Abstract
Clostridium perfringens is capable of producing up to 15 toxins, including alpha-toxin (CPA), beta-toxin (CPB), epsilon-toxin (ETX), enterotoxin, beta2-toxin (CPB2), and perfringolysin O. Type B isolates, which must produce CPA, CPB, and ETX, are associated with animal illnesses characterized by sudden death or acute neurological signs, with or without intestinal damage. Type B pathogenesis in ruminants is poorly understood, with some animals showing lesions and clinical signs similar to those caused by either type C or type D infections. It is unknown whether host or environmental conditions are dominant for determining the outcome of type B disease or if disease outcomes are determined by variable characteristics of type B isolates. To help clarify this issue, 19 type B isolates were evaluated for toxin production during late-log-phase growth via quantitative Western blotting and by biological activity assays. Most type B isolates produced CPB levels similar to those produced by type C isolates in vitro and have the potential to produce genotype C-like disease. The lethality of type B isolate supernatants administered intravenously to mice was evaluated with or without prior trypsin treatment, and monoclonal antibody neutralization studies also were performed. Correlation analyses comparing toxin levels in type B supernatants versus lethality and neutralization studies both found that the main contributor to lethality without pretreatment with trypsin was CPB, whereas neutralization studies indicated that CPB and ETX were both important after trypsin pretreatment. At least part of the CPB produced by type B isolates remained active after trypsin treatment. However, the overall lethalities of most supernatants were lower after trypsin pretreatment. Also, there was a significant association between ETX, CPB2, and CPA production in vitro among type B isolates. However, our results suggest that both CPB and ETX are likely the most important contributors to the pathogenesis of C. perfringens type B infections in domestic animals.
The gram-positive, spore-forming anaerobe Clostridium perfringens is a major human pathogen and also is considered the most important cause of clostridial enteric disease in domestic animals (17, 24). This microorganism is classified into five toxinotypes based upon the production of four major lethal toxins (alpha-toxin [CPA], beta-toxin [CPB], epsilon-toxin [ETX], and iota-toxin). However, the virulence of C. perfringens is very complex because this bacterium produces combinations of up to 15 different toxins, including the typing toxins and other lethal toxins such as perfringolysin O (PFO), enterotoxin (encoded by cpe), and beta2-toxin (CPB2) (24).
C. perfringens type B isolates produce disease in several animal species and also can be found, although infrequently, in the intestines of healthy animals (13, 25). In sheep, C. perfringens type B produces enterotoxemias that initiate with the proliferation of the microorganism in the gut and are accompanied by production of toxins that act locally but also are absorbed through the intestinal mucosa (18). C. perfringens type B isolates also are associated with disease in goats, calves, and foals (24). For reasons that are not yet clear, diseases caused by C. perfringens genotype B isolates have been reported only in limited regions of the world (2, 16, 24).
By definition, C. perfringens type B isolates must produce, at minimum, the potent and lethal exotoxins CPA, CPB, and ETX. However, the pathological role of these toxins during C. perfringens type B-associated infections is mostly unknown. CPB is reportedly very sensitive to trypsin digestion, and animals with low levels of intestinal trypsin (such as newborn animals) are usually the most susceptible to infection by C. perfringens isolates producing CPB (24). In contrast, ETX requires proteolysis via trypsin or other intestinal or bacterial proteases to become completely active (19). These opposing effects of trypsin on ETX and CPB activity may indicate that when both toxins are present in the intestine during type B-associated infections, different intestinal conditions might select for the predominant activity of ETX over CPB or vice versa.
Most knowledge about the pathogenesis of C. perfringens type B infections comes from descriptions of natural disease produced by this microorganism, especially in sheep. In sheep, illness is characterized by sudden death or acute neurological signs with or without hemorrhagic diarrhea (2, 16). If present, intestinal lesions are characterized by diffuse or multifocal necrohemorrhagic enteritis, predominantly in the ileum, with serosanguineous fluid in the abdominal cavity and serosal petechiae (2). These findings are similar to those observed during infections caused by C. perfringens type C isolates (18), which produce CPA and CPB but not ETX. Alternatively, type B-associated diseases also can result in sudden death or neurological signs (18, 24) similar to those observed in ovine enterotoxemia caused by C. perfringens type D, which produces CPA and ETX but not CPB (2, 8). When sheep with type B enterotoxemia survive for a few days, focal symmetrical encephalomalacia may be present. As in chronic type D ovine enterotoxemia, focal symmetrical encephalomalacia of chronic type B infection is thought to be mediated by ETX (2, 16). Thus, while C. perfringens type B infections can reproduce the characteristics of both type C and D enterotoxemias, it is unknown whether conditions imposed by the host or the environment or differences in toxin production by individual type B isolates are the dominant factors determining disease outcome.
The present study was performed to evaluate the lethalities of trypsin-treated and non-trypsin-treated culture supernatants of C. perfringens type B isolates from clinical cases of animal enterotoxemia by use of a model involving intravenous (i.v.) injection of supernatants into mice. Quantifying the levels of toxins produced by type B isolates during vegetative growth allowed the lethalities of these supernatants to be dissected into the major toxin components. Correlation analysis of toxin concentrations and neutralizing monoclonal antibody (MAb) studies were then used to assess the contribution of each toxin to mouse lethality.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
The 26 putative C. perfringens type B strains examined in this study originated from different sources as follows: the 22 Burroughs-Wellcome (BW) isolates (kindly provided by Russell Wilkinson, University of Melbourne, Australia) were collected from diseased animals throughout the United Kingdom during the 1940s to 1960s and have been stored in a lyophilized form since then, one isolate was kindly provided by J. Glenn Songer (University of Arizona), and the remaining three isolates were from our laboratory collections.
All C. perfringens isolates were initially grown overnight at 37°C under anaerobic conditions on TSC agar medium (SFP agar [Difco Laboratories], 0.04% d-cycloserine [Sigma Aldrich Co.]) to ensure culture purity. FTG (fluid thioglycolate medium [Difco Laboratories]) or TGY (3% tryptic soy broth [Becton, Dickinson and Company], 2% glucose [Sigma Aldrich Co.], 1% yeast extract [Becton, Dickinson and Company], 0.1% l-cysteine [Sigma Aldrich Co.]) were used for growing broth cultures.
Multiplex PCR.
To determine the toxin genotype of the putative 26 type B isolates, a multiplex PCR assay that detects genes encoding the four typing toxins plus the enterotoxin and the beta2-toxin was used. Brain heart infusion agar (Becton, Dickinson and Company) plates were inoculated with a putative genotype B isolate, which was then grown anaerobically overnight at 37°C. Three or four colonies were picked from each plate and used to prepare template DNA, as described previously (29). Those DNA preparations were then mixed with TaqComplete master mix (Genechoice) and subjected to multiplex PCR (10). Products from each multiplex PCR were run on 2% agarose gels. After electrophoresis, those gels were stained with ethidium bromide for visualization. Isolates carrying cpa, cpb, and etx genes are genotypically B and henceforth are referred to as type B isolates.
PCR detection and sequencing of the pfoA gene.
To determine if selected PFO− type B isolates carry the pfoA gene, two separate PCRs were used to amplify internal portions of this gene. Primer sequences and amplification conditions were the same as those used previously (9). PCR products were separated on 1.5% agarose gels and stained with ethidium bromide. In addition, the entire pfoA gene (including the promoter region, the coding sequence, and the 3′ terminus) also was sequenced from selected PFO− and PFO+ genotype B isolates to determine if mutations were present that could explain the lack of PFO production for those isolates that were PCR positive for the pfoA gene. PCR products were generated and sequenced using the primers and amplification conditions used previously (9). Sequencing was performed at the University of Pittsburgh core sequencing facility (http://www.genetics.pitt.edu/services.html), and sequences were then analyzed and aligned using BioEdit.
Optimization of vegetative growth conditions for CPB and ETX production.
Isolated genotype B colonies on a TSC plate were inoculated into FTG medium, which was then incubated overnight at 37°C. A 0.1-ml aliquot of this overnight FTG culture was transferred into 10 ml of FTG, TGY, brain heart infusion broth (Difco Laboratories), or differential reinforced clostridial broth (EM Science). Those cultures were incubated at 37°C until late log phase, when samples were removed for CPB and ETX Western blot analysis (see below). Cultures were monitored for growth by measuring the optical density at 600 nm. Samples of cultures grown in TGY (determined to be the most consistent medium for CPB and ETX production [see Results]) were taken at 1-hour intervals; after centrifugation of those samples, supernatants were mixed 1:1 with 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, boiled for 5 min, and then loaded on 12% SDS-PAGE gels for CPB or ETX Western blotting (see below).
Preparation of culture supernatants from type B isolates for toxin quantification.
Single isolated colonies were inoculated into 10 ml of FTG medium, which was incubated overnight at 37°C. A 0.1-ml aliquot then was inoculated into 10 ml of TGY and grown at 37°C to late log phase. Levels of lethal toxins produced during vegetative growth became maximal during late log phase, based upon pilot experiments for CPB and ETX (see Results) and previous reports for CPA, PFO, and CPB2 (9, 23). Therefore, genotype B isolates were grown in TGY medium to late log phase. Bacteria were removed from each TGY culture by centrifugation, and the resultant supernatants were filter sterilized with a 0.45-μm filter. The filtered supernatants were used to quantify the production of the different toxins as well as to determine the lethal activity in mice.
Quantification of toxin levels in culture supernatants from type B isolates.
Because specific activity assays are not available for CPB, ETX, or CPB2, the presence of those three lethal toxins in the culture supernatants was assessed by Western blotting (9, 23). Phospholipase C activity on egg yolk agar plates and hemolytic activity against horse erythrocytes were measured (23) to determine, respectively, CPA and PFO activities in culture supernatants of our collection of genotype B isolates.
Comparative analysis of lethal toxin levels in culture supernatants from different C. perfringens toxinotypes.
Toxin levels in genotype B culture supernatants were compared against the toxin levels produced by genotype A, genotype C, and genotype D isolates analyzed by use of the same conditions and methods described before (9, 23). The average toxin production levels between genotypes were evaluated by the Student t test. A P value of <0.05 was considered significant.
Mouse lethality assays performed with type B culture supernatants.
For initial toxicity testing, each sterile culture supernatant was aliquoted into two portions. Because trypsin treatment is necessary for complete ETX activation (19), one of the aliquots was treated with 0.05% trypsin (Sigma) for 30 min at 37°C, while the other was incubated without trypsin. Pilot studies performing Western blotting of trypsin-treated and non-trypsin-treated type B culture supernatants (data not shown) confirmed that this trypsin pretreatment fully activated all ETX in the supernatants. Two BALB/c mice (male or female, ∼17 to 20 g; Charles River Laboratories) each received an i.v. injection (via the tail vein) of 0.5 ml of the trypsin-treated supernatant, while two other mice each received a similar 0.5-ml i.v. injection of the non-trypsin-treated supernatant. All mice were observed for up to 48 h to monitor for death or the development of neurological distress (at which point those mice were immediately euthanized with CO2). Neurological signs included circling, twitching of the hind legs, or severe depression preceding death. Supernatants that killed or produced neurological signs within 48 h were considered to possess lethal activity.
For supernatants having lethal activity, a 50% lethal dose per milliliter (LD50/ml) was determined as described previously (9) using the up-and-down method (4). Negative control mice received an i.v. injection of 1% peptone water with or without trypsin (two mice each). Positive control mice received i.v. injections containing twofold dilutions of a filtered C. perfringens genotype B (enterotoxin-negative and CPB2-negative) culture supernatant of known toxicity.
For these lethality assays, at least two independent batches of culture supernatants were prepared and tested in mice, as described above. In a few cases in which the LD50/ml values for different culture supernatant preparations from the same isolate showed a greater-than-twofold dilution difference, the samples were retested. LD50/ml results were averaged for each isolate. All experimental procedures were approved by the Animal Care and Use Committee of the University of California, Davis (permit 04-11593) and the Animal Care and Use Committee of the California Animal Health and Food Safety Laboratory (permit 34).
MAb neutralization of type B culture supernatant lethality.
To further identify which toxins are responsible for the lethal activity of supernatants prepared from genotype B cultures, MAb neutralization experiments were performed as follows. A portion (1.2 ml) of an undiluted genotype B culture supernatant, prepared as described above, was divided into two aliquots that either were or were not treated with trypsin, as before. A 0.1-ml aliquot of a solution containing 2 mg/ml of anti-ETX (5B7), anti-CPB (CPCN10A2), or anti-CPA neutralizing MAb was then added to each aliquot. Aliquots of culture supernatants were also incubated with all the possible combinations of MAb mentioned above. Mixtures were then brought to 1.2 ml with 1% peptone water and incubated for 30 min at room temperature. A 0.5-ml aliquot of each mixture was injected i.v. into two mice, while an additional pair of mice received a similar i.v. injection of the same sterile trypsin-treated or non-trypsin-treated supernatants that had been identically prepared, except for the omission of the MAb. Previous work using semipurified CPB (9) and pure ETX (23) demonstrated that the MAbs used in this study were specific and neutralizing for the intended toxin.
Nucleotide sequence accession numbers.
Results for the sequencing of the pfoA gene were deposited in GenBank under accession numbers EF165972, EF165973, and EF165974.
RESULTS
PCR analysis of C. perfringens type B isolates.
Multiplex PCR analyses of 26 isolates that had been previously classified as C. perfringens type B isolates by use of either multiplex PCR or the classical toxin neutralization method confirmed that 19 of these isolates were of genotype B (they carried the cpa, cpb, and etx genes). None of those 19 isolates were positive for cpe, but all 19 carried the cpb2 gene. The remaining seven isolates, which had been classified initially as type B by classical toxin-neutralizing methods, were genotyped by multiplex PCR as follows: cpb2-positive genotype A (one isolate), cpe-positive genotype A (one isolate), genotype D (one isolate), or genotype C (four isolates). Those seven isolates were not studied further.
Lethal toxin levels in vegetative culture supernatants of type B strains.
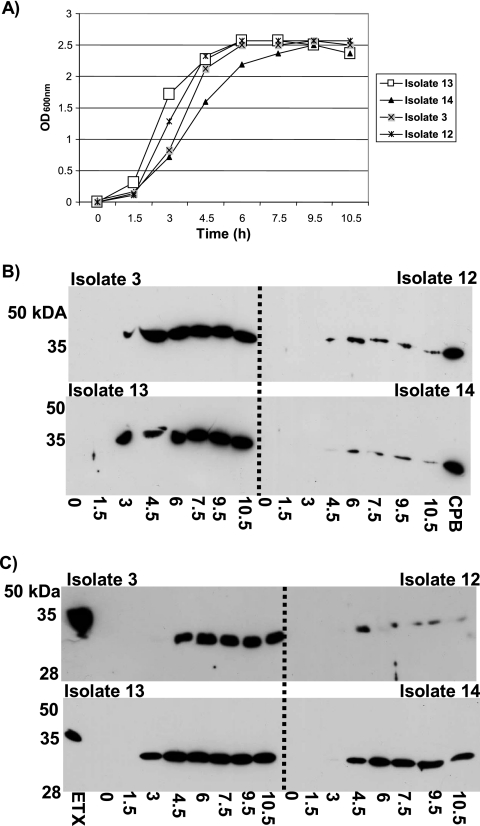
Since CPB and ETX production by C. perfringens type B isolates is not well understood, a few randomly selected isolates were initially grown in four different vegetative growth media (FTG, TGY, brain heart infusion broth, or differential reinforced clostridial broth) to identify the optimal vegetative growth conditions for CPB and ETX production. Similar to previous results for CPB production by genotype C isolates (9) and ETX production by genotype D isolates (23), Western blot analyses (using specific MAbs) determined that TGY was the most consistent broth medium for obtaining strong ETX and CPB production by genotype B isolates (data not shown). Therefore, cultures were grown in TGY medium to determine at which growth phase (Fig. 1A) the type B isolates produce maximum levels of ETX and CPB. Those studies showed that both CPB and ETX production reach a maximum during late log phase (Fig. 1B and C), which is consistent with previous observations concerning CPB and ETX production by genotype C and D isolates, respectively (9, 23).
FIG. 1.
Growth versus production of CPB and ETX. (A) TGY was inoculated with an overnight starter culture and incubated at 37°C. Supernatant samples were collected at the indicated time intervals and measured for turbidity (optical density at 600 nm [OD600nm]). Samples were then electrophoresed on a 12% SDS-polyacrylamide gel and then Western blotted with MAb-CPB (B) or MAb-ETX (C). The times (in h) of samples used for Western blotting are listed underneath the blots in panels B and C. The locations of protein molecular mass standards are shown on the left of each blot (B and C). Purified CPB or ETX was run on each gel as a positive control for Western blotting.
Since late-log-phase TGY cultures of type B isolates showed maximal production of ETX and CPB and, as determined in previous studies, also contain maximal or significant levels of CPB2, PFO, and CPA (23), this growth condition was used to quantify toxin levels for our comparative study. The vegetative culture supernatants from these genotype B isolates would eventually be tested for their lethalities by use of the mouse i.v. injection model, so toxin levels were compared using only this growth condition to minimize the number of animals required for those lethality studies.
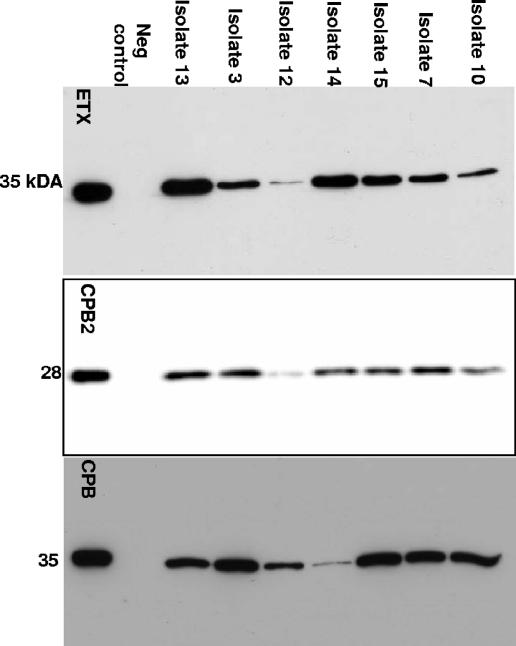
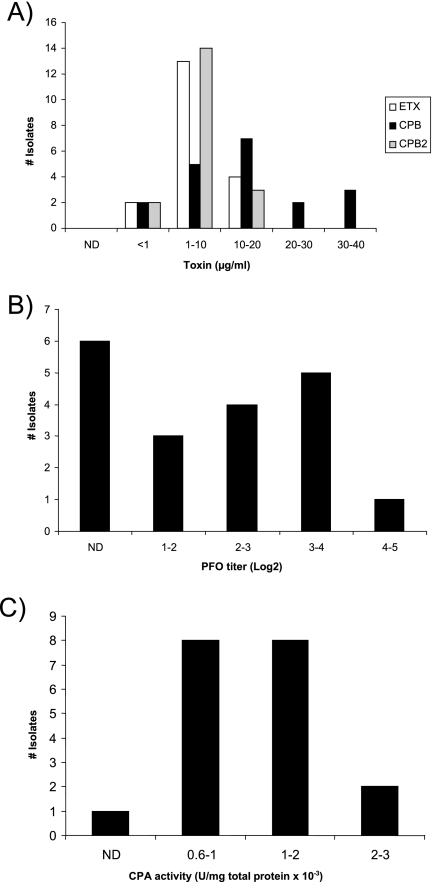
When CPB, ETX, and CPB2 production were quantified by densitometry of Western blot bands by use of a purified toxin standard curve, variations in production levels of these three toxins were observed for our genotype B isolates (Fig. 2). All of the culture supernatants from genotype B isolates contained detectable levels of CPB, ETX, and CPB2 (Fig. 3), usually at more than 1 μg/ml of ETX, CPB, and CPB2. However, a few type B supernatants contained only a low (<1-μg/ml) level of at least one of these three toxins.
FIG. 2.
Western blots for ETX, CPB2, and CPB production by representative type B isolates. Isolates were grown in TGY medium to late log phase, and supernatants were collected and run on 12% SDS-PAGE gels. Detection was performed with MAb-ETX, polyclonal Ab-CPB2, or MAb-CPB. The migration of protein standards are shown to the left of each blot, and isolates are indicated at the top. Purified toxin was run on the far left of each blot as a positive control, and the genotype A cpb2-negative strain ATCC3624 was used as a negative (neg) control for ETX, CPB, and CPB2. MAb-ETX and MAb-CPB blots were visualized with film, whereas the anti-CPB2 blot was visualized with a Bio-Rad imager.
FIG. 3.
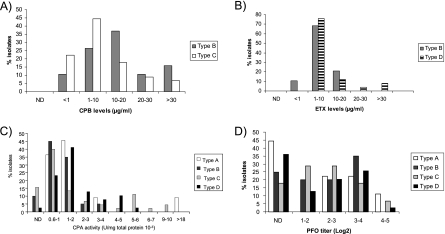
Toxin levels produced by genotype B isolates. Toxin levels in late-log-phase culture supernatants were quantified as described in Materials and Methods. Charts shown are for (A) ETX, CPB, and CPB2 (all quantified via Western blots) and for (B) PFO and (C) CPA (quantified via toxin activity assays). PLC, phospholipase C.
By use of the same toxin production conditions, supernatant levels of CPA and PFO (whose structural gene [pfoA] is not detected by the conventional multiplex PCR assay) were measured using biological assays. While most type B isolates produced CPA and PFO, considerable variations were noted in supernatant PFO and CPA activity levels among different isolates. PFO activity in these supernatants varied from not detectable for six isolates to a log2 titer of 4.82 (Fig. 3B), while CPA levels (Fig. 3C) ranged from not detectable (one isolate) to 2.51 units/mg × 10−3. A poor correlation (R2 < 0.5) was observed between production levels of CPA and CPB, CPA and PFO, CPB and ETX, CPB and CPB2, CPB and PFO, PFO and ETX, and PFO and CPA for the surveyed genotype B isolates. However, some correlation (R2 > 0.5) was observed between their production levels of ETX and CPA, ETX and CPB2, and CPA and CPB2.
Six genotype B isolates did not produce PFO. To determine whether those isolates lacked the pfoA gene, two pfoA PCR assays were performed. Those PCR assays amplified the expected products from all six nonproducing genotype B isolates (data not shown). Subsequent sequencing of the pfoA gene (including the promoter region [VirR binding sites, −35 and −10 boxes, and ribosomal binding site], the structural gene, and the 3′ terminus) revealed no mutations within the promoters or 3′ termini of these six isolates, that is, their failure to produce PFO activity. These sequencing studies did identify several silent mutations and missense mutations within the pfoA open reading frames of genotype B isolates compared to the PFO sequence from strain 13 (a type A strain). These missense mutations resulted in the following amino acid sequence changes in the PFO produced by type B isolates: A71T (also identified in all sequenced pfoA genes from genotype C isolates [9]), A437S, and A449V. Since these differences were also found in the pfoA genes of PFO-producing genotype B isolates, they do not explain why these six type B isolates failed to produce detectable PFO levels.
The toxin production profiles of the type B isolates were then compared (Fig. 4) against the equivalent profiles of the type A, type C, and type D isolates similarly analyzed in previous reports (9, 23). Those comparisons showed that the proportion of isolates producing high (>10-μg/ml) levels of CPB was greater among type B isolates than among genotype C isolates (Fig. 4A). However, the mean CPB production levels did not significantly vary between type B isolates and type C isolates (Table 1). Similarly, type B isolates did not produce higher ETX levels on average than type D isolates (Fig. 4B and Table 1). Most genotype B strains produced CPA levels similar to other those of C. perfringens strains, including genotype A (Fig. 4C and Table 1). From all the isolates analyzed in this and previous studies, C. perfringens type A and C isolates contained the highest CPA producers (Fig. 4C). No differences were observed between PFO production levels of type B isolates and those of the other types (Fig. 4D and Table 1).
FIG. 4.
Comparative analysis of toxin production by different genotypes of C. perfringens isolates in late-log-phase culture supernatants. Results shown are for CPB (A), ETX (B), CPA (C), and PFO (D) as relative percentages of surveyed isolates found in each range of toxin levels, quantified as described in Materials and Methods. For all panels, ND indicates that toxin could not be detected in culture supernatants. Total numbers of isolates were 10 (type A), 19 (type B), 34 (type C), and 39 (type D).
TABLE 1.
Toxin production by C. perfringens genotypes A, B, C, and D
| Toxin | Production by indicated genotype
|
|||
|---|---|---|---|---|
| A (n = 10) | B (n = 19) | C (n = 34) | D (n = 39) | |
| CPAa | 1.6 ± 0.3 | 1.3 ± 0.2 | 2.1 ± 0.8 | 1.9 ± 0.4 |
| PFOa | 2.6 ± 0.6 | 2.3 ± 0.3 | 2.2 ± 0.3 | 1.9 ± 0.4 |
| ETXb | NDc | 6.5 ± 1.2 | ND | 9.9 ± 3.1 |
| CPBb | ND | 16.5 ± 2.3 | 12.3 ± 2.2 | ND |
| CPB2b | ND | 7.6 ± 0.8d | 2.9 ± 0.8e | 1.2 ± 0.4 |
Values are activity levels (U/mg total protein, 10−3) for CPA or titers (log2) for PFO.
Values are micrograms of protein/ml.
ND, not determined.
Statistically significant when compared with genotype C or D.
Statistically significant when compared with genotype D.
Intravenous mouse lethality of genotype B culture supernatants.
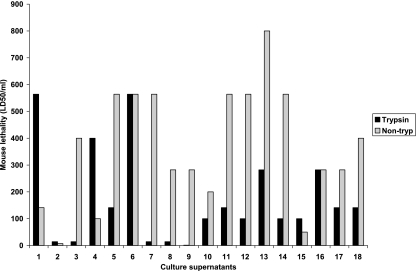
The analysis of toxin production showed that late-log-phase cultures of different type B isolates contain various levels of multiple lethal toxins (Fig. 3 and Table 2) . To assess their lethalities, sterile, filtered dilutions of vegetative culture supernatants of type B isolates were either trypsin treated or not trypsin treated and then injected i.v. into mice to determine their LD50/ml values. The results (Fig. 5) were then compared, for both trypsin treatment and non-trypsin treatment groups, against the amount of each lethal toxin (CPB, CPB2, ETX, PFO, or CPA) present in the tested culture supernatants to evaluate possible correlations between the levels of a particular toxin and type B supernatant lethality.
TABLE 2.
Neutralization of genotype B toxins with MAb
| Isolatea | Genotype | Phenotypeb | Supernatant lethality neutralization with:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No trypsin
|
Trypsin
|
||||||||
| Anti-CPA | Anti-CPB | Anti-ETX | Anti-CPA | Anti-ETX | Anti-CPA + anti-ETX | Anti-CPB + anti-ETX | |||
| 1 | B | ETX<1.0 CPB17.7 CPB2<1.0 CPA0 PFO3.5 | NDc | + | − | ND | − | ND | + |
| 2 | B | ETX8.1 CPB12.2 CPB25.7 CPA1.13 PFO1.6 | − | + | − | − | − | − | + |
| 3 | B | ETX1.2 CPB32.4 CPB27.1 CPA0.87 PFO3.6 | − | + | − | ND | − | ND | + |
| 4 | B | ETX1.3 CPB13.5 CPB29.3 CPA0.5 PFO2.5 | ND | + | − | ND | − | ND | + |
| 5 | B | ETX1.2 CPB8.6 CPB25.4 CPA1.0 PFO4.4 | − | + | − | − | − | − | + |
| 6 | B | ETX13.7 CPB15.8 CPB27.4 CPA1.3 PFO2.5 | ND | + | − | ND | − | ND | ND |
| 7 | B | ETX7.4 CPB23.3 CPB29.3 CPA1.2 PFO0 | ND | + | − | ND | − | ND | ND |
| 8 | B | ETX8.0 CPB36.6 CPB214.0 CPA1.8 PFO3.8 | − | + | − | − | − | − | + |
| 9 | B | ETX4.7 CPB34.1 CPB27.7 CPA0.84 PFO0 | ND | + | − | ND | − | ND | + |
| 10 | B | ETX2.7 CPB5.3 CPB29.0 CPA0.9 PFO1.6 | ND | + | − | ND | − | ND | + |
| 11 | B | ETX4.7 CPB19.5 CPB28.5 CPA1.15 PFO2.1 | − | + | − | − | − | − | + |
Since ETX is an USDA/CDC overlap select toxin, the identities of type B isolates are not listed to avoid misuse due to the identification of high-ETX/CPB-producing isolates.
Values shown in subscript for ETX, CPB, and CPB2 are micrograms of protein/ml, and those for PFO and CPA are activity levels (log2 titer or U of phospholipase C/mg of total protein, 10−3, respectively).
ND, not determined.
FIG. 5.
Lethalities of non-trypsin-treated (non-tryp) and trypsin-treated C. perfringens genotype B isolate culture supernatants expressed as LD50/ml values. Pairs of mice were injected i.v. through the tail vein with dilutions of culture supernatants, and mice were observed for up to 48 h for the development of clinical signs of distress.
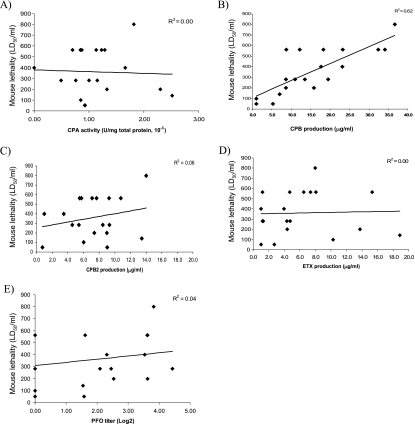
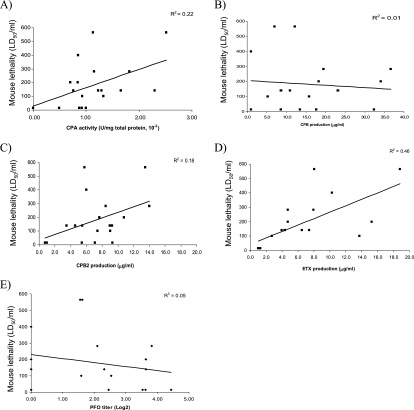
These comparative analyses demonstrated that, for 16 of the 19 type B supernatants, lethal activity either was not affected or was reduced after trypsin treatment (Fig. 5). LD50/ml values for those non-trypsin-treated genotype B supernatants showed a marked correlation (R2 = 0.62) with CPB levels (Fig. 6B) but low or negligible correlation with ETX, CPB2, CPA, or PFO production (Fig. 6A and C to E). In contrast, LD50/ml values of trypsin-treated supernatants showed a marked correlation (R2 = 0.46) with ETX levels (Fig. 7D) and a moderate correlation (R2 = 0.22) with CPA levels (Fig. 7A) but only a low correlation with CPB, CPB2, or PFO levels (Fig. 7B, C, and E).
FIG. 6.
Correlation analyses of genotype B toxin levels versus mouse i.v. LD50/ml values for non-trypsin-treated culture supernatants. LD50/ml values were correlated with the amount of CPA (A), CPB (B), CPB2 (C), ETX (D), and PFO (E) present in each vegetative culture supernatant. A linear equation was then used to draw a best-fit line based on the data points, and the R2 value for this line is reported for each graph.
FIG. 7.
Correlation analyses of genotype B toxin levels versus mouse i.v. LD50/ml values for trypsin-treated culture supernatants. LD50/ml values were correlated with the amounts of CPA (A), CPB (B), CPB2 (C), ETX (D), and PFO (E) present in the vegetative culture supernatants prior to trypsin treatment. A linear equation was then used to draw a best-fit line based on the data points, and the R2 value for this line is reported for each graph.
Correlations between LD50/ml values and different toxin combinations were not stronger than the correlations obtained for ETX or CPB alone, under either trypsin-treated or non-trypsin-treated conditions. For this analysis, toxin data values were standardized to the toxin levels of a reference isolate producing detectable levels of all tested toxins and then expressed as a percentage amount versus that for the reference isolate (this was necessary to compensate for the different methods used to quantify toxin levels, i.e., Western blotting versus activity assays). Those transformed values were then added together in different combinations and correlated with LD50/ml. As examples, two categories analyzed were CPB plus ETX versus LD50/ml or CPB plus ETX plus CPA versus LD50/ml (data not shown).
Neutralization of genotype B culture supernatant lethality with MAb.
To further probe the lethality of the supernatants in the mouse i.v. injection model, neutralization experiments with MAb were performed where 14 pairs of non-trypsin-treated or trypsin-treated genotype B culture supernatants were preincubated with neutralizing MAb specific for either CPB or ETX; a randomly selected group of these supernatants was also preincubated with anti-CPA MAb. For non-trypsinized supernatants, only preincubation with a MAb against CPB neutralized lethality (Table 2). In contrast, the lethality of the trypsin-treated supernatants was neutralized by pretreatment of supernatants with both ETX and CPB MAb (Table 2) but not by pretreatment with any single MAb or combinations of the CPA and ETX or ETX MAbs. The specificities and neutralizing properties of the MAbs used in this study have been demonstrated previously (9, 23).
DISCUSSION
The pathogenesis of C. perfringens type B-associated disease in ruminants is poorly understood. Although CPB is necessary for diseases produced by type C strains and ETX is necessary for type D enterotoxemias (24), little is known about the respective roles of CPB and ETX in the development of type B diseases. Sheep suffering from type B infections can show clinical signs and lesions similar to genotype C infections (e.g., necrotic enteritis) or signs and lesions characteristic of genotype D disease (e.g., neurological alterations) (2, 16, 18). It could be argued that particular conditions in the intestine of the host, such as variations in trypsin activity, are important in determining the final outcome of type B infections. However, another possibility is that variations in the toxinogenic characteristics of individual strains are important in determining the lesions and signs of disease during a type B infection. The main objective of the present study was to analyze the toxinogenic capabilities and lethality of type B isolates.
The precise intestinal conditions under which C. perfringens type B isolates cause disease is unknown, as is the mechanism by which the synthesis and secretion of CPB and ETX are regulated in vitro and in vivo. However, like many C. perfringens diseases, type B diseases may occur under circumstances that maximize toxin production. Based on this concept, our comparative analysis of toxin production by genotype B isolates was performed in vitro using culture conditions that maximize CPB and ETX production.
It could be argued that more-stringent growth conditions might better mimic what happens during natural disease in the natural host (e.g., sheep). However, no information is available in the literature about the levels of CPB and ETX present in the intestine of animals with type B infection. Also, the results of this paper are based on a model using an artificial host (i.e., mouse) and an artificial route of inoculation (i.e., intravenous) in which the objective was to compare the net effects of culture supernatants with the least possible influence of other factors.
In our experiments, the culture supernatants of most type B isolates contained higher levels of CPB than of ETX. However, since activated ETX is about 5 to 30 times more lethal than CPB, it is interesting that trypsin treatment reduced the overall LD50/ml of supernatants from what was seen for non-trypsin-treated samples. These results contrast with the increased supernatant lethality observed after trypsin treatment of type D supernatants (23). In addition, if intestinal absorption of CPB occurs, our results may suggest that intestinal conditions favoring low trypsin activity are more harmful than a trypsin-rich, highly ETX-activating condition during a type B infection. However, our MAb neutralization results could indicate that, for most genotype B strains, intestinal trypsin conditions optimizing the activation of ETX, while retaining some CPB activity, represent the most lethal scenario.
Our current report indicates that most type B isolates tested in this study produce CPB levels similar to those of type C isolates when cultured in vitro under similar conditions. A previous report (7) demonstrated that CPB is the main contributor to lethality for type C isolates. Collectively, these results suggest that most type B isolates have the potential to produce genotype C-like disease. Hemorrhagic enteritis, a sporadic condition affecting lambs up to 3 weeks of age, is caused by C. perfringens isolates of either genotype B or genotype C, consistent with a role for CPB in type B disease. Also, sudden death, hemorrhagic diarrhea, and lesions such as hemorrhagic necrosis of the intestine can be found in animals affected by C. perfringens type B or type C (2, 16).
In this study, a number of C. perfringens isolates previously characterized as type B by the mouse toxin neutralization method were reanalyzed using the multiplex PCR assay. About 27% of these isolates were found to lack the cpb or etx genes required of a genotype B isolate. Some C. perfringens type C and type D isolates previously typed using the classical typing methods were also found to lack the expected typing toxin genes when analyzed by multiplex PCR in previous studies (9, 23). These differences between typing by toxin neutralization methods and that by multiplex PCR could be due to incorrect neutralization typing or a lack of selective pressure for maintaining these toxin genes during in vitro growth and long term storage.
The role of CPB2, a recently discovered toxin (11), in disease is unknown and its biological effects have not been well characterized, but epidemiological studies support a relationship between this toxin and intestinal diseases in several animal species (18). Therefore, it is notable that all genotype B isolates surveyed in this study were positive for cpb2, which contrasts with the ∼40% or 20% of the genotype C or D isolates, respectively, that carry the cpb2 gene (9, 23). Most of the genotype B isolates analyzed here produced CPB2 in vitro, as do most cpb2-positive type A and C isolates obtained from the intestine of pigs with enteric disease (5). These findings contrast with previous observations of C. perfringens type A isolates from livestock with enteritis (5) or horses with gastrointestinal disease (28), where those isolates failed to produce detectable levels of CPB2. On average, the surveyed cpb2-positive genotype B isolates produced a statistically significantly greater amount of CPB2 in vitro than cpb2-positive genotype C and D isolates did (9, 23). While this observation could suggest that CPB2 is involved in the pathogenesis of type B infections, it should be mentioned that none of 54 genotype B isolates recovered from diseased animals in another recent study were positive for cpb2 (12); that is, CPB2 does not appear to be necessary for type B disease. The reasons for the discrepancy between our results and those of the study referred to (12) are unknown, although it is possible that geographical differences are at least partly responsible for these differences. This discrepancy highlights the need for further studies to clarify the connection between CPB2 and pathogenesis.
In terms of toxin gene regulation, it is worth mentioning that we found a significant association between ETX production, CPB2 production, and CPA production in vitro among type B isolates. This association could correspond to coregulated expression of production of these toxins, possibly via the global regulatory cascade of the VirS/VirR system, which controls the expression of both chromosomal virulence genes (including cpa and pfoA [3, 21]) and plasmid-borne virulence genes (including cpb2 and cna [20]). The possible involvement of VirR/VirS in the regulation of toxin genes in type B isolates is currently under study.
The results presented in this report show that although most supernatants of C. perfringens genotype B isolates contain CPB2, CPA, or PFO, these toxins are not essential for lethality in the i.v. mouse model, at least under the conditions used in our studies. In contrast, a marked degree of correlation between CPB levels and the lethality of non-trypsin-treated supernatants was revealed. However, the correlation was weaker than that between CPB and lethality of type C supernatants, which had an r2 value of 0.75 (9). After trypsin treatment, a marked degree of correlation was also observed between ETX levels of type B supernatants and lethality (r2 value of 0.46), although this coefficient of determination was lower than the coefficient of determination (r2 value of 0.90) between ETX levels and the lethality of type D supernatants (23). The trypsin concentration used in this study is known to activate all of the ETX present in culture supernatants (19). However, for all isolates analyzed here, trypsin-treated culture supernatant lethality was abolished only when both anti-CPB and anti-ETX MAbs were used simultaneously, while preincubation with anti-CPB (but not with anti-CPA or anti-ETX) neutralizing MAbs was sufficient to neutralize the lethal activity of non-trypsin-treated supernatants. These results suggest that CPB plays a major role in the lethality of type B supernatants under both low- or non-trypsin conditions and high-trypsin conditions but that ETX becomes important only in the presence of trypsin.
It was surprising that CPB present in genotype B supernatants remained active after trypsin treatment (as demonstrated by neutralization experiments with CPB and ETX MAbs), since CPB purified from type C isolates is very trypsin sensitive (15, 22). Moreover, the same trypsin treatment conditions used in the present study rapidly destroyed the lethal properties of type C culture supernatants (7). Therefore, our present results may suggest that CPB from type B isolates is more trypsin resistant than CPB from type C isolates, possibly due to amino acid sequence variations that remove or hide trypsin hydrolysis sites within the three-dimensional structure of CPB. This hypothesis is currently being explored. Type B disease is a rare condition reported in only a few countries, and literature on this condition is scant. More research is needed to characterize CPB from type B isolates and C. perfringens type B disease in animals.
Type B-mediated diseases are better characterized in sheep than in any other species. Disease in young animals produces hemorrhagic enteritis, with or without diarrhea, and death apparently from toxemia. In older animals, the disease is chronic and involves abdominal pain without diarrhea (24). While C. perfringens type B isolates produce many lethal toxins, the effects of these toxins on the intestine are not similar. ETX does not induce morphological damage to the small intestine of sheep under experimental or natural conditions (2, 6, 26), suggesting that ETX is probably not directly responsible for the intestinal mucosal damage occurring during type B infections. Experimental data for the intestinal effects of purified CPB are not available, although type C culture supernatants containing CPB reportedly induced fluid accumulation and damage to the intestine of rabbits (30). Similar histological findings are described for the intestines of young and adult sheep with type B- and C-mediated diseases (18, 24). While CPB could produce or contribute to small intestinal changes, other toxins such as CPA may also be involved in these changes. Although it remains controversial whether CPA plays a role in necrotic enteritis in chickens (1, 14), CPA can produce fluid accumulation and morphological damage in a sheep intestinal loop model (7). C. perfringens type B can produce a large number of toxins, the pathogenic mechanisms of most of which have not been elucidated. Although we have shown that ETX and CPB are the most important toxins in terms of lethality, we cannot rule out that one or more of those toxins act synergistically with ETX and/or CPB pathogenesis in nature.
On a theoretical basis, C. perfringens genotype B isolates should be the most dangerous of all C. perfringens genotypes, at least from a toxinogenic point of view. The current study confirms that on average, type B isolates produce the greatest number of lethal toxins (typically five different lethal toxins/isolate) of all C. perfringens isolates. The simultaneous production of many lethal toxins provides a broad virulence potential under diverse intestinal protease conditions. Although diseases caused by C. perfringens type B have been reported in limited areas of the world (2, 16, 24), type B isolates can be the most recurrent C. perfringens isolates isolated from lambs with dysentery in those particular areas (12). The limited geographic scope of endemic type B disease may reflect the difficulty in retaining the cpb and etx genes. However, genotype B isolates also have been isolated from healthy animals in a geographical region where cases of diseases produced by this organism have not been reported (27). This finding could indicate that type B disease manifests itself following changes in the normal conditions of the infected animal; that is, type B-associated disease could be an opportunistic event in the biology of C. perfringens.
Additional research is clearly needed to understand the roles of the different toxins, bacterial components, and host factors during intestinal infection of ruminants with C. perfringens type B isolates. Current studies in our laboratories are aimed at using a genetic approach to solving this pathogenesis problem. The varied levels of CPB and ETX present in the supernatants from individual genotype B isolates highlight the importance of optimizing growth conditions and strain selection when C. perfringens vaccines are prepared for use in domestic animals. Finally, our results suggest that type B vaccines should induce immunity against both CPB and ETX to provide complete protection against type B disease.
Acknowledgments
National Institute of Allergy and Infectious Diseases grants AI056177-03, T32 AI49820 (Molecular Microbial Persistence and Pathogenesis Graduate Training Program), and T32 AI060525-01A1 (Ruth L. Kirschstein National Service Award) supported this research. Research at Monash University was further supported by a grant from the Australian Research Council to the ARC Centre of Excellence in Structural and Functional Microbial Genomics.
We thank P. Hauer for supplying monoclonal antibodies against CPB and CPA and Jon Brazier for providing information regarding the BW strains.
Editor: D. L. Burns
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Al-Sheikhly, F., and R. B. Truscott. 1977. The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum. Avian Dis. 21:241-255. [PubMed] [Google Scholar]
- 2.Barker, I. K., A. A. Van Dreumel, and N. Palmer. 1993. The alimentary system, p. 237-247. In K. V. F. Jubb, P. C. Kennedy, and N. Palmer (ed.), Pathology of domestic animals, 4th ed. Academic Press, New York, NY.
- 3.Ba-Thein, W., M. Lyristis, K. Ohtani, I. T. Nisbet, H. Hayashi, J. I. Rood, and T. Shimizu. 1996. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J. Bacteriol. 178:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce, R. D. 1985. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 5:151-157. [DOI] [PubMed] [Google Scholar]
- 5.Bueschel, D. M., B. H. Jost, S. J. Billington, H. T. Trinh, and J. G. Songer. 2003. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121-129. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez Miyakawa, M. E., and F. A. Uzal. 2003. The early effects of Clostridium perfringens type D epsilon toxin in ligated intestinal loops of goats and sheep. Vet. Res. Commun. 27:231-241. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez Miyakawa, M. E., and F. A. Uzal. 2005. Morphologic and physiologic changes induced by Clostridium perfringens genotype A alpha toxin in the intestine of sheep. Am. J. Vet. Res. 66:251-255. [DOI] [PubMed] [Google Scholar]
- 8.Finnie, J. W. 2004. Neurological disorders produced by Clostridium perfringens type D epsilon toxin. Anaerobe 10:145-150. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, D. J., M. E. Fernandez-Miyakawa, S. Sayeed, V. Adams, R. Poon, J. I. Rood, F. A. Uzal, and B. A. McClane. 2006. Dissecting the lethality contributions of Clostridium perfringens genotype C toxins in the mouse intravenous injection model. Infect. Immun. 74:5200-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garmory, H. S., N. Chanter, N. P. French, D. Busechel, J. G. Songer, and R. W. Titball. 2000. Occurrence of Clostridium perfringens β2-toxin amongst animals determined using genotyping and subtyping PCR assays. Epidemiol. Infect. 124:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibert, M., C. Jolivet-Reynaud, and M. R. Popoff. 1997. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 203:65-73. [DOI] [PubMed] [Google Scholar]
- 12.Gkiourtzidis, K., J. Frey, E. Bourtzi-Hatzopoulou, N. Iliadis, and K. Sarris. 2001. PCR detection and prevalence of α, β, β2, ɛ, ι, and enterotoxin genes in Clostridium perfringens isolated from lambs with clostridial dysentery. Vet. Microbiol. 82:39-43. [DOI] [PubMed] [Google Scholar]
- 13.Itodo, A. E., A. A. Adesiyun, J. O. Adekeye, and J. U. Umoh. 1986. Toxin-genotypes of Clostridium perfringens strains isolated from sheep, cattle and paddock soils in Nigeria. Vet. Microbiol. 12:93-96. [DOI] [PubMed] [Google Scholar]
- 14.Keyburn, A. L., S. A. Sheedy, M. E. Ford, M. M. Williamson, M. M. Awad, J. I. Rood, and R. J. Moore. 2006. The alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74:6496-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence, G., and R. Cooke. 1980. Experimental pigbel: the production and pathology of necrotizing enteritis due to Clostridium welchii genotype C in the guinea-pig. Br. J. Exp. Pathol. 61:261-271. [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, C. J. 2000. Clostridial diseases, p. 131-143. In W. B. Martin and I. D. Aitkin (ed.), Diseases of sheep, 3rd ed. Blackwell Science, Oxford, United Kingdom.
- 17.McClane, B. A., and J. I. Rood. 2001. Clostridial toxins involved in human enteric and histotoxic infections, p. 169-209. In H. Bahl and P. Duerre (ed.), Clostridia: biotechnology and medical applications. Wiley-VCH, Weinheim, Germany.
- 18.McClane, B. A., F. A. Uzal, M. F. Miyakawa, D. Lyerly, and T. Wilkins. 2006. The enterotoxic clostridia, p. 698-752. In M. Dworkin, S. Falkow, E. Rosenburg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 4. Springer-Verlag, New York, NY. [Google Scholar]
- 19.Miyata, S., O. Matsushita, J. Minami, S. Katayama, S. Shimamoto, and A. Okabe. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J. Biol. Chem. 276:13778-13783. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani, K., H. I. Kawsar, K. Okumura, H. Hayashi, and T. Shimizu. 2003. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol. Lett. 222:137-141. [DOI] [PubMed] [Google Scholar]
- 21.Rood, J. I. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52:333-360. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai, J., and C. L. Duncan. 1978. Some properties of beta-toxin produced by Clostridium perfringens genotype C. Infect. Immun. 21:678-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayeed, S., M. E. Fernandez-Miyakawa, D. J. Fisher, V. Adams, R. Poon, J. I. Rood, F. A. Uzal, and B. A. McClane. 2005. Epsilon-toxin is required for most Clostridium perfringens genotype D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect. Immun. 73:7413-7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tschirdewahn, B., S. Notermans, K. Wernars, and F. Untermann. 1991. The presence of enterotoxigenic Clostridium perfringens strains in faeces of various animals. Int. J. Food Microbiol. 14:175-178. [DOI] [PubMed] [Google Scholar]
- 26.Uzal, F. A., and W. R. Kelly. 1998. Experimental Clostridium perfringens type D enterotoxemia in goats. Vet. Pathol. 35:132-140. [DOI] [PubMed] [Google Scholar]
- 27.Uzal, F. A., and R. B. Marcellino. 2002. Abstr. 6th Bienn. Cong. Anaerobe Soc. Amer. Anaerobe 8:168. [Google Scholar]
- 28.Waters, M., D. Raju, H. S. Garmory, M. R. Popoff, and M. R. Sarker. 2005. Regulated expression of the beta2-toxin gene (cpb2) in Clostridium perfringens type A isolates from horses with gastrointestinal diseases. J. Clin. Microbiol. 43:4002-4009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Wen, Q., and B. A. McClane. 2004. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 70:2685-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamagishi, T., Y. Gyobu, K. Sakamoto, S. Ishisaka, K. Saito, S. Morinaga, S. Katsuda, T. Umei, and K. Konishi. 1987. Response of ligated rabbit ileal loop to Clostridium perfringens type C strains and their toxic filtrates. Microbiol. Immunol. 31:859-868. [DOI] [PubMed] [Google Scholar]