Abstract
Surfactant protein A (SP-A) enhances phagocytosis of Pseudomonas aeruginosa. Two functional genes, SP-A1 and SP-A2, encode human SP-A. As we showed before, baculovirus-mediated insect cell-expressed SP-A2 enhances the association of P. aeruginosa with rat alveolar macrophages (rAMs) more than does SP-A1. However, true phagocytosis (internalization) was not shown, and insect cell derived proteins lack or are defective in certain mammalian posttranslational modifications that may be important for SP-A1 and SP-A2 activity and specificity. Here we used SP-A1 (6A2, 6A4) and SP-A2 (1A0, 1A1) allele variants expressed by CHO (Chinese hamster ovary) mammalian cells to study their effect on association and/or internalization of P. aeruginosa by rAMs and/or human AMs (hAMs) and to study if phagocytosis can be modulated differentially and/or more effectively by CHO cell-expressed SP-A variants than by insect-cell expressed SP-A variants. For cell association and internalization assessments, light microscopy and fluorescence-activated cell sorter analyses were used, respectively. We found the following for the first time. (i) SP-A2 variants enhanced phagocytosis (cell association and/or internalization) of P. aeruginosa more than SP-A1 variants did, and the cell association correlated with internalization. (ii) Differences in the activities of SP-A variants were observed in the following order: 1A1>1A0>6A2>6A4. (iii) rAMs, although more active than hAMs, are an appropriate model, as SP-A2 variants exhibited activity higher than that seen for SP-A1 variants with either rAMs or hAMs. (iv) CHO cell-expressed SP-A was considerably more active than insect cell-expressed variants. We conclude that SP-A2 variants stimulate phagocytosis of P. aeruginosa more effectively than SP-A1 variants and that posttranslational modifications positively influence the phagocytic activity of SP-A.
As essential elements of host defense, nonspecific innate immune mechanisms play a critical role in the primary response to invading pathogenic microorganisms. In healthy individuals, there is a balance that favors the host with regards to respiratory host defense and microorganisms that arrive via inhaled ambient air and colonize the airway mucosal surface (53). This balance can be overcome in cystic fibrosis (CF) patients.
CF, an autosomal recessive disease, is associated with airway obstruction, excessive inflammation, and chronic bacterial infections. Patients with CF become infected with a limited spectrum of bacteria, including Pseudomonas aeruginosa, an opportunistic gram-negative bacilliform bacterium (19). The developments of P. aeruginosa infection in patients with CF may occur in three steps: no P. aeruginosa, initial nonmucoid P. aeruginosa, and finally, mucoid P. aeruginosa strains overexpressing the exopolysaccharide alginate. After initial colonization, P. aeruginosa has to adapt to certain challenges, i.e., to various factors of the innate immune system, antibiotics, and growth in the lung microenvironment. P. aeruginosa bacteria may in turn undergo phenotypic conversion from nonmucoid to mucoid (5, 41). In this study, a nonmucoid P. aeruginosa strain was used as a model to study differences in the abilities of SP-A variants to enhance phagocytosis of P. aeruginosa by alveolar macrophages.
The immune defenses of the lung involve pulmonary surfactant, a complex system of lipids and proteins, which lines the alveolar epithelial surfaces of the lungs. Surfactant protein A (SP-A) is a member of a family of collagenous calcium-dependent defense lectins, or collectins. One of the major biological roles of the collectins is to bind to targets (e.g., microorganisms) and to enhance phagocytosis/clearance of the target (22). SP-A plays an important role in the modulation of innate host defense in the lung (13). It has been shown to stimulate the chemotaxis of macrophages (67), influence the proliferation of immune cells (3, 36) and the production of proinflamatory cytokines (4, 35, 64, 65), induce the generation of reactive oxidants (58), regulate nitric oxide production (2, 23), and enhance phagocytosis (11, 34, 44, 46). SP-A can interact directly with phagocytic cells (29, 58) and can bind to the lipid A portion of rough lipopolysaccharide (59). It has also been reported that genetically modified mice lacking SP-A are highly susceptible to challenge with experimental pneumonia (39).
Two functional genes, SP-A1 and SP-A2, encode human SP-A (hSP-A). There are more than 30 alleles characterized for the SP-A genes (10, 32). Of these, four SP-A1 alleles (6A, 6A2, 6A3, 6A4) and six SP-A2 alleles (1A, 1A0, 1A1, 1A2, 1A3, 1A5) have been observed at a high frequency in the general population (10). SP-A variants with both qualitative (functional, biochemical, and/or structural) and quantitative (regulatory) differences have been identified. Nucleotide differences within the coding sequence are likely to contribute to qualitative differences. These include differences in their abilities to inhibit surfactant secretion (61) and to stimulate production of tumor necrosis factor alpha by macrophage-like THP-1 cells (27, 64, 65) and in their aggregation and oligomerization properties (18, 56, 61). Nucleotide and/or splice differences within regulatory regions (3′ untranslated region [UTR] and 5′ UTR) (32, 37, 54) between the SP-A1 and SP-A2 genes and/or alleles may contribute to quantitative differences. Differences in basal mRNA levels and in mRNA levels in response to dexamethasone have been observed as assessed by in vitro or explant culture studies (30, 38, 55, 63), and differences in protein levels have been observed for lung tissues and bronchoalveolar lavage (BAL) fluids from different individuals (56a). Splice 5′ UTR variants were recently shown to differentially affect translation and mRNA stability (62). Differences in SP-A protein content levels among individuals have been observed in cases of lung disease, including CF (20, 24, 52, 57), or in physiological changes in lung activity (12). In these cases, no distinction was made between SP-A1 and SP-A2 content or SP-A genotype.
Previously, we have shown that baculovirus-mediated insect cell-derived SP-A2 variants enhance P. aeruginosa cell association with rat alveolar macrophages more effectively than do SP-A1 variants. However, true phagocytosis (internalization) was not shown at that time, and the cell association enhancement was severalfold lower than that observed for human SP-A obtained from alveolar proteinosis BAL fluids (46). We speculated that incomplete posttranslational modification of the insect cell-expressed proteins accounts for the lower level of cell association. Insect cell-derived proteins lack or are defective in certain mammalian posttranslational modifications (16, 40), such as proline hydroxylation and complex N-linked glycosylation, that may be important for enhancement of cell association. In the present study, we expanded on our previous findings. We used CHO (Chinese hamster ovary) mammalian cell-expressed SP-A variants, in which posttranslational modifications are more similar to natural human SP-A, to investigate for the first time whether mammalian cell-produced SP-A2 variants stimulate phagocytosis (internalization) or cell association of P. aeruginosa by rat or human AMs more than do SP-A1 variants and whether SP-A variants expressed in CHO cells exhibit phagocytic ability comparable to that observed with SP-A variants from BAL fluids.
MATERIALS AND METHODS
Reagents and media.
Tryptic soy agar and RPMI 1640 (HEPES modification) was from Sigma (St. Louis, MO), and Dulbecco's phosphate-buffered saline was from Invitrogen (Life Technologies, Grand Island, NY). Sterile saline solution (0.9% NaCl) was purchased from Baxter Healthcare, Corp. (Deerfield, IL), and fluorescein-5-isothiocyanate (FITC) was from Molecular Probes, Inc. (Eugene, OR). Bronchoalveolar lavage fluid was obtained from patients with alveolar proteinosis who were undergoing routine therapeutic lavage. Normal human lungs (n = 4) were obtained from the Gift of Life Donor Program (Philadelphia, PA). The protocol for the use of human lung tissue in the present study was approved by the Institutional Review Board of the Pennsylvania State University College of Medicine.
Growing and preparation of bacteria.
