062939 and P20 RR0164443 and by a bridging grant from the University of Kansas Medical Center Research Institute.
We thank Jing Yu for invaluable technical contributions, Joyce Slusser for assistance with flow cytometry, Guillermo Madico for typing the clinical F. tularensis strains, and Jeannine Petersen for providing the F. tularensis LVS.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Alcamí, A., J. A. Symons, and G. L. Smith. 2000. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 74:11230-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, L. S., E. Ghadirian, F. P. Nestel, and P. A. Kongshavn. 1989. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7:421-428. [DOI] [PubMed] [Google Scholar]
- 3.Anthony, L. S., P. J. Morrissey, and F. E. Nano. 1992. Growth inhibition of Francisella tularensis live vaccine strain by IFN-γ-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J. Immunol. 148:1829-1834. [PubMed] [Google Scholar]
- 4.Baker, C. N., D. G. Hollis, and C. Thornsberry. 1985. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J. Clin. Microbiol. 22:212-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biet, F., C. Locht, and L. Kremer. 2002. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 80:147-162. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A. 2001. Interferons α and β as immune regulators—a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 8.Bosio, C. M., and K. L. Elkins. 2001. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 69:194-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colamonici, O. R., P. Domanski, S. M. Sweitzer, A. Larner, and R. M. Buller. 1995. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon α transmembrane signaling. J. Biol. Chem. 270:15974-15978. [DOI] [PubMed] [Google Scholar]
- 10.Cole, L. E., K. L. Elkins, S. M. Michalek, N. Qureshi, L. J. Eaton, P. Rallabhandi, N. Cuesta, and S. N. Vogel. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 176:6888-6899. [DOI] [PubMed] [Google Scholar]
- 11.Conlan, J. W., W. Chen, H. Shen, A. Webb, and R. KuoLee. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34:239-248. [DOI] [PubMed] [Google Scholar]
- 12.Conlan, J. W., R. KuoLee, H. Shen, and A. Webb. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32:127-134. [DOI] [PubMed] [Google Scholar]
- 13.Conlan, J. W., and R. J. North. 1992. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect. Immun. 60:5164-5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello, C. A., and G. Fantuzzi. 2003. Interleukin-18 and host defense against infection. J. Infect. Dis. 187(Suppl. 2):S370-S384. [DOI] [PubMed] [Google Scholar]
- 15.Dreisbach, V. C., S. Cowley, and K. L. Elkins. 2000. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect. Immun. 68:1988-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duckett, N. S., S. Olmos, D. M. Durrant, and D. W. Metzger. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 73:2306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkins, K. L., T. Rhinehart-Jones, C. A. Nacy, R. K. Winegar, and A. H. Fortier. 1993. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect. Immun. 61:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forestal, C. A., J. L. Benach, C. Carbonara, J. K. Italo, T. J. Lisinski, and M. B. Furie. 2003. Francisella tularensis selectively induces proinflammatory changes in endothelial cells. J. Immunol. 171:2563-2570. [DOI] [PubMed] [Google Scholar]
- 21.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjöstedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golovliov, I., G. Sandström, M. Ericsson, A. Sjöstedt, and A. Tärnvik. 1995. Cytokine expression in the liver during the early phase of murine tularemia. Infect. Immun. 63:534-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosselin, E. J., D. R. Gosselin, and S. A. Lotz. 2005. Natural killer and CD8 T cells dominate the response by human peripheral blood mononuclear cells to inactivated Francisella tularensis live vaccine strain. Hum. Immunol. 66:1039-1049. [DOI] [PubMed] [Google Scholar]
- 27.Happel, K. I., E. A. Lockhart, C. M. Mason, E. Porretta, E. Keoshkerian, A. R. Odden, S. Nelson, and A. J. Ramsay. 2005. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect. Immun. 73:5782-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawless, V. A., S. Zhang, O. N. Ozes, H. A. Bruns, I. Oldham, T. Hoey, M. J. Grusby, and M. H. Kaplan. 2000. Stat4 regulates multiple components of IFN-γ-inducing signaling pathways. J. Immunol. 165:6803-6808. [DOI] [PubMed] [Google Scholar]
- 29.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López, M. C., N. S. Duckett, S. D. Baron, and D. W. Metzger. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell. Immunol. 232:75-85. [DOI] [PubMed] [Google Scholar]
- 31.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastroeni, P., J. A. Harrison, J. H. Robinson, S. Clare, S. Khan, D. J. Maskell, G. Dougan, and C. E. Hormaeche. 1998. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect. Immun. 66:4767-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray, H. W. 1997. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J. Infect. Dis. 175:1477-1479. [DOI] [PubMed] [Google Scholar]
- 34.Neumann, D., and M. U. Martin. 2001. Interleukin-12 upregulates the IL-18Rβ chain in BALB/c thymocytes. J. Interferon Cytokine Res. 21:635-642. [DOI] [PubMed] [Google Scholar]
- 35.Pammit, M. A., V. N. Budhavarapu, E. K. Raulie, K. E. Klose, J. M. Teale, and B. P. Arulanandam. 2004. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob. Agents Chemother. 48:4513-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polsinelli, T., M. S. Meltzer, and A. H. Fortier. 1994. Nitric oxide-independent killing of Francisella tularensis by IFN-γ-stimulated murine alveolar macrophages. J. Immunol. 153:1238-1245. [PubMed] [Google Scholar]
- 37.Rhoades, E. R., and I. M. Orme. 1997. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect. Immun. 65:1189-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romani, L., A. Mencacci, E. Cenci, R. Spaccapelo, C. Toniatti, P. Puccetti, F. Bistoni, and V. Poli. 1996. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sareneva, T., I. Julkunen, and S. Matikainen. 2000. IFN-α and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J. Immunol. 165:1933-1938. [DOI] [PubMed] [Google Scholar]
- 40.Schricker, R. L., H. T. Eigelsbach, J. Q. Mitten, and W. C. Hall. 1972. Pathogenesis of tularemia in monkeys aerogenically exposed to Francisella tularensis 425. Infect. Immun. 5:734-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjöstedt, A., M. Eriksson, G. Sandström, and A. Tärnvik. 1992. Various membrane proteins of Francisella tularensis induce interferon-γ production in both CD4+ and CD8+ T cells of primed humans. Immunology 76:584-592. [PMC free article] [PubMed] [Google Scholar]
- 42.Surcel, H. M., J. Ilonen, K. Poikonen, and E. Herva. 1989. Francisella tularensis-specific T-cell clones are human leukocyte antigen class II restricted, secrete interleukin-2 and gamma interferon, and induce immunoglobulin production. Infect. Immun. 57:2906-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Symons, J. A., A. Alcamí, and G. L. Smith. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81:551-560. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 45.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren, M. K., and S. N. Vogel. 1985. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J. Immunol. 134:982-989. [PubMed] [Google Scholar]
- 47.Williams, D. M., B. G. Grubbs, T. Darville, K. Kelly, and R. G. Rank. 1998. A role for interleukin-6 in host defense against murine Chlamydia trachomatis infection. Infect. Immun. 66:4564-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimoto, T., K. Takeda, T. Tanaka, K. Ohkusu, S. Kashiwamura, H. Okamura, S. Akira, and K. Nakanishi. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J. Immunol. 161:3400-3407. [PubMed] [Google Scholar]