The nonmucoid P. aeruginosa strain (ATCC 39018) was obtained from the American Tissue Culture Collection (Rockville, MD). A bacterial suspension was prepared as described previously (46). Briefly, bacteria were grown overnight (20 to 24 h) on tryptic soy agar plates at 30°C and resuspended in the RPMI medium. The CFU-per-ml value was determined with a calibration curve based on the optical density of a bacterial suspension at 660 nm, and the bacterial suspension was spread on agar plates to control for quantification of bacteria. The bacterial suspension was then used immediately.
FITC labeling of bacteria.
P. aeruginosa bacteria from agar plates were resuspended at 109 CFU/ml in 0.1 M sodium carbonate buffer (pH 9.0). FITC was then added to a final concentration of 0.1 mg/ml. The mixture was incubated on a LABQUAKE shaker (Labindustries, Berkeley, CA) for 1 h at room temperature in the dark. Bacteria were washed to remove free FITC four times with cold phosphate-buffered saline (PBS) with centrifugations at 4°C at 2,000 × g for 20 min. Final washes were performed with RPMI medium. Bacteria were resuspended at 5 × 108 CFU/ml in RPMI and used immediately for phagocytosis.
Purification of SP-A from human BAL fluid.
SP-A was purified (21) from bronchoalveolar lavage fluid obtained from a patient with alveolar proteinosis, as described previously (26). The concentration of SP-A was determined using a Micro bicinchoninic acid protein assay (Pierce, Rockford, IL) with RNase A as a standard, and the purity of the SP-A preparation was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining and by Western blotting and two-dimensional gel analysis.
Preparation and purification of human SP-A genetic variants expressed in CHO cells.
Human SP-A variants SP-A1(6A2 and 6A4) and SP-A2(1A1 and 1A0) observed frequently in the general population were expressed from stably transfected CHO-K1 mammalian cells as described previously (65). Briefly, human SP-A1 and SP-A2 cDNAs were cloned into expression vector pEE14. SP-A gene transcription was driven by a cytomegalovirus promoter. The recombinant constructs with SP-A variants were transfected into CHO cells. Stably transfected cell lines were established based on SP-A expression. SP-A variants were purified from the media of cultured CHO cells by use of mannose affinity chromatography as described previously (65). CHO cell-expressed SP-A variants were dialyzed and concentrated. The purity of SP-A was checked by silver staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The level of purity was approximately 95 to 98%. In this work, we used different concentrations (from 1.56 to 14 μg/ml) of SP-A variants for phagocytosis assays. Similar results were seen at higher SP-A concentrations (data not shown; the highest concentration tested was 33 μg/ml). However, at all SP-A concentrations tested, the activity of SP-A2 relative to that of SP-A1 was the same. Three different preparations of 1A0 and 6A2 and two preparations of 1A1 and 6A4 were tested with the same effect.
Isolation of rat alveolar macrophages.
Male adult pathogen-free Sprague-Dawley rats (Harlan, Indianapolis, IN) were used as a source for alveolar macrophages. The Penn State University Institutional Animal Care and Use Committee approved all procedures involving animals. Alveolar macrophages were isolated as described previously (46). Briefly, animals were anesthetized with an intramuscular injection of a mixture of ketamine HCl (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (XYLA-JECT; Phoenix Pharmaceuticals Inc., St. Joseph, MO). Rats were sacrificed and bled, the tracheas were cannulated, and the lungs were subjected to lavage three times with sterile saline (0.9% NaCl). Lavage fluids were centrifuged, and the cell pellets were washed three times with RPMI medium. Cells were counted using a hemacytometer and resuspended in RPMI medium. The viability of cells was determined by trypan blue dye exclusion, and only cell suspensions with greater than 95% viable cells were used.
Isolation of human alveolar macrophages.
Human lungs unable to be utilized for lung transplantation were obtained from The Gift of Life Donor Program en bloc. For the collection of alveolar macrophages, the entire left lung underwent bronchoalveolar lavage (BAL) with 1,000 ml of sterile saline (0.9% NaCl) at room temperature. The BAL fluid was centrifuged at 150 × g for 5 min at 4°C to pellet the cells, and cells were counted using a hemacytometer and plated at 5 × 106 cells per 100-mm tissue culture dish in 10 ml of RPMI. Dishes were incubated at 37°C for 3 h in the presence of 5% CO2 for the attachment of AMs. After 3 h, the attached cells were washed twice with 10 ml of PBS to remove any unattached cells, mucus, and tissue debris, and 10 ml of RPMI with 10% fetal calf serum was added to each plate for an overnight incubation of the attached AMs. After the overnight incubation, AMs were washed twice with 10 ml of PBS, 5 ml of cell dissociation solution (Sigma, St. Louis, MO) per plate was added, and plates were incubated at 37°C with gentle shaking to detach the AMs from the plates. AMs were centrifuged at 4°C at 250 × g for 3 min and washed three times with 10 ml of RPMI with centrifugations at 4°C at 250 × g for 3 min. AMs were counted using a hemacytometer. The viability of cells was determined by trypan blue dye exclusion, and only cell suspensions with greater than 95% viable cells were used. More than 95% of cells recovered were AMs. In a separate experiment, human alveolar macrophages (isolated from one of the BAL specimens processed) were also used without overnight incubation with identical results.
PI calculation.
To calculate the phagocytic index (PI), we assessed the association (attachment and internalization steps of phagocytosis) of P. aeruginosa with alveolar macrophages. The assay was performed as described previously (46). Briefly, suspensions of rat or human AMs (106 cells per ml) and bacteria (107 CFU per ml) in RPMI medium were mixed (50 μl plus 50 μl) to obtain a 1:10 ratio. SP-A was then immediately added to the mixture, and the mixture was incubated for 1 h at 37°C for phagocytosis on a LABQUAKE shaker. One milliliter of ice-cold PBS was then added to stop phagocytosis. AMs were sedimented by centrifugation at 4°C at 250 × g for 8 min and washed twice with 1 ml of cold PBS followed by sedimentation. Final suspensions of AMs were resuspended in 200 μl of PBS and applied to slides using a cytocentrifuge. The slides were stained using a Hema-3 stain kit (Fisher Scientific, Pittsburgh, PA) for analysis by light microscopy. Two hundred randomly selected AMs were analyzed for each experimental point at a magnification of ×1,000 under oil immersion (9). The phagocytic index was calculated according to the following formula: PI = percentage of bacterium-positive macrophages (that associated with at least one bacterium) × average number of bacteria per bacterium-positive macrophage (7). The values for the phagocytic index were expressed as a percentage of the negative control (i.e., without SP-A), which was set to 100%.
Internalization of P. aeruginosa by rat alveolar macrophages.
Suspensions of rat AMs (2 × 106 cells per ml, 250 μl) and FITC-labeled bacteria (5 × 108 CFU per ml, 50 μl) in RPMI medium were mixed to get a 1:50 proportion. SP-A was then immediately added to the mixture, and the mixture was incubated for 1 h at 37°C for phagocytosis on a LABQUAKE shaker. Two milliliters of ice-cold PBS was then added to stop phagocytosis. AMs were sedimented by centrifugation at 4°C at 250 × g for 8 min and washed once more with 2 ml of cold PBS followed by sedimentation. One milliliter of cold PBS was then added, and the cell suspension was divided into two aliquots of 500 μl. One was used for trypan blue treatment to quench external fluorescence for internalization analysis (500 μl of cold trypan blue at a 0.4-mg/ml concentration in PBS was added), and the other was mixed with 500 μl of cold PBS (for cell association analysis). After 5 min of incubation on ice, 1 ml of cold PBS was added, and cells were sedimented by centrifugation. AMs were washed twice with 2 ml of PBS, fixed in 300 μl of 1% paraformaldehyde in PBS, and analyzed with fluorescence-activated cell sorter (FACS) analysis. Data from ∼10,000 cells per condition were collected and analyzed with CellQuest software (Becton Dickson Immunometry Systems).
Statistics.
Paired t tests were performed for comparison of the effects of SP-A at the same concentration, and two-sample t tests were used for comparison of the effects of SP-A at different concentrations and to compare the activities of rat and human AMs. Data were expressed as mean ± standard error (SE) for three or more independent experiments. Differences were considered significant when the P value was <0.05.
RESULTS
Phagocytosis.
To study the phagocytic activity of CHO cell-derived SP-A variants, we used live P. aeruginosa and rat or human alveolar macrophages. A traditional light microscopy method was used, as we described previously (46). With this method, the attachment and internalization steps, two steps of the phagocytosis process (6) that we previously referred to as “cell association” of intact live bacteria (46), are assessed together. To measure cell association, we calculated the PI, as described in Materials and Methods (7). For internalization assessment, we used freshly prepared FITC-labeled live P. aeruginosa bacteria and flow cytometry (FACS) analysis with quenching of extracellular fluorescence. FACS allows for a more accurate differentiation between internalized and cell-associated bacteria.
Association of P. aeruginosa with rat alveolar macrophages stimulated by CHO cell-produced SP-A1 and SP-A2 variants.
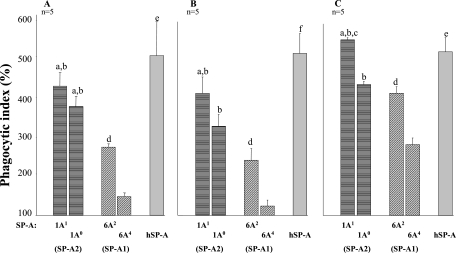
We used three different concentrations (1.56, 3.12, and 6.25 μg/ml) of SP-A1 (6A2, 6A4) and SP-A2 (1A0, 1A1) variants to compare their activities (we also used higher SP-A concentrations [highest concentration tested, 33 μg/ml] with results the same as those found for the lower concentrations [data not shown]). At all concentrations, SP-A variants significantly increased the PI compared to the negative control, except for the 6A4 variant at the 3.12-μg/ml concentration (Fig. 1).
FIG. 1.
Enhancement of association of P. aeruginosa with rat alveolar macrophages by SP-A1 and SP-A2 variants. Rat alveolar macrophages were mixed with P. aeruginosa in the presence or absence (negative control) of SP-A. After 1 h of incubation at 37°C, cell association was assessed. Two hundred AMs were counted at each experimental point. The negative control was set at 100%, and the data were presented as a percentage of the negative control. Shown are values for 1.56 (A), 3.12 (B), and 6.25 (C) μg/ml of SP-A. The phagocytic index was calculated as described in Materials and Methods. Data are expressed as means ± SE for five independent experiments. Differences were considered significant when the P value was <0.05. All variants significantly differed from the negative control (without SP-A), except for 6A4 at the 3.12-μg/ml concentration. Symbols: a, SP-A2 variants (1A1 or 1A0) significantly differed from 6A2; b, SP-A2 variants (1A1 or 1A0) significantly differed from 6A4; c, 1A1 significantly differed from 1A0; d, 6A2 significantly differed from 6A4; e, hSP-A differed from 6A4 only; f, hSP-A differed from all SP-A variants tested.
At the 1.56-μg/ml concentration, the SP-A2 variants (PIs of 420 ± 37 and 372 ± 23 for 1A1 and 1A0, respectively) significantly increased the PI more than either of the SP-A1 variants studied (PIs of 268 ± 11 and 144 ± 9 for 6A2 and 6A4, respectively) (Fig. 1A). 6A2 was significantly more active than 6A4, and the activities of 1A1 and 1A0 did not differ significantly. The activity of hSP-A (PI, 499 ± 85) differed significantly only from the 6A4 variant activity.
At the 3.12-μg/ml concentration, 1A1 increased the PI (413 ± 43) significantly more than either of the SP-A1 variants (PIs of 242 ± 30 and 125 ± 14 for 6A2 and 6A4, respectively), whereas 1A0 (PI, 330 ± 30) was more active than 6A4 only. The 6A2 variant was more active than 6A4, and hSP-A increased the phagocytic index (515 ± 52) significantly more than all variants tested. The PI of 1A1 did not differ from that of 1A0 (Fig. 1B).
At the 6.25-μg/ml concentration, the activity of 1A1 (PI, 555 ± 5) was significantly higher than the activity of either the 6A2 or the 6A4 variant (PIs of 416 ± 17 and 283 ± 18 for 6A2 and 6A4, respectively), and the activities of 1A0 (PI, 438 ± 9) and hSP-A (PI, 523 ± 38) were significantly greater than that of 6A4 (Fig. 1C).
Overall comparison of phagocytic indices between SP-A1 and SP-A2 variants.
Comparisons of the overall phagocytic activities of SP-A1 and SP-A2 variants expressed by the CHO cell system revealed (depending on the concentration used) that (i) the phagocytic activity of 6A2 was 73 to 95% of the 1A0 activity and 61 to 75% of the 1A1 activity (Table 1) , (ii) the phagocytic activity of 6A4 was 39 to 65% of the 1A0 activity and 32 to 51% of the 1A1 activity (Table 1), and (iii) the abilities of different SP-A variants to increase PI comparable to hSP-A were as follows: 65 to 85% for 1A0, 81 to 109% for 1A1, 51 to 82% for 6A2, and 26 to 55% for 6A4 (Table 2). Based on the data presented, the activities for SP-A variants tested were in the following order: 1A1>1A0>6A2>6A4.
TABLE 1.
Comparison of phagocytic indices between SP-A1 (6A4, 6A2) and SP-A2 (1A0, 1A1) variantsa
| Comparison (SP-A1 variant vs SP-A2 variant) | Activity at indicated concn of SP-A (μg/ml)b
|
||
|---|---|---|---|
| 1.56 | 3.12 | 6.25 | |
| 6A2 vs 1A0 | 72.70 ± 3.97* | 77.37 ± 12.61 | 95.24 ± 5.18 |
| 6A2 vs 1A1 | 64.67 ± 3.48* | 61.27 ± 9.45* | 74.89 ± 3.09* |
| 6A4 vs 1A0 | 39.24 ± 2.70* | 40.52 ± 7.89* | 64.57 ± 3.71* |
| 6A4 vs 1A1 | 34.77 ± 1.99* | 32.24 ± 6.13* | 51.01 ± 3.46* |
For phagocytosis of P. aeruginosa, rat alveolar macrophages were used. The phagocytic index values were compared between SP-A1 and SP-A2 variants at the same concentrations according to the following formula: SP-A1:SP-A2 (ratio) × 100% ± SE. The number of experiments for each test and concentration is shown in Fig. 1.
*, P < 0.05, indicating significant differences between phagocytic index values for SP-A1 and SP-A2 variants at the same concentrations.
TABLE 2.
Comparison of phagocytic indices between SP-A variants and hSP-Aa
| SP-A variant compared with hSP-A | Activity at indicated concn of SP-A (μg/ml)b
|
||
|---|---|---|---|
| 1.56 | 3.12 | 6.25 | |
| 1A0 | 85.27 ± 15.59 | 64.80 ± 3.13* | 85.09 ± 4.64 |
| 1A1 | 102.66 ± 26.94 | 81.20 ± 5.37* | 108.65 ± 8.70 |
| 6A2 | 64.23 ± 15.56 | 50.08 ± 8.52* | 81.65 ± 8.04 |
| 6A4 | 34.96 ± 8.85* | 26.45 ± 5.80* | 55.39 ± 5.25* |
For phagocytosis of P. aeruginosa, rat alveolar macrophages were used. The phagocytic index values were compared between each SP-A variant tested and the positive control (hSP-A) at the same concentration, according to the following formula: SP-A variant:hSP-A (ratio) × 100% ± SE. The number of experiments for each test is shown in Fig. 1.
*, P < 0.05, indicating significant differences between phagocytic indices induced by hSP-A and SP-A variants at the same concentrations.
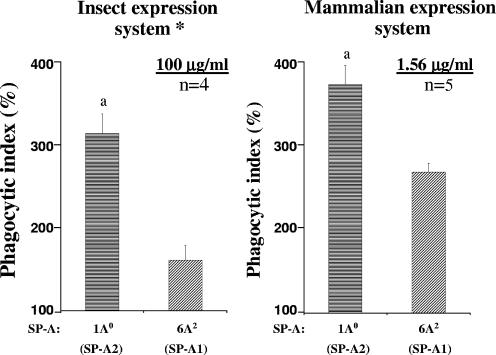
Comparison of the activities of mammalian cell- and insect cell-produced SP-A1 and SP-A2 variants.
As we published previously (46), insect cell-derived SP-A2 variants increase the PI significantly more than do SP-A1 variants. Comparison of the activities of SP-A1 and SP-A2 gene products expressed in CHO cells with those of SP-A1 and SP-A2 gene products expressed in insect cells (46) revealed that the SP-A2 (1A0) variant increases the PI significantly more than the SP-A1 (6A2) variant, irrespective of the cell type (insect or mammalian) in which they were expressed. The activity of 6A2 was about 52% of the 1A0 activity (46) for insect cell-expressed SP-A (at the highest SP-A concentration tested [100 μg/ml]) and about 73% (Table 1 and Fig. 2) for the CHO cell-expressed SP-A (at the lowest SP-A concentration tested [1.56 μg/ml]). Thus, for insect cell-expressed SP-A to exhibit activity similar to that observed for CHO cell-expressed SP-A, a concentration more than 60 times higher is required.
FIG. 2.
Comparison of the activities of SP-A1 and SP-A2 variants expressed in two different systems. Both insect and CHO cell-expressed SP-A variants were tested with rat AMs. The experimental designs were the same and the phagocytic indices were calculated the same way for both systems, as described in the legend for Fig. 1. The numbers of independent experiments were four for insect cell-expressed variants and five for CHO cell-expressed variants. The proportions of AMs to bacteria were 1:100 and 1:10 in insect and CHO cell systems, respectively. All variants significantly differed from the negative control (without SP-A). Symbols: a, SP-A2 variants significantly differed in terms of activity from SP-A1; *, data (for baculovirus-mediated insect cell expression system) are from our previously published paper (46).
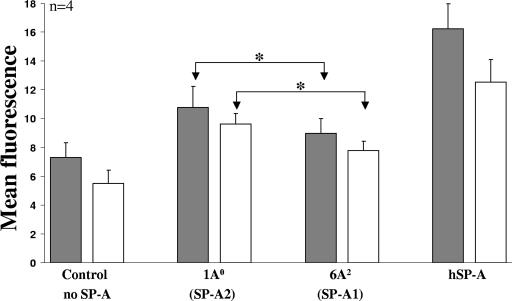
Enhancement of internalization of P. aeruginosa by rat alveolar macrophages in the presence of CHO cell-produced SP-A1 and SP-A2 variants.
A FACS-based analysis was used to determine the effect of SP-A variants on both cell association (attached and internalized bacteria) and internalization. For this, 1A0 (SP-A2), 6A2 (SP-A1), and hSP-A (positive control) at 14-μg/ml concentrations were used with freshly FITC-labeled P. aeruginosa. For all SP-As tested, both cell association (10.8 ± 1.5, 9.0 ± 1.0, and 16.2 ± 1.7 for 1A0, 6A2, and hSP-A, respectively) and internalization (9.7 ± 0.7, 7.7 ± 0.7, and 12.5 ± 1.6 for 1A0, 6A2, and hSP-A, respectively) were significantly higher than that for the negative control (no SP-A) (7.3 ± 1.0 and 5.5 ± 0.9 for cell association and for internalization, respectively) (Fig. 3). Differences between cell association and internalization values were observed (P < 0.05) for the negative control (no SP-A) and the positive control (hSP-A), but no significant differences were observed between cell association and internalization for 1A0 and 6A2. However, differences (P < 0.05) were observed between 1A0 and 6A2 both for cell association and for internalization. The activity of hSP-A was higher than that for either 1A0 or 6A2 for cell association and higher for internalization for 6A2 only.
FIG. 3.
Internalization of P. aeruginosa by rat alveolar macrophages stimulated by SP-A1 and SP-A2 variants. Rat alveolar macrophages were mixed with FITC-labeled P. aeruginosa at the proportion of 1:50 in the presence or absence (negative control) of SP-A (14 μg/ml). After 1 h of incubation at 37°C, the internalization of bacteria was measured by FACS analysis. Ten thousand cells were analyzed for the presence of fluorescent bacteria. The data are expressed as the mean fluorescence of all cells counted after the subtraction of background fluorescence (macrophages only). Trypan blue treatment was performed to quench external fluorescence. Solid bars, mean fluorescence of attached and internalized bacteria (cell association); open bars, mean fluorescence of internalized bacteria only. Each experiment was performed in triplicate, and the data are expressed as means ± SE for four independent experiments. Differences were considered significant when the P value was <0.05. Asterisks indicate significant differences between SP-A2- and SP-A1-induced cell association or internalization data.
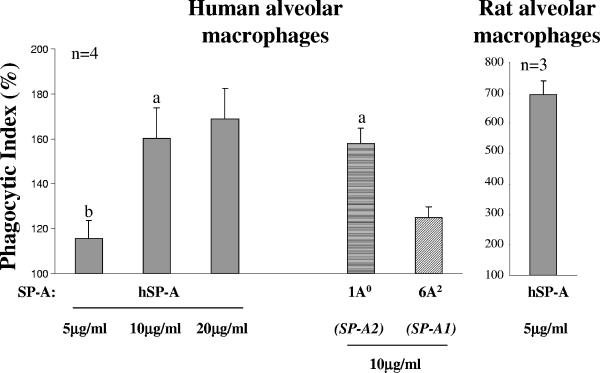
Association of P. aeruginosa with human alveolar macrophages in the presence of CHO cell-produced SP-A1 and SP-A2 variants and comparison of the activities of human and rat alveolar macrophages.
We used 1A0 (for SP-A2) and 6A2 (for SP-A1) at 10 μg/ml to test their phagocytic activities with human AMs. To compare the activities of human and rat AMs, the hSP-A was used at three different concentrations (5, 10, and 20 μg/ml) with human AMs and at a single 5-μg/ml concentration with rat AMs. The PIs of all SP-As tested differed significantly from that for the negative control, with the exception of that for 5 μg/ml of hSP-A with human AMs (Fig. 4). The activities of 1A0 and hSP-A were significantly higher than the 6A2 variant activity, and the PIs of 1A0 and hSP-A did not differ at the same concentration (10 μg/ml). The PI of hSP-A with human AMs at the 5 μg/ml concentration differed from that at 10 or 20 μg/ml. The PIs of the SP-As under study were as follows: 158 ± 7 and 125 ± 5 for 1A0 and 6A2, respectively, and 115 ± 8, 160 ± 14, 169 ± 13 for hSP-A at 5, 10, and 20 μg/ml, respectively, when tested with human AMs. The phagocytic activity of the 6A2 was 79% ± 4% of the 1A0 activity, and the activities of 1A0 and 6A2 were 96% ± 9% and 75% ± 5% of the hSP-A activity, respectively, at the 10-μg/ml concentration. Comparison of the PIs of rat and human AMs when hSP-A at the 5-μg/ml concentration was used revealed that the PI in rat AMs was about six times higher than the PI in hAMs (PIs of 694 ± 46 and 115 ± 8 for rat and human AMs, respectively) (Fig. 4).
FIG. 4.
Enhancement of association of P. aeruginosa with human alveolar macrophages by SP-A1 and SP-A2 variants: comparison of the activities of human and rat alveolar macrophages. The experimental design and phagocytic index calculations were performed as described in the legend for Fig. 1. Human or rat AMs were used. The numbers of independent experiments were four for human AMs and three for rat AMs. SP-A variants (1A0 and 6A2) were used at the concentration of 10 μg/ml, and hSP-A was used at 5, 10, and 20 μg/ml. Both, SP-A variants and hSP-A differed significantly (P < 0.05) in their activity from the negative control (without SP-A), with the exception of hSP-A at 5 μg/ml with human AMs. Symbols: a, the 1A0 or hSP-A activity significantly differed from the activity of the 6A2 variant at the same concentration; b, human AMs activity differed significantly from that of rat AMs in the presence of hSP-A at a 5-μg/ml concentration.
DISCUSSION
SP-A is a multifunctional protein involved in lung host defense. SP-A enhances phagocytosis of P. aeruginosa. In our previous study (46), we showed that insect cell-derived SP-A2 variants enhance cell association more effectively than do insect cell-derived SP-A1 variants. However, both insect-derived SP-A1 and SP-A2 variants enhanced cell association at levels considerably lower than those for hSP-A, and true phagocytosis (internalization) was not shown. In this report, we investigated the hypothesis that mammalian (CHO) cell-expressed SP-A variants exhibit activities similar to those of hSP-A and studied differences in their phagocytic indices by use of rat and human AMs. The results showed the following. (i) The activity of the CHO cell-expressed SP-A was similar to that of native SP-A, and mammalian (CHO) cell-expressed SP-A2 enhanced phagocytosis (internalization or cell association) of P. aeruginosa by rat or human alveolar macrophages more than did SP-A1. These observations indicate that although posttranslational modifications positively influence SP-A activity, they are not responsible for gene-specific differences, as these differences are maintained regardless of the presence (this study) or absence (46) of posttranslational modifications. (ii) Internalization of P. aeruginosa by alveolar macrophages stimulated by SP-A1 and SP-A2 variants correlated with cell association. (iii) Differences in the activities of among SP-A variants, as assessed by their phagocytic indices, were observed in the following order: 1A1>1A0>6A2>6A4. (iv) There exist species differences in AM phagocytic activity in response to human SP-A variants, with rat AMs exhibiting an activity significantly higher (∼6×) than that seen for hAMs. However, SP-A2 was more active than SP-A1 for both rat and human AMs, indicating that rat AMs are an appropriate model for human AMs to study SP-A variant activity in phagocytosis.
Consistent findings were observed regarding the abilities of SP-A1 and SP-A2 variants to enhance phagocytosis by use of light microscopy (46) and flow cytometry (FACS) with host alveolar macrophage cells from two species—rat and human. Freshly isolated live and FITC-labeled bacteria (P. aeruginosa) were used to better reflect occurrences in vivo, as discussed previously (46). The light microscopy assay is very sensitive, because it enables one to account for every bacterium phagocytized by each single cell analyzed, whereas flow cytometry allows one to better differentiate internalization from cell association. The results from both types of analyses (microscopy and FACS) showed for the first time that the CHO cell-expressed SP-A2 enhances both the cell association and the internalization steps of phagocytosis more than SP-A1 does and that the level of phagocytosis enhancement by the SP-A2 variants was similar to that seen for hSP-A from BAL specimens. However, the level of enhancement by SP-A1 or SP-A2 differed significantly from that observed previously for insect cell-expressed SP-As (46).
The difference in the levels of activity of insect cell- and mammalian cell-expressed SP-As is likely due to differences in posttranslational modifications that can considerably influence protein function. A role for glycosylation (40) or hydroxylation of prolines (17) in protein folding, oligomer assembly, structural stability, specific signal transduction, recognition and secretion processes, or clearance of glycoproteins has been described previously. Insect cells are unable to produce several modifications, including proline hydroxylation and complex N-linked side chains with terminal sialic acid (28, 43). SP-A is subject to several posttranslational modifications, including proline hydroxylation, N-linked glycosylation, sialylation of N-glycans, and others (14, 50, 51). SP-A expressed by insect cells lacks proline hydroxylation (17, 45) and presumably complex N glycosylation, as shown for other proteins (28). Although proline hydroxylation of the collagen-like domain of SP-A has been shown to be required for the assembly of oligomers that may be necessary for increasing the effectiveness of SP-A binding to receptors, this type of modification seemingly does not affect the general carbohydrate-binding ability of SP-A, because insect cell-expressed SP-A was still able to bind to immobilized mannose with an efficiency of greater than 95% (17, 45). Whether proline hydroxylation, lack of complex N-linked glycosylation, or other modifications (i.e., O-linked glycosylation, C mannosylation) that insect cells are not capable of performing (16, 40) account for the increased activity of CHO cell-expressed SP-As remains to be determined.
In spite of significant differences in the posttranslational modifications between the two systems, the results showed that the SP-A2 gene products stimulate phagocytosis more effectively than SP-A1, irrespective of the type of the expression system used (Fig. 2) (46). SP-A variants expressed either in CHO cells or in insect cells showed the same pattern of differences, even though insect cells are unable to produce several posttranslational modifications (8, 28, 43) produced by CHO cells. However, a concentration more than 60 times higher was required for insect cell-expressed SP-A variants to show a phagocytic activity comparable to that seen for the CHO cell-expressed variants. These observations indicate that although posttranslational modifications influence the activity from a quantitative point of view, they do not seem to alter the activity from a qualitative point of view with regard to phagocytosis. Therefore, intrinsic differences, such as differences in the amino acid sequence, may most likely contribute to the observed gene-specific activity differences. These may include oligomerization and/or thermal stability differences (17, 18, 45). SP-A1 has been shown to exhibit a thermal stability lower than that of SP-A2. The measured differences in thermal stability could indicate significant structural differences that may influence the overall functional activity of SP-A1 and SP-A2. Similar observations have been made for the cystic fibrosis transmembrane conductance regulator (CFTR) (49). Both CHO cell- and insect cell-expressed CFTR exhibited functional CFTR channel activity, but the activity of the insect-expressed CFTR was lower (1, 33, 48). The state of glycosylation of CFTR appeared to affect its stability at the plasma membrane (42, 66).
It has been proposed that the trimeric form of human SP-A consists of two SP-A1 molecules and one SP-A2 molecule (60). However, given that the ratio of SP-A1 to SP-A2 mRNA among individuals differs considerably from the anticipated 2:1 ratio (31), single gene products may exist, and they may form functional homotrimers and/or homo-oligomers. Indeed, it has been shown recently that single gene products are functional and that the SP-A2 product is more active than the SP-A1 product in its ability to modulate cytokine production, inhibit surfactant secretion, and enhance bacterium-cell association (46, 61, 64, 65). It was also found that the SP-A2 product is structurally more stable than the SP-A1 (18). Amino acid differences among SP-A variants are located within the signal peptide, the collagen-like domain, and the carbohydrate recognition domain (CRD) regions of SP-A. For functional studies, differences in the collagen domain and CRD are important because they are found in the mature protein.
Differences in the activities of SP-A1 and SP-A2 could be associated with differences within the collagen-like region where SP-A1 and SP-A2 gene-specific amino acid differences are located. One of the key differences between SP-A1 and SP-A2 is amino acid 85 (Cys85), where SP-A1 has a cysteine and SP-A2 has an arginine (Table 3) . The additional Cys85 in SP-A1 may be involved in the formation of an SP-A intertrimeric or intratrimeric disulfide bond and may account in part for the observed oligomerization pattern differences between SP-A1 and SP-A2 variants (61). These, in turn, may account for the SP-A1 and SP-A2 functional differences. Indeed, we have recently observed that a substitution of Arg85 to Cys85 in SP-A2 results in a functional activity similar to that observed for SP-A1, and substitution of Cys85 to Arg85 in SP-A1 changed the activity of SP-A1 to one similar to that of SP-A2 (our unpublished data). Alternatively, amino acid Cys85/Arg85 may alter the structural stability of SP-A1 and SP-A2 (18); structural differences, in turn, may have an impact on the functional capabilities of the CRD region.
TABLE 3.
SP-A1 and SP-A2 amino acid differencesa
| SP-A variant or SNPc | Nucleotide or codon corresponding to indicated amino acid positionb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 19 | 50 | 66* | 73* | 81* | 85* | 91 | 219 | 223 | |
| SP-A1 | ||||||||||
| 6A2 | A | T | G | TG | G | A | T | C | C | C |
| 6A4 | A | T | C | TG | G | A | T | C | T | C |
| SP-A2 | ||||||||||
| 1A0 | A | C | G | CA | A | G | C | G | C | C |
| 1A1 | C | C | G | CA | A | G | C | G | C | A |
| SNP | AAC (Asn) | GCG (Ala) | CTC (Leu) | ATG (Met) | GAT (Asp) | ATC (Ile) | TGT (Cys) | CCT (Pro) | CGG (Arg) | CAG (Gln) |
| SNP | ACC (Thr) | GTG (Val) | GTC (Val) | ACA (Thr) | AAT (Asn) | GTC (Val) | CGT (Arg) | GCT (Ala) | TGG (Trp) | AAG (Lys) |
See reference 15 for more details.
Boldface indicates a critical position. In the last two rows (SNPs), underlining indicates the nucleotide changes among SP-A1 and SP-A2 variants, and the encoded amino acids are in parentheses. Amino acids 1 to 18/20 comprise a signal peptide; the carboxy terminus of the signal peptide exhibits heterogeneity, therefore resulting in SP-A variants that differ at the N-terminal amino acid (61). Amino acids 19/21 to 27 comprise an N-terminal region with interchain disulfide bonds, amino acids 28 to 100 a collagen-like domain, amino acids 101 to 133 a triple-helical neck region, and amino acids 134 to 248 a C-terminal carbohydrate recognition domain. Amino acid positions indicated by asterisks (66, 73, 81, and 85) are SP-A1 and SP-A2 gene specific.
SNP, single nucleotide polymorphism.
Moreover, differences between variants of SP-A1 or SP-A2 may be due to amino acid differences in CRD (Table 3). The SP-A2 variants, 1A0 and 1A1, differ in CRD at position 223 (Gln for 1A0 and Lys for 1A1), and the SP-A1 6A2 differs from 6A4 at CRD amino acid position 219 (Arg for 6A2 and Trp for 6A4). Although the nature of functional differences between variants of a given SP-A gene is not known, the Arg219/Trp219 has been shown to alter SP-A protein behavior, as assessed by use of absorption spectra (56), and in an another system, a single Gln-to-Lys mutation resulted in a change of cofactor specificity (25).
Alveolar macrophages are important components of the lung innate host defense. However, AMs isolated from different species can differ in their abilities to phagocytize bacteria (47). Although the responses of rat and human AMs to SP-A1 and SP-A2 stimulation were similar qualitatively, species-specific differences in terms of quantity were observed. The SP-A2 variants enhanced the association of P. aeruginosa with both rat and human AMs more effectively than did SP-A1 (for comparison, see Fig. 1, 3, and 4), but the overall activity of rat AMs in response to SP-A variants was higher than that of human AMs. Therefore, although species differences may exist in the degree of activity, qualitatively the SP-A1 and SP-A2 responses are similar with both rat and human AMs, indicating that rat AMs are an appropriate model for human AMs when the phagocytic effects of SP-A variants are studied.
In conclusion, we have shown that although differences exist among SP-A variants, SP-A2 gene-specific variants stimulate phagocytosis of P. aeruginosa by rat or human alveolar macrophages more than do SP-A1 variants and that mammalian posttranslational modifications positively influence the activity of SP-A in phagocytosis. We speculate that given the differences in function, the overall SP-A functional activity in the lung depends upon the relative levels or the ratio of SP-A1 to SP-A2 rather than upon the total SP-A content (i.e., without regard to the SP-A1 and SP-A2 proportions). We further speculate that a derangement in the regulation of SP-A1 or SP-A2 gene expression could result in an “inadequate” (or “unfavorable”) SP-A1-to-SP-A2 ratio for normal host defense and that this putative functional compromise contributes to lung disease severity.
Acknowledgments
This work was supported by the National Institutes of Health Grant (NIH HL 68947).
We gratefully acknowledge the Gift of Life Donor Program (Philadelphia, PA) and the generosity of the organ donor families for allowing these organs, which were not suitable for transplantation, to be utilized to advance the understanding of human disease.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Bear, C. E., C. H. Li, N. Kartner, R. J. Bridges, T. J. Jensen, M. Ramjeesingh, and J. R. Riordan. 1992. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 68:809-818. [DOI] [PubMed] [Google Scholar]
- 2.Blau, H., S. Riklis, J. F. Van Iwaarden, F. X. McCormack, and M. Kalina. 1997. Nitric oxide production by rat alveolar macrophages can be modulated in vitro by surfactant protein A. Am. J. Physiol. Lung Cell. Mol. Physiol. 272:L1198-L1204. [DOI] [PubMed] [Google Scholar]
- 3.Borron, P., F. X. McCormack, B. M. Elhalwagi, Z. C. Chroneos, J. F. Lewis, S. Zhu, J. R. Wright, V. L. Shepherd, F. Possmayer, K. Inchley, and L. J. Fraher. 1998. Surfactant protein A inhibits T cell proliferation via its collagen-like tail and a 210-kDa receptor. Am. J. Physiol. Lung Cell. Mol. Physiol. 275:L679-L686. [DOI] [PubMed] [Google Scholar]
- 4.Borron, P., J. C. McIntosh, T. R. Korfhagen, J. A. Whitsett, J. Taylor, and J. R. Wright. 2000. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L840-L847. [DOI] [PubMed] [Google Scholar]
- 5.Bragonzi, A., D. Worlitzsch, G. B. Pier, P. Timpert, M. Ulrich, M. Hentzer, J. B. Andersen, M. Givskov, M. Conese, and G. Doring. 2005. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J. Infect. Dis. 192:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E. J., and D. Gresham. 2003. Phagocytosis, p. 1108. In W. E. Paul (ed.), Fundamental immunology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 7.Campbell, P., B. P. Canono, and D. A. Drevets. 1994. Measurement of bacterial ingestion and killing by macrophages, p. 14.6.1-14.6.13. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, National Institutes of Health, vol. 3, suppl. 12. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 8.Davidson, D. J., M. J. Fraser, and F. J. Castellino. 1990. Oligosaccharide processing in the expression of human plasminogen cDNA by lepidopteran insect (Spodoptera frugiperda) cells. Biochemistry 29:5584-5590. [DOI] [PubMed] [Google Scholar]
- 9.De Brauwer, E., J. Jacobs, F. Nieman, C. Bruggeman, and M. Drent. 1999. Test characteristics of acridine orange, Gram, and May-Grunwald-Giemsa stains for enumeration of intracellular organisms in bronchoalveolar lavage fluid. J. Clin. Microbiol. 37:427-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiAngelo, S., Z. Lin, G. Wang, S. Phillips, M. Ramet, J. Luo, and J. Floros. 1999. Novel, non-radioactive, simple and multiplex PCR-cRFLP methods for genotyping human SP-A and SP-D marker alleles. Dis. Markers 15:269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding, J., T. M. Umstead, J. Floros, and D. S. Phelps. 2004. Factors affecting SP-A-mediated phagocytosis in human monocytic cell lines. Respir. Med. 98:637-650. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, I. R., M. E. Jones, H. A. Barr, S. Orgeig, A. J. Crockett, C. F. McDonald, and T. E. Nicholas. 1994. Composition of human pulmonary surfactant varies with exercise and level of fitness. Am. J. Respir. Crit. Care Med. 149:1619-1627. [DOI] [PubMed] [Google Scholar]
- 13.Floros, J., and D. S. Phelps. 2002. Pulmonary surfactant protein A; structure, expression, and its role in innate host defense, p. 87-102. In G. Nakos and M. Lekka (ed.), Surfactant-update of intensive care medicine, vol. 6, University of Ioannina, Ioannina, Greece. [Google Scholar]
- 14.Floros, J., R. Steinbrink, K. Jacobs, D. Phelps, R. Kriz, M. Recny, L. Sultzman, S. Jones, H. W. Taeusch, H. A. Frank, et al. 1986. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J. Biol. Chem. 261:9029-9033. [PubMed] [Google Scholar]
- 15.Floros, J., G. Wang, and Z. Lin. 2005. Genetic diversity of human SP-A, a molecule with innate host defence and surfactant-related functions; characteristics, primary function, and significance. Curr. Pharmacogenomics 3:87-95. [Google Scholar]
- 16.Furmanek, A., and J. Hofsteenge. 2000. Protein C-mannosylation: facts and questions. Acta Biochim. Pol. 47:781-789. [PubMed] [Google Scholar]
- 17.Garcia-Verdugo, I., F. Sanchez-Barbero, F. U. Bosch, W. Steinhilber, and C. Casals. 2003. Effect of hydroxylation and N187-linked glycosylation on molecular and functional properties of recombinant human surfactant protein A. Biochemistry 42:9532-9542. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Verdugo, I., G. Wang, J. Floros, and C. Casals. 2002. Structural analysis and lipid-binding properties of recombinant human surfactant protein a derived from one or both genes. Biochemistry 41:14041-14053. [DOI] [PubMed] [Google Scholar]
- 19.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 20.Griese, M., R. Essl, R. Schmidt, E. Rietschel, F. Ratjen, M. Ballmann, and K. Paul. 2004. Pulmonary surfactant, lung function, and endobronchial inflammation in cystic fibrosis. Am. J. Respir. Crit. Care Med. 170:1000-1005. [DOI] [PubMed] [Google Scholar]
- 21.Haagsman, H. P., S. Hawgood, T. Sargeant, D. Buckley, R. T. White, K. Drickamer, and B. J. Benson. 1987. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J. Biol. Chem. 262:13877-13880. [PubMed] [Google Scholar]
- 22.Hickling, T. P., H. Clark, R. Malhotra, and R. B. Sim. 2004. Collectins and their role in lung immunity. J. Leukoc. Biol. 75:27-33. [DOI] [PubMed] [Google Scholar]
- 23.Hickman-Davis, J. M., J. Gibbs-Erwin, J. R. Lindsey, and S. Matalon. 2004. Role of surfactant protein-A in nitric oxide production and mycoplasma killing in congenic C57BL/6 mice. Am. J. Respir. Cell Mol. Biol. 30:319-325. [DOI] [PubMed] [Google Scholar]
- 24.Honda, Y., H. Takahashi, Y. Kuroki, T. Akino, and S. Abe. 1996. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest 109:1006-1009. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh, J. Y., G. Y. Liu, G. G. Chang, and H. C. Hung. 2006. Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial NAD(P)+-dependent malic enzyme: functional roles of glutamine 362. J. Biol. Chem. 281:23237-23245. [DOI] [PubMed] [Google Scholar]
- 26.Huang, W., G. Wang, D. S. Phelps, H. Al-Mondhiry, and J. Floros. 2002. Combined SP-A-bleomycin effect on cytokines by THP-1 cells: impact of surfactant lipids on this effect. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L94-L102. [DOI] [PubMed] [Google Scholar]
- 27.Huang, W., G. Wang, D. S. Phelps, H. Al-Mondhiry, and J. Floros. 2004. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L546-L553. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis, D. L. 2003. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology 310:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabha, K., J. Schmegner, Y. Keisari, H. Parolis, J. Schlepper-Schaeffer, and I. Ofek. 1997. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am. J. Physiol. 272:L344-L352. [DOI] [PubMed] [Google Scholar]
- 30.Karinch, A. M., G. Deiter, P. L. Ballard, and J. Floros. 1998. Regulation of expression of human SP-A1 and SP-A2 genes in fetal lung explant culture. Biochim. Biophys. Acta 1398:192-202. [DOI] [PubMed] [Google Scholar]
- 31.Karinch, A. M., D. E. deMello, and J. Floros. 1997. Effect of genotype on the levels of surfactant protein A mRNA and on the SP-A2 splice variants in adult humans. Biochem. J. 321:39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karinch, A. M., and J. Floros. 1995. 5′ splicing and allelic variants of the human pulmonary surfactant protein A genes. Am. J. Respir. Cell Mol. Biol. 12:77-88. [DOI] [PubMed] [Google Scholar]
- 33.Kartner, N., O. Augustinas, T. J. Jensen, A. L. Naismith, and J. R. Riordan. 1992. Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat. Genet. 1:321-327. [DOI] [PubMed] [Google Scholar]
- 34.Khubchandani, K. R., R. E. Oberley, and J. M. Snyder. 2001. Effects of surfactant protein A and NaCl concentration on the uptake of Pseudomonas aeruginosa by THP-1 cells. Am. J. Respir. Cell Mol. Biol. 25:699-706. [DOI] [PubMed] [Google Scholar]
- 35.Kremlev, S. G., and D. S. Phelps. 1994. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am. J. Physiol. Lung Cell. Mol. Physiol. 267:L712-L719. [DOI] [PubMed] [Google Scholar]
- 36.Kremlev, S. G., T. M. Umstead, and D. S. Phelps. 1994. Effects of surfactant protein A and surfactant lipids on lymphocyte proliferation in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 267:L357-L364. [DOI] [PubMed] [Google Scholar]
- 37.Krizkova, L., R. Sakthivel, S. A. Olowe, P. K. Rogan, and J. Floros. 1994. Human SP-A: genotype and single-strand conformation polymorphism analysis. Am. J. Physiol. Lung Cell. Mol. Physiol. 266:L519-L527. [DOI] [PubMed] [Google Scholar]
- 38.Kumar, A. R., and J. M. Snyder. 1998. Differential regulation of SP-A1 and SP-A2 genes by cAMP, glucocorticoids, and insulin. Am. J. Physiol. 274:L177-L185. [DOI] [PubMed] [Google Scholar]
- 39.LeVine, A. M., K. E. Kurak, M. D. Bruno, J. M. Stark, J. A. Whitsett, and T. R. Korfhagen. 1998. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 19:700-708. [DOI] [PubMed] [Google Scholar]
- 40.Li, P., X. G. Gao, R. O. Arellano, and V. Renugopalakrishnan. 2001. Glycosylated and phosphorylated proteins-expression in yeast and oocytes of Xenopus: prospects and challenges-relevance to expression of thermostable proteins. Protein Expr. Purif. 22:369-380. [DOI] [PubMed] [Google Scholar]
- 41.Li, Z., M. R. Kosorok, P. M. Farrell, A. Laxova, S. E. West, C. G. Green, J. Collins, M. J. Rock, and M. L. Splaingard. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581-588. [DOI] [PubMed] [Google Scholar]
- 42.Lukacs, G. L., X. B. Chang, C. Bear, N. Kartner, A. Mohamed, J. R. Riordan, and S. Grinstein. 1993. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J. Biol. Chem. 268:21592-21598. [PubMed] [Google Scholar]
- 43.Marchal, I., D. L. Jarvis, R. Cacan, and A. Verbert. 2001. Glycoproteins from insect cells: sialylated or not? Biol. Chem. 382:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariencheck, W. I., J. Savov, Q. Dong, M. J. Tino, and J. R. Wright. 1999. Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of P. aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 277:L777-L786. [DOI] [PubMed] [Google Scholar]
- 45.McCormack, F. X., H. M. Calvert, P. A. Watson, D. L. Smith, R. J. Mason, and D. R. Voelker. 1994. The structure and function of surfactant protein A. Hydroxyproline- and carbohydrate-deficient mutant proteins. J. Biol. Chem. 269:5833-5841. [PubMed] [Google Scholar]
- 46.Mikerov, A. N., T. M. Umstead, W. Huang, W. Liu, D. S. Phelps, and J. Floros. 2005. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L150-L158. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen, B. Y., P. K. Peterson, H. A. Verbrugh, P. G. Quie, and J. R. Hoidal. 1982. Differences in phagocytosis and killing by alveolar macrophages from humans, rabbits, rats, and hamsters. Infect. Immun. 36:504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Riordan, C. R., A. Erickson, C. Bear, C. Li, P. Manavalan, K. X. Wang, J. Marshall, R. K. Scheule, J. M. McPherson, S. H. Cheng, et al. 1995. Purification and characterization of recombinant cystic fibrosis transmembrane conductance regulator from Chinese hamster ovary and insect cells. J. Biol. Chem. 270:17033-17043. [DOI] [PubMed] [Google Scholar]
- 49.O'Riordan, C. R., A. L. Lachapelle, J. Marshall, E. A. Higgins, and S. H. Cheng. 2000. Characterization of the oligosaccharide structures associated with the cystic fibrosis transmembrane conductance regulator. Glycobiology 10:1225-1233. [DOI] [PubMed] [Google Scholar]
- 50.Phelps, D. S., and J. Floros. 1988. Proline hydroxylation alters the electrophoretic mobility of pulmonary surfactant-associated protein A. Electrophoresis 9:231-233. [DOI] [PubMed] [Google Scholar]
- 51.Phelps, D. S., J. Floros, and H. W. Taeusch, Jr. 1986. Post-translational modification of the major human surfactant-associated proteins. Biochem. J. 237:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postle, A. D., A. Mander, K. B. Reid, J. Y. Wang, S. M. Wright, M. Moustaki, and J. O. Warner. 1999. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 20:90-98. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds, H. Y. 2002. Modulating airway defenses against microbes. Curr. Opin. Pulm. Med. 8:154-165. [DOI] [PubMed] [Google Scholar]
- 54.Rishi, A., D. Hatzis, K. McAlmon, and J. Floros. 1992. An allelic variant of the 6A gene for human surfactant protein A. Am. J. Physiol. Lung Cell. Mol. Physiol. 262:L566-L573. [DOI] [PubMed] [Google Scholar]
- 55.Scavo, L. M., R. Ertsey, and B. Q. Gao. 1998. Human surfactant proteins A1 and A2 are differentially regulated during development and by soluble factors. Am. J. Physiol. Lung Cell. Mol. Physiol. 275:L653-L669. [DOI] [PubMed] [Google Scholar]
- 56.Selman, M., H. M. Lin, M. Montano, A. L. Jenkins, A. Estrada, Z. Lin, G. Wang, S. DiAngelo, X. Guo, T. M. Umstead, C. M. Lang, A. Pardo, D. S. Phelps, and J. Floros. 2003. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum. Genet. 113:542-550. [DOI] [PubMed] [Google Scholar]
- 56a.Tagaram, H. R., G. Wang, T. M. Umstead, A. N. Mikerov, N. J. Thomas, G. R. Graff, J. C. Hess, M. J. Thomassen, M. S. Kavuru, D. S. Phelps, and J. Floros. 22December2006. Characterization of a human surfactant protein A1 (SP-A1) gene-specific antibody; SP-A1 content variation among individuals of varying age and pulmonary health. Am. J. Physiol. Lung Cell Mol. Physiol. [Epub ahead of print.] [DOI] [PubMed]
- 57.Takahashi, H., Y. Honda, Y. Kuroki, K. Imai, and S. Abe. 1995. Pulmonary surfactant protein A: a serum marker of pulmonary fibrosis in patients with collagen vascular diseases. Clin. Chim. Acta 239:213-215. [DOI] [PubMed] [Google Scholar]
- 58.van Iwaarden, F., B. Welmers, J. Verhoef, H. P. Haagsman, and L. M. van Golde. 1990. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2:91-98. [DOI] [PubMed] [Google Scholar]
- 59.van Iwaarden, J. F., J. C. Pikaar, J. Storm, E. Brouwer, J. Verhoef, R. S. Oosting, L. M. van Golde, and J. A. van Strijp. 1994. Binding of surfactant protein A to the lipid A moiety of bacterial lipopolysaccharides. Biochem. J. 303:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voss, T., K. Melchers, G. Scheirle, and K. P. Schafer. 1991. Structural comparison of recombinant pulmonary surfactant protein SP-A derived from two human coding sequences: implications for the chain composition of natural human SP-A. Am. J. Respir. Cell Mol. Biol. 4:88-94. [DOI] [PubMed] [Google Scholar]
- 61.Wang, G., S. R. Bates-Kenney, J. Q. Tao, D. S. Phelps, and J. Floros. 2004. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry 43:4227-4239. [DOI] [PubMed] [Google Scholar]
- 62.Wang, G., X. Guo, and J. Floros. 2005. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L497-L508. [DOI] [PubMed] [Google Scholar]
- 63.Wang, G., X. Guo, and J. Floros. 2003. Human SP-A 3′-UTR variants mediate differential gene expression in basal levels and in response to dexamethasone. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L738-L748. [DOI] [PubMed] [Google Scholar]
- 64.Wang, G., D. S. Phelps, T. M. Umstead, and J. Floros. 2000. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L946-L954. [DOI] [PubMed] [Google Scholar]
- 65.Wang, G., T. M. Umstead, D. S. Phelps, H. Al-Mondhiry, and J. Floros. 2002. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ. Health Perspect. 110:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei, X., R. Eisman, J. Xu, A. D. Harsch, A. E. Mulberg, C. L. Bevins, M. C. Glick, and T. F. Scanlin. 1996. Turnover of the cystic fibrosis transmembrane conductance regulator (CFTR): slow degradation of wild-type and delta F508 CFTR in surface membrane preparations of immortalized airway epithelial cells. J. Cell. Physiol. 168:373-384. [DOI] [PubMed] [Google Scholar]
- 67.Wright, J. R., and D. C. Youmans. 1993. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophage. Am. J. Physiol. Lung Cell. Mol. Physiol. 264:L338-L344. [DOI] [PubMed] [Google Scholar